Abstract
The cytokine thymic stromal lymphopoietin (TSLP) has been implicated in the development and progression of allergic inflammation in both humans and mice. TSLP has been shown to promote Th2-type response through upregulation of OX40L on dendritic cells, and through direct induction of IL-4 production in naïve CD4 T cells. However, its direct effect on effector Th cells has not been extensively investigated. In this study, we show that the level of TSLPR expression on mouse effector Th2 cells is higher than on Th1 and Th17 cells, and that TSLP induced proliferation of effector Th2, but not Th1 and Th17 cells. TSLP also induced the phosphorylation of Signal Transducer and Activator of Transcription (Stat) 5, and expression of anti-apoptotic factor Bcl-2 in Th2 cells. Finally, TSLP-mediated proliferation on Th2 cells was enhanced by TCR stimulation, through IL-4-mediated induction of TSLPR expression. Taken together, these results indicate that TSLP is involved in exacerbation of mouse Th2-mediated allergic inflammation in a Th2 environment through direct stimulation of Th2 effector cells.
Keywords: TSLPR, TSLP, Th2 cell, IL-4
Introduction
TSLP is a type I cytokine that most closely related to IL-7. TSLP is expressed primarily by epithelial cells at barrier surfaces, with the highest levels seen in the skin, gut, and lung [1]. Importantly, TSLP expression was markedly elevated in the lesional skin of human atopic dermatitis (AD) patients and in the epithelium from asthmatic human patients [2, 3]. Consistent with these observations, mice over-expressing TSLP or treated with TSLP in the skin or lung developed helper type (Th) 2-type allergic inflammation in the site [4–8]. These data demonstrate that TSLP is an initiation factor for allergic inflammation.
TSLP exerts its biological activities by binding to a heterodimeric receptor consisting of the IL-7 receptor α-chain (IL-7Rα) and the TSLP receptor (TSLPR) [9–11]. TSLPR is expressed on a variety of cell types, including T cells, B cells, dendritic cells (DCs), and monocytes [12, 13]. TSLP polarizes human DCs to induce the differentiation of naive T cells into Th2 cells, mediated in part by induction of OX40L expression on DCs [14, 15]. In addition, TSLP, in conjunction with TCR stimulation, can act directly on naïve CD4 T cells to promote Th2 differentiation through induction of IL-4 gene transcription [16, 17] . However, TSLPR expression and direct effects of TSLP on differentiated effector Th2 cells have not been determined.
In this report we show that TSLPR expression is elevated on Th2, but not Th1 or Th17 cells, and that TSLP acts to drive the proliferation and cytokine production from committed Th2 cells. We also show that IL-4, acting through IL-4Rα, acts to increase TSLPR expression. Taken as a whole, these data suggest that TSLP driving the proliferation and survival of effector Th2 cells provides another means by which TSLP participates in and promotes Th2-type inflammatory responses.
Results
Expression of TSLP receptor (TSLPR) is increased on Th2 cells in an IL-4-Stat6-dependent manner
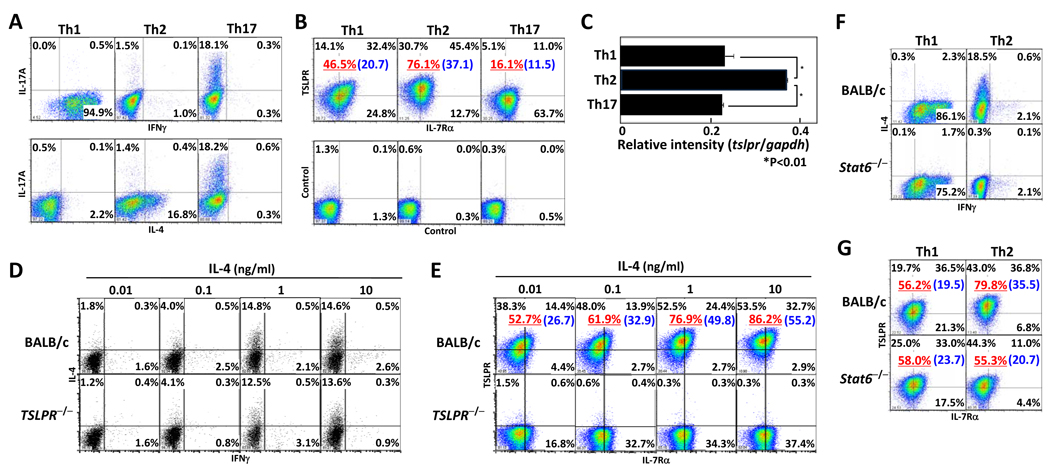
To investigate the level of TSLPR expression on cells from CD4 effector T cell lineages, we differentiated CD4 T cells under Th1, Th2, or Th17 conditions for 5 days, and confirmed expression of IFNγ, IL-4, and IL-17 from effecter Th cells after restimulation by intracellular staining (Fig. 1A). Each subset was analyzed for expression of surface TSLPR and IL-7Rα chain by flow cytometry and expression of tslpr mRNA by quantitative RT-PCR (Fig. 1B and C). The surface expression of TSLPR on Th2 cells (76.1%) was higher than Th1 (46.5%) and Th17 cells (16.1%), and IL-7Rα on Th subsets was expressed similarly. Corresponding to surface expression of TSLPR, tslpr mRNA expression in Th2 cells was significantly higher than in Th1 and Th17 cells. To confirm the TSLPR expression on Th2 cells, CD4 T cells from BALB/c and TSLPR−/− mice were differentiated into Th2 cells by IL-4 titration (Fig. 1 D and E). IL-4 production from Th2 cells from TSLPR−/− mice was equivalent to those from wild-type mice. Expression of TSLPR on wild-type Th2 cells was increased by IL-4 treatment in a dose-dependent manner.
