Abstract
Objective
Patients on dialysis maintain extremely low levels of physical activity. Prior studies have demonstrated a direct correlation between nutrition and physical activity but provide conflicting data on the link between inflammation and physical activity. Using a cohort of patients new to dialysis from the Comprehensive Dialysis Study (CDS), we examined associations of self-reported physical activity with laboratory markers of nutrition and inflammation.
Design, setting & patients
Between September 2005 and June 2007, CDS collected data on self-reported physical activity, nutrition and health-related quality of life from patients starting dialysis in 296 facilities located throughout the United States. Baseline serum samples were collected from participants in a nutrition sub-study of CDS.
Measures
Serum albumin and prealbumin were measured as markers of nutrition and C-reactive protein and α-1-acid glycoprotein as markers of inflammation. Self-reported physical activity was characterized by the maximal activity score (MAS) and adjusted activity score (AAS) of the Human Activity Profile.
Results
The mean age of participants in the analytic cohort (n=201) was 61 years. The MAS and AAS were below the 10th and first percentile respectively in comparison to healthy 60 year-old norms. Both activity scores were directly correlated with albumin (r2=0.3, p<0.0001) and prealbumin (r2=0.3, p<0.0001), and inversely correlated with C-reactive protein (r2=-0.2, p=0.01 for AAS and r2 = -0.1, p=0.08 for MAS). In multivariable analyses adjusting for age, sex, race/ethnicity, diabetes status and center, both activity scores were directly correlated with prealbumin and inversely correlated with C-reactive protein.
Conclusions
Patients new to dialysis with laboratory-based evidence of malnutrition and/or inflammation are likely to report lower levels of physical activity.
Keywords: Physical activity, albumin, prealbumin, C-reactive protein, inflammation
Introduction
Physical activity substantially improves health and function in the general population. Higher levels of physical activity are associated with lower mortality in a dose-dependent manner [1-4]. Underlying this survival benefit is the reduced impact of many major diseases in physically active individuals.
The incidence of primary and secondary events related to coronary heart disease, diabetes, osteoporosis and cancer is 25-50% lower in physically active adults [1, 5-8]. Patients with diabetes who walk at least two hours per week experience all-cause and cardiovascular mortality one-third of that seen among those who remain sedentary [7]. Older adults who maintain higher levels of physical activity are subject to less disability and experience lower rates of cognitive decline [1, 9].
Patients on dialysis have very low levels of physical activity, even when compared to sedentary individuals [10]. Moreover patients on dialysis carry an immense burden of physical disability and chronic disease. Sedentary patients starting dialysis have 60% higher one-year mortality than their physically active counterparts [11].
Research into reasons for low levels of physical activity in patients on dialysis has identified muscle atrophy as a potential contributing cause [12-14]. Muscle atrophy is further linked with both malnutrition and inflammation [15]. Studies examining associations among malnutrition, inflammation and physical activity are few in number and small in size [16-20]. These studies reported a direct correlation between nutritional status and physical activity [16-17, 19-20], but yielded conflicting data on the link between inflammation and physical activity [16, 18-20].
In a well-characterized cohort of patients new to dialysis, we examined the relations among self-reported physical activity, nutritional markers (albumin and prealbumin) and inflammatory markers (C-reactive protein (CRP) and α-1-acid glycoprotein (AAG)). We hypothesized that in patients on dialysis, laboratory proxies of malnutrition would be directly associated and laboratory proxies of inflammation inversely associated with self-reported physical activity.
Methods
Comprehensive Dialysis Study
The Comprehensive Dialysis Study (CDS) is a United States Renal Data System (USRDS) Special Study implemented cooperatively by the USRDS Coordinating Center and the Special Studies Centers in Nutrition and Rehabilitation/Quality of Life. The study was approved by institutional review boards at University of California San Francisco and Davis, Emory University, Stanford University and University of Minnesota. Details of the study protocol and data elements are described elsewhere [21]. Briefly, a total of 1,677 patients starting dialysis between September 2005 and June 2007 were recruited from 296 dialysis units throughout the United States. Participants answered questions about demographics, nutrition, physical activity and health-related quality of life via a telephone interview.
