SUMMARY
The adaptor protein Src homology 2 domain-containing leukocyte-specific protein of 76 kDa (SLP-76) is central to the organization of intracellular signaling downstream of the T cell receptor (TCR). Evaluation of its role in mature, primary T cells has been hampered by developmental defects that occur in the absence of wild-type SLP-76 protein in thymocytes. Following tamoxifen-regulated conditional deletion of SLP-76, mature, antigen-inexperienced T cells maintain normal TCR surface expression but fail to transduce TCR generated signals. Conditionally deficient T cells fail to proliferate in response to antigenic stimulation or a lymphopenic environment. Mice with induced deletion of SLP-76 are resistant to induction of the CD4+ T cell mediated autoimmune disease experimental autoimmune encephalomyelitis. Our findings demonstrate the critical role of SLP-76-mediated signaling in initiating T cell-directed immune responses both in vitro and in vivo and highlight the ability to analyze signaling processes in mature T cells in the absence of developmental defects.
Keywords: T Cells, Signal Transduction, Transgenic/Knockout Mice
INTRODUCTION
Signals generated by ligation of the T cell receptor (TCR) are critical in both thymocyte development and peripheral activation and differentiation. TCR signaling mechanisms have been explored using both in vitro [1] and in vivo [2] systems. These studies have led to a model in which crosslinking of the cell surface TCR leads to phosphorylation and activation of both Src and Syk family kinases resulting in phosphorylation of the lipid raft resident-transmembrane adaptor protein linker for T cell activation (LAT) [3]. Phosphorylated of LAT recruits cytosolic SLP-76/Grb2-like adaptor downstream of Shc (GADS) and PLCγ1 [4]. Formation of a complex composed of LAT, SLP-76, GADS, and PLCγ1 is critical for propagation of signals to downstream second messengers. A deficiency in any one of these components results in defects in biochemical markers of T cell activation in cell lines [5–8] and T cell developmental defects in genetically modified mice [9–12].
The study of SLP-76 in mature T cell signaling has only been partially addressed due to the critical role for SLP-76 in thymocyte development in the absence of SLP-76. Germline deletion of SLP-76 leads to a complete block at the CD4−CD8−CD25+CD44− (DN3) to CD4+CD8+ (DP) transition [9, 11]. Transgenic expression or knock-in rescue of germline SLP-76 deficiency with mutant forms of SLP-76 result in varied degrees of developmental defects, but generate single positive thymocytes and mature T cells for study [13–15]. Cre-loxP mediated conditional deletion of SLP-76 with a tissue-specific CD4-Cre transgenic (SLP-76CD4Cre) revealed a requirement for SLP-76 in positive selection [16]. These mice exhibit varying degrees of peripheral T lymphopenia with abnormal cell surface expression of TCR/CD3 and cell surface activation markers [14–16]. In addition, we have recently shown that sustained expression of wild-type SLP-76 is required for normal memory T cell generation [17–19]. A systematic evaluation of SLP-76 requirements in antigen-inexperienced peripheral T cells has not been described.
To study TCR-mediated signaling in normally developed, non-antigen experienced T lymphocytes, we employ a system in which temporally-controlled deletion of SLP-76 can be induced after normal T cell development. Efficient deletion of SLP-76 is achieved in adult mice using a tamoxifen (tam)-responsive Cre recombinase (CreT2). In contrast to the effect observed with CD4Cre-mediated deletion, naïve peripheral T cells from mice with tam-induced SLP-76 deletion maintain cell surface expression of TCR but remain unable to propagate TCR generated signals. Primary immune responses are dramatically reduced using an in vivo model of experimental autoimmune encephalomyelitis (EAE) induction. Lastly, timed activity of Cre induction can be used to “switch” expression of wild-type to a mutant form of SLP-76 in peripheral T cells, allowing in vivo structure-function studies confined to mature cell compartments. Our studies establish a model for regulated deletion of SLP-76 after thymic development and reveal a central role for SLP-76 in initiating immune responses by naïve T cells.
RESULTS
To directly address whether peripheral T lymphocytes lacking SLP-76 are capable of transducing signals generated by TCR cross-linking, we used an inducible bi-transgenic system that could delete in a temporally controlled fashion (FIG. 1A). Conditional SLP-76 mice (SLP-76Flox) with the Rosa26-based reporter R26RYFP [20] were intercrossed with SLP-76+/− mice expressing a tam-regulated Cre recombinase (CreT2) [21]; a modified form of the Cre recombinase that is inactive unless exposed to the estrogen receptor antagonist tam [22]. Prior to tam administration, CreT2 is inactive; there is no evidence of YFP expression or SLP-76 germline deficiency. Specifically, thymic and peripheral T lymphocytes exhibit wildtype numbers, phenotypes and functions (data not shown). Mice lacked gross evidence of the abnormal lymphatic/vascular separation that has been described in SLP-76null mice [23]. For clarity, SLP-76Flox/-R26RYFPCreT2+ mice delete SLP-76 are designated SLP-76cKO, control littermates with a wild-type allele of SLP-76 (SLP-76Flox/+R26RYFPCreT2+) are designated SLP-76cHET.