Figure 1. Increased TSLPR expression on Th2 cells by IL-4-Stat6 dependent pathway.
CD4+CD62hi T cells from BALB/c mice were stimulated with immobilized anti-CD3 mAb and anti-CD28 mAb under Th1/Th2/Th17 conditions for 5 days. (A) Cells were re-stimulated by anti-CD3 mAbs for 6hr and then assessed for IFNγ, IL-4 and IL-17 production by intracellular staining. (B) Cell-surface levels of TSLPR and IL-7Rα were measured by flow cytometry and compared to isotype staining samples. (C) mRNA levels of tslpr and gapdh were determined by quantitative RT-PCR. The relative intensity (/gapdh) is shown with standard deviation ( n=3). (D, E) CD4+CD62hi T cells from BALB/c and TSLPR−/−mice were differentiated into Th2 cells and cultured in varying doses of IL-4 for 5 days. (F, G) CD4+CD62hi T cells from BALB/c and Stat6−/−mice were differentiated into Th1 orTh2 cells. Cells were measured for IFNγ and IL-4 production by intracellular staining (D, F), and for cell-surface expression of TSLPR and IL-7Rα (E, G). The underlined value represents the percentage of TSLPR+ cells and the value in parenthesis represents the Mean Fluorescence Intensity of TSLPR+ cells. Three independent experiments were performed with similar results. *p<0.01 by Student’s t test.
The data in figure 1E suggested that IL-4 signaling is involved in regulating TSLPR expression on Th2 cells. We extended these studies by examining TSLPR expression on Th2-like cells from Stat6−/− mice. TSLPR expression on Stat6−/− Th2 cells was reduced compared to TSLPR expression on wild-type Th2 cells (Fig. 1F and G). The decreased expression appeared to be due to lack of IL-4 signaling as TSLPR expression was similar to wild-type and Stat6−/− Th1 cells. These results indicated that TSLPR expression on Th2 cells was higher than on Th1 and Th17 cells, and that IL-4 treatment increased TSLPR expression on Th2 cells in a Stat6-dependent manner.
TSLP induced proliferation, phosphorylation of Stat5, and expression of Bcl-2 in Th2 cells
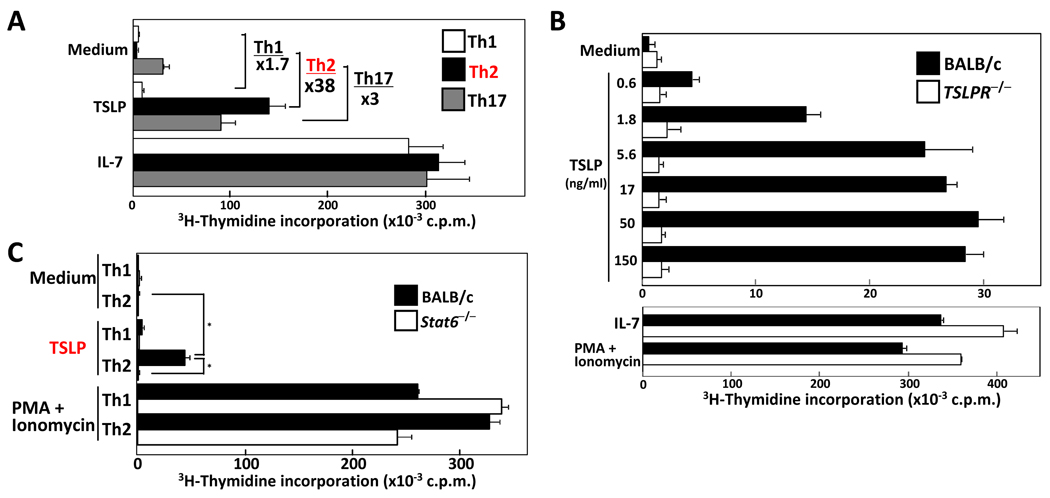
TSLP has been shown to enhance proliferation of human and mouse naïve CD4 T cells after TCR stimulation [16, 17]. To examine direct TSLP-mediated proliferation of Th subsets, Th1, Th2, and Th17 cells were cultured with medium, TSLP, or IL-7 for 24 h. Cells were pulsed with tritium-thymidine for an additional 16 h. Wild-type Th2-skewed cells proliferated in the presence of TSLP in a dose-dependent manner, while Th1 and Th17 cells showed very little response (Fig. 2A and B). IL-7 stimulation induced equal proliferation in these cells. The response of Th2 cells to TSLP treatment was specific as TSLPR-deficient Th2 cells did not respond to TSLP, but did proliferate following stimulation with IL-7 or PMA plus ionomycin. Carboxyflorescein diacetate succinimidyl ester (CFSE)-labeled Th2 cells were stimulated with medium, TSLP, or IL-7, and 2 and 4 days later, the cell division was assessed (Supporting Information Fig. 1). Consistent with the previous data, TSLP-treated Th2 cells underwent more extensive proliferation as compared to cells cultured in medium alone. These results clearly indicate that TSLP significantly induced the proliferation of Th2 cells, but not Th1 or Th17 cells. Consistent with the reduced TSLPR expression, TSLP-mediated proliferation of Stat6−/− Th2 cells was reduced compared to wild-type Th2 cells (Fig. 2C). These results indicated that TSLP-mediated proliferation of Th2 cells is enhanced through an IL-4-Stat6 pathway.
