Abstract
Sarcolipin (SLN), a key regulator of cardiac sarco(endo)plasmic reticulum (SR) Ca2+ ATPase, is predominantly expressed in atria and mediates β-adrenergic responses. Studies have shown that SLN mRNA expression is decreased in human chronic atrial fibrillation (AF) and in aortic banded mouse atria; however, SLN protein expression in human atrial pathology and its role in atrial SR Ca2+ uptake are not yet elucidated. In the present study, we determined the expression of major SR Ca2+ handling proteins in atria of human AF patients and in human and in a mouse model of heart failure (HF). We found that the expression of SR Ca2+ uptake and Ca2+ release channel proteins are significantly decreased in atria but not in the ventricles of pressure-overload induced HF in mice. In human AF and HF, the expression of SLN protein was significantly decreased; whereas the expressions of other major SR Ca2+ handling proteins were not altered. Further, we found that the SR Ca2+ uptake was significantly increased in human AF. The selective downregulation of sarcolipin and enhanced SR Ca2+ uptake in human AF suggest that SLN downregulation could play an important role in abnormal intracellular Ca2+ cycling in atrial pathology.
Keywords: Atrial Fibrillation, Calcium, Heart failure, Sarcolipin, SERCA
1. Introduction
Sarcolipin (SLN), a 31 amino acid sarco(endo)plasmic reticulum (SR) membrane protein is expressed predominantly in atria [1,2]. Overexpression of SLN in the adult rat ventricular myocytes [3] or in the mouse ventricles [4,5,6] demonstrates that SLN is an inhibitor of cardiac sarcoplasmic reticulum Ca2+-ATPase (SERCA). The inhibitory effect of SLN is independent of phospholamban (PLN) and relieved upon isoproterenol treatment [4,5,6]. Recently, using adenoviral mediated gene expression in adult rat ventricular myocytes; it has been shown that the conserved threonine 5 at the N-terminus of SLN critically regulates SLN function and mediates β-adrenergic responses [7]. Together, these studies suggest that SLN is a key regulator of cardiac SERCA pump and a mediator of β-adrenergic responses.
The expression of SLN both at mRNA and protein levels are shown to be altered in the diseased atrial myocardium [1,8,9]. SLN protein level was found to be increased in atria of canine heart failure (HF) and decreased in atria of ischemic dog hearts [1]. Sarcolipin mRNA levels are decreased in atria of patients with chronic atrial fibrillation (AF) [9] and in pressure-overload mouse models of cardiac hypertrophy [8]. The functional significance of SLN downregulation in atrial Ca2+ homeostasis was demonstrated using a gene knockout mouse model [10]. Ablation of SLN selectively increases the atrial SERCA pump activity [10], SR Ca2+ load and Ca2+ transients, but results in structural and electrical remodeling [11] suggesting that loss of SLN function can cause abnormal intracellular Ca2+ cycling and atrial remodeling. However, altered SLN protein expression and its role in atrial Ca2+ handling in human atrial pathology are yet to be reported. The present study is, therefore, aimed to determine the SLN protein expression in human AF and HF and how it affects the SR Ca2+ uptake in human AF.
2. Materials and Methods
We examined right atrial biopsies obtained from three patients with normal sinus rhythm, three patients with AF and three patients with normal sinus rhythm with HF. All protocols for obtaining human cardiac tissue were approved by the Ethical Committee of the Institut Hospitalier Jacques Cartier, France and were conducted in accordance with the Declaration of Helsinki principles. Informed consent was obtained before cardiac surgery from each patient.
Animals used in this study were 3-4 months old C57/BL6 mice. All animal procedures were performed with the approval of Institute Animal Care and Use Committee (IACUC) in the UMDNJ-Newark campus in accordance with the provision of the animal welfare act, the PHS policy on Human Care and Use of Laboratory Animals.
2.1. Western blot analysis
Total protein extracts from atria and ventricles were used for Western blot analyses using protein-specific antibodies as described earlier [12]. Signals detected by Super Signal WestDura substrate (Pierce) were quantitated by densitometry and then normalized to calsequestrin (CSQ) or sarcomeric α-actin levels.