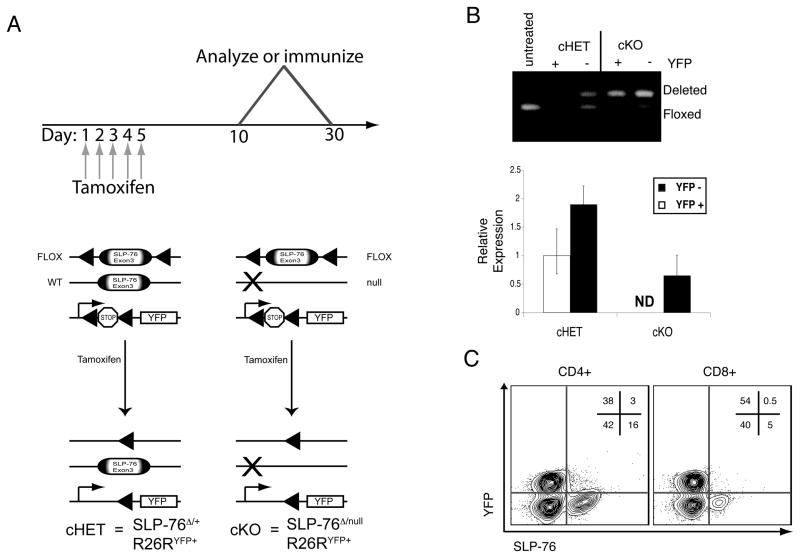
Figure 1. SLP-76 deletion in peripheral T cells.
(A) Schematic of temporal deletion approach: Mice with the SLP-76 gene floxed were crossed to mice expressing a tamoxifen-regulated Cre recombinase (CreT2). (Top) Tamoxifen is administered daily for five consecutive days by oral gavage and mice are analyzed from day ten to thirty from the start of tamoxifen. (Bottom) Prior to tamoxifen, a portion of the SLP-76 gene is floxed; a transcriptional stop cassette that blocks transcription of YFP from the Rosa26 locus is also floxed. In the presence of tamoxifen, CreT2 is active resulting in recombination between loxP sites, deletion of SLP-76 and of the stop cassette. This results in cells lacking SLP-76 but expressing YFP. (B) Following tam treatment, splenocytes from SLP-76cHET and SLP-76cKO mice were sorted based on YFP and CD4+ expression. Deletion was assessed at the genomic DNA level by PCR (top) and the RNA level by Tacman based real-time PCR (bottom). Error bars represent standard deviation of triplicate reactions for each cDNA sample (ND=non-detectable). Representative of three individual experiments. (C) Contour plot of CD4 and CD8 gated splenocytes from a SLP-76CKO mouse. Relative percentages of cells within the gated regions are shown. Representative of greater than 10 individual mice.
SLP-76 can be efficiently deleted from mature T lymphocytes
To confirm the efficiency of SLP-76 excision and generation of SLP-76-deficient T cells, lymphocytes from spleen and lymph nodes were harvested from tam-treated mice. PCR analysis of genomic DNA confirms deletion at the floxed locus in CD4+YFP+ cells (FIG. 1B). YFP negative cells isolated from the same mice contain a mixture of deleted and non-deleted alleles complicating interpretation of this population. Steady state RNA expression is also substantially reduced in cells isolated from the SLP-76cKO mice relative to those from the SLP-76cHET mice and non-detectable in YFP+SLP-76cKO cells (FIG 1B, bottom).
To confirm that individual T cells lacked detectable SLP-76 protein, we utilized flow cytometry to assess protein levels. Approximately 15–20% of CD4+ gated cells and 3–5% of CD8+ gated cells from SLP-76cKO mice retain SLP-76 protein after tam-induced deletion (FIG. 1C). Expression of YFP is highly correlative with loss of SLP-76 protein; YFP-positive cells rarely stained positive for SLP-76. T lymphocytes from SLP-76cHET animals treated with tamoxifen express equivalent levels of SLP-76 in the YFP+ and YFP− populations, which are slightly less than those found in SLP-76+/− cells (FIG. S1 and FIG 2A). Importantly, SLP-76cKO mice have normal numbers of splenic T lymphocytes. Cell surface levels of TCR, CD44, CD62L and CD25 are similar to those from SLP-76cHET and tamoxifen treated C57BL/6 (Fig. S2). Thus, effective inducible in vivo deletion of SLP-76 can be achieved resulting in phenotypically normal, naive, SLP-76 deficient T cells for study.
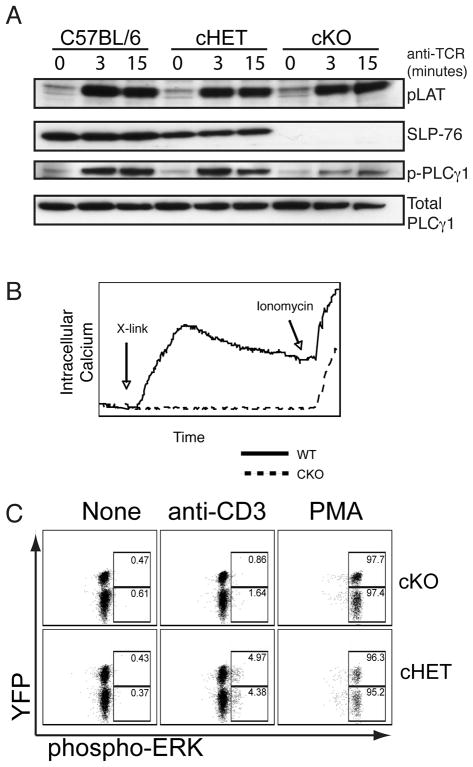
Figure 2. SLP-76CKO T cells are unable to transduce TCR-mediated signals.
(A) FACS-sorted CD90.2+YFP+ T cells were left unstimulated or stimulated with anti-CD3 (500A2) for the time periods indicated followed by lysis and western blotting to detect SLP-76, pLAT and pPLCγ1. Total PLCγ1 is shown as a loading control. Representative of three individual experiments. (B) Cell suspensions from lymph node of indicated mice loaded with Indo1 were stimulated by co-crosslinking cell surface CD3 and CD4 (indicated by arrow). Intracellular calcium was measured as a ratio of FL4/FL5. Ionomycin was added to each sample during the last 30 seconds of acquisition. Representative of two experiments (C) Splenocytes stimulated for 15 minutes with 500A2 or PMA were assessed for phopsphorylation of ERK. Dot plots are gated on CD4+ cells. Numbers within the gated areas are relative percentages of YFP+ and YFP− gated cells that stain positive for pERK. Representative of cells from 6 SLP-76cHET and 4 SLP-76cKO mice.