All participants in the CDS provided informed consent. As participants in the nutrition sub-study of the CDS, 266 patients from 68 of the 296 dialysis units selected via probability sampling proportional to estimated facility size also consented to provide quarterly serum specimens. We further restricted the current analysis to individuals whose baseline serum samples were obtained within 120 days of patient questionnaire administration.
Measure of self-reported physical activity
The Human Activity Profile (HAP) is a questionnaire that ranks 94 physical tasks according to level of energy consumption [22-23]. The least strenuous activity (transferring out of bed or chair without assistance) is assigned the first rank and running 3 miles in 30 minutes or less is ranked number 94. The activities covered in the HAP range from 1 to 10 Metabolic Equivalent of Task (METs). Respondents are asked to indicate whether they are still doing, have stopped doing or never did each activity. Two measures are then calculated: the maximal activity score (MAS) and adjusted activity score (AAS).
The MAS represents the highest ranked activity that the participant is still performing, and thus indicates the participant's maximal potential energy expenditure. The AAS subtracts from MAS the total number of activities that the participant has stopped performing and rank below his or her MAS. For example if a participant's MAS indicates that he or she can walk a half block uphill non stop (rank 40) but he or she has stopped climbing nine steps (rank 37) and carrying groceries (rank 36) then his or her AAS would be 38. Thus, the AAS more accurately reflects the participant's daily activities.
Laboratory markers
Samples of the four laboratory proxies (albumin, prealbumin, CRP and AAG) were collected at the participants' dialysis units at the time of their routine monthly labs and held on dry ice. The serum specimens were then delivered overnight to, and processed at, a central laboratory at the University of California Davis. All four laboratory markers were measured nephelometrically and the measurements made in duplicate as described previously [24-25]. Data presented here utilize the mean of two measurements.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median with 10th and 90th percentile range; categorical data are presented as proportions. We compared continuous variables with unpaired Student's t-tests and categorical variables with the chi square or Fisher Exact test.
Univariate correlation among HAP scores and laboratory markers was assessed using the Pearson (parametric) correlation coefficient. Since CRP was heavily skewed, we utilized the natural log transformed CRP for inference testing. In multivariable analyses, we adjusted for age, sex, race/ethnicity and diabetes status using a random effects linear regression model to account for clustering of individuals within dialysis facilities. Because all facilities did not have an equal probability of being included in the nutrition sub-study of the CDS, multivariable analyses also included a weight for each facility based on its probability of inclusion. Companion models also adjusted for modality (hemodialysis versus peritoneal dialysis) or vascular access in addition to age, sex, race/ethinicity, diabetes status and facility. We considered 2-tailed p-values <0.05 as statistically significant. All analyses were conducted using SAS 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Two hundred and one patients provided serum samples within 120 days of HAP questionnaire administration (Figure 1). There were no statistically significant differences in demographic characteristics between the analytic cohort and the larger CDS sample (Table 1). In the analytic cohort, the mean age was 61 ± 14 years with a slight male predominance and an average Quetélet's (body mass) index in the overweight category (Table 1). About half of patients undergoing hemodialysis were using a catheter for access, and more than half had diabetes mellitus. The mean MAS score of 63 (out of possible 94) on the HAP questionnaire falls below the 10th percentile in comparison to healthy 60 year-old norms; the mean AAS score of 45 falls below the first percentile [23].
Figure 1. Analytic cohort for current study in relation to larger Comprehensive Dialysis Study.
A total of 265 participants agreed to participate in the nutrition sub-study and provide serum samples. Of these, data were available from both patient questionnaires and serum samples from 225 participants. The current analysis is limited to 201 participants who completed the questionnaire within 120 days of providing serum samples. Abbreviations: CDS-Comprehensive Dialysis study; HAP-Human activity profile.