2.2. SR Ca2+ uptake assays
The SR Ca2+ uptake was measured by the Millipore filtration technique as described earlier [12]. The rate of SR Ca2+ uptake and the Ca2+ concentration required for half maximal velocity of Ca2+ uptake (EC50) were determined by non-linear curve fitting analysis using Graph Pad PRISM 5.0 software.
2.3. Aortic banding
Pressure-overload in mice was induced by transverse aortic constriction against a 28G needle as described previously [12]. Corresponding sham-operated animals were used as controls. The echocardiography was performed after three weeks of aortic banding as described earlier [12]. The pressures in left ventricle and abdominal aorta were measured simultaneously using two separate 1.4F Millar catheters and the pressure gradients were calculated.
2.4. Statistical Analysis
All data reported as mean± SEM of at least three independent experiments. Statistical analysis was performed with two-tailed ANOVA or Student's t test. Significance was assigned at P<0.05.
3. Results
3.1. Selective downregulation of SLN in atria from AF and HF patients
Table 1 shows the clinical parameters of three groups of patients (sinus rhythm, AF and HF) studied. Mean age values were not significantly different in all three groups. Data obtained from atrial tissues of patients with normal sinus rhythm (NSR) without HF were used as control and compared to data obtained from AF and HF patients.
Table 1.
Clinical characteristics of patients
| Patient | Cardiac frequency (min−1) |
Rhythm | RA Size | LA Size | RV Size | LV Size | EF % |
Age, Sex |
Diagnosis | Kind of surgery |
Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NSR | 60 | SR | Normal | Normal | Normal | Normal | 60 | 72, M |
AVS, CABG |
||
| NSR | 75 | SR | Normal | Normal | Normal | Normal | 70 | 77, M |
Aortic stenosis |
AVS, CABG |
ACE Inhibitors; Diuretics; Statines |
| NSR | 55 | SR | Normal | Normal | Normal | Normal- hypertrophic |
49- 53 |
86, M |
Light mitral insufficiency |
CABG | β blockers; Statines |
| AF | 83 | cAF | Normal | Dilated | Normal | Normal- hypertrophic |
55 | 75, M |
Aortic insufficiency, CAR |
AVS, CABG |
ACE Inhibitors; Diuretics; β blockers |
| AF | 90 | cAF | Normal | Dilated | Normal | Normal- hypertrophic |
80 | 84, M |
AVS | Diuretics Digoxine |
|
| AF | 69 | AF | Normal | Normal | Normal | Normal | 60 | 75, M |
Light mitral insufficiency |
AVS, CABG |
β blockers; Statines |
| HF | 67 | SR | Normal | Normal | Normal | Normal- hypertrophic |
48 | 77, M |
HF, AHT, CAR |
AVS, CABG |
Diuretics |
| HF | 80 | SR | Normal | Normal | Normal | Normal | 35- 40 |
61, M |
Moderate- grave mitral insufficiency |
CABG | β blockers; Statines |
| HF | SR | Normal | Normal | Normal | Dilated | 30 | 79, M |
CABG | Antiarrhythmics |
AF, atrial fibrillation; AHT, arterial hypertension; AVS, Aortic valve surgery; CABG, coronary artery bypass graft; cAF, chronic AF; CAR, coronary artery revascularization; EF, ejection fraction; LA, left atrium; LV, left ventricle; NSR, normal sinus rhythm; RA, right atrium; RV, right ventricle.
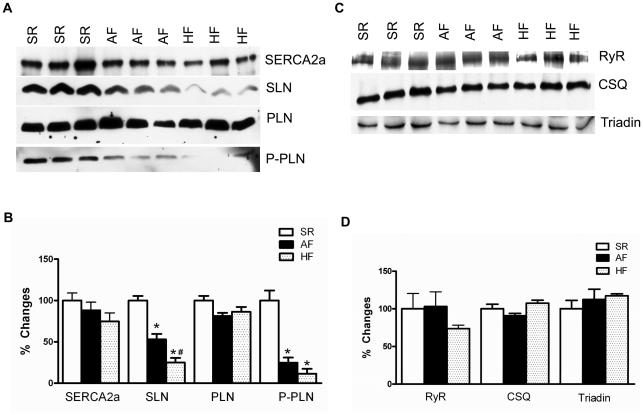
To determine the protein expression of various SR Ca2+ handling proteins, total protein prepared from atria of patients in NSR, AF and HF was studied by quantitative Western blot analysis. Results shown in Figure 1 point out that the level of SLN protein is significantly decreased both in AF (NSR: 100.0±5.5% vs. AF: 53.1±6.5%; P= 0.0053) and in HF (25.1±5.5%; P= 0.0006) atrial tissues compared to NSR patients. The protein levels of SERCA2a, PLN, ryanodine receptor (RyR), calsequestrin (CSQ) and triadin were not significantly different between all three groups of patients. Although PLN protein levels were not altered, PLN phosphorylation at Ser16 was significantly decreased in the atrial tissues of patients with AF (NSR: 100.0±1.0% vs. AF: 25.0±%) and HF (11.6±6.0%) compared to NSR controls.
Fig. 1.
Western blot analysis of SR Ca2+ uptake (A) and SR Ca2+ release (B) proteins in atria of human NSR, AF and HF patients. The fold change in expression levels of these proteins are shown in panel B and D; n=3 for each group. The expression level of CSQ was used as a loading control. Asterisks (*) indicate statistically significant differences in SLN levels or PLN phosphorylation in AF or HF samples compared to NSR controls; # from AF. P<0.05.
3.2. The SR Ca2+ uptake is increased in atria of patients with AF
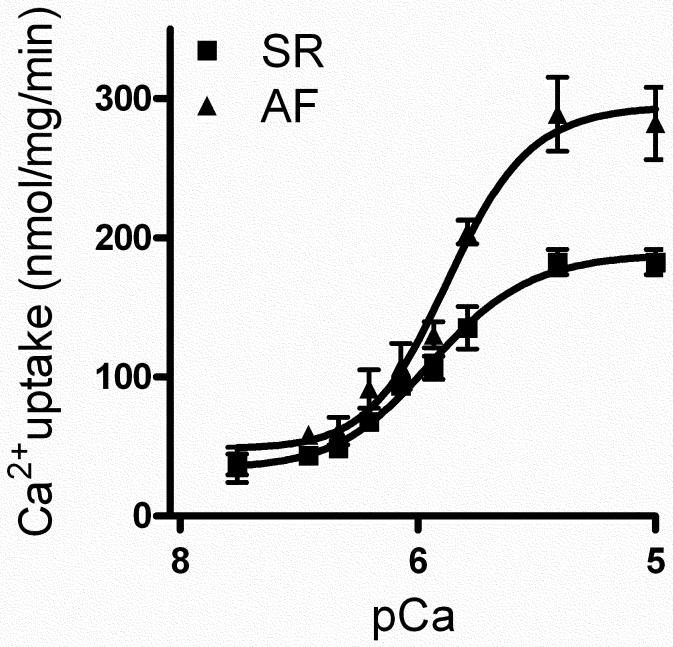
We next determined the rate as well as the maximum velocity (Vmax) of SR Ca2+ uptake in atria of patients with AF. The rates of Ca2+-dependent Ca2+ uptake was significantly increased in atria of patients with AF compared to the samples from NSR controls (Fig. 2). The EC50 value for Ca2+ uptake was significantly decreased in AF (NSR: 159±10 vs. AF: 129±9 nmol/L; P < 0.05) indicating an increased Ca2+ affinity of the SERCA pump. The Vmax of Ca2+ uptake was also significantly increased (NSR: 135±15 vs. AF: 204±8 nmol of Ca2+ per mg·min− 1; P < 0.05).
Fig. 2.
SR Ca2+ uptake. Ca2+-dependent SR Ca2+ uptake assays were performed by using total homogenates from atria of human AF and NSR patients. n=3 for each group. The Vmax of Ca2+ uptake was obtained at pCa 6.0.
3.3. Decreased expression of SR Ca2+ handling proteins in atria of mouse model of HF
To determine the expression of SR Ca2+ handling proteins in animal models of HF, we studied the expression of major SR Ca2+ handling proteins in atria and in the ventricles of pressure-overloaded mice hearts. Pressure-overload was imposed on the mouse heart by transverse aortic banding. After 3 weeks of aortic banding, the left ventricular ejection fraction decreased significantly compared to sham operated control mice (from 75±0.01% to 44±0.02%; N=4; P<0.05) indicating a cardiac dysfunction. The increased ratio of left ventricle (LV) weight to tibia length (from 3.0±0.2 to 4.7±0.6; P<0.05), and lung weight to body weight ratio (from 3.8±0.2 to 10.6±2.6; P<0.05) further confirmed that 3 weeks of aortic banding induced cardiac hypertrophy/heart failure. The left (LA) and right atrial (RA) weights were also significantly increased in the aortic banded mice (LA: from 4.9±0.7 to 10.8±1.3 mg; RA: from 4.0±0.4 to 8.0±1.5 mg; P<0.05)
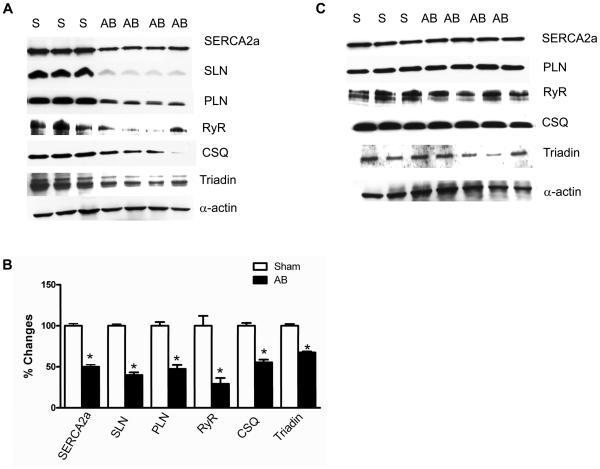
To determine the expression levels of SR Ca2+ handling proteins, we quantitated SERCA2a, SLN, PLN, RyR, CSQ and triadin protein levels in atria and in the ventricles of aortic banded mice. Results in Fig. 3A and 3B show that the expression of all the SR Ca2+ handling proteins analyzed were significantly decreased in atria of aortic banded mice. However in the LV of aortic banded mice, the expression of these proteins were not altered compared to the sham-operated controls (Fig. 3C).
Fig. 3.
Western blot analysis of SR Ca2+ handling proteins in atria (Panel A) and in the ventricles (Panel C) of 3 weeks aortic banded and sham-operated control mice. Panel B shows the quantitative analysis of SR Ca2+ handling protein levels in atria of 3 weeks aortic banded and sham-operated control mice. The expression level of sarcomeric α-actin was used as control. Asterisks (*) indicate statistically significant differences from sham-operated controls. S-sham-operated; AB- aortic banded. n=4; P<0.05.
4. Discussion
The key findings of the present study are: (i) sarcolipin protein expression is specifically decreased in human atria from AF and HF patients; (ii) the Ca2+ sensitivity as well as the Vmax of SR Ca2+ uptake are significantly increased in human atria from AF patients; and (iii) the expression of SR Ca2+ handling proteins is significantly decreased in atria but not in the ventricles of mouse models of HF.
Our studies demonstrate that SLN protein expression is selectively downregulated and the SR Ca2+ uptake is increased in the atria of AF patients. Our data corroborate the decreased SLN mRNA levels reported in atria of patients with chronic AF [9]. Studies using transgenic mouse models have suggested that unlike PLN, SLN can affect the Vmax of SR Ca2+ uptake. Transgenic overexpression of SLN in the mouse heart has been shown to decrease the Vmax of SR Ca2+ uptake [4]. Whereas, loss of SLN function in atria is associated with an increased Ca2+ sensitivity and an increased Vmax of SR Ca2+ uptake [10]. Thus, the increased Ca2+ sensitivity and Vmax of SR Ca2+ uptake in human AF could be due to the downregulation of SLN protein. The decreased basal level of PLN phosphorylation in AF and HF may be a compensatory alteration for the loss of SLN function.
Several studies have suggested abnormal Ca2+ handling as one of the major causes of atrial remodeling and arrhythmogenesis [13,14,15,16,17,18,19,20,21,22,23,24,25]. The atrial tachycardia remodeling which promotes AF in a goat model causes contractile dysfunction, mainly via Ca2+ handling abnormalities [23]. The reduced SR Ca2+ load and diastolic Ca2+ levels are suggested to cause atrial dilatation and AF in this model [16,20]. Studies from congestive HF dog model susceptible to sustained AF suggest that the increased SR Ca2+ load can contribute to the generation of delayed afterdepolarizations (DAD) and triggered activities in atrial myocytes [18]. Similarly, atrial arrhythmias observed during acidosis are associated with an increase in SR Ca2+ load [13]. Using a SLN knockout mouse model, we have shown that loss of SLN in atria is associated with increased SR Ca2+ load and atrial remodeling [10,11]. Thus, the increased SR Ca2+ uptake can cause an increased atrial SR Ca2+ load in AF patients. Altogether, these studies suggest that SLN is a key regulator of atrial SERCA pump and its downregulation in AF could lead to an increased SR Ca2+ load and contribute to abnormal intracellular Ca2+ handling and associated atrial remodeling.
Our studies also demonstrate that the expression pattern of SLN and other SR Ca2+ handling proteins in atria of human HF patients is similar to that of AF patients. These data suggest that the selective downregulation of SLN can enhance the SERCA activity and SR Ca2+ uptake in atria in HF as observed in AF. On the other hand, in mouse models of cardiac hypertrophy/heart failure, the expression level of all major SR Ca2+ handling proteins tested was significantly decreased in atria but not in ventricles. This is in contrast to studies showing a decrease in SERCA levels and SR Ca2+ uptake in hypertrophic ventricles [26,27]. Although the reason for this discrepancy is unknown, this could be due to the greater atrial structural remodeling including fibrosis and decreased muscle content in aortic banded mice. The supportive evidence also came from a recent study which reported that atrial fibroblasts are consistently more reactive than the ventricular fibroblasts and contribute to increased atrial fibrosis during HF [28]. These studies along with our data showing atrial dilatation in aortic banded mice suggest that atria may be more sensitive to pressure-overload than ventricles.
In conclusion, the present study is the first report to demonstrate the decreased levels of SLN protein and its effect on SR Ca2+ uptake in human atria of AF patients. Our studies suggest that the specific downregulation of SLN in AF and HF could contribute for the abnormal Ca2+ handling and atrial remodeling.
Highlights.
Sarcolipin, a key regulator of atrial SERCA pump, is specifically downregulated in human atrial fibrillation and heart failure
The SR Ca2+ uptake is significantly increased in human atria from atrial fibrillation patients
The expression of SR Ca2+ handling proteins is decreased in atria but not in the ventricles of aortic banded mice
Acknowledgements
This work was supported by the funds from the Department of Cell Biology and Molecular Medicine, UMDNJ-NJMS, Newark, NJ (to GJB), the Fondation Leducq for the Transatlantic Network of Excellence cycAMP grant 06CVD02 (to RF), and the Fondation Lefoulon-Delalande (to CEM).