Figure 2. TSLP induces proliferation of Th2 cells.
(A) Naïve CD62LhiCD4 T cells were differentiated into Th1, Th2, and Th17 lineages. The cells were cultured with medium, TSLP (50ng/ml), or IL-7 (10ng/ml) for 24 hr, then pulsed with 3H-thymidine for an additional 16 h and proliferation measured. (B) Wild-type and TSLPR−/−Th2 cells and (C) BALB/c and Stat6−/−Th1/Th2 cells were cultured in the presence or absence of the indicated concentration of TSLP, IL-7, or with PMA +ionomycin, and proliferation measured as in panel A. Data shown are mean+/− SD of threeindependent experiments. *p<0.01 by Student’s t test.
TSLP induces tyrosine-phosphorylation of Stat5 in human and mouse naïve CD4 T cells [16, 17]. To examine phosphorylation of Stat5, Th2 cells were cultured with TSLP or IL-7 for indicated time, the Stat5 was detected by western blotting and flow cytometry (Supporting Information Fig. 2A and B). Phosphorylation of Stat5 in wild-type Th2 cells was detected by TSLP stimulation, but not in TSLPR−/− Th2 cells.
IL-7 mediated Stat5 phosphorylation has an important function in T cell survival by increasing the expression of anti-apoptotic molecules, such as Bcl-2 [18, 19]. To determine whether TSLP affected expression of Bcl-2 in Th2 cells, we examined the Bcl-2 mRNA expression in Th2 cells following TSLP treatment. Bcl2 mRNA expression was increased in Th2 cells by both TSLP and IL-7 treatment compared to medium alone (data not shown). Furthermore, the increased expression of Bcl-2 protein in IL-7- and TSLP-treated Th2 cells was detected by flow cytometry (Supporting Information Fig. 2C). These results indicate that TSLP induces the phosphorylation of Stat5 and Bcl-2 expression in Th2 cells in the absence of TCR stimulation.
TSLP co-stimulation increases Th2 cell proliferation in an IL-4 dependent manner
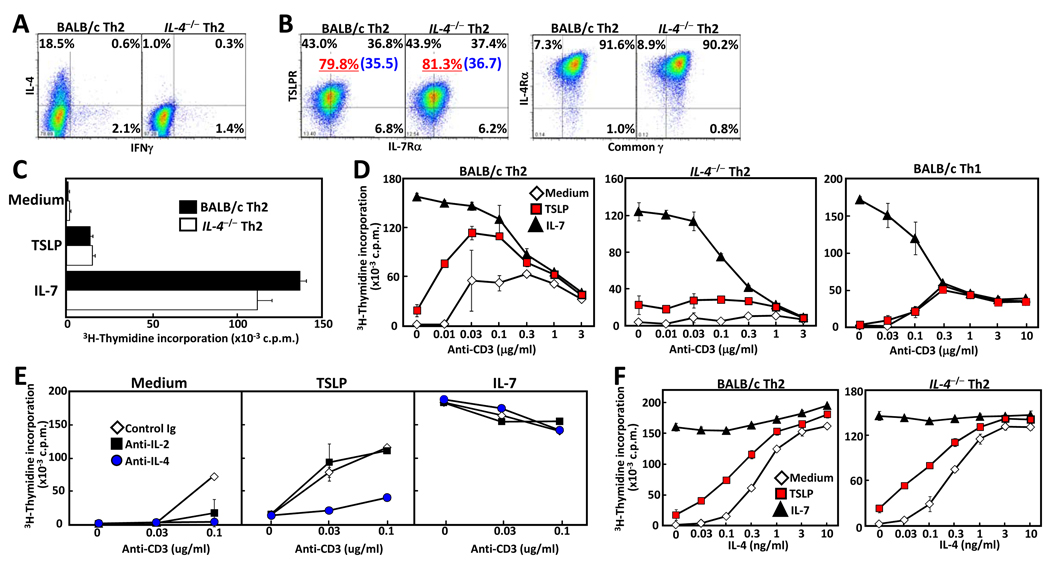
The data shown above suggests a role for IL-4 in regulating functional cell-surface TSLPR levels. To further examine the interplay between these cytokines, naïve CD4 T cells from IL-4-deficient (IL-4−/−) mice were differentiated under Th2 conditions, and expression of TSLPR, IL-7Rα, common γ chain(γc), and IL-4Rα was measured. All receptors were equally expressed on wild-type and IL-4 deficient Th2-skewed cells due to the addition of exogenous IL-4, while, as expected, only the wild-type Th2 cells produced IL-4 (Fig. 3A and B). In addition, TSLP-mediated proliferation was unaffected (Fig. 3C). TSLP was also able to co-stimulate with TCR engagement to increase proliferation of wild-type, but not IL-4-deficient, Th2 cells (Fig. 3D). This co-stimulation was especially apparent at low concentrations of anti-CD3 (0.01–0.1µg/ml), while activation induced cell death was induced at high concentrations of anti-CD3 (over 0.3µg/ml). To determine the mechanism of TSLP-mediated co-stimulation, IL-2 or IL-4 was neutralized in the cultures and proliferation measured as before. As shown in Figure 3E, blockade of IL-4, but not IL-2, abrogated the TSLP co-stimulatory effect. As a control, IL-7-mediated co-stimulation was unaffected by blockade of either cytokine. Consistent with synergy between TSLP and IL-4 in the promotion of Th2 proliferation , IL-4, in a dose-dependent fashion, increased TSLP-mediated proliferation of both wild-type and IL-4-deficient Th2 cells (Fig. 3F). Taken as a whole, these data suggest that IL-4, induced by TCR engagement, acts synergistically in an autocrine or paracrine fashion with TSLP to drive the proliferation of Th2 cells.