Table 1. Characteristics of Comprehensive Dialysis Study participants.
| Participants with physical activity data | Participants with physical activity and laboratory data* | ||
|---|---|---|---|
| Characteristic | Mean ± standard deviation or N (%) (n=1424) | Mean ± standard deviation or N (%) (n=201) | p value |
| Age (years) | 60.3 ± 14.2 | 61.4 ± 14.1 | 0.31 |
| Sex, male | 787 (55.3%) | 104 (51.7%) | 0.35 |
| Race, white | 969 (68.1%) | 148 (73.6%) | 0.11 |
| Current smoker | 221 (15.5%) | 27 (13.4%) | 0.44 |
| College education or more | 626 (44.0%) | 97 (48.3%) | 0.27 |
| Body mass index (kg/m2) | 29.8 ± 8.1 | 29.9 ± 7.8 | 0.78 |
| Peritoneal dialysis | 144 (10.1%) | 24 (11.9%) | 0.43 |
| Hemodialysis access via catheter† | 719 (56.6%) | 89 (50.9%) | 0.33 |
| Co-morbidity | |||
| Diabetes | 823 (57.8%) | 116 (57.7%) | 0.98 |
| Congestive Heart Failure | 430 (30.2%) | 67 (33.3%) | 0.37 |
| Atherosclerotic disease‡ | 485 (34.1%) | 69 (34.3%) | 0.94 |
| Human Activity Profile scores | |||
| Maximal activity score | 60.8 ± 16.0 | 62.9 ± 13.8 | 0.08 |
| Adjusted activity score | 43.0 ± 18.7 | 45.3 ± 17.3 | 0.10 |
This group is comprised of patients whose laboratory data was obtained within 120 days of completing the patient questionnaire. All other CDS participants with Human Activity Profile scores are included in the first group (n=1424).
Proportions expressed in this row are based on patients on hemodialysis only.
Atherosclerotic disease includes: coronary heart disease, cerebrovascular disease, peripheral vascular disease or amputation.
The mean and median time between survey administration and acquisition of serum sample was 50 and 30 days, respectively. Participants' mean (± standard deviation) albumin and prealbumin concentrations were 3.5 (± 4.6) g/dL, and 31.4 (± 9.2) mg/dL respectively. Albumin and prealbumin were directly correlated (r2 = 0.5, p<0.0001). C-reactive protein concentrations were right skewed with a median of 7.5 (10th percentile 2.3, 90th percentile 25.2) mg/L and mean of 12.6 (± 17.1) mg/L. Both values are above the 90th percentile for the general population [26]. C-reactive protein was inversely correlated with both albumin (r2 = -0.2, p = 0.01) and prealbumin (r2 = -0.3, p < 0.01). The mean AAG concentration was 115.0 (± 34.7) mg/dL and as expected, AAG was directly correlated with CRP (r2=0.5, p<0.0001). There were no statistically significant correlations between AAG and albumin (r2 =-0.01, p=0.90), or AAG and prealbumin (r2=-0.002, p=0.97).