T cells deficient in SLP-76 fail to transduce TCR-mediated signals
To assess the effect of SLP-76 deficiency on TCR signaling in mature T lymphocytes, CD90+YFP+ FACS-sorted T lymphocytes were stimulated with soluble anti-CD3 antibody and assayed at various time points. Crosslinking of the TCR with anti-CD3 antibody results in phosphorylation of LAT suggesting that proximal signaling is grossly intact (Fig. 2A). In contrast, despite normal cell surface expression of TCR and LAT phosphorylation in SLP-76cKO peripheral T cells, crosslinking with anti-CD3 antibody did not induce appreciable phosphorylation of PLCγ1 or increases in intracellular calcium (FIG. 2A,B). Similarly, single-cell phosphoflow analysis shows minimal (<2 fold increase over unstimulated) ERK phosphorylation in YFP+CD4+ and YFP+CD8+ cells following TCR crosslinking (FIG. 2C and FIG S3A, respectively). Conversely, the number of cells with detectable phospho-ERK increased approximately 10-fold in YFP+ cells from SLP-76cHET mice. Stimulation with phorbol ester bypassed the proximal block resulting in phosphorylation of ERK in both SLP-76cKO and SLP-76cHET cells. These results demonstrate that mature peripheral T cells lacking SLP-76 cannot transduce appropriate intracellular signals following TCR crosslinking.
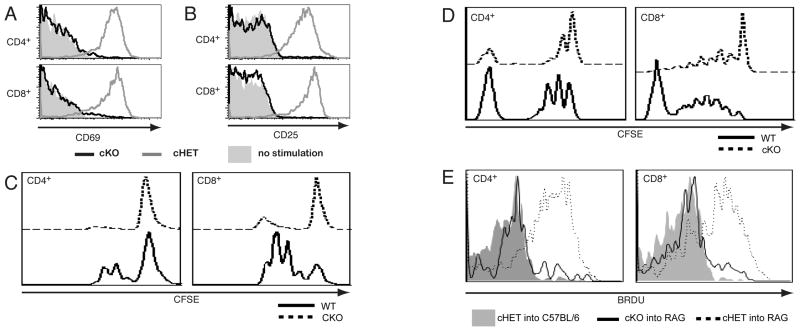
We next assessed downstream indicators of TCR-mediated signaling in SLP-76CKO cells, including upregulation of activation markers and proliferative expansion. TCR crosslinking results in the upregulation of both CD69 and CD25 on YFP+ gated cells from SLP-76cHET but not SLP-76cKO T cells (Fig. 3A,B). Consistent with incomplete deletion, a small percentage of YFPneg T cells from SLP-76cKO upregulate the activation markers (FIG. S4A). Comparison of SLP-76cHET with C57BL/6 controls had no defect in activation marker upregulation suggesting that acute conversion from two to one allele of SLP-76 has no effect on these functions (Fig. S4B). To assess proliferative capacity, splenocytes from SLP-76cKO and SLP-76cHET mice lacking the YFP reporter were labeled with CFSE and stimulated with anti-CD3 in vitro. CD4+ and CD8+ SLP-76cKO T cells fail to proliferate (FIGURE 3C), regardless of the concentration of antibody used, addition of anti-CD28 co-stimulation, or IL-2 supplementation (Fig S4A and data not shown). In contrast, bypassing proximal signaling with PMA and ionomycin stimulation is sufficient to upregulate activation markers and rescue proliferation (data not shown). Overall, mature T cells lacking SLP-76 are incapable of effective activation or proliferation in response to TCR crosslinking.
Figure 3. SLP-76 T is required in mature T cells for up-regulation of activation markers and for proliferation.
Splenocytes isolated from SLP-76cHET and SLP-76cKO mice were left unstimulated (shaded histograms) or were stimulated overnight with soluble anti-CD3 (open histograms). Cells were surface stained with CD4, CD8, CD69 and CD25. Expression of CD69 (A) and CD25 (B) on CD4+YFP+ and CD8+YFP+ are shown. Representative of greater than five experiments. (C) Splenocytes were labeled with CFSE and stimulated in vitro for seventy-two hours then stained with CD4 and CD8. Proliferation measured by dilution of CFSE in CD4 gated (left panel) and CD8 gated (right panel) are shown. SLP-76cKO cells are shown with a dotted line and SLP-76cHET by solid line. Representative of two experiments (D) CFSE-labeled Thy1.2+ T cells from SLP-76cHET and SLP-76cKO mice were adoptively transferred into RAG2−/−CD45SJL mice. Suspensions taken from lymph nodes seven days after transfer gated on CD45B6 donor, B220− then CD4 (left) or CD8 (right) are shown. Representative of two experiments. (E) Splenocytes from SLP-76cHet (dashed line) and SLP-76cKO (solid line) were adoptively transferred into CD45SJL and RAG2−/− CD45SJL recipients. BRDU was administered from during days 5 through 7. Lymph node suspensions gated on CD45B6 donor, YFP+ then CD4 (left) or CD8 (right) are shown. Shaded histograms represent BRDU incorporation into cells transferred into non-lymphopenic CD45SJL mice, where no LIP is expected. Representative of 2 experiments with a total of 9 recipient mice in each.