Figure 3. IL-4 enhances TSLP-mediated co-stimulation of Th2 cells.
Naïve CD62LhiCD4 T cells from BALB/c and IL-4−/−mice were differentiated under Th2 conditions for 5 days. After washing of cells, cytokine production was assessed by intracellular staining (A). (B) TSLPR, IL-7Rα, IL-4Rα, and common γ chain expression were detected by flow cytometry. The underlined value represents the percentage of TSLPR+ cells and the value in parenthesis represents the Mean Fluorescence Intensity of TSLPR+ cells. (C) Proliferation was measured by 3H-thymidine incorporation following culture with medium, TSLP, or IL-7. (D) Wild-type and IL-4−/−Th2, and wild-type Th1 cells were cultured with medium, TSLP, or IL-7 in the presence or absence of plate-bound anti-CD3 (at indicated concentration) and proliferation was measured by 3H-thymidine incorporation. (E) Wild-type Th2 cells were cultured with medium, TSLP, or IL-7 with either control Ig, anti-IL-2, or anti-IL-4 Abs (each 2mg / well), and proliferation was measured by 3H-thymidine incorporation. (F) Wild-type and IL-4−/−Th2 cells were cultured with medium, TSLP, or IL-7, in the presence or absence of increasing amounts of IL-4, and proliferation was measured by 3H-thymidine incorporation. Data shown are mean +/− SD of three independent experiments.
TSLP co-stimulation increases cytokine production
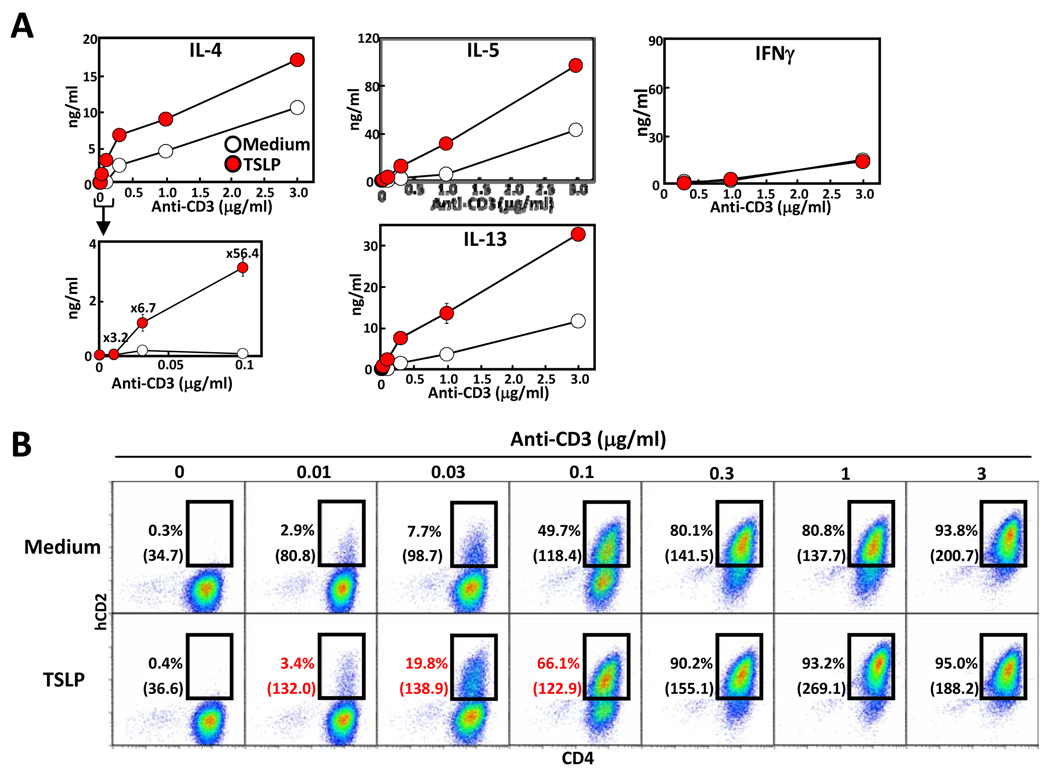
Our previous work showed that TSLP was capable of directly inducing IL-4 production from TCR-stimulated naïve CD4 T cells [16]. To assess whether TSLP increases CD3-mediated IL-4 production from differentiated Th2 cells, Th2 cells were generated in vitro, rested, and then cultured with medium or TSLP with or without anti-CD3 stimulation, and IL-4 mRNA and Th2 cytokines were determined. Six hours later, the expression levels of IL-4 mRNA was significantly increased in the TSLP containing cultures (Supporting Information Fig 3). In addition, production of IL-4, IL-5, and IL-13 was markedly increased in cultures containing TSLP, while the level of IFNγ was unaffected (Fig. 4A).
Figure 4. Enhanced IL-4 production by TSLP co-stimulation.
Effector Th2 cells were cultured with medium or TSLP in the presence or absence of increasing plate-bound anti-CD3 antibody. (A) After 48 hours of culture IL-4, IL-5, IL-13 and IFNγ concentration in the culture supernatant was measured by ELISA. (B) KN2/KN2 Th2 cells were cultured with medium or TSLP in the presence or absence of increasing anti-CD3 antibody for 24 hours, then stained with human CD2 and mouse CD4 antibodies and analyzed by flow cytometry. The value in parenthesis represents the Mean Fluorescence Intensity of CD4+ KN2+ cells. Data shown are representative of three independent experiments.