Relations among nutritional markers, inflammatory markers and self-reported physical activity
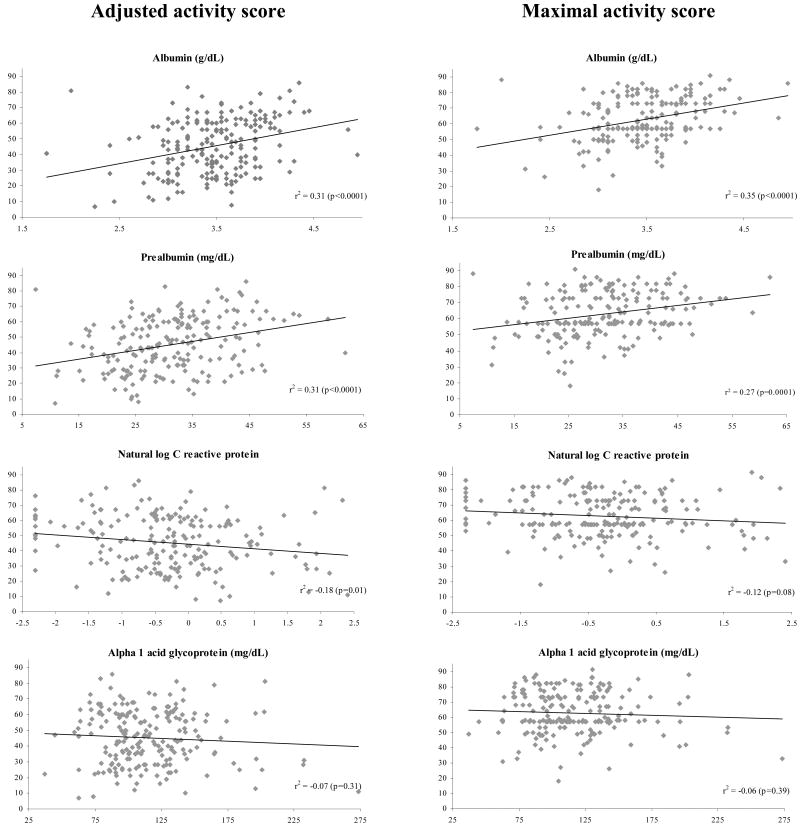
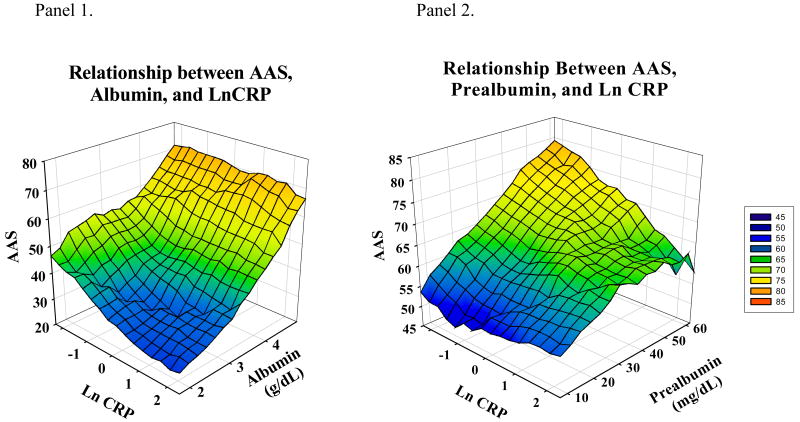
On unadjusted analysis, serum albumin and prealbumin were directly correlated with AAS and MAS scores (Figure 2). C-reactive protein was inversely correlated with AAS; the correlation between CRP and MAS was not statistically significant. AAG was not significantly correlated with AAS or MAS. To show the relations among malnutrition, inflammation and self-reported physical activity, Figure 3 graphically displays AAS scores by albumin and CRP (panel 1) and prealbumin and CRP (panel 2).
Figure 2. Correlation of AAS and MAS with laboratory markers.
Activity scores can range from 0-94. Pearson r2 and corresponding p values are displayed. Significant correlation was seen between AAS and albumin, prealbumin and CRP; and between MAS and albumin and prealbumin. Abbreviations: AAS-Adjusted activity score; MAS-Maximal activity score; CRP-C-reactive protein.
Figure 3. Relationship between AAS, albumin or prealbumin and ln CRP.
In unadjusted analyses, AAS levels were highest when prealbumin or albumin were high and CRP low. Lowest AAS levels were seen when albumin or prealbumin were low and ln CRP was high. In Panel 2, AAS levels remained relatively low when ln CRP was high, despite high concentrations of prealbumin. Abbreviations: AAS-adjusted activity score, Ln-Natural log; CRP-C-reactive protein.
In multivariable analyses adjusting for age, sex, race/ethnicity, diabetes status and center, albumin remained independently associated with MAS (Table 3). Prealbumin and CRP were independently associated with AAS and MAS. Alpha-1-acid glycoprotein was not significantly associated with either AAS or MAS.
Similar results were obtained when the analytic sample was restricted to the 158 participants whose laboratory draw was within 60, rather than 120 days of the administration of the HAP questionnaire. In this smaller, more proximate sample, the association between albumin and AAS was statistically significant, whereas the associations among CRP and both activity scores, while still negative in trend and similar in magnitude, failed to reach statistical significance. Additional analyses adjusting for modality or vascular access resulted in no change in the directionality and minimal change in the magnitude of correlation between each laboratory marker and AAS or MAS (data not shown).
Discussion
In a geographically diverse cohort of hemodialysis and peritoneal dialysis patients, we found that nutritional and inflammatory markers were significantly associated with self-reported physical activity. To put these results in context, a prealbumin concentration higher by three mg/dL or CRP concentration lower by 40-50% was associated with roughly a one point higher AAS and MAS. For a participant with a mean AAS score of 45, a one point difference would reflect the difference between whether he or she can walk two blocks on level ground rather than one. The largest incremental differences in self-reported activity scores were noted in relation to age and sex. A 10-year difference in age was associated with a four point difference in activity scores; men had five to six point higher activity scores compared with women.
Serum albumin and prealbumin (also known as transthyretin) are hepatic secretory proteins, widely used as indicators of nutritional status, specifically as reflective of visceral protein stores [27]. The direct correlation between albumin and physical activity has been demonstrated previously [10, 17, 19, 28]. In one of the largest analyses of self-reported physical activity in dialysis patients, participants in the Dialysis Morbidity and Mortality study (n=2,264) with higher levels of albumin were less likely to report limitations in moderate physical activity [28]. Herein we demonstrate that prealbumin—which has a shorter half life than albumin (2 days versus 22 days, respectively)—is associated with maximal and usual levels of physical activity.
Since albumin and prealbumin are both acute phase reactants, whether the correlations among these proteins and physical activity are uniquely attributable to malnutrition, or whether inflammation also contributes to lower levels of physical activity in the dialysis population has been difficult to resolve [12-13, 16, 18-20, 29]. Malnourished individuals have lower protein stores and decreased muscle mass—which in turn has been linked to lower levels of physical activity [13-14]. But inflammatory cytokines also stimulate a catabolic state that induces proteolysis within muscles and prevents restoration of lost muscle protein mass [30]. Kaizu et al. demonstrated that muscle area was correlated directly with serum albumin and inversely with interleukin-6 and CRP [15]. Hepatic synthesis of CRP and AAG is increased in response to inflammation [31-32]. In our study CRP concentrations were, not unexpectedly, very high; only 18% of patients fell below the proposed laboratory cut point for “normal” (< 3.0 mg/L) [26]. Similarly high concentrations of CRP (median concentrations around 8 mg/L) have been reported in other cross sectional and longitudinal studies of the dialysis population [33-35].
Prior cross sectional studies have failed to confirm a relation between physical activity and proxies of inflammation [18-20]. These studies were small, with sample sizes of 60 or fewer participants. In the CDS, multivariable analyses confirmed an independent association of C-reactive protein with self-reported physical activity, suggesting that both inflammation and malnutrition are associated with lower levels of physical activity in the dialysis population.
Given that our analysis is cross sectional, we cannot determine whether inflammation causes lower levels of physical activity, or if the reverse is true. In a small randomized controlled trial of progressive resistance training in patients on hemodialysis, CRP concentrations decreased and were significantly lower by the end of a 12 week trial period in the treatment group relative to controls—suggesting that the process of resistance exercise and/or improved muscle strength may reduce inflammation [29].
This study has several strengths. It represents the largest experience linking laboratory markers and self-reported physical activity in the dialysis population to date. Further, the CDS included hemodialysis and peritoneal dialysis patients and employed a geographically diverse, stratified random sampling strategy to recruit participants from dialysis facilities throughout the United States. We incorporated multiple laboratory markers and a well established instrument to assess physical activity—one that we and others had validated in the dialysis population [36-37]. Finally, we incorporated widely recommended statistical approaches to account for clustering by facility and for the differential probability of inclusion of patients by facility. There are also several limitations. We employed an indirect, self-reported measure of physical activity. Accelerometry was not feasible here, given the national scope of the CDS and the training, expense and quality control that would have been required for implementation. Nevertheless, we were confident that the HAP would provide a reasonable estimate of true physical activity [22, 38-40]. Although we adjusted for geographical clustering and unequal sampling probability according to size of facilities, the tendency for HAP scores to be slightly higher in participants of the nutrition sub-study as compared to the larger CDS sample may decrease the generalizability of our results. Finally, a shorter time period between HAP administration and serum sample collection would have been ideal in order to minimize confounding by changes in participants' clinical status. However, restricting the analytic sample to patients with laboratory and HAP assessments within 60 rather than 120 days did not materially alter results.
In summary, patients new to dialysis with laboratory markers indicative of malnutrition or inflammation report lower levels of physical activity. While statistically significant, the relations are modest, explaining less than five percent of the variation in self-reported physical activity. Further research is need to delineate other modifiable risk factors for low levels of physical activity in the dialysis population and to determine whether correcting malnutrition, inflammation or both can increase physical activity, or alternatively whether increasing physical activity can improve nutritional status or inflammation.
Table 2. Multivariate analyses the associations among AAS and MAS and laboratory markers.
| Adjusted activity score | Maximal activity score | |||
|---|---|---|---|---|
| Model components | β coefficient ± SE | p value | β coefficient ± SE | p value |
| Age (per 10 years) | -3.9 ± 0.9 | <0.01 | -1.5 ± 0.9 | 0.0753 |
| Sex, male | 5.7 ± 3.1 | 0.07 | 5.0 ± 2.7 | 0.07 |
| Race, white | -1.1 ± 3.6 | 0.75 | 1.2 ± 2.2 | 0.59 |
| Diabetes | -3.7 ± 2.5 | 0.14 | -2.1 ± 1.8 | 0.23 |
| Albumin (g/L) | 0.6 ± 0.3 | 0.06 | 0.8 ± 0.2 | <0.01 |
| Adjusted activity score | Maximal activity score | |||
|---|---|---|---|---|
| Model components | β coefficient ± SE | p value | β coefficient ± SE | p value |
| Age (per 10 years) | -3.9 ± 0.8 | <0.01 | -1.8 ±0.9 | 0.05 |
| Sex, male | 5.7 ± 3.0 | 0.06 | 5.5 ± 2.8 | 0.05 |
| Race, white | -0.5 ± 3.4 | 0.89 | 2.0 ± 2.2 | 0.38 |
| Diabetes | -2.7 ± 2.2 | 0.24 | -1.9 ± 1.9 | 0.34 |
| Prealbumin (mg/dL) | 0.4 ± 0.1 | <0.01 | 0.3 ±0.1 | 0.04 |
| Adjusted activity score | Maximal activity score | |||
|---|---|---|---|---|
| Model components | β coefficient ± SE | p value | β coefficient ± SE | p value |
| Age (per 10 years) | -4.3 ± 0.8 | <0.01 | -2.1 ±0.7 | <0.01 |
| Sex, male | 6.0 ± 3.0 | <0.01 | 5.7 ± 2.7 | <0.01 |
| Race, white | 0.5 ± 3.1 | 0.88 | 2.6 ± 2.1 | 0.26 |
| Diabetes | -3.8 ± 2.4 | 0.11 | -2.7 ± 1.8 | 0.15 |
| Ln C-reactive protein | -0.3 ± 0.1 | 0.02 | -0.2 ±0.1 | 0.03 |
| Adjusted activity score | Maximal activity score | |||
|---|---|---|---|---|
| Model components | β coefficient ± SE | p value | β coefficient ± SE | p value |
| Age (per 10 years) | -4.2 ± 0.8 | <0.0001 | -2.1 ±0.8 | 0.01 |
| Sex, male | 5.4 ± 2.9 | 0.07 | 5.2 ± 2.8 | 0.07 |
| Race, white | -0.5 ± 3.2 | 0.88 | 2.8 ± 2.2 | 0.21 |
| Diabetes | -4.3 ± 2.3 | 0.07 | -2.9 ± 1.8 | 0.10 |
| α-1-acid glycoprotein (mg/dL) | -0.1 ± 0.04 | 0.06 | -0.1 ± 0.03 | 0.08 |
β coefficient can be interpreted as change in activity scores for a 10% difference in C-reactive protein. Abbreviations: SE-standard error; Ln-natural log
Acknowledgments
This work was funded by contract N01-DK-7-0005 from the NIDDK. Dr. Anand was supported by F32 DK 084697. Dr. Chertow was supported by K24 DK 080645. Drs. Chertow, Johansen, Grimes, and Kaysen were supported by N01 DK012450.
Footnotes
Institution where work was primarily conducted: Division of Nephrology, Stanford University School of Medicine 780 Welch Road, Suite 106 Palo Alto CA 94304
The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shuchi Anand, Division of Nephrology, Department of Medicine, Stanford UniversitySchool of Medicine.
Glenn M. Chertow, Division of Nephrology, Department of Medicine, StanfordUniversity School of Medicine.
Kirsten L. Johansen, Division of Nephrology, Department of Medicine, University of California, San Francisco and Department of Veterans Affairs Medical Center, San Francisco, California.
Barbara Grimes, Department of Epidemiology and Biostatistics, University of California, San Francisco.
Manjula Kurella Tamura, Division of Nephrology, Department of Medicine, Stanford University School of Medicine.
Lorien S. Dalrymple, Division of Nephrology, Department of Medicine, University of California Davis.
George A. Kaysen, Division of Nephrology, Department of Medicine, and Department of Biochemistry and Molecular Medicine, University of California, Davis and Department of Veterans Affairs Medical Center, Mather, California.
References
- 1.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–9. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haskell WL, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 3.Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;49(1):122–8. doi: 10.1002/art.10907. [DOI] [PubMed] [Google Scholar]
- 4.Kodama S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 5.Tanasescu M, et al. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham AJ, et al. A randomized controlled trial of the effects of group psychological therapy on survival in women with metastatic breast cancer. Psychooncology. 1998;7(6):508–17. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<508::AID-PON376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, et al. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163(12):1440–7. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 8.Shea B, et al. Cochrane Review on exercise for preventing and treating osteoporosis in postmenopausal women. Eura Medicophys. 2004;40(3):199–209. [PubMed] [Google Scholar]
- 9.Scarmeas N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–37. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen KL, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–70. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 11.O'Hare AM, et al. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–54. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 12.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9(3):218–35. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 13.Johansen KL, et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63(1):291–7. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre CW, et al. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006;21(8):2210–6. doi: 10.1093/ndt/gfl064. [DOI] [PubMed] [Google Scholar]
- 15.Kaizu Y, et al. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis. 2003;42(2):295–302. doi: 10.1016/s0272-6386(03)00654-1. [DOI] [PubMed] [Google Scholar]
- 16.Johansen KL, et al. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77(4):842–6. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 17.Johansen KL, et al. Determinants of physical performance in ambulatory patients on hemodialysis. Kidney Int. 2001;60(4):1586–91. doi: 10.1046/j.1523-1755.2001.00972.x. [DOI] [PubMed] [Google Scholar]