SLP-76 deficient T cells fail to undergo lymphopenia induced proliferation
T cells can undergo lymphopenia-induced proliferation (LIP) that requires both cytokine and TCR signals [24, 25]. LIP was assessed using CFSE dye dilution and alternatively with bromodeoxyurine (BRDU) incorporation in conjuction with YFP expression. T cells isolated from SLP-76cHET and SLP-76cKO mice lacking the YFP reporter were labeled with CFSE and adoptively transferred into RAG2-deficient mice expressing the CD45SJL congenic marker. One week later, lymph node CD45B6 donor cells gated on CD4 or CD8 were assessed for LIP (FIG. 3D). A majority of both CD4+ and CD8+ T cells from SLP-76cHET donors underwent one or more rounds of division. In contrast, a majority of CD4 and CD8 cells derived from SLP-76cKO donors fail to undergo cell division as measured by CFSE dilution. A small percentage of SLP-76cKO-derived CD4+ T cells underwent one round of division and CD8+ cells underwent more than one division. This may be due to a small number of SLP-76 deficient cells proliferating in response to lymphopenia or the non-deleted cells. To differentiate these possiblilites, additional adoptive transfers were done using cells containing the YFP reporter and RAG2−/− recipients and BRDU was administered during the final two days of potential LIP. YFP+ T cells originating from SLP-76cKO mice failed to incorporate BRDU (Fig 3E) and represented a small percentage of cells found in the secondary lympoid organs of recipient mice (data not shown). Recipients of SLP-76cHET T cells had greater numbers of donor-derived cells that had efficiently incorporated BRDU (Fig. 3E). Taken together, these data strongly argue that SLP-76 expression is a cell-intrinsic requirement for LIP.
SLP-76 is required for induction of EAE
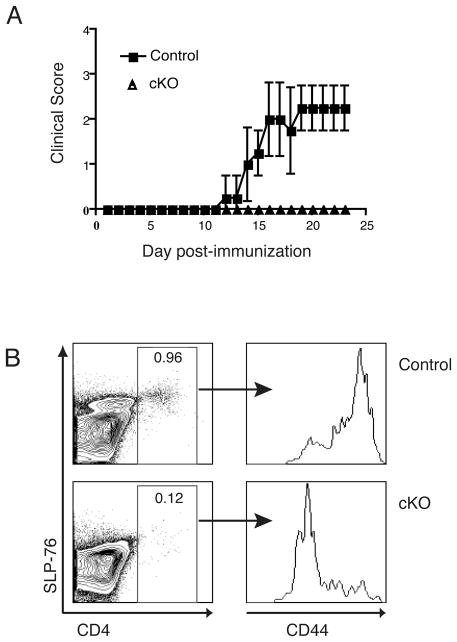
To determine the extent to which SLP-76 contributes to a functional immune response in vivo, we examined the effect of SLP-76 absence during active EAE. All mice, both SLP-76cKO and control (which included SLP-76cHET, C57BL/6 and (C57BL/6 x 129)F1), received tam. Following tam treatment, mice were immunized with MOG35-55 emulsified in complete freund’s adjuvant (CFA) according to standard protocol [26] and observed for evidence of clinical disease. None of the SLP-76cKO mice immunized with MOG peptide showed evidence of disease, while 83% of the control mice developed hind-limb weakness (FIG. 4A and TABLE 1). Onset of clinical disease in control mice was characteristic of MOG35-55-induced EAE in C57BL/6 and B6.129 mice[27] (FIG. 4A). Histological examination of brain and spinal cords from wild-type mice revealed inflammatory infiltration and demyelination, while minimal inflammation of CNS tissue was observed in SLP-76cKO mice (data not shown). CD4+ T cells isolated from the central nervous system tissue of control mice expressed high levels of CD44, consistent with previous activation (FIG. 4B). Conversely, the few CD4+ cells found within the CNS of SLP-76cKO mice had lower levels of CD44, indicative of limited prior activation. Thus, mice with conditional deletion of SLP-76 lack both lymphocytic CNS infiltrates as well as clinical signs of EAE, suggesting a central role for SLP-76 in the initiation of autoimmune pathology and disease.
Figure 4. SLP-76CKO mice are resistant to active EAE induction.
(A) Mice were treated with tam for five consecutive days prior to immunization with MOG35-55 and assessed for clinical signs of EAE. Control mice (SLP-76WT, C57Bl/6 or C57Bl/6×129 mice) (squares; n=4) developed typical ascending paralysis starting at day 11. In contrast, SLP-76CKO mice (triangles; n=3) were asymptomatic throughout. Representative clinical scores from one of three individual experiments are shown. (B) Mononuclear cells were isolated from the CNS of mice at day 28 post-immunization with MOG35-55. Cells were stained and analyzed by flow cytometry. Left panels are gated on live, mononuclear cells and a representative contour plot is shown. Right panels are gated on CD4+ as indicated and histograms indicate levels of cell surface CD44. Representative of data from two separate experiments with a total of six mice.
Table 1.
EAE in SLP-76cKO and SLP-76control mice
| Incidence | Onset (mean ± SEM) | Maximal Score | Mean Score** | |
|---|---|---|---|---|
| * SLP-76control | 10/12 | 15.5 ± 0.7 | 4 | 1.8 ± 0.3 |
| SLP-76cKO | 0/13 | N/A | 0 | 0 |
Combined data from a total of three individual experiments
The SLP-76control group includes CreT2− and non-transgenic mice in addition to SLP-76cHET
assessed at day 21 ± SEM; two-tailed student t-test was performed for comparison between SLP-76WT and SLP-76CKO mice assessed at day 21 (p < 0.0001)
Disease severity was scored on a scale of 0 – 5: 0, no illness; 1, limp tail; 2, hindlimb paresis; 3, partial hindlimb paralysis; 4, complete hindlimb paralysis; 5, moribund or dead.
Tam/CreT2 induced deletion can be used for structure-function analysis in mature T lymphocytes
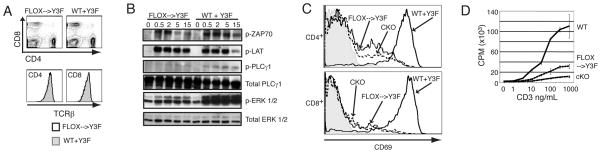
We next applied the conditional deletion model of SLP-76 to the study of structure-function in normally developed primary cells. TCR crosslinking of Jurkat cells expressing only a mutant form of SLP-76 in which three tyrosines have been mutated to phenylalanine (Y3F) results in impaired activation of PLCγ1 and calcium influx [28, 29]. Similarly, in SLP-76-deficient mice expressing a T cell specific SLP-76Y3F transgene, thymocyte cellularity is significantly decreased and TCR mediated signaling in thymocytes is markedly abnormal [15]. To address whether the presence of the Y3F mutation in normally developed, primary T cells leads to abnormal function of T cell signaling, we bred mice expressing the SLP-76Y3F transgene onto the conditional knockout background. Adult mice were fed tam to induce deletion of the wild-type allele, leaving the mutated SLP-76Y3F form of the protein as the only remaining SLP-76 molecule expressed (SLP-76Flox→Y3F). Examination of splenocytes from SLP-76Flox→Y3F mice revealed normal proportions of CD4 and CD8 T cells and normal levels of cell surface TCR (FIG. 5A). Biochemical analysis of SLP-76Flox→Y3F cells stimulated with anti-CD3 revealed wild-type levels of phosphorylation of ZAP-70 in response to TCR cross-linking, but virtually absent PLCγ1 phosphorylation. Additionally, ERK phosphorylation was detectable but reduced (FIG. 5B). In response to TCR crosslinking, CD69 is moderately upregulated in CD8+ cells but only minimally in CD4+ T cells from SLP-76Flox→Y3F mice (FIG. 5C). This subtle difference between CD4 and CD8 cells suggests that the mutant form of the protein may be differentially required. Lastly, proliferation of SLP-76Flox→Y3F T cells was substantially less that wild-type levels, but increased when compared to SLP-76cKO cells (FIG. 5D). These results indicate that T cells restricted to signaling through the SLP-76Y3F mutant form of the adaptor have moderate signaling defects and suggest a decrease in TCR signal strength in SLP-76Flox→Y3F T cells.
Figure 5. Peripheral T cells expressing a mutant form of SLP-76 fail to transduce TCR-mediated signals despite normal development and TCR levels.
WT and SLP-76flox→Y3F mice were treated with tam for five days. (A) No differences in the expression of CD4 and CD8 (top panels) and TCRβ (bottom panels) on splenocytes analyzed by FACS was detected. Representative of four experiments (B) Cell lysates prepared from splenocytes stimulated with anti-CD3 for the indicated times were used for western blotting to detect phospho-ZAP-70 (p-ZAP-70), phospho-LAT (p-LAT), phospho-PLCγ1 (p-PLCγ1), total PLCγ, phospho-ERK (p-ERK), and total ERK. Representative of two experiments (C) Splenocytes from SLP-76WT (solid line), SLP-76flox→Y3F (dotted line), SLP-76CKO (dashed line) with anti-CD3 overnight and CD69 levels were assessed by flow cytometry on gated CD4+ T cells (top panel) and CD8+ T cells (bottom panel) Shaded areas represent staining of unstimulated cells. Representative of two experiments. (D) Thymidine incorporation was measured after overnight anti-CD3 stimulation of splenocytes from SLP-76WT (solid line), SLP-76CKO (dashed line), and SLP-76flox→Y3F (dotted line) mice. Representative of two experiments.
DISCUSSION
We describe an inducible, conditional system for in vivo deletion of SLP-76 expression in mature, antigen-inexperienced T lymphocytes, enabling novel analysis of the role of this critical signal transducer in a primary response in vivo. In order to circumvent the developmental abnormalities associated with SLP-76 absence or mutation, we use a peripherally expressed, drug inducible form of the Cre recombinase. Using this system, we delete SLP-76 in normally developed, peripheral, mature T cells that are phenotypically similar to wild-type control lymphocytes both before and after deletion. SLP-76 deficient T cells expressing wildtype levels of cell surface TCR exhibit defective TCR-mediated proximal signal transduction and immune response.
This report highlights the advantage of a temporal deletion strategy to assess signal transduction in antigen-inexperienced cells. While tissue-specific conditional deletion approaches allow for cell lineage specificity, they still suffer from potential developmental alterations. For example, low cell surface TCR levels are found in SLP-76CD4Cre mice and other models in which TCR signaling has been disrupted during development [15, 16, 30, 31]. Similar to conditional loss of Lck and Fyn [32, 33], deletion of SLP-76 from mature T cells does not alter cell surface TCR levels. Normal cell surface levels of TCR are maintained at least as long as one month post tam-induced deletion and up to a year following deletion of SLP-76 in virally-induced memory T cell populations [19]. Normal cell surface TCR levels suggest an Lck/Fyn/SLP-76 independent maintenance of cell surface TCR levels and add support to a model in which so called “tonic” TCR signals may be qualitatively different than antigen-induced TCR mediated signals.
The use of a temporal deletion system also allowed us to evaluate the role of SLP-76 in vivo using EAE as a model system. EAE is a CD4 T cell-dependent inflammatory demyelinating disease of the CNS involving a complex inflammatory cascade of events [34–36]. Increased TCR signaling resulting from deletion of negative regulators hematopoietic progenitor kinase 1 or Sts1/Sts2 worsens EAE [37, 38]. The lack of EAE induction seen here suggests that SLP-76 dependent TCR signaling is required for development of CNS autoimmune disease, but does not rule out alternative SLP-76 dependent mechanisms.