To further examine the affect of TSLP on IL-4 production from Th2 cells, we took advantage of the KN2 mouse strain. This strain is a knockin/knockout of the IL-4 gene, with a cDNA encoding human CD2 (hCD2) inserted into the first exon of the IL-4 gene [20]. Homozygous KN2/KN2 mice are IL-4 deficient, but the ability to produce IL-4 can be determined by measuring cell-surface hCD2 by flow cytometry. Naïve CD4 T cells from KN2/KN2 mice were differentiated into Th2 cells and then cultured in the presence or absence of TSLP and increasing amounts of anti-CD3 for 24 hours, and then examined for hCD2 expression. Consistent with the data shown in Figure 4A, at low concentrations of anti-CD3 stimulation the number of hCD2+, and thus IL-4 producing cells, in KN2/KN2 Th2 cells were clearly increased in cultures containing TSLP, but not Th1 cells (Fig 4B and data not shown). Addition of exogenous IL-4, in the absence of TCR stimulation, did not increase IL-4 producing cells (data not shown). In addition, the amount of IL-4, as measured by the mean fluorescent intensity (MFI) of hCD2 staining, was increased by TSLP treatment. Again, similar to what was seen with naïve CD4 T cells, the ability of TSLP to increase IL-4 production required TCR engagement as TSLP treatment alone did not induce IL-4 production. These results indicate that TSLP increases TCR-mediated Th2 cytokine production through TSLPR signaling pathway.
As we showed previously (Fig. 1), TSLPR expression on Th2 cells is regulated by the IL-4-Stat6 signaling pathway. Next, we examined whether IL-4 produced by TCR-stimulated Th2 cells regulated TSLPR expression. To confirm the TSLPR expression on wild-type and IL-4−/− Th2 cells, CD4 T cells from BALB/c and IL-4−/− mice were differentiated into Th2 cells and then cultured in the presence or absence of TSLP and increasing amounts of anti-CD3 for 24 hours. At that time the cells were examined for TSLPR and IL-7Rα expression. Interestingly, the surface expression of TSLPR on wild-type Th2 cells at TSLP plus low concentration of CD3 stimulation (71.4%) was clearly higher than on the IL-4−/− Th2 cells (15.9%; Fig. 5A). TSLPR expression on Th2 cells from both strains was increased by CD3 stimulation in a dose-dependent manner either in the presence or absence of TSLP. To further determine a role of IL-4 on surface TSLPR expression, wild-type Th2 cells were cultured in the presence or absence of TSLP and increasing amounts of IL-4 for 24 hours, and then TSLPR and IL-7Rα expression was measured. Consistent with our previous data, IL-4 treatment increased the surface expression of TSLPR on Th2 cells in a dose-dependent manner, in the presence or absence of exogenous TSLP (Fig. 5B), and TSLPR expression was increased by CD3 stimulation in the absence of TSLP. In addition, IL-4 treatment also modestly increased the surface expression of TSLPR on Th1 and Th17 cells in the presence or absence of exogenous TSLP, although the level of increase on Th1 and Th17 cells were lower than on Th2 cells (Fig. 5C). This result indicated that IL-4 can increase TSLPR expression, with the effect on Th2 cells greater than that seen on other Th subsets.
Figure 5. Enhanced TSLPR expression by IL-4 treatment on Th1, Th2, and Th17 cells.
(A) Wild-type and IL-4−/− Th2 cells were cultured in medium alone or with TSLP in the presence or absence of plate-bound anti-CD3 (at indicated concentration) for 24 hours. (B) Wild-type Th2 cells were cultured in medium alone or with TSLP in the presence or absence of IL-4 (at indicated concentration) for 24 hours. (C) Th1, Th2, and Th17 cells were cultured in medium alone or with TSLP in the presence or absence of IL-4 for 24 hours. Then, TSLPR and IL-7Rα expression was detected by flow cytometry. The underlined value represents the percentage of TSLPR+ cells and the value in parenthesis represents the Mean Fluorescence Intensity of TSLPR+ cells. Data shown are representative of three independent experiments.
Discussion
TSLP has been shown to be an important initiation factor for Th2-type allergic inflammation. Elevated TSLP expression has been seen in the epidermis of lesional skin from patients with atopic dermatitis, and in the lungs of asthmatics [2, 3]. In addition, TSLP has also been shown to be both necessary and sufficient in mouse models of airway inflammation [7, 21], and to drive a spontaneous atopic dermatitis-like disease when expressed in the epidermis [6].
What is somewhat less clear is which cell type is responding to TSLP in these disease settings. Studies in the human demonstrate that myeloid-derived dendritic cells respond to TSLP, and promote the Th2 differentiation of naïve CD4 T cells following TSLP treatment [2, 15]. In addition, Zhou et al have shown that mouse DCs also respond to TSLP through the production of CCL17 [7]. On the other hand, transfer of wild-type CD4 T cells into TSLPR-deficient mice restored responses in models of airway inflammation and food allergy [22, 23]. Consistent with a role for CD4 T cells responding to TSLP, Rochman et al showed that human CD4 cells up-regulated TSLPR upon TCR stimulation [17], with TSLP subsequently augmenting the response of these cells to low amounts of IL-2.