- 18.Hung AM, et al. Inflammatory markers are unrelated to physical activity, performance, and functioning in hemodialysis. J Ren Nutr. 2002;12(3):170–6. doi: 10.1053/jren.2002.33513. [DOI] [PubMed] [Google Scholar]
- 19.Zamojska S, et al. Correlates of habitual physical activity in chronic haemodialysis patients. Nephrol Dial Transplant. 2006;21(5):1323–7. doi: 10.1093/ndt/gfi323. [DOI] [PubMed] [Google Scholar]
- 20.Majchrzak KM, et al. Physical activity patterns in chronic hemodialysis patients: comparison of dialysis and nondialysis days. J Ren Nutr. 2005;15(2):217–24. doi: 10.1053/j.jrn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Kutner NG, et al. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4(3):645–50. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson M, de Morton N. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21(2):151–62. doi: 10.1177/0269215506069475. [DOI] [PubMed] [Google Scholar]
- 23.Fix A, Daughton D. Human Activity Profile Professional Manual. Odessa, FL: Psychological Assessment Resources Inc.; 1988. [Google Scholar]
- 24.Kaysen GA, et al. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 2004;65(4):1408–15. doi: 10.1111/j.1523-1755.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 25.Don BR, et al. The effect of etanercept on suppression of the systemic inflammatory response in chronic hemodialysis patients. Clin Nephrol. 2010;73(6):431–8. doi: 10.5414/cnp73431. [DOI] [PubMed] [Google Scholar]
- 26.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem. 2003;49(4):666–9. doi: 10.1373/49.4.666. [DOI] [PubMed] [Google Scholar]
- 27.Fauci AS. Harrison's Principles of Internal Medicine. McGraw-Hill Companies; 2008. [Google Scholar]
- 28.Stack AG, Murthy B. Exercise and limitations in physical activity levels among new dialysis patients in the United States: an epidemiologic study. Ann Epidemiol. 2008;18(12):880–8. doi: 10.1016/j.annepidem.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Cheema B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 30.Lang CH, et al. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282(2):E336–47. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 31.Kaysen GA. Malnutrition and the acute-phase reaction in dialysis patients-how to measure and how to distinguish. Nephrol Dial Transplant. 2000;15(10):1521–4. doi: 10.1093/ndt/15.10.1521. [DOI] [PubMed] [Google Scholar]
- 32.Hochepied T, et al. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003;14(1):25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 33.deFilippi C, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290(3):353–9. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrom J, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial. 2000;13(3):163–75. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaysen GA, et al. The acute-phase response varies with time and predicts serum albumin levels in hemodialysis patients. The HEMO Study Group. Kidney Int. 2000;58(1):346–52. doi: 10.1046/j.1523-1755.2000.00172.x. [DOI] [PubMed] [Google Scholar]
- 36.Johansen KL, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59(3):1121–7. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 37.Wellard S. Validation of physical activity measurement for people on dialysis treatment. EDTNA ERCA J. 2003;29(3):140–2. doi: 10.1111/j.1755-6686.2003.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira-Salmela LF, Devaraj R, Olney SJ. Validation of the human activity profile in stroke: a comparison of observed, proxy and self-reported scores. Disabil Rehabil. 2007;29(19):1518–24. doi: 10.1080/09638280601055733. [DOI] [PubMed] [Google Scholar]
- 39.Bennell KL, et al. Is the Human Activity Profile a useful measure in people with knee osteoarthritis? J Rehabil Res Dev. 2004;41(4):621–30. doi: 10.1682/jrrd.2003.07.0103. [DOI] [PubMed] [Google Scholar]
- 40.Nield M, et al. Usefulness of the human activity profile, a functional performance measure, in people with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25(2):115–21. doi: 10.1097/00008483-200503000-00012. [DOI] [PubMed] [Google Scholar]