Abstract
In adults, obesity has been associated with disinhibited eating, decreased cortical gray matter volume, and lower performance on cognitive assessments. Much less is known about these relationships in adolescence and there are no studies assessing behavioral, cognitive, and neurostructural measures in the same group of study participants. This study examined the relationship between obesity, executive function, disinhibition, and brain volumes in relatively healthy youth. Participants included 54 obese and 37 lean adolescents. Participants received a cognitive battery, questionnaires of eating behaviors, and magnetic resonance imaging (MRI). Neuropsychological assessments included tasks targeting frontal lobe function. Eating behaviors were determined using the Three Factor Eating Questionnaire (TFEQ), and structural MRIs were performed on a 1.5 T Siemens Avanto MRI System (Siemens, Erlangen, Germany) to determine brain gray matter volumes. Lean and obese adolescents were matched on age, years of education, gender, and socioeconomic status. Relative to lean adolescents, obese participants had significantly higher ratings of disinhibition on the TFEQ, lower performance on the cognitive tests, and lower orbitofrontal cortex volume. Disinhibition significantly correlated with Body Mass Index, Stroop Color-Word score, and orbitofrontal cortex volume. This is the first report of these associations in adolescents and point to the importance of better understanding the associations between neurostructural deficits and obesity.
Keywords: Obesity, Adolescents, Disinhibition, MRI, Frontal Lobe, Cognition, Orbitofrontal Cortex
Introduction
The prevalence of child and adolescent obesity in the US has more than tripled since 1970. Although recent evidence suggests that childhood obesity may have leveled off, the present high rates predict an impending public health problem involving cardiovascular and endocrine illness (1).
Disinhibition in eating behavior, which is characterized in part as the propensity to eat opportunistically in response to environmental cues, has long been associated with obesity both in youth and adults (2). The related control failure in caloric intake that leads to eventual obesity may occur at several levels in the brain including the hypothalamus (3) and, according to more recent work, in cerebral cortex (4). A series of functional neuroimaging studies of lean and obese individuals in both hungry and fed states have shown several cortical regions including the anterior cingulate, medial prefrontal (5), insula, posterior cingulate, temporal, and orbitofrontal cortices (6) to be differentially activated depending on the level of satiety and BMI, suggesting their involvement in the regulation of caloric intake. The understanding of OFC as a key area in inhibition of behavior stretches back to the case of Phineas Gage, the unfortunate 19th century rail worker who survived an accident likely damaging his orbitofrontal cortex, which resulted in personality changes and increased impulsivity (7).
Neurostructural findings have also been correlated with Body Mass Index (BMI). In a small study of women 55 years of age and older, which employed voxel-based morphometry (VBM), BMI was negatively correlated with gray matter volumes in several frontal areas including the left orbitofrontal, right inferior frontal, and right precentral gyri in addition to other regions including the right cerebellum as well as a large right posterior region encompassing the parahippocampal, fusiform, and lingual gyri (8). A larger study of 1,428 adults found a negative correlation among males between BMI and overall gray matter as well as specific brain regions such as bilateral medial temporal lobes, occipital lobes, frontal lobes, precuneus, midbrain and anterior lobe of the cerebellum (9). Another VBM study showed that obese adults have lower gray matter density in areas such as frontal operculum, middle frontal gyrus, post-central gyrus, as well as putamen (10). Our group has described neurostructural abnormalities among obese adolescents with type 2 diabetes mellitus (T2DM) (26), but to our knowledge no such deficits have been described among obese youth without T2DM.
In addition to structural findings, cognitive assessments have demonstrated that executive functioning and response inhibition may be compromised in both adult and adolescent obese individuals. One study employing positron emission tomography (PET) and cognitive tests found obese adults to have decreased baseline prefrontal glucose metabolism as well as diminished performance on the Stroop task, a test of selective attention and executive function (11). Other studies of executive function and response inhibition in adults have shown a negative association of those variables with BMI (12-14). Furthermore, extremely obese adolescents show decreased functioning on executive tasks compared to normative data (15).
We hypothesized that consistent with previous findings using the Three Factor Eating Questionnaire (TFEQ), obese adolescents would have higher ratings of self-reported disinhibition in eating behaviors. We further hypothesized that obese adolescents would have lower scores on assessments of executive function and reduced integrity in neurostructural measures of frontal lobe (MRI-based gray matter volumes as well as regional brain volumes). In addition, we posited that disinhibition on the TFEQ will be negatively associated with cognitive scores on relevant domains as well as with MRI-based measurements of brain areas involved in response inhibition and executive control.
Methods
Participants and Procedures
Ninety-one youth (14-21y/o), 37 lean (BMI<25 kg/m2 or Waist to Height ratio <0.5) and 54 obese (BMI≥30 kg/m2 or>95 percentile for BMI for age and gender) participated in the study. Eighty-one of these (36 lean, 45 obese) received an MRI. Ten adolescents did not receive an MRI for the following reasons: two did not keep their appointments, one was pregnant and we chose to err on the side of safety, one could not tolerate the MRI (claustrophobia), and six had a BMI > 50 kg/m2 and exceeded the body size that could be accommodated by the scanner.
Lean participants had a mean age of 17.3 ± 1.6 years and obese 17.5 years ± 1.6 years. The two groups were also matched on years of education, gender, and socio-economic status and were all in the cognitively normal range. Evidence of neurological, medical (other than dyslipidemia, insulin resistance short of T2DM, polycystic ovary disease, or hypertension), or psychiatric (including depression and alcohol or other substance abuse) illness excluded individuals from participation in the study. T2DM also excluded individuals from participation. Participants and their parents gave written informed consent and were compensated for their time and inconvenience. The study protocol was approved by the New York University School of Medicine Institutional Review Board.
All study participants had a blood sample taken after a 10-hour overnight fast for the assessment of glucose, insulin, lipid, and inflammatory marker (high sensitivity C-Reactive Protein; hs-CRP) levels. Glucose was measured using a glucose oxidase method (VITROS 950 AT, Amersham, England), insulin by chemiluminescence (Advia Centaur, Bayer Corporation), and CRP was measured in plasma using an enzymatic immunoassay (Vitros CRP slide, Ortho Clinical Diagnostics). Insulin sensitivity was estimated using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR).
Assessments
Neuropsychological assessment
We conducted a broad evaluation of neurocognitive functions, including intellectual achievement, recent memory, working memory, attention, and executive function. We hypothesized that there would be differences in frontal lobe functions between lean and obese adolescents and therefore restricted our analyses to neurocognitive tests that reflect frontal lobe integrity and intact executive functions, namely the Controlled Oral Word Association Test (COWAT), Trail Making Test parts A & B, Stroop Task, Attention/Concentration Index of the Wide Range Assessment of Learning and Memory ( WRAML) and Working Memory Index of the WRAML. With the exception of the WRAML and the Stroop, which provide age-corrected standard scores, raw scores are reported. All tests administered are standard neuropsychological instruments described in detail elsewhere (16).
Three Factor Eating Questionnaire (TFEQ)
Characteristics of eating behavior were assessed by using the TFEQ. The TFEQ is a 51-item instrument, composed of three subscales measuring restraint (i.e., cognitive control of eating behavior; 21 items), disinhibition (i.e., the susceptibility of eating in response to emotional factors and sensory cues; 16 items), and hunger (i.e., the susceptibility of eating in response to feelings of hunger; 14 items). The TFEQ was administered approximately one hour after subjects had lunch.
MRI Acquisition and Image Analyses
All subjects were studied on the same 1.5 T Siemens Avanto MRI System, which has a 65 inch diameter bore and a table suitable for up to a 400 pound individual. We acquired T1-weighted magnetization-prepared rapid acquisition gradient echo images (MPRAGE; TR 1300 ms; TE 4.38 ms; TI 800 ms; FOV 250 × 250; slice thickness 1.2 mm; NEX 1; Flip angle 15°; matrix size 256 × 256; 192 coronal slices).
WM/GM Volumetric Analysis
Spatial normalization and segmentation of MPRAGE images utilized automated procedures as described in (17) the statistical parametric mapping software (SPM5). MPRAGE images were first corrected for signal non-uniformities and spatially normalized to the standard T1 Montreal Neurological Institute template. Using the tissue classification algorithm in SPM5, we segmented the normalized MPRAGE images into their gray matter (GM), white matter (WM), and cerebro-spinal fluid (CSF) partitions, which are maps representing the probability for each voxel being classified as GM, WM or CSF. These segmented partitions were subsequently normalized to their respective standard templates. In addition to performing whole brain assessment, and given that during adolescence frontal lobe myelination is still ongoing, we utilized two different templates to derive the regions of interest (ROI) in the frontal lobe. These were the SPM Automatic Anatomic Labeling (AAL) (18) template and our published reliable frontal lobe parcelation method (19). The AAL template was used to derive a total frontal lobe, an anterior cingulate region, and an orbitofrontal region. Our own parcelation method was used to derive a prefrontal region (frontal lobe minus the supplementary motor region). We quantified the proportions of WM, GM, CSF volumes in the whole brain and frontal regions at the case level by first mapping the regions to each segmented partition and then averaging the values across subjects for each of the two groups.
Statistical Analyses
We conducted two-tailed independent samples t-tests examining group differences in demographics, endocrine data, cognitive data, and brain volumes as well as Pearson correlations between the TFEQ disinhibition score and BMI, Stroop color word score, and orbitofrontal cortex gray matter volume. Data that were further than 2 standard deviations from the mean of the group for that variable were excluded. Given that there is individual variability in regional brain volumes related to overall head size, we measured each individual's intra-cranial vault (ICV) size and utilized the ICV values to adjust the regional brain volumes. Therefore, to allow comparability to other studies and give the reader a sense of the size of the brain regions studied, the table describing regional brain volumes shows the raw (non-residualized) volumes. However, statistical comparison and the significance and effect size for all imaging presented utilized the volumes adjusted (residualized) brain volumes.
Results
Demographics and Endocrine Data
Subject groups were matched for age, gender, school grade, and Hollingshead socio-economic-status (SES). Obese participants were, by definition, higher in BMI, and as expected also had higher systolic and diastolic blood pressure, fasting insulin and glucose levels (but all in the normoglycemic range) as well as the homeostatic model assessment of insulin resistance (HOMA-IR), triglycerides, low density lipoprotein (LDL) cholesterol, and high sensitivity C-reactive protein (CRP). Obese subjects also had significantly lower levels of high density lipoprotein (HDL). Please refer to Table 1.
Table 1.
Demographics and Endocrine Characteristics of Lean and Obese Adolescent Groups
| Lean n = 37 |
Obese n = 54 |
||||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t | p | d | |
| Age (years) | 17.32 ± 1.59 | 17.50 ± 1.59 | 0.538 | 0.592 | 0.08 |
| Education (years) | 11.77 ± 1.99 | 11.63 ± 1.71 | 0.322 | 0.748 | 0.10 |
| Gender (% F) | 56.8% | 63.6% | |||
| Hollingshead SES | 2.5 ± 1.59 | 2.1 ± 1.17 | 1.62 | 0.109 | 0.37 |
| BMI (kg/m2)* | 21.67 ± 2.49 | 39.86 ± 9.46 | −11.41 | <0.01 | 2.45 |
| Systolic BP (mm/Hg)* | 101.0 ± 8.68 | 114.4 ± 12.46 | −6.00 | <0.01 | 1.29 |
| Diastolic BP (mm/Hg)* | 62.97 ± 7.72 | 69.51 ± 9.33 | −3.73 | <0.01 | 0.80 |
| HOMA-IR* | 1.03 ± 1.80 | 3.06 ± 3.70 | −3.50 | <0.01 | 0.73 |
| Fasting Insulin (U/mL)* | 6.95 ± 2.99 | 19.43 ± 11.82 | −6.02 | <0.01 | 1.59 |
| Fasting Glucose (mg/dL)* | 74.44 ± 7.02 | 77.90 ± 8.00 | −2.11 | 0.038 | 0.48 |
| Triglycerides (mg/dL)* | 66.20 ± 24.08 | 85.29 ± 35.58 | −2.77 | <0.01 | 0.61 |
| HDL (mg/dL)* | 52.56 ± 9.55 | 43.50 ± 7.44 | 4.88 | <0.01 | 1.09 |
| LDL (mg/dL)* | 85.24 ± 22.17 | 98.29 ± 21.26 | −2.73 | <0.01 | 0.60 |
| C-reactive protein (mg/dL)* | 0.53 ± 0.56 | 3.41 ± 2.19 | −7.53 | <0.01 | 1.73 |
Three Factor Eating Questionnaire
Obese adolescents scored significantly higher than lean participants on the disinhibition factor of the Three Factor Eating Questionnaire (6.85 ± 3.55 vs. 3.91 ± 1.96, p<0.000, cohen's d (d) = 1.07), as well as the hunger factor (6.60 ± 3.37 vs. 4.68 ± 2.84, p = 0.008, d = 0.81) and the cognitive restraint factor (9.19 ± 4.30 vs. 6.78 ± 4.11, p = 0.012, d = 0.57). Please note that we repeated these analyses for the subset of 81 participants with an MRI and the results were essentially unchanged (data not shown).
Cognitive Measures
Relative to lean adolescents, obese adolescents had worse cognitive performance in every frontal lobe task, most pronounced for the Stroop (a measure of inhibition), and the Working Memory Index of the WRAML, even when we controlled for estimated IQ. Please refer to Table 2.
Table 2.
Cognitive Differences between Lean and Obese Adolescent Groups
| Frontal Lobe Tasks | Lean Mean ± SD |
Obese Mean ± SD |
t | p | d |
|---|---|---|---|---|---|
| Stoop Color-Word Score (no. correct/min.) |
46.3 ± 8.3 | 38.9 ± 7.6 | 4.30 | <0.001 | 0.94 |
| (Response Inhibition) | |||||
| Trail Making Test Part A (secs.) | 22.6 ± 5.2 | 28.2 ± 8.1 | −3.90 | <0.001 | 0.88 |
| (Attention) | |||||
| Trail Making Test Part B (secs.) | 51.3 ± 16.3 | 68.1 ± 32.0 | −2.71 | 0.008 | 0.62 |
| (Cognitive Flexibility) | |||||
| COWAT – Sum of F, A, S | 39.3 ± 13.1 | 33.8 ± 7.9 | 2.21 | 0.032 | 0.50 |
| (Verbal Fluency) | |||||
| WRAML Working Memory Index | 110.7 ± 13.3 | 99.4 ± 13.8 | 3.79 | <0.001 | 0.83 |
| WRAML Attn/Concent. Index | 108.6 ± 15.6 | 100.8 ± 12.4 | 2.58 | 0.012 | 0.56 |
| Estimated Full-Scale IQ | 108.6 ± 12.5 | 102.6 ± 10.9 | 2.37 | 0.020 | 0.51 |
Because 10 subjects did not receive an MRI evaluation (for details please refer to the participant and procedures sections above), we repeated our analyses for the subgroup of 81 adolescents who had an MRI and the direction and significance of the cognitive results remained unchanged (data not shown).
Brain imaging
Frontal lobe grey matter volume (in cubic centimeters) trended smaller, although not at a level of statistical significance, among obese adolescents (265.3 ± 29.5 vs. 269.6 ± 26.7; residualized 0.00369 ± 0.018312 vs. −0.00609 ± 0.014076, p = 0.139, d = 0.35). Please note that although the absolute differences between these volumes was small, the analyses were conducted after residualizing to the ICV and significance values and effect sizes reflect these analyses. Furthermore in order to control for the possible developmental effects of age on frontal and cerebral volumes we re-ran our analyses co-varying for age. We found significantly lower grey matter volumes for obese youth in orbitofrontal cortex (32.3 ± 3.68 vs. 33.3 ± 3.99; residualized 0.00781 ± 0.024944 vs. −0.01227 ± 0.018947, p = 0.005, d = 0.66). The OFC volume group differences were unchanged after controlling for systolic blood pressure or HOMA-IR. Other brain regions assessed, including prefrontal cortex and anterior cingulate cortex were not significantly different between obese and lean participants. Co-varying for age did not change any of these relationships.
Associations
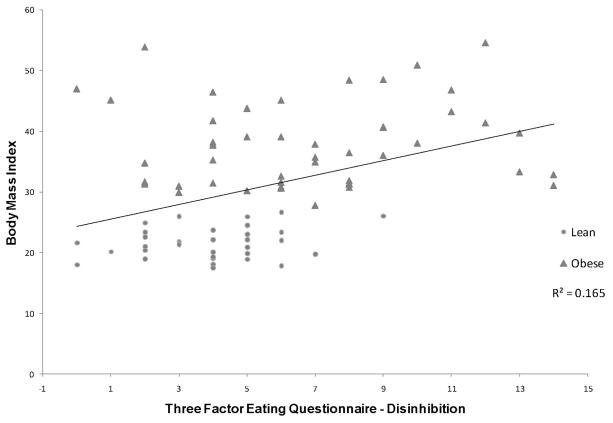
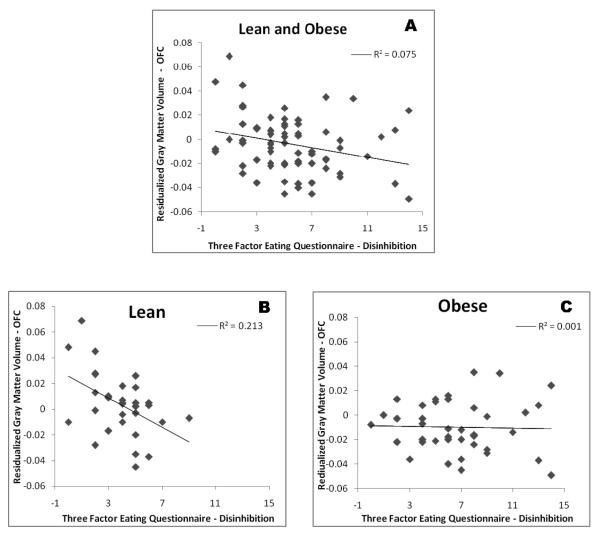
We found significant associations between the TFEQ and cognitive, BMI, and MRI volume measures. Specifically, the disinhibition factor score on the TFEQ showed a significant correlation with BMI(r(81) = 0.406, p < 0.001), Stroop Color-Word score (r(77) = −0.272, p = 0.017), and OFC gray matter volume (r(71) = −0.273, p = 0.021). In order to further understand the relationship between OFC volume and disinhibition we explored the association separately for the two groups. We found that there was no association between disinhibition and OFC volume for obese individuals (r(40) = −0.028, p = 0.864), whereas there was a strong association for the lean group (r(31) = −0.460, p = 0.009). The associations between the disinhibition factor score and the BMI and Stroop remained significant for the subset of individuals with MRI (data not shown).
Discussion
As expected, obese adolescents had significantly higher ratings of disinhibition, hunger, and cognitive restraint on the TFEQ. Although higher levels of cognitive restraint among obese adolescents would on first inspection appear counterintuitive, it conforms to the described model of “rigid restraint” in which an individual with disinhibited eating and cognitive restraint may tend to restrict food in some situations but grossly overeat in others (20).
Our novel neurostructural results among obese adolescents are consistