Abstract
Changes in metabolic and myofilament phenotypes coincide in developing hearts. Posttranslational modification of sarcomere proteins influences contractility, affecting the energetic cost of contraction. However, metabolic adaptations to sarcomeric phenotypes are not well understood, particularly during pathophysiological stress. This study explored metabolic adaptations to expression of the fetal, slow skeletal muscle troponin I (ssTnI). Hearts expressing ssTnI exhibited no significant ATP loss during 5 minutes of global ischemia, while non-transgenic littermates (NTG) showed continual ATP loss. At 7 min ischemia TG-ssTnI hearts retained 80±12% of ATP vs. 49±6% in NTG (P<0.05). Hearts expressing ssTnI also had increased AMPK phosphorylation. The mechanism of ATP preservation was augmented glycolysis. Glycolytic end products (lactate and alanine) were 38% higher in TG-ssTnI than NTG at 2 min and 27% higher at 5 min. This additional glycolysis was supported exclusively by exogenous glucose, and not glycogen. Thus, expression of a fetal myofilament protein in adult mouse hearts induced elevated anaerobic ATP production during ischemia via metabolic adaptations consistent with the resistance to hypoxia of fetal hearts. The general findings hold important relevance to both our current understanding of the association between metabolic and contractile phenotypes and the potential for invoking cardioprotective mechanisms against ischemic stress.
Keywords: Troponin; Glycolysis; Adenosine Triphosphate (ATP); AMP Activated Protein Kinase, (AMPK); NMR Spectroscopy
INTRODUCTION
The understanding of switches in gene expression in the heart, either developmental or in response to stress, is just recently emerging to include the role of programmatic changes to the expression profiles of entire families of related genes. Such programmatic changes have been noted in the metabolic profiles of both the developing and stressed myocardium, which can coincide with changes in cardiac contractility and even chemical modification of the contractile proteins in the cardiomyocyte (1–5). The resulting phenotypes confer responses to stress that can be interpreted as either adaptive or maladaptive. Even much less understood, however, is the level of reciprocal adaptation between phenotypes of the metabolic and contractile activity. This study explores the adaptations in metabolic phenotype in the heart that are induced by switching of sarcomeric protein isoforms, thereby altering myofilament activity and sensitivity to Ca++, and the resulting stress response of the myocardium during ischemic insult.
In the adult cardiomyocyte, the isoform of the heterotrimeric sarcomeric protein, troponin, contains the cardiac variant of troponin I (cTnI), while the fetal and neonatal troponin contains slow skeletal muscle troponin I (ssTnI). Myofilaments regulated by ssTnI are characterized by an increased sensitivity to intracellular Ca++ in comparison to cTnI (6). Thus, hearts of transgenic (TG) mice expressing ssTnI, in place of the adult cTnI, demonstrate a constitutive increase in the myofilament response to Ca++. This ssTnI-TG mouse heart exhibits resistance to post-ischemic contractile dysfunction, which has been attributed to reduced phosphorylation and a consequential preservation of Ca++ sensitivity during reperfusion (7). What had remained unstudied, was the potential for a shift in metabolic phenotype to occur in response to expression of the fetal isoform, ssTnI, in the adult mouse heart and a contribution from metabolic protection to ischemic stress.
This study investigates the potential for metabolic adaptations in the phenotype of adult TG mouse hearts, expressing the fetal, ssTnI isoform, to mediate the response to ischemic stress. The normal metabolic response of the adult, mammalian heart to ischemia is very well characterized, and includes a shift away from the mitochondrial production of ATP, primarily via long chain fatty acid oxidation, to the anaerobic metabolism of glucose to produce ATP through glycolysis (8–11). However, the adult heart displays a limited capacity for sustaining anaerobic ATP synthesis during ischemic stress. In contrast, the fetal heart demonstrates a greater resistence to anaerobic conditions, with an increased level of glucose metabolism and glycolytic activity (12–15). The findings of the current study suggest a functional link between metabolic phenotype and myofilament activity, in which the expression of a fetal myofilament regulatory protein induces a metabolic profile that is consistent with the resistance of the fetal heart to hypoxia through augmented anaerobic energy production. A precedent is demonstrated for the metabolism of the heart to reciprocate to altered modes of myofilament activity, leading to remodeled energy synthesis pathways.
MATERIALS AND METHODS
Heart Model and Experimental Protocols
Two experimental groups of male mice (CS strain) were studied at 5 months of age: a transgenic mouse model of cardiac expression of slow skeletal muscle troponin I (TG-ssTnI) and nontransgenic littermates (NTG). The TG-ssTnI model has been previously described in detail (6,7). Hearts were excised from mice that were heparizined (50 U/10 g, i.p.) and anesthetized (ketamine, 80 mg/kg, plus xylazine, 12 mg/kg, i.p.). Isolated hearts were retrogradely perfused (60 mm Hg) with modified Krebs-Henseleit buffer (118.5 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4 and 1.2 mM KH2PO4) equilibrated with 95% O2/5% CO2, at 37°C, and containing 0.4 mM palmitate/ fatty acid free albumin complex (3:1 molar ratio) and 10 mM glucose. A water-filled latex balloon was fitted into the left ventricle and set to a diastolic pressure of 5 mmHg. Left ventricular developed pressure (LVDP) and heart rate (HR) were continuously recorded with a pressure transducer and digital recording system (Powerlab, AD Instruments, Colorado Springs, CO). Rate-pressure product (RPP) was calculated as the product of heart rate and developed pressure. Hearts were studied with or without zero flow, global ischemia at 37°C. All investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85–23, Revised 1985) and were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Groups of isolated hearts were subjected to separate protocols: 1) 31P NMR of high energy phosphate content in the intact heart during 25 minutes of normal perfusion followed by 10 minutes of global ischemia (NTG, n=6; TG-ssTnI, n=6); 2) Perfusion with 10 mM glucose plus 0.4 mM palmitate for 25 minutes followed by 2 minutes of zero flow global ischemia after which hearts were frozen in liquid nitrogen cooled tongs (NTG, n=8; TG-ssTnI, n=8); 3) Perfusion with 10 mM glucose plus 0.4 mM palmitate for 15 minutes of stabilization followed by perfusion with 10 mM [1,6-13C2] glucose plus 0.4 mM unlabeled palmitate for 10 minutes and then either immediate freezing (NTG, n=4; TG-ssTnI, n=4) or 5 minutes of global, zero flow ischemia after which hearts were frozen in liquid nitrogen cooled tongs (NTG, n=9; TG-ssTnI, n=9); 4) Similar perfusion and freeze clamping was performed with 5 minutes of ischemia for assay of P-AMPK/AMPK (ssTnI, n=5; NTG, n=6) and glycogen content (ssTnI, n=5; NTG, n=5), and glucose transporter expression and translocation (ssTnI, n=4; NTG, n=4). Additional hearts were excised from anesthetized mice and frozen, without perfusion, for baseline measurements of P-AMPK/AMPK (ssTnI, n=4; NTG = 6), glucose transporter expression and translocation (ssTnI, n=4; NTG = 4) and glycogen content (ssTnI, n=8; NTG = 6).
31P NMR Spectroscopy
Isolated, perfused mouse hearts were placed within a 10 mm broadband NMR probe that was situated in a 14.1 T NMR magnet for phosphorus-31 (31P) NMR spectroscopy to monitor myocardial ATP content before and during 10 minutes of global ischemia. 31P spectra were sequentially acquired in 2 minute intervals (ns= 64), using previously described pulse parameters, and corrected for partial saturation effects (16). Relative ATP content of the pre-ischemic and ischemic heart was determined by the integrated signal intensity of the β-phosphate resonance at −16 ppm, using NMR dedicated software (Topspin 2.0, Bruker Biospin) and expressed as a percent of pre-ischemic content.
Tissue Biochemistry
Tissue metabolites were extracted from frozen, powdered ventricular myocardium with 7% perchloric acid and neutralized with KOH (16). Tissue lactate content was assayed by UV spectrophotometric analysis (17). Alanine content was determined by 1H NMR relative to the signal from known lactate concentration from assays (18,19,20). Glycogen was extracted and content measured following digestion with amyloglucosidase and detection of glycosyl units, as previously described (21).
P-AMPK/AMPK ratios were determined from total ventricular homogenates (20–30 mg) using previously described methods to isolated ventricular proteins (22,23). Proteins (20–40 µg) were electrophoretically transferred to 0.20 µM PVDF for western blot. AMPKα activation was determined by probing the blot with the monoclonal antibody 40H9 (Cell Signaling) specific for AMPKα phosphorylated at Thr-172. Following washes and incubation with HRP labeled secondary, phosphorylated AMPKα was detected by enhanced chemiluminescence. The resultant blot was subsequently stripped and re-probed with a polyclonal antibody against total AMPKα (Cell signaling). Densities were quantified using Image Quant TL software. To ensure samples were loaded within the linear range for quantitation, a linear loading control was included. Ventricular myocardium proteins from NTG animals were loaded onto 1D-PAGE in a linear range from 0 through 45 µg total protein. Western blot analysis was performed as described above for both APMKα and P-AMPK and data were fit to a linear regression line.
Assays for glucose transporter expression, of GLUT1 and GLUT4 and of GLUT4 translocation, were performed on samples from frozen myocardium that was processed and fractionated, as previously described (23–25). The primary antibodies used were: mouse anti-Na+/K+ATPase 1:3,000 (upstate), rabbit anti-Glut-4 1:5,000 (Millipore), and mouse anti- Glut-1 1:1,000 (Abcam). The secondary antibodies used were goat anti-mouse IgG conjugated to peroxidase 1:100,000 (Sigma) and goat anti-rabbit IgG conjugated to peroxidase 1:40,000 (GE Healthcare). Western blots were developed with ECL-Plus detection reagents and Hyperfilm both from GE Healthcare. Hyperfilm was imaged with an Imagescanner III from GE Healthcare, and subsequently analyzed with ImageQuant TL (GE Healthcare) software to determine optical density values of bands for relative comparisons.
Protein levels for glycolytic proteins were determined as previously described (26). Whole-cell protein extracts were resolved by SDS-PAGE, and after transfer to polyvinylidene difluoride, membranes were probed with antibodies against hexokinase (Millipore, Billerica, MA), PFK-1 cardiac, PFK-2, PFK-2 cardiac, FBPase (Santa Cruz Biotechnology, Santa Cruz, CA) and tubulin (Sigma-Aldrich, St. Louis, MO) was used as a loading control. Detection and quantification were performed by measuring fluorescently labeled secondary antibodies using the Odyssey Infrared Imaging System and accompanying software (version 3.0; LI-COR Biosciences, Lincoln, NE). Values were corrected for loading to tubulin and normalized to wild-type controls without ischemia (a.u. = 1.0). Protein concentrations were determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL).
RNA extraction and quantitative RT-PCR were performed to detect transcript levels for glycolytic enzymes as previously described (27). Total RNA was extracted from hearts with TRizol reagent (Invitrogen Corporation, Carlsbad, CA) and 3 µg of RNA were reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) (28). The resulting cDNA were subjected to quantitative real-time RT-PCR. All reactions were performed in triplicate. Relative quantification was performed by interpolating crossing point data on an independent standard curve. Product size was confirmed by melting curve and agarose gel electrophoresis with ethidium bromide staining. Data were corrected for loading relative to the levels of the invariant transcript Ppia (NM_018874) and normalized to wild-type controls without ischemia (a.u. = 1.0).
NMR Spectroscopy of Tissue Extracts
Lyophilized tissue extracts were reconstituted in 0.5 mL D2O and proton (1H) NMR spectra were collected using a 5 mm 1H probe (Bruker Instruments, Billerica, MA) as described elsewhere (19,20). 1H spectra of the 3-carbon methyl group resonances of lactate and alanine revealed the fractional 13C enrichment of each metabolite from the observed splitting of the proton resonances due to proton-carbon-13 coupling, distinguishing 12CH3 from 13CH3 groups, as previously described (18–20).
Statistical Analysis
Inter-group statistics were analyzed using Student t-test for comparison of two mean values and one-way ANOVA analysis with the Tukey post-test for comparison of multiple groups means. Statistical significance was established at 5% probability (P < 0.05). All reported values are reported as averages ± SEM.
RESULTS
ATP content in ischemic myocardium
Prior to ischemia, contractile function of isolated hearts was similar for both the transgenic and NTG groups: ssTnI, RPP = 37,015 ± 4524; NTG, RPP = 40,524 ± 8652. 31P NMR spectra (Figure 1) displayed relative levels of phosphocreatine (PCr) and ATP at baseline that were also similar between the hearts of TG-ssTnI and NTG mice, NTG PCr/ATP = 1.73 ± 0.08; TG-ssTnI PCr/ATP = 1.74 ± 0.09. Consistent with many previous studies of ischemic hearts, PCr content was rapidly depleted within the first 3 min of ischemia in both groups of hearts (29,30). However, sequential 31P NMR spectra of the pre-ischemic and ischemic hearts revealed an attenuated loss of ATP in hearts expressing ssTnI during global ischemia as compared to NTG. ATP content was retained at preischemic levels for the first 5 minutes of global ischemia in hearts of TG-ssTnI mice, whereas NTG hearts showed the expected, progressive loss of ATP from over the entire 10 minutes of ischemia. Figure 2 displays ATP loss during ischemia in both groups. In addition to delayed ATP loss during the first 5 minutes of ischemia, TG-ssTnI hearts retained higher ATP content over the first 7 minutes of ischemia.
Figure 1. 31P NMR of high energy phosphates.
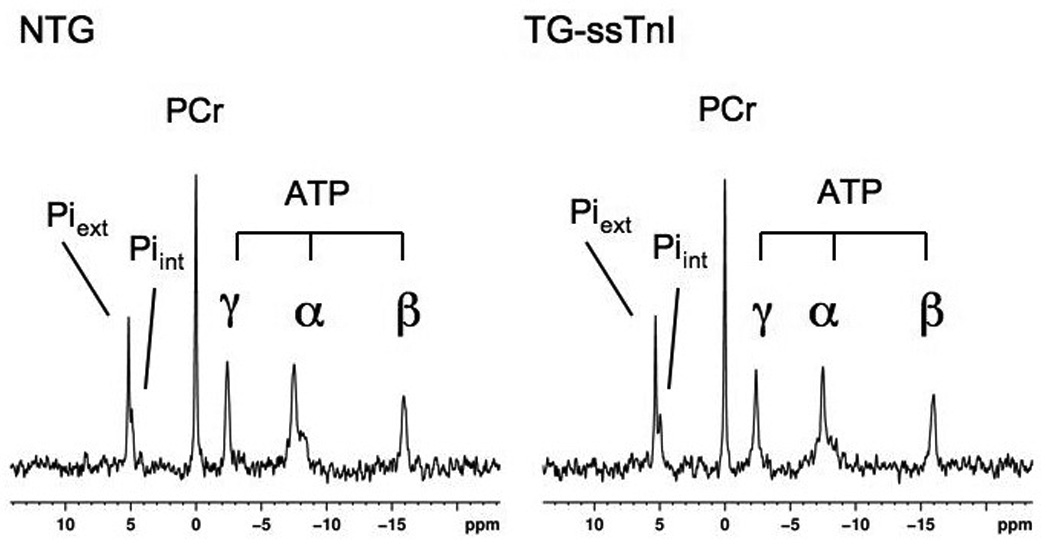
31P NMR spectra display relative high energy phosophate content in perfused hearts of non-transgenic littermates (NTG, left) and ssTnI expressing hearts of transgenic mice (TG-ssTnI, right). Piext, inorganic phosphate signal from extracellular buffer; Piint, intracellular inorganic phosphate; PCr, phosphocreatine; ATP, adenosine triphosphate groups.
Figure 2. ATP loss during ischemia.
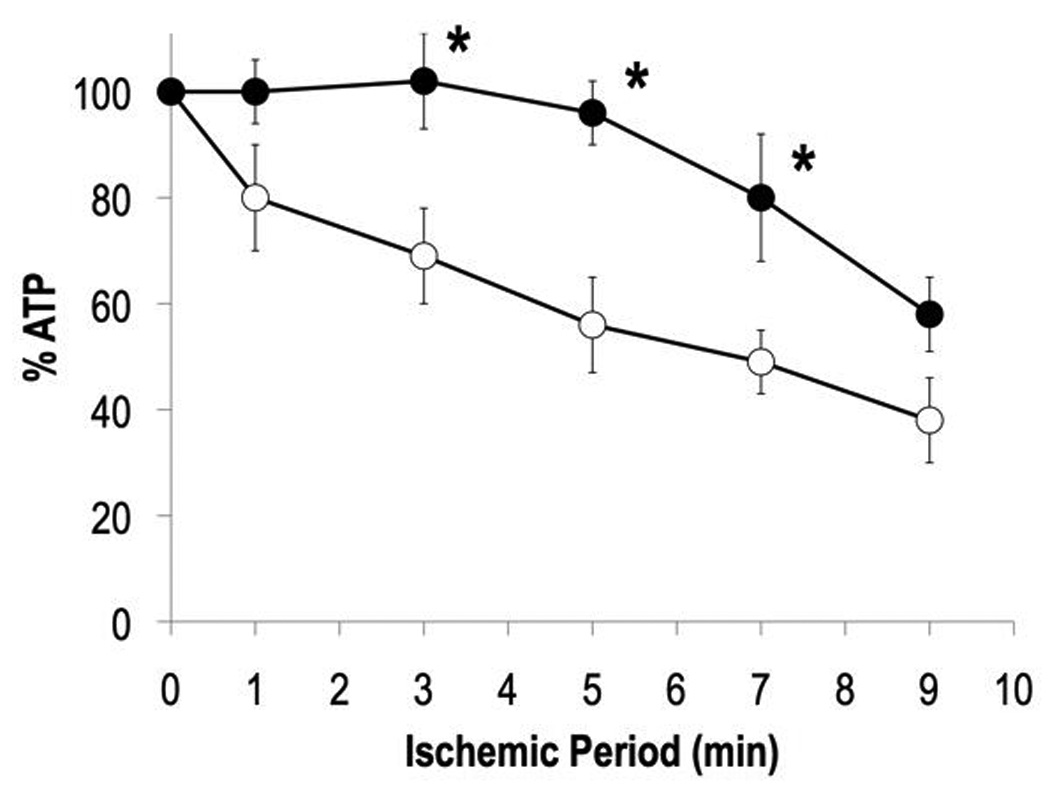
Note attenuated ATP loss in heart of TG-ssTnI mouse (mean ± SE). Open circles, NTG hearts; Filled circles, TG-ssTnI hearts. *, P< 0.05 versus NTG.
The improved levels of ATP that persisted for the majority of the 10 minute ischemic period indicate a metabolic protection from energy depletion during ischemia and thus a reduced severity of insult. Preservation of ATP levels implies improved ability to support cell function and maintenance during ischemia. The consequential reduction in the severity of ischemic insult in the ssTnI expressing heart is likely to contribute to the previously reported improvement in post-ischemic contractile recovery during reperfusion of the ssTnI expressing heart (7).