None of the studies mentioned above were focused on the potential role of TSLP on effector Th2 cells. Our lab had previously shown that naïve CD4 T cells, in the presence of TCR stimulation, responded to TSLP through induction of IL-4 gene transcription [16]. In this study, we demonstrated that TSLPR expression and TSLP-mediated proliferation on Th2 cells was higher than on Th1 and Th17 cells, and that TSLP stimulation induced the phosphorylation of Stat5 and Bcl-2 expression in Th2 cells. TSLP also enhanced TCR-mediated proliferation of Th2 cells through increased IL-4 production. In an atopic dermatitis model, He et al suggest that TSLP is essential for Th2 cytokine secretion by antigen-specific skin infiltrating CD4 T cells, although TSLPR expression on the T cells was not examined [24]. In addition, Th2 cytokines such as IL-4 and IL-13 have been shown to synergize with innate stimuli (allergens, pro-inflammatory cytokines and TLR agonists) to increase TSLP expression from epithelial cells of the skin, lung, and gut [25–28]. Interestingly, in our work shown above, IL-4 was capable of maintaining TSLPR levels on Th2 cells, demonstrating a potential positive feedback loop involving these two cytokines. Our findings are consistent with a role for TSLP in both the initiation and progression of allergic inflammatory diseases.
In addition to TSLP and Th2 cells, IL-17 and Th17 cells have been associated with allergic disease. In asthmatic patienst, the level of IL-17A expression in the lung, bronchoalveolar lavage, or sera was correlated with the severity of airway hypersensitivity [29], and the number of Th17 cells in peripheral blood was correlated the severity of acute Atopic dermatitis [30]. However, it is unclear whether TSLP and IL-17 cooperate to drive allergic inflammation. Interestingly, exogenous IL-4 was also capable of maintaining TSLPR levels on Th17 cells (Fig.5C) ,suggesting the possibility of a connection.
Treatment with IL-7 induced down-modulation of the IL-7Rα for signal attenuation, and also induces a negative feedback molecules for IL-7 signaling, such as Gfi-1 and SOCS-1 [31, 32]. Interestingly, TSLP induces down-modulation of TSLPR and slightly IL-7Rα (Fig.5) though expression of TSLPR mRNA in TSLP-treated Th2 cells was not affected (unpublished observation), suggesting the loss of cell surface expression reflected receptor internalization. This hypothesis is supported by the finding that TSLP induced the proliferation of proliferation of Th2 cells (Fig.2A). These results may suggest that TSLP down-regulates surface TSLPR and IL-7Rα expression through internalization, which then leads to subsequent signaling, both positive and negative as TSLP has been shown to induce the expression of the SOCS-family gene Cis [33].
TCR and IL-4R signaling has been shown to reduce IL-7Rα expression [31, 34]. On the other hand, we found that TCR and IL-4R signaling increased TSLPR expression in a cooperative manner (Figs. 3 and 5). These results indicate that responses to TSLP and IL-7 are differentially regulated by TCR and IL-4 signaling. Also, as IL-7Rα is shared by both TSLP and IL-7 receptor complexes, these data suggest that these stimulated Th2 cells are poised to respond to TSLP through expression of TSLPR, but still require additional signals to express IL-7Rα. Taken together one possible scenario is that TSLP responsiveness is not required during active stimulation of Th2 cells in lymph nodes where TCR and IL-4 can provide appropriate growth and survival signals. However, at tissue sites where these factors are absent, signaling through the TSLP receptor complex can provide these signals. IL-7Rα expression has been shown to resume in the absence of TCR stimulation [35, 36], thus allowing responsiveness to TSLP that is present in the tissue.
As described previously, we had shown that naïve CD4 T cells were capable of Th2-type differentiation following TCR engagement in the presence of TSLP [16]. In this system, TSLP acted to induce IL-4 gene transcription, with subsequent Th2 differentiation dependent on IL-4. These data suggest that TSLP is acting as a differentiation factor, possibly through remodeling of the chromatin at the IL-4 locus. In the current study we show here that, under conditions of low TCR stimulation, TSLP enhanced Th2 cell proliferation through induction of IL-4. Thus, TSLP can affect Th2 cells both at their induction and following their differentiation into effector cells.
The mechanism by which TSLP enhances IL-4 expression in Th2 cells is IL-4-independent as IL-4-deficient KN2/KN2 mice showed increased cell-surface hCD2 expression (Fig. 4B). Zhu et al. reported that IL-2 could promote IL-4 gene transcription through a Stat5-dependent remodeling of the IL-4 locus [37]. We showed TSLP induces the phosphorylated Stat5 in Th2 cells (Supporting information Fig. 2A and B), and that TSLP-mediated proliferation of Th2 cells is IL-2 independent (Fig. 3E). Taken together, these results show that TSLP can replace IL-2 as a co-stimulator of Th2 cell proliferation through its ability to activate Stat5.
An interesting aspect of this study is the finding that IL-4 signaling maintains TSLPR expression on Th2 cells (Fig. 1). Little is known as to the regulation of TSLPR gene expression. These data suggest that Stat6 signaling, in the presence of TCR stimulation, can induce and/or maintain TSLPR expression. Consistent with this observation, CD4 T cells from Stat6-deficient mice showed no increase in TSLPR expression when cultured in the presence of IL-4, displaying the same level of expression as either wild-type or Stat6−/− CD4 T cells grown under Th1 conditions (Fig. 1). Recently, Wei et al. reported that the TSLPR locus is a Stat6-target gene in Th2 cells, using genome-wide analyses. They also found that the pattern of TSLPR expression on wild-type Th1, Th2, and Sta6−/− Th2 cells was similar to our data [38]. As TSLPR is expressed by Stat6−/− Th2 cells this suggests that IL-4-dinducible, but not basal, expression of TSLPR is Stat6-dependent. In addition, TSLP-mediated proliferation of Stat6-deficient Th2 cells was dramatically reduced when compared to wild-type Th2 cells (Fig. 2). Thus, Stat6 signaling appears to promote TSLP-mediated Th2 cell proliferation indirectly through increased TSLPR levels and possibly directly by through activation following TSLP exposure.