The requirements for SLP-76 expression for chemokine mediated trafficking have only begun to be addressed experimentally. Initial studies in SLP-76 deficient Jurkat T cells suggested that SDF1 mediated signaling required expression of SLP-76 [39], but a recent study was unable to extend this finding into primary T murine T cells [40]. In addition, preliminary studies have shown no differences in trafficking of SLP-76cKO and SLP-76cHET cells in a non-inflammatory setting (E.C. and J.S.M., unpublished). Alternatively, because Cre-mediated SLP-76 deletion is effective in all cell types, antigen presenting cell function may also be affected following tam treatment. Alterations in dendritic cell function have been reported in SLP-76-deficient mice [41], including impaired adhesion downstream of integrin activation. A combination of events attributable to the pleiotropic effects of SLP-76 deletion are probably responsible for the abrogation in clinical and pathologic EAE. Memory T cell differentiation and homeostasis is altered by timing deletion of SLP-76 after acute LCMV infection[19] suggesting that temporal targeting of SLP-76 after induction of EAE may alter the clinical course. Studies to address this questions using temporal deletion at various time points during an immune response are currently ongoing in the laboratory.
Our use of a ubiquitously expressed CreT2 transgenic has several advantages and disadvantages. Following tam administration, Cre activity is seen in all hematopoetic cell types that we have evaluated (data not shown). For example, following tam administration, we find decreased thymic cellularity with a majority of thymocytes expressing markers consistent with a DN3 developmental block. This results in little to no ongoing T cell development and makes thymectomy unnecessary for the studies presented here. In addition, ubiquitous expression of CreT2 allows for the evaluation of SLP-76 deficient cells in other hematopoetic lineages. On the other hand, widespread deletion does have several disadvantages. Specifically, determining that defects in signaling and function are T cell intrinsic cannot be formally assessed without adoptive transfer or mixed chimera experiments. Interpretation of in vivo studies, such as evaluation of EAE, are complicated by potential defects in SLP-76 deficient antigen presenting cells.
Our result of deficient immune responses following conditional deletion of a TCR proximal adaptor are in contrast to a recent report in which conditional deletion of LAT in peripheral T cells [42] resulted in Th2 mediated autoimmune pathology similar to that seen with the LATY136F knockin [30, 31]. We believe that the divergent functional consequences of SLP-76 deletion reported here compared with LAT deletion in mature T cells are most likely due to differences in the method of Cre delivery and previous activation state of the cells evaluated. Specifically, our studies delete SLP-76 in resting lymphocytes and assess the immune response in a lymphoreplete animal, the LAT studies utilized ex vivo activation followed by exposure to the elevated cytokine levels in lymphodepleted mice. Further experiments will be needed to determine if this technical difference is the sole cause of the disparate results or if the roles of these two critical adaptors in peripheral T cells are truly so distinct.
The functional defects resulting from SLP-76 deletion in mature T cells are as profound as those resulting from deletion in developing thymocytes. Loss of SLP-76 at any stage of T cell development or differentiation leads to a block in signal transduction and loss of effector function. These data suggest that SLP-76cKO mice generated through a temporal deletion strategy behave functionally as inducible TCR-knockouts and that this system will be useful to address fundamental questions regarding the requirements for TCR signaling requirements in the function and homeostasis of multiple T cell lineages.
MATERIALS AND METHODS
Mice and Induction of in vivo SLP-76 Deletion
The generation of SLP-76Flox mice, in which exon 3 is flanked by loxP sites, has been previously described [16, 17, 19, 43]. SLP-76Δ mice lack exon 3 of SLP-76 in the germline and were generated by breeding SLP-76Flox mice with protamine-1-Cre transgenic mice [44]. SLP-76null mice, containing a germline deletion of SLP-76 generated by insertion of Neomycin at exon 1 [9], and SLP-76Y3F mice, expressing the Y3F mutant form of SLP-76 under the transcriptional control of the CD2 promoter/enhancer [15] were obtained from Gary Koretzky (University of Pennsylvania). R26RYFP [20] were obtained from F. Costantini (Columbia University). SLP-76Flox/Flox mice were inter-crossed with CreT2 transgenic mice [21] to generate mice with tam-inducible deletion of SLP-76. All conditional SLP-76 mice were crossed with C57BL/6 mice for at least 2 generations. All mice studied received tam treatment. RAG2−/−CD45SJL mice were initially purchased from Taconic (model #000461-M/F). Mice were housed and bred in the University of Pennsylvania mouse facility. All animal experiments were performed in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee guidelines.
Tam administration
Tamoxifen (Sigma: T-5648) was resuspended in ethanol at a concentration of 1mg/μL then diluted into corn oil with frequent vortexing for a final stock concentration of 20mg/mL. Using a weight based regimen, 200μg tam/gm mouse body weight was administered by oral gavage each day for five consecutive days.
Flow cytometry
Fluorochrome conjugated antibodies to CD4, CD8, CD44, CD25, CD69, CD62L, TCRβ were purchased from Becton-Dickenson or eBioscience. FITC-labeled anti-SLP-76 was purchased from Becton-Dickenson, P-Erk from cell signaling, and PE-anti-rabbit from Jackson Immunologicals. PE-labeled sheep anti-SLP-76 antisera was used as described [9]. Surface and intracellular staining was performed using standard methods. Data was acquired using either FACS-Calibur or LSR-II instruments (Becton-Dickinson). FACS analysis was performed using FlowJo (Tree Star, Inc.). Splenocyte YFP+ and YFP− populations were obtained using flow cytometric sorting on a BD FACSAria.
Real Time PCR
Cells were sorted by high-speed FACS directly into Trizol Reagent (Invitrogen). RNA was isolated according to the manufacture’s instructions. cDNA was made using a SuperScriptII First Strand kit (Invitrogen). Real-time PCR reactions were performed using murine SLP-76 and Actin primer/probe mix from Applied Biosystems. Reactions were performed in 10μL total volume using a 7500 Fast Real-Time PCR System with FastTaq Master Mix (Applied Biosystems). For analysis, samples were normalized to actin levels and then set relative to normalized YFP+ SLP-76cHET levels. Each sample was performed in triplicate.
Calcium Flux
Calcium flux assays were performed as described [16]. Briefly, lymphocytes were loaded with 2μg/ml of Indo-1 (Molecular Probes) at 30°C for 30 minutes and concurrently stained with biotinylated anti-CD3 (clone RMA4-4), PE-labeled anti-CD4 and APC-labeled-anti-CD8 antibodies. Baseline levels were measured for 30 seconds before addition of streptavidin (Molecular Probes) as a cross-linker. Cytoplasmic calcium was measured by a change in Indo-1 fluorescence. Ionomycin was added to all samples during the last thirty seconds of data collection. A BD-LSR (Becton Dickinson) flow cytometer was used for data acquisition and FlowJo software (TreeStar) was used for analysis.
Western Blotting
For experiments with sorted cells, splenocytes +/− lymph node cells were B220-depleted using B220 MACs purification (Miltenyi biotech) or BioMag Goat Anti-Rat IgG magnetic beads (Qiagen) bound to purified anti-B220 (eBioscience). Cells then underwent flow cytometric sorting for expression of YFP and/or CD90.2 using a FACs Aria (BD) or MoFlo (DAKO-Cytomation) sorter. Alternatively, four million bulk splenocytes were left unstimulated or were stimulated by the addition of 5μg/mL final anti-CD3 (500A2, Pharmingen) for the indicated times. Cells were then lysed in buffer containing 1% Nonidet-P40, 150mM NaCl, 50mM Tris, pH 7.4 with 1mM Na3VO4, 5mM NaF, 1mM PMSF, 5mM Na pyrophosphate and Protease Inhibitor Cocktail (Sigma). Proteins were resolved by SDS-PAGE and transferred to a Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories). Antibodies used for blotting included phospho-PLCγ-1 (Tyr783), phospho-pp44/42 MAPK (Thr202/Tyr202), phospho-AKT (Ser-473), phospho-LAT (Tyr191), and PLCγ-1 from Cell Signaling Technology and ERK1/2 (Santa Cruz) and SLP-76 (eBioscience).
Phospho-flow Analysis
2×106 splenocytes were incubated with α–CD3 (500A2, BD Pharmingen) at 2.5 μg/mL or PMA (Sigma) at 1 μg/mL for 15 minutes in reaction volumes of 200 μL. Reactions were stopped with 3 mL of 1× BD Phosflow lyse/fix buffer prewarmed to 37 °C. Cells were washed and surface stained in FACS buffer (PBS with 3% FCS and 0.01% Sodium azide), and then equilibrated and probed for pERK in BD Perm/wash buffer. Primary α–pERK (Cell Signaling) was used at 1:200, and secondary α-rabbit-Ax488 (Molecular Probes) was used at 1:100. Flow cytometry was performed with an LSR II cytometer (BD) and analyzed using FlowJo software (Treestar).
Proliferation and Activation Marker Upregulation
Splenocytes (0.5×106) were cultured in a flat-bottom 96-well plate with titrated doses of anti-CD3 (2C11) for 4 days. Cells were pulsed with 3H-thymidine for the last 20 hours of the culture and harvested the following day. To measure the upregulation of CD69, splenocytes were cultured as above. Eighteen hours later, cells were stained with anti-CD4, anti-CD8, anti-CD69, and anti-CD25 and evaluated by flow cytometry.
Lymphopenia-induced proliferation
Cells derived from mice lacking the R26RYFP reporter were Thy1.2+ purified using MACS magnetic beads (Miltenyi Biotech), labeled with CFSE and 2 × 106 cells were intravenously injected into RAG2−/− CD45SJL mice. Seven days later, single cell suspensions from lymph node and spleen were analyzed by flow cytometry. Alternatively, 2 × 106 SLP-76cHet and SLP-76cKO splenocytes were injected intravenously into RAG2−/−CD45SJL or CD45SJL non-lymphopenic control mice. On days five through seven, recipient mice were administered BrdU. On day seven, single cell suspensions from lymph node and spleen were assessed for BRDU incorporation using a BRDU Flow Cytometry Assay Kit (BD Biosciences) according to the manufactures protocol.
Induction and Evaluation of EAE
Seven to nine week-old mice were immunized with myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (CS Bio; Menlo Park, CA) according to standard protocol [26]. Briefly, subcutaneous injections of 200 μg MOG35-55 emulsified in 500 μg complete Freund’s adjuvant (Sigma; St. Louis, MO) were divided over the four flanks of each mouse. On the day of immunization and 48 hours later, mice were injected intraperitoneally with 200 ng pertussis toxin (List Biological laboratories; Campell, CA) diluted in 200 μl of PBS. Clinical evaluation was undertaken on subsequent days based on the following scores: 0 = no weakness, 1 = limp tail, 2 = mild hind limb paresis, 3 = severe hind limb paresis, 4 = hind limb paralysis, 5 = quadraplegia or moribund. Scoring was undertaken in a blinded fashion by one examiner (GFW).
Mononuclear cell isolation from the CNS
CNS mononuclear cells were isolated as previously described [45]. Briefly, mice were perfused with ice-cold PBS. Spinal cord and brain tissue were isolated and homogenized in RPMI 1640 medium with 10% FCS, followed by filtration through nytex (Sefar America; Depew, NY). Percoll (GE Healthcare Biosciences; Uppsala, Sweden) was added to a final concentration of 30% and the suspension was centrifuged at 1300 × g for 30 min at 4°C. The Percoll and lipid layers were aspirated and the cell pellet was washed in 5 ml RPMI 1640 medium with 10% FCS.
Acknowledgments
We thank F. Costantini and G. Koretzky for providing mouse lines. We thank L. Kovoor for technical assistance. We thank D. Farber for critical review of this manuscript. This work was supported in part by grants from the NIH/NIAID (K08 AI055806 and R01 AI085160) and the John Merrill Award from the American Society of Nephrology/American Society of Transplantation (to JSM). GFW and TML receive support from the National Multiple Sclerosis Society.
Abbreviations used in this paper
- SLP-76
Src homology 2 domain-containing leukocyte protein of 76 kDa
- LAT
Linker for activated T cells
- GADS
Grb2-like adaptor downstream of Shc
- DN3
double negative3
- DP
double positive
- tam
tamoxifen
- EAE
experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
- YFP
Yellow fluorescent protein
- LIP
lymphopenia induced proliferation
Footnotes
CONFLICTS OF INTEREST
The authors have no financial conflict of interest.
References
- 1.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell DA. Transgenic analysis of thymocyte signal transduction. Nat Rev Immunol. 2002;2:20–27. doi: 10.1038/nri703. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 5.Irvin BJ, Williams BL, Nilson AE, Maynor HO, Abraham RT. Pleiotropic contributions of phospholipase C-gamma1 (PLC-gamma1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma1-deficient Jurkat T-cell line. Mol Cell Biol. 2000;20:9149–9161. doi: 10.1128/mcb.20.24.9149-9161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 7.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 8.Abraham RT. Mutant T cell lines as model systems for the dissection of T cell antigen receptor signaling pathways. Immunol Res. 2000;22:95–117. doi: 10.1385/IR:22:2-3:95. [DOI] [PubMed] [Google Scholar]
- 9.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi K, Kawasaki Y, Ishii N, Sasaki Y, Asao H, Takeshita T, Miyoshi I, et al. Suppression of thymic development by the dominant-negative form of Gads. Int Immunol. 2001;13:777–783. doi: 10.1093/intimm/13.6.777. [DOI] [PubMed] [Google Scholar]
- 11.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 13.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar L, Pivniouk V, de la Fuente MA, Laouini D, Geha RS. Differential role of SLP-76 domains in T cell development and function. Proc Natl Acad Sci U S A. 2002;99:884–889. doi: 10.1073/pnas.022619199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myung PS, Derimanov GS, Jordan MS, Punt JA, Liu QH, Judd BA, Meyers EE, et al. Differential requirement for SLP-76 domains in T cell development and function. Immunity. 2001;15:1011–1026. doi: 10.1016/s1074-7613(01)00253-9. [DOI] [PubMed] [Google Scholar]
- 16.Maltzman JS, Kovoor L, Clements JL, Koretzky GA. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J Exp Med. 2005;202:893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, et al. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 23.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racke MK. Experimental autoimmune encephalomyelitis (EAE) Curr Protoc Neurosci. 2001;Chapter 9(Unit9):7. doi: 10.1002/0471142301.ns0907s14. [DOI] [PubMed] [Google Scholar]
- 27.Hilliard B, Samoilova EB, Liu TS, Rostami A, Chen Y. Experimental autoimmune encephalomyelitis in NF-kappa B-deficient mice:roles of NF-kappa B in the activation and differentiation of autoreactive T cells. J Immunol. 1999;163:2937–2943. [PubMed] [Google Scholar]
- 28.Jordan MS, Sadler J, Austin JE, Finkelstein LD, Singer AL, Schwartzberg PL, Koretzky GA. Functional hierarchy of the N-terminal tyrosines of SLP-76. J Immunol. 2006;176:2430–2438. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- 29.Yablonski D, Kadlecek T, Weiss A. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol Cell Biol. 2001;21:4208–4218. doi: 10.1128/MCB.21.13.4208-4218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 31.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 32.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 33.Seddon B, Zamoyska R. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol. 2002;169:2997–3005. doi: 10.4049/jimmunol.169.6.2997. [DOI] [PubMed] [Google Scholar]
- 34.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 36.Waldor MK, Sriram S, Hardy R, Herzenberg LA, Herzenberg LA, Lanier L, Lim M, et al. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985;227:415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- 37.Shui JW, Boomer JS, Han J, Xu J, Dement GA, Zhou G, Tan TH. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat Immunol. 2007;8:84–91. doi: 10.1038/ni1416. [DOI] [PubMed] [Google Scholar]
- 38.Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, Doherty P, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- 39.Kremer KN, Humphreys TD, Kumar A, Qian NX, Hedin KE. Distinct role of ZAP-70 and Src homology 2 domain-containing leukocyte protein of 76 kDa in the prolonged activation of extracellular signal-regulated protein kinase by the stromal cell-derived factor-1 alpha/CXCL12 chemokine. J Immunol. 2003;171:360–367. doi: 10.4049/jimmunol.171.1.360. [DOI] [PubMed] [Google Scholar]
- 40.Baker RG, Hsu CJ, Lee D, Jordan MS, Maltzman JS, Hammer DA, Baumgart T, et al. The adapter protein SLP-76 mediates “outside-in” integrin signaling and function in T cells. Mol Cell Biol. 2009;29:5578–5589. doi: 10.1128/MCB.00283-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luckashenak NA, Ryszkiewicz RL, Ramsey KD, Clements JL. The Src homology 2 domain-containing leukocyte protein of 76-kDa adaptor links integrin ligation with p44/42 MAPK phosphorylation and podosome distribution in murine dendritic cells. J Immunol. 2006;177:5177–5185. doi: 10.4049/jimmunol.177.8.5177. [DOI] [PubMed] [Google Scholar]
- 42.Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Clemens RA, Lenox LE, Kambayashi T, Bezman N, Maltzman JS, Nichols KE, Koretzky GA. Loss of SLP-76 expression within myeloid cells confers resistance to neutrophil-mediated tissue damage while maintaining effective bacterial killing. J Immunol. 2007;178:4606–4614. doi: 10.4049/jimmunol.178.7.4606. [DOI] [PubMed] [Google Scholar]
- 44.O’Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]