Abbreviations
- AF
atrial fibrillation
- CSQ
calsequestrin
- HF
heart failure
- LV
left ventricle
- NSR
normal sinus rhythm
- PLN
phospholamban
- RA
right atrium
- RyR
ryanodine receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+ ATPase
- SLN
sarcolipin
- SR
sarco(endo)plasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu GJ, Zheng Z, Natarajan P, Wheeler D, Janssen PM, Periasamy M. Overexpression of sarcolipin decreases myocyte contractility and calcium transient. Cardiovasc Res. 2005;65:177–186. doi: 10.1016/j.cardiores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem. 2006;281:3972–3979. doi: 10.1074/jbc.M508998200. [DOI] [PubMed] [Google Scholar]
- 5.Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini AO, Trivieri MG, Oudit GY, Morita T, Kusakari Y, Hirano S, Hongo K, Hirotani S, Yamaguchi O, Peterson A, Backx PH, Kurihara S, Hori M, MacLennan DH. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci U S A. 2004;101:9199–9204. doi: 10.1073/pnas.0402596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gramolini AO, Trivieri MG, Oudit GY, Kislinger T, Li W, Patel MM, Emili A, Kranias EG, Backx PH, Maclennan DH. Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc Natl Acad Sci U S A. 2006;103:2446–2451. doi: 10.1073/pnas.0510883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhupathy P, Babu GJ, Ito M, Periasamy M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol. 2009;47:723–729. doi: 10.1016/j.yjmcc.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimura M, Minamisawa S, Yokoyama U, Umemura S, Ishikawa Y. Mechanical stress-dependent transcriptional regulation of sarcolipin gene in the rodent atrium. Biochem Biophys Res Commun. 2005;334:861–866. doi: 10.1016/j.bbrc.2005.06.186. [DOI] [PubMed] [Google Scholar]
- 9.Uemura N, Ohkusa T, Hamano K, Nakagome M, Hori H, Shimizu M, Matsuzaki M, Mochizuki S, Minamisawa S, Ishikawa Y. Down-regulation of sarcolipin mRNA expression in chronic atrial fibrillation. Eur J Clin Invest. 2004;34:723–730. doi: 10.1111/j.1365-2362.2004.01422.x. [DOI] [PubMed] [Google Scholar]
- 10.Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie LH, Shanmugam M, Park JY, Zhao ZH, Tian B, Periasamy M, Babu GJ. Loss of Sarcolipin is Associated With Structural and Electrical Remodeling of Atria. Circulation. 2009;120:S675–S676. [Google Scholar]
- 12.Shanmugam M, Gao S, Hong C, Fefelova N, Nowycky MC, Xie LH, Periasamy M, Babu GJ. Ablation of phospholamban and sarcolipin results in cardiac hypertrophy and decreased cardiac contractility. Cardiovasc Res. 2011;89:353–361. doi: 10.1093/cvr/cvq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol. 2008;295:H1669–1683. doi: 10.1152/ajpheart.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stambler BS, Fenelon G, Shepard RK, Clemo HF, Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 15.Dobrev D. Atrial Ca2+ signaling in atrial fibrillation as an antiarrhythmic drug target. Naunyn Schmiedebergs Arch Pharmacol. 2009 doi: 10.1007/s00210-009-0457-1. [DOI] [PubMed] [Google Scholar]
- 16.Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008;52:293–299. doi: 10.1097/FJC.0b013e318171924d. [DOI] [PubMed] [Google Scholar]
- 17.Dobrev D, Teos LY, Lederer WJ. Unique atrial myocyte Ca2+ signaling. J Mol Cell Cardiol. 2009;46:448–451. doi: 10.1016/j.yjmcc.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 19.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Gaspo R, Leblanc N, Nattel S. Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation. 1998;98:719–727. doi: 10.1161/01.cir.98.7.719. [DOI] [PubMed] [Google Scholar]
- 21.Fenelon G, Shepard RK, Stambler BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:1093–1102. doi: 10.1046/j.1540-8167.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 23.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 24.Dobrev D, Wehrens XH. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med. 20:30–34. doi: 10.1016/j.tcm.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llach A, Molina CE, Fernandes J, Padro J, Cinca J, Hove-Madsen L. Sarcoplasmic reticulum and L-type Ca2+ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J Physiol. doi: 10.1113/jphysiol.2010.197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarain-Herzberg A. Regulation of the sarcoplasmic reticulum Ca2+-ATPase expression in the hypertrophic and failing heart. Can J Physiol Pharmacol. 2006;84:509–521. doi: 10.1139/y06-023. [DOI] [PubMed] [Google Scholar]
- 27.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 28.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]