Cell surface TSLPR expression by Th1 and Th17 cells differed, with somewhat lower expression in Th17 cells, in spite of both subsets having similar levels of tslpr mRNA and similar proliferative responses to TSLP (Fig. 1). Thus, these results may indicate that surface TSLPR expression is specifically down modulated on Th17 cells. In support of this notion, TGFβ treatment of resting Th2 cells resulted in reduced levels of surface TSLPR (unpublished observation). As TGFβ is involved in Th17 differentiation, these data suggest an explanation for the reduced levels of cell surface TSLPR expression on Th17 cells.
These data suggest a model for the role of TSLP in inducing and maintaining Th2 cells (Supporting information Fig. 4). In naïve CD4 T cells, TSLP can begin Th2 differentiation through direct induction of IL-4 gene transcription. Once CD4 T cells undergo full differentiation to Th2 effectors, TSLP can co-stimulate their proliferation through increased IL-4 production. IL-4, in turn, can act in a positive feedback loop to maintain, and possibly increase, TSLPR levels, allowing for continued responsiveness to TSLP present in the microenvironment. As TSLP is primarily expressed by epithelial cells at barrier surfaces [39], this provides a mechanism for maintaining Th2 cells at these sites. These results have implications in normal barrier homeostasis, where a Th2 environment maintains barrier integrity, as well as in allergic-type inflammation, where TSLP may act on Th2 cell directly to drive the inflammatory response [2, 3, 6, 7].
Materials and methods
Animals
Female BALB/c, and Stat6-deficient (Stat6−/−) and IL-4-deficient (IL-4−/−) mice on BALB/c background were purchased from Taconic Farms and Jackson Laboratory. Kn2 mice on BALB/c background were provided by Dr. Richard Locksley (UCSF; [20]). Tslpr-deficient (TSLPR−/−) mice were backcrossed to BALB/c for 12 generations. All mice for this study were maintained under pathogen-free conditions. All animal procedures were performed in accordance with IACUC guidelines at the Benaroya Research.
Flow Cytometry Analysis
In general, one million cells were stained with antibodies as indicated according to a standard method [16, 40, 41]. For surface staining, the Abs, allophycocyanin-conjugated anti-TSLPR mAb [33], and PE-conjugated anti-IL-7Rα mAb , FITC-conjugated anti-CD25 mAb , FITC-conjugated anti-CD44 mAb , PE-conjugated anti-common gamma chain mAb and Biotin-conjugated anti-IL-4Rα mAb, and allophycocyanin -conjugated streptavidin (eBioscience or BioLegend, ) were used. For cytokine intracellular staining, the Abs, allophycocyanin-conjugated anti-IL-4 mAb , FITC-conjugated anti-IFNγ mAb , PE-conjugated anti-IL17A mAb (eBioscience) were used. For the phosphorylated Stat5 intracellular staining, Alexa 488-conjugated anti-Stat5 (Y694) mAb (BD Biosciences) was used. For the Bcl-2 intracellular staining, FITC-conjugated anti-Bcl-2 mAb was used (BD Biosciences).
In vitro Th1/Th2/Th17 cell differentiation cultures
Effector Th1/Th2 cells were generated as previously described [16, 42, 43]. Briefly, splenic CD62LhighCD4 T cells were purified and stimulated with immobilized anti-CD3ε mAb (2C11; 3µg/ml; eBioscience) and anti-CD28 mAb (1µg/ml; eBioscience) under Th1, Th2, and Th17 conditions for 5 days: Th1 cell differentiation, IL-2 (25U/ml), IL-12 (1ng/ml), anti-IL-4 (11B11; 1µg/ml; National Institute of Health); Th2 cell differentiation, IL-2 (25U/ml), IL-4 (0.01–10ng/ml; eBioscience), anti-IFNγ mAb culture supernatant (R4-6A2); for Th17 cell differentiation, IL-6 (30ng/ml), TGFβ (5ng/ml), anti-IFNγ mAb culture supernatant (R4-6A2), anti-IL-4 mAb (11B11; 1µg/ml; National Institute of Health), anti-IL-2 mAb (JES6-1A2; 1µg/ml; eBioscience), IL-1β (10ng/ml; eBioscience) and IL-23 (40ng/ml; eBioscience).
Quantitative RT-PCR analysis
Total RNA was isolated from the cells using the GenElute Mammalian Total RNA Kit (SIGMA), and reverse transcription was performed with Superscript II RT (Invitrogen) according to the manufacture’s protocol. PCR amplification was performed on ABI 7700Sequence Detector (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed as described [16]. The expression was normalized by the gapdh signal. The primers used as follows:
tslpr forward, 5’-GAGAGCAATGACGATGAGGAC-3’; tslpr Reverse,
5’-GAAAGCCTTGTCACCGCTGT-3’; bcl-2 forward,
5’-CATGTGTGTGGAGAGCGTCAA-3’; bcl-2 reverse,
5’-GTCTTCAGAGACAGCCAGGA-3’; gapdh forward,
5’-TGCACCACCAACTGCTTAG -3’; gapdh Reverse,
5’-GGATGCAGGGATGATGTTC -3’;
Proliferation assay
For some experiments, Th2 cells were stimulated in 200 µl cultures for 24 h with medium, TSLP (50ng/ml), IL-7 (10ng/ml), PMA (20ng/ml) plus ionomycin (500nM) with or without titrated IL-4 (0.03–10ng/ml; eBioscience) or plate-bound anti-CD3ε antibody (2C11; 0.01–3µg/ml, eBioscience). Proliferation was measured either by [3H] thymidine (37 kBq/ well) incorporation for the final 16h of culture, or by CFSE dilution following labeling of cells with 5µM CFSE (Molecular Probes) for 8 min at 37 °C before stimulation. Then, cells were cultured with medium, TSLP (50ng/ml), and IL-7 (10ng/ml) for 2 or 4 days.
Western blotting analysis
Rested Th2 cells were stimulated with TSLP (50ng/ml) or IL-7 (10ng/ml) for 0.5, 1, 2 hours. Cell extracts were prepared using triple-detergent lysis buffer including complete protease inhibitor (Sigma-Aldrich). Cell extracts were resolved by 10% SDS-PAGE gel and transferred to nitrocellulose membrane (Bio-Rad). The membranes were immunoblotted with anti-phospho-Stat5 mAb (Cell Signaling) and anti-Stat5 mAb (Cell Signaling). The levels of protein were visualized with HPR-conjugated rabbit anti-mouse IgG by ECL detection system (Amersham Pharmacia).
Statistical analysis
The significance between two groups was determined by two-tailed Student’s t test.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Daniel J Campbell and Mark B. Headley for helpful comments and constructive criticism in the preparation of the manuscript. We also thank Matt Warren for assistance with preparing the manuscript.
Abbreviations
- IL-4−/−
IL-4-deficient
- Stat6−/−
Stat-6-deficient
- Tg
transgenic
- OVA
Ovalbumin
Footnotes
This work was supported by grants from NIH (R01 AI068731, R01 AR055695, R01 AR056113 and U19 AI071130) and The Food Allergy Initiative to SFZ, and by the Chiba University Global COE Program (Global Center for Education and Research in Immune System Regulation and Treatment) (MK and TN).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nature Immunology. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 3.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. The Journal of Immunology. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 4.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. The Journal of Immunology. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, Ziegler SF, Comeau MR. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. The Journal of Immunology. 2008;181:4311–4319. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 6.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. The Journal of experimental medicine. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature Immunology. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 8.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. The Journal of Immunology. 2008;181:6557–6562. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujio K, Nosaka T, Kojima T, Kawashima T, Yahata T, Copeland NG, Gilbert DJ, Jenkins NA, Yamamoto K, Nishimura T, Kitamura T. Molecular cloning of a novel type 1 cytokine receptor similar to the common gamma chain. Blood. 2000;95:2204–2210. [PubMed] [Google Scholar]
- 10.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. The Journal of Immunology. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 11.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. The Journal of experimental medicine. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nature Immunology. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu YJ, Romani N. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. The Journal of allergy and clinical immunology. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. The Journal of Experimental Medicine. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. The Journal of Immunology. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 17.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. The Journal of Immunology. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 18.Boothby M, Mora AL, Stephenson LM. Lymphokine-dependent proliferation of T-lymphoid cells: regulated responsiveness and role in vivo. Critical reviews in immunology. 2001;21:487–522. [PubMed] [Google Scholar]
- 19.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. The Journal of Immunology. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 20.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. The Journal of experimental medicine. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. The Journal of Experimental Medicine. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blazquez AB, Mayer L, Berin MC. Thymic Stromal Lymphopoietin is Required for Gastrointestinal Allergy but not Oral Tolerance. Gastroenterology. 2010;139:1301–1309. doi: 10.1053/j.gastro.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 24.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. The Journal of Immunology. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 26.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. The Journal of Immunology. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. The Journal of Immunology. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka J, Saga K, Kido M, Nishiura H, Akamatsu T, Chiba T, Watanabe N. Proinflammatory Th2 cytokines induce production of thymic stromal lymphopoietin in human colonic epithelial cells. Digestive diseases and sciences. 2010;55:1896–1904. doi: 10.1007/s10620-009-0979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. American Journal of Respiratory Cell and Molecular Biology. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 30.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. Journal of Investigative Dermatology. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nature Reviews Immunology. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 33.Isaksen DE, Baumann H, Zhou B, Nivollet S, Farr AG, Levin SD, Ziegler SF. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. The Journal of Immunology. 2002;168:3288–3294. doi: 10.4049/jimmunol.168.7.3288. [DOI] [PubMed] [Google Scholar]
- 34.Franchimont D, Galon J, Vacchio MS, Fan S, Visconti R, Frucht DM, Geenen V, Chrousos GP, Ashwell JD, O'Shea JJ. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor alpha. The Journal of Immunology. 2002;168:2212–2218. doi: 10.4049/jimmunol.168.5.2212. [DOI] [PubMed] [Google Scholar]
- 35.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nature Immunology. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, Takahashi H, Liang J, Gutierrez-Cruz G, Zang C, Peng W, O'Shea JJ, Kanno Y. Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity. 2010;32:840–851. doi: 10.1016/j.immuni.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. The Journal of Immunology. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima H, Iwamoto I, Tomoe S, Matsumura R, Tomioka H, Takatsu K, Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. The American review of respiratory disease. 1992;146 doi: 10.1164/ajrccm/146.2.374. 374-347. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, Nakayama T. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, Hasegawa A, Nakayama T. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24:611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita M, Shinnakasu R, Nigo Y, Kimura M, Hasegawa A, Taniguchi M, Nakayama T. Interleukin (IL)-4-independent maintenance of histone modification of the IL-4 gene loci in memory Th2 cells. The Journal of biological chemistry. 2004;279:39454–39464. doi: 10.1074/jbc.M405989200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.