Glycolysis in the Ischemic Heart
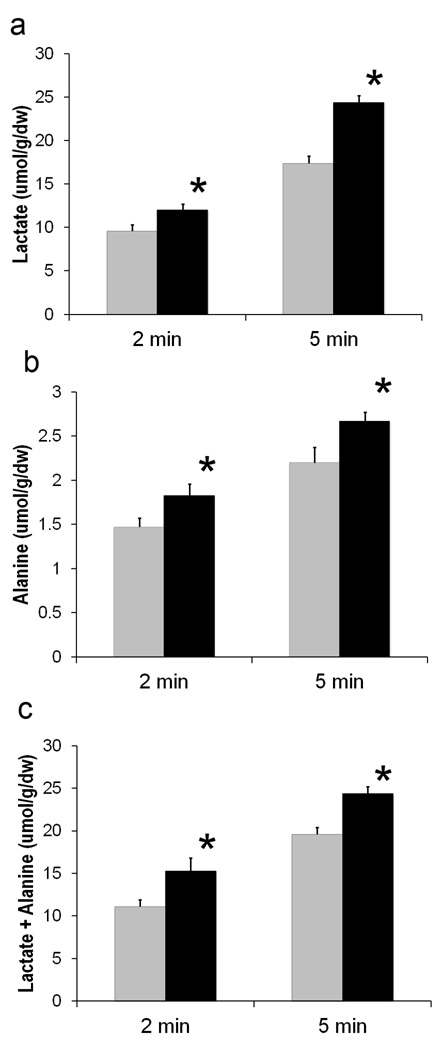
The protocol for zero-flow, global ischemia enabled the relative glycolytic activity to be assessed in the ischemic hearts from the tissue content of both alanine and lactate (18,31). Figure 3 displays values for glycolytic end product accumulation in both TG-ssTnI and NTG hearts at 2 and 5 minutes of ischemia, both time points corresponding to attenuated ATP loss (Figure 2). At both 2 and 5 minutes of ischemia, tissue levels of lactate and alanine were elevated in ischemic ssTnI hearts over those of ischemic NTG hearts. Total accumulation of these glycolytic products was also elevated in ssTnI hearts over NTG hearts by 38% at 2 minutes of ischemia and 27% at 5 minutes of ischemia (Figure 3).
Figure 3. Elevated accumulation of glycolytic end products in TG-ssTnI hearts during ischemia.
a) Lactate accumulation at 2 and 5 minutes of ischemia. b) Alanine accumulation at 2 and 5 minutes of ischemia. c) Total end product (lactate + alanine) accumulation at 2 and 5 minutes of ischemia. All values shown as mean ± SE. Gray bar, NTG; Black bar, TG-ssTnI. *, P< 0.05 versus NTG.
Resonance signals within 1H NMR spectra of tissue extracts distinguished between methyl group protons bonded to 12C and 13C (Figure 4). 13C enrichment of lactate and alanine after 5 min of ischemia, as shown in Table 1, were also elevated in the ssTnI hearts relative to NTG during ischemia. The increased isotopic enrichment from the metabolism of [1,6-13C2] glucose, indicates a greater distribution of exogenous glucose being metabolized in ssTnI hearts than in NTG hearts, which instead metabolized a greater percentage of unlabeled glucose units derived from the endogenous glycogen supply.
Figure 4. Proton (1H) NMR spectra of lactate and alanine content in acid extract of heart.
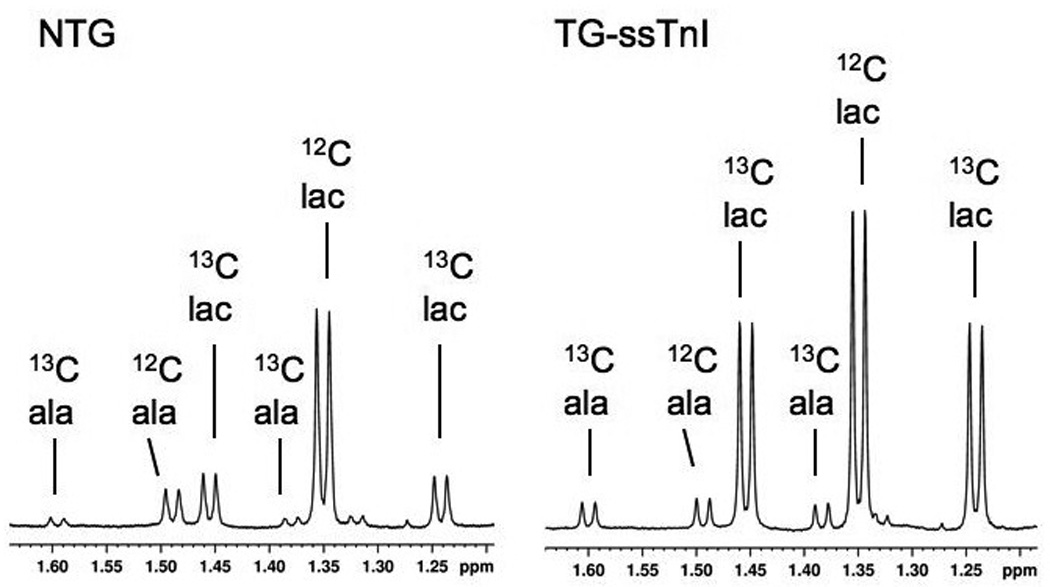
Spectra are shown from non-transgenic heart (NTG, left) and a ssTnI expressing heart (TG-ssTnI) subjected to 5 minutes ischemia. Doublet resonance signals from protons of 3-carbon methyl groups in lactate (lac) and alanine (ala) arise from 1H-1H J-coupling between methyl and vicinyl protons. Methyl group 1H signals from 13C enriched groups are split by 1H -13C J-coupling (13C lac; 13C ala), and distinguish products of [1,6-13C2] glucose metabolism from unenriched metabolites (12C lac; 12C ala).
TABLE 1.
13C enrichment percentages and content of 13C enriched glycolytic end products from [1,6-13C2] glucose at 5 minutes of ischemia.
| Metabolite | 13C% | NTG [µmole /g dry wt] |
13C% | ssTnI [µmole /g dry wt] |
|---|---|---|---|---|
| Lactate | 29 ± 3 | 5.24 ± 0.60 | 44 ± 6 * | 9.43 ± 1.45 * |
| Alanine | 29 ± 5 | 0.65 ± 0.11 | 46 ± 6 * | 1.16 ± 0.14 * |
P<0.05 versus NTG.
Despite an appreciable fraction of unlabeled glucose derived from endogenous glycogen supporting glycolysis in the ischemic hearts, the quantitative differences in 13C enriched lactate and alanine between ssTnI and NTG hearts (Table 1 and Figure 3) are similar to the difference between total lactate and alanine accumulation in these two groups. The difference in mean lactate content between ssTnI and NTG was 4.3 µmoles units/g dry weight, while the difference in mean 13C lactate was a strikingly similar 4.2 µmoles/g dry weight. The difference in mean alanine content was 0.46 µmoles/g dw, while the difference in 13C alanine content was only 0.05 µmoles/g dry weight higher at 0.51. Thus, the balance of 13C enriched alanine and lactate that was increased in the ssTnI hearts appears to account for the total elevated content of these metabolites in ssTnI hearts. From these data, the elevated levels of glycolytic end products observed in the ssTnI hearts can be attributed almost exclusively to an increased recruitment of additional exogenous glucose, above the combined contributions to glycolysis from both exogenous glucose and endogenous glycogen in NTG hearts.
In contrast, normal perfusion conditions did not reveal any difference in 13C-enrichment of either lactate or alanine between NTG and TG-ssTnI hearts (NTG lactate = 30 ± 8%; TG-ssTnI lactate = 44 ± 6; NTG alanine = 43 ± 5; TG-ssTnI alanine = 51 ± 10). Therefore, the response of increased glucose metabolism in TG-ssTnI hearts was specific to ischemic stress. While lactate content was similar in normally perfused NTG and TG-ssTnI, alanine content in the TG-ssTnI was elevated (NTG lactate = 4.07 ± 0.65 µmol/g/dw; TG-ssTnI lactate = 3.49 ± 0.61; NTG alanine = 1.52 ± 0.08; TG-ssTnI alanine = 3.04 ± 0.56). However, while this increased alanine suggests a different balance between glycolytic activity and glucose oxidation, glycolytic activity cannot be inferred from mere content of end products during normal perfusion and with tissue washout, unlike the condition of zero flow ischemia.
Despite obvious differences in glycolytic metabolism during ischemia between NTG and TG-ssTnI hearts, RNA levels and protein content of key glycolytic enzymes were not different between groups (See Supplemental Data Figure 1S). However, regulatory mechanisms controlling glycolytic enzyme activity would not be reflected by these content measurements.
AMPK phosphorylation and tissue glycogen
To examine a potential source for increased glucose metabolism, particularly that of exogenous glucose, in the ischemic ssTnI hearts, levels of AMPK activation were examined via the ratio of phosphorylated AMPK to total AMPK (P-AMPK/AMPK). Figure 5 displays the comparison of P-AMPK/AMPK levels in ssTnI and NTG hearts frozen at baseline conditions, immediately following cardiectomy, and in isolated, perfused hearts frozen at 5 minutes of zero flow ischemia. As anticipated, the percentage of P-AMPK was elevated by ischemia in both groups. Importantly, hearts expressing ssTnI had markedly higher AMPK phosphorylation at baseline (Figure 5). However, ischemia induced increases to similar levels of P-AMPK/AMPK in both groups (Figure 5). This finding suggests that higher levels of AMPK activation in ssTnI hearts prior to ischemia may contribute to the observed increases in the glycolysis and glucose metabolism upon the onset of ischemia but not by 5 min of ischemia.
Figure 5. Phosphorylation of AMPK.
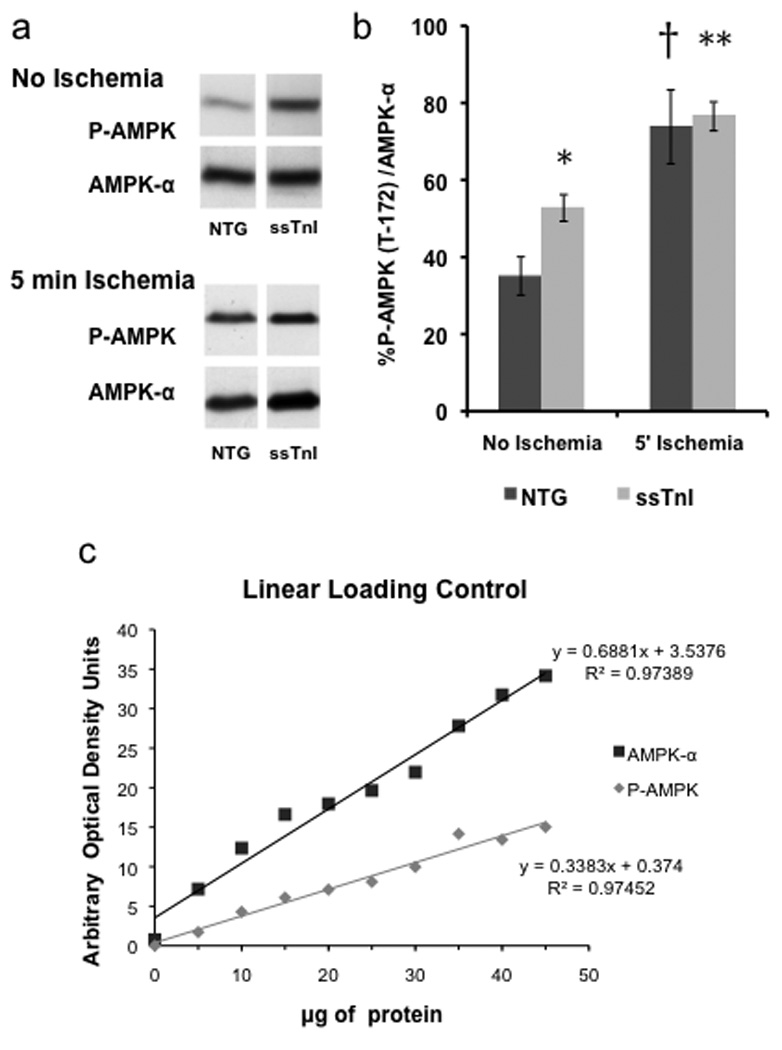
a) Western blots of phosphorylated AMPK (P-AMPK) and total AMPK from hearts at baseline (no ischemia) and during ischemia are shown. b) Bar graph displays percentages of phosphorylated AMPK in non-transgenic (NTG) and ssTnI expressing hearts (TG-ssTnI) harvested under baseline conditions and after 5 minutes of ischemia (mean ± SE). Dark bar, nontransgenic (NTG); Gray bar, TG-ssTnI (ssTnI). *, P< 0.05 versus NTG. †, P<0.05 versus NTG No Ischemia. **, P<0.01 versus ssTnI No Ischemia. c) Linear loading control for both AMPK-α and P-AMPK. All samples were loaded within this linear range (20–40 µg of ventricular proteins).
Similar glycogen levels in TG-ssTnI and NTG hearts, both at baseline and after ischemia, are consistent with the additional anaerobic glycolytic metabolism, in ssTnI expressing hearts, being derived from exogenous glucose, as shown above. Glycogen content at baseline and after 5 minutes of ischemia were similar between the ssTnI and NTG hearts: NTG baseline glycogen = 146 ± 17 µmoles/g/dw glycosyl units; ssTnI baseline = 131 ± 23; NTG ischemic glycogen = 32 ± 12; ssTnI ischemic = 33 ± 9. As expected, glycogen content was significantly lowered by ischemia in both groups.
Although the contribution of exogenous glucose was markedly increased in ischemic ssTnI hearts over that of NTG, no difference in the expression levels of either the glucose transporters, GLUT-1 or GLUT-4, was observed between groups at baseline preischemic or ischemic conditions. No difference in GLUT-4 translocation was detected between ssTnI and NTG hearts (See Supplemental Data Figure 2S).
DISCUSSION
Earlier work by Arteaga, et al (7) tested the hypothesis that hearts with increased myofilament response to Ca2+ are less susceptible to damage from ischemia/reperfusion (I/R) in a transgenic (TG) mouse heart that expresses the embryonic/neonatal isoform ssTnI in place of cTnI. TG ssTnI hearts demonstrated a significant reduction in postischemic contractile dysfunction (7). While this beneficial effect of ssTnI expression in response to I/R was initially interpreted solely with respect to the known increased myofilament sensitivity to Ca2+ in the TG ssTnI hearts, this current study demonstrates that expression of ssTnI in these hearts is also related to changes in metabolic phenotype that are protective during the ischemic phase of the I/R protocol. However, no difference in metabolism was evident in the absence of ischemia, and the metabolic adaptations to expression of ssTnI are actuated in response to ischemic stress. Our findings indicate that the delay in ATP depletion in the ischemic TG ssTnI hearts compared to controls may have been a significant factor in protecting the hearts in the study by Arteaga et al (7) by shortening the exposure of the myocardium to low concentrations of ATP.
Surprisingly, TG ssTnI hearts exhibited virtually no ATP loss during the first five minutes of the ischemic insult, as shown by 31P NMR spectroscopy of isolated perfused hearts during zero-flow, normothermic ischemia. In contrast, and as expected, hearts of NTG age-matched littermates lost ATP at a constant rate during the 10 minute ischemic episode. Of course, glycolytic activity can not be sustained indefinitely during zero flow ischemia, and the eventual accumulation of end products to inhibitory levels resulted in ATP loss during prolonged ischemia in both groups. Nevertheless, due to an initial increased rate of glycolysis, the resulting retarded ATP loss during ischemia appears to preserve the energy content of the cardiomyocyte for a prolonged period, reducing the severity of the ischemic insult; in a sense, “buying time” for the ischemic myocardium (32). These data show that ssTnI expression in adult mouse hearts induces resistance to ATP loss during the early phase of an ischemic insult.
Work by He, et al (33) clearly demonstrates that mutations in another regulatory protein in the sarcomere, troponin T, that are associated with contractile dysfunction can also have negative effects on the energetic state of the whole heart. However, the current study indicates that alterations in the regulatory proteins in the sarcomere that have no discernable effect on baseline energetics, as in the case of ssTnI isoform expression, can also be associated with improved energetic status under extreme conditions of pathophysiological stress. Regarding TnI, an important observation by Day, et al (34) of hearts that possess a substitution of a single, pH sensitive histidine residue in the adult cTnI, is that this mutation confers enhanced cardiac performance under acute stress also without differences in high energy phosphate content. The significance of the normal energetic state, despite this mutation in cTnI that mimics the histidine button of the fetal TnI isoform, is that the energetic efficiency of contractile function is therefore also improved during ischemia/reperfusion. Therefore, while changes in the regulatory proteins of the sarcomere can improve contractility and the energetic cost of contraction, the current study, demonstrates direct and profound effects of fetal ssTnI expression on the actual content of ATP in the cell that was preserved during ischemic stress. These results advance our understanding beyond the important finding that energetic efficiency of contraction is influenced by the sensitivity of TnI to the intracellular milieu, to a new level of realization that the expression of these regulatory proteins can have reciprocal effects on the actual metabolic activity of the cardiomyocyte. Our results also extend data reported Rice, et al demonstrating the influence of expression of a mutant sarcomeric protein, in their case cardiac TnT, that induced remodeling of another distant pathway, control of intracellular Ca2+ fluxes (35).
The novelty of this current study is that not only was the energetic state of the ischemic heart preserved, albeit transiently, in hearts expressing ssTnI, but that this preservation of ATP content is linked to increased anaerobic glycolysis. Evidence of glycolytic activity in the ischemic tissue content of the metabolites of the end-product pyruvate that accumulate in hypoxic and ischemic myocardium, alanine and lactate (18,19,31), were elevated in TG ssTnI hearts over NTG at both 2 and 5 minutes of ischemia (Figure 3). This increase in glycolytic production of alanine and lactate corresponds to the time course of elevated ATP content in TG ssTnI hearts versus NTG hearts during ischemia (Figure 2). Because the protocol employed zero flow ischemia, the increased accumulation of glycolytic end products in the transgenic ssTnI mouse hearts indicates that the preservation of ATP content during ischemia is sustained by increased glycolytic rates. After the initial 5 minutes of ischemia ATP loss became significant in TG-ssTnI hearts. This loss of ATP coincides with the time frame for glycolytic end product accumulation, which became similar between groups after 5 minutes of ischemia, and is consistent with the anticipated, eventual inhibition of glycolytic activity in the ischemic tissue. Interestingly, the additional glycolytic metabolism observed in the TG-ssTnI hearts over that of the NTG hearts, can be attributed to increased metabolism of exogenous glucose and not endogenous glucosyl units from glycogen stores. Thus, the expression of ssTnI is the heart is associated with increased glucose metabolism during ischemic stress.
These findings demonstrate a resistance to ischemic stress through anaerobic ATP production in hearts expressing this fetal isoform of troponin I. The ssTnI hearts either exhibit increased glycolytic capacity, enabling augmented glycolytic ATP production prior to the eventual inhibition by negative feedback of end products, or the glycolytic enzymes are less susceptible to end product inhibition. In either case, the expression of the fetal/neonatal isoform of cTnI, ssTnI, induces the metabolic phenotype of enhanced glycolysis and improved ATP content during ischemic stress. The increased capacity for glycolysis, even if transient during the ischemic event, provides a protective metabolic response. However, the most intriguing aspect of these findings is that the expression of ssTnI induces a metabolic phenotype, of increased glycolytic capacity, that is consistent with that of the metabolic phenotype of the fetal/neonatal cardiomyoyte (12–15,36,37).
Elevated levels of phosphorylated AMPK were detected in hearts expressing ssTnI. However, the similarity of baseline PCr/ATP ratio within these hearts, versus that of NTG hearts, does not suggest that AMPK activation resulted from chronically elevated AMP (38,39). The acute drop in bioenergetic potential in both groups of hearts during ischemia produced similarly elevated levels of P-AMPK, and thus belied the difference in AMPK activation prior to ischemia. Thus, the initial preservation of ATP content during ischemia, which appears to be supported by augmented glycolytic ATP synthesis during the first few minutes of ischemia in the hearts of TG-ssTnI mice, may be more closely linked to the baseline, preischemic levels of AMP activation. While the increased glucose metabolism is consistent with elevated P-AMPK, neither glucose transporter translocation nor glycogen content, both reported consequences of AMPK activation, were altered in the TG-ssTnI hearts (40). However, the near exclusive use of exogenous glucose to account for the increased glycolytic activity during ischemia is, in fact consistent with limited glucose storage into glycogen that is a response to AMPK activation. Thus, elevated levels of P-AMPK/AMPK at baseline in TG-ssTnI may have contributed to the availability of exogenous glucose to fuel glycolysis during ischemia as a result of limited glucose storage in TG-ssTnI hearts (41).
Conclusions
The response to ischemic stress in adult mouse hearts with expression of the fetal, ssTnI isoform, is a transient increase in anaerobic glycolysis during the initial minutes of the insult. This metabolic phenotype affords some measure of protection against the deleterious consequences of ATP loss and energetic supply during ischemia, and is reminiscent of the greater reliance on glycolytic metabolism and comparative resistance to hypoxia in the fetal heart (12–15,32). The findings elucidate, at least in part, the mechanism responsible for improved contractile recovery during reperfusion following ischemia in these TG-ssTnI mouse hearts (7). Interestingly, the increased component of glycolytic activity was supported, almost exclusively, by exogenous glucose, as opposed to the available endogenous glucose supply in the form of glycogen. While a significant difference in the metabolic response to ischemic stress was obviated in these studies of hearts from TG-ssTnI mice as compared to hearts of non-transgenic littermates, no noticeable distinctions in the energetic state were evident in the normoxic hearts prior to ischemia. Indeed, PCr/ATP levels were very similar between the TG-ssTnI and nontransgenic hearts. However, elevated levels of AMPK phosphorylation at baseline, despite no evidence of elevated AMP in the normoxic ssTnI heart, indicate that a fundamental difference in the metabolic reactivity, and potentially metabolic signaling, is induced by ssTnI expression. However, given that ATP loss was significantly attenuated in the TG-ssTnI hearts, perhaps even minimized, then the relative level of P-AMPK/AMPK to the ATP content of the heart was disproportionably higher in the TG-ssTnI hearts. The provocative findings of an increased anaerobic response for ATP generation during ischemia and elevated levels of AMPK phosphorylation in hearts expressing the fetal ssTnI, suggest a heightened metabolic response to stress due to changes in a regulatory sarcomeric protein that should be subject to further investigation.
Highlights.
Isoform changes in myofilament proteins induce changes in cardiac metabolic phenotype
Expression of the ssTnI, in place of adult cTnI, induces metabolic protection to ischemic stress
Adult mouse hearts expressing ssTnI exhibit augmented glycolytic ATP production during ischemia
Expression of fetal ssTnI induces resistance to hypoxia, due to ischemia, similar to fetal hearts
Supplementary Material
Glucose transporter content of GLUT-1 and GLUT-4 and of GLUT-4 translocation were all similar in both transgenic ssTnI expressing mouse hearts (TG-ssTnI, n=4) and non-transgenic littermate hearts (NTG, n=4). GLUT-1 and GLUT-4 signals were normalized to Na+/K+ATPase signals. Representative Western blots are shown above bar graph representing each group. NTG, non-transgenic; TG-ssTnI, transgenic. A. GLUT-1 abundance in whole mouse myocardium. B. GLUT-4 abundance following fractionation of myocardium into pre-fractionation pellet containing both surface membranes plus intracellular membranes (P2) and post-fractionation pellet containing only sarcolemmal membrane (P3).
a) Relative mRNA levels of glycolytic enzymes relative to the invariant Ppia gene and normalized to NTG no ischemia (mean ± SE; n = 5 per group). Gene names are presented in Table S1. b) Representative western blot images for key glycolytic enzymes at baseline (no ischemia) and during ischemia. HKII, hexokinase (Hk2); PFK-1, phosphofructokinase, muscle (Pfkm); PFK-2 car, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 heart (Pfkfb2); FBPase, fructose bisphosphatase 2, muscle (Fbp2). c) Protein content of key glycolytic enzymes from Western blot normalized to NTG-no ischemia (mean ± SE; n = 4 per group). No statistically significant differences reported.
Acknowledgments
ROLE OF THE FUNDING SOURCE
Supported by NIH Grants 2PO1HL062426, RO1 HL22231, and RO1 HL62702. The funding source played no role in the production of this report.
Abbreviations
- TG
transgenic
- NTG
nontransgenic
- ppm
parts per million
- PCr
phosphocreatine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have no disclosures to report.
REFERENCES
- 1.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 3.Du J, Nan C, Huang JJ, Zhang C, Liu J, Jia P, Abers M, Huang XP. Functional characterization of mouse fetal TnI gene promoters in myocardial cells. J Biomed Sci. 2008;15:605–613. doi: 10.1007/s11373-008-9246-y. [DOI] [PubMed] [Google Scholar]
- 4.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardio. 2008;45:185–192. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann NY Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H2183–H2192. doi: 10.1152/ajpheart.00520.2005. [DOI] [PubMed] [Google Scholar]
- 8.Pauly DF, Kirk KA, McMillin JB. Carnitine palmitoyltransferase in cardiac ischemia: a potential site for altered fatty acid metabolism. Circ Res. 1991;68:1085–1094. doi: 10.1161/01.res.68.4.1085. [DOI] [PubMed] [Google Scholar]
- 9.Apstein CS. Increased glycolytic substrate protection improves ischemic cardiac dysfunction and reduces injury. Am Heart J. 2000;139:S107–S114. doi: 10.1067/mhj.2000.103920. [DOI] [PubMed] [Google Scholar]
- 10.Lopaschuk G. Regulation of carbohydrate metabolism in ischemia and reperfusion. Am Heart J. 2000;139:S115–S119. doi: 10.1067/mhj.2000.103919. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski ED, Kudej RK, White LT, O'Donnell JM, Vatner SF. Mitochondrial preference for short chain fatty acid oxidation during coronary artery constriction. Circulation. 2002;105:367–372. doi: 10.1161/hc0302.102594. [DOI] [PubMed] [Google Scholar]
- 12.Hoerter J. Changes in the sensitivity to hypoxia and glucose deprivation in the isolated perfused rabbit heart during perinatal development. Pfugers Arch. 1976;363:1–6. doi: 10.1007/BF00587394. [DOI] [PubMed] [Google Scholar]
- 13.Hoerter J, Opie LH. Perinatal changes in glycolytic function in response to hypoxia in the incubated or perfused rat heart. Biol Neonate. 1978;33:144–161. doi: 10.1159/000241064. [DOI] [PubMed] [Google Scholar]
- 14.Ascuitto RJ, Ross-Ascuitto NT. Substrate metabolism in the developing heart. Semin Perinatol. 1996;20:542–563. doi: 10.1016/s0146-0005(96)80068-1. [DOI] [PubMed] [Google Scholar]
- 15.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–666. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewandowski ED. Nuclear magnetic resonance evaluation of metabolic and respiratory support of workload in intact rabbit hearts. Circ Res. 1992;70:576–582. doi: 10.1161/01.res.70.3.576. [DOI] [PubMed] [Google Scholar]
- 17.Gutmann I, Wahlefeld AW. L-(+)-Lactate. Determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1994. pp. 1464–1468. [Google Scholar]
- 18.Lewandowski ED, Johnston DL, Roberts R. Effects of inosine on glycolysis and contracture during myocardial ischemia. Circ Res. 1991;68:578–587. doi: 10.1161/01.res.68.2.578. [DOI] [PubMed] [Google Scholar]
- 19.Damico LA, White LT, Yu X, Lewandowski ED. Chemical versus isotopic equilibrium and the metabolic fate of glycolytic end products in the heart. J Mol Cell Cardiol. 1996;28:989–999. doi: 10.1006/jmcc.1996.0092. [DOI] [PubMed] [Google Scholar]
- 20.Lewandowski ED. Metabolic heterogeneity of carbon substrate utilization in mammalian heart: NMR determinations of mitochondrial versus cytosolic compartmentation. Biochemistry. 1992;31:8916–8923. doi: 10.1021/bi00152a031. [DOI] [PubMed] [Google Scholar]
- 21.Taegmeyer H, Roberts AF, Raine AE. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J Am Coll Cardiol. 1985;6:864–870. doi: 10.1016/s0735-1097(85)80496-4. [DOI] [PubMed] [Google Scholar]
- 22.Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983;168:123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- 23.Abel ED, Graveleau C, Betuing S, Pham M, Reay PA, Kandror V, Kupriyanova T, Xu Z, Kandror KV. Regulation of insulin-responsive aminopeptidase expression and targeting in the insulin-responsive vesicle compartment of glucose transporter isoform 4-deficient cardiomyocytes. Mol Endocrinol. 2004;18:2491–2501. doi: 10.1210/me.2004-0175. [DOI] [PubMed] [Google Scholar]
- 24.Warren CM, Arteaga GM, Rajan S, Ahmed RP, Wieczorek DF, Solaro RJ. Use of 2-d dige analysis reveals altered phosphorylation in a tropomyosin mutant (glu54lys) linked to dilated cardiomyopathy. Proteomics. 2008;8:100–105. doi: 10.1002/pmic.200700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 26.Wende AR, Soto J, Olsen CD, Pires KM, Schell JC, Larrieu-Lahargue F, Litwin SE, Kakoki M, Takahashi N, Smithies O, Abel ED. Loss of bradykinin signaling does not accelerate the development of cardiac dysfunction in type 1 diabetic akita mice. Endocrinology. 2010;151(8):3536–3542. doi: 10.1210/en.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banke NH, Wende AR, Leone TC, O’Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARα. Circ Res. 2010;107(2):233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED. Cardiac hypertrophy caused by PPARγ agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology. 2007;148(12):6047–6053. doi: 10.1210/en.2006-1559. [DOI] [PubMed] [Google Scholar]
- 29.Lewandowski ED, Devous MD, Nunnally RL. High energy phosphates and function in isolated, working rabbit hearts. Am J Physiol Heart Circ Physiol. 1987;253:H1215–H1223. doi: 10.1152/ajpheart.1987.253.5.H1215. [DOI] [PubMed] [Google Scholar]
- 30.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995;91:2071–2079. doi: 10.1161/01.cir.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 31.Taegtmeyer H, Peterson MB, Ragavan VV, Ferguson AG, Lesch M. De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium. J Biol Chem. 1971;252:5010–5018. [PubMed] [Google Scholar]
- 32.Vanoverschelde JL, Janier MF, Bakke JE, Marshall DR, Bergmann SR. Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol. 1994;267:H1785–H1794. doi: 10.1152/ajpheart.1994.267.5.H1785. [DOI] [PubMed] [Google Scholar]
- 33.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J. 2007;93:1834–1844. doi: 10.1529/biophysj.107.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D'Alecy LG, Ingwall JS, Metzger JM. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nature Med. 2006;12:181–189. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 35.Rice R, Guinto P, Dowell-Martino C, He H, Hoyer K, Krenz M, Robbins J, Ingwall JS, Tardiff JC. J Mol Cell Cardiol. 2010;48:979–988. doi: 10.1016/j.yjmcc.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin J, O’Donnell JM, White LT, Hajjar RJ, Lewandowski ED. Postnatal expression and activity of the 2-oxoglutarate malate carrier in intact hearts. Am J Physiol: Cell Physiol. 2000;279:C1704–C1709. doi: 10.1152/ajpcell.2000.279.6.C1704. [DOI] [PubMed] [Google Scholar]
- 37.Yatscoff MA, Jaswal JS, Grant MR, Greenwood R, Lukat T, Beker DL, Rebeyka IM, Lopaschuk GD. Myocardial hypertrophy and the maturation of fatty acid oxidation in the newborn human heart. Pediatr Res. 2008;64:643–647. doi: 10.1203/PDR.0b013e318184d281. [DOI] [PubMed] [Google Scholar]
- 38.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 39.Frederich M, Balschi JA. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- 40.Russell RR, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol: Heart Circ. Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 41.Jørgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2 5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;5:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glucose transporter content of GLUT-1 and GLUT-4 and of GLUT-4 translocation were all similar in both transgenic ssTnI expressing mouse hearts (TG-ssTnI, n=4) and non-transgenic littermate hearts (NTG, n=4). GLUT-1 and GLUT-4 signals were normalized to Na+/K+ATPase signals. Representative Western blots are shown above bar graph representing each group. NTG, non-transgenic; TG-ssTnI, transgenic. A. GLUT-1 abundance in whole mouse myocardium. B. GLUT-4 abundance following fractionation of myocardium into pre-fractionation pellet containing both surface membranes plus intracellular membranes (P2) and post-fractionation pellet containing only sarcolemmal membrane (P3).
a) Relative mRNA levels of glycolytic enzymes relative to the invariant Ppia gene and normalized to NTG no ischemia (mean ± SE; n = 5 per group). Gene names are presented in Table S1. b) Representative western blot images for key glycolytic enzymes at baseline (no ischemia) and during ischemia. HKII, hexokinase (Hk2); PFK-1, phosphofructokinase, muscle (Pfkm); PFK-2 car, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 heart (Pfkfb2); FBPase, fructose bisphosphatase 2, muscle (Fbp2). c) Protein content of key glycolytic enzymes from Western blot normalized to NTG-no ischemia (mean ± SE; n = 4 per group). No statistically significant differences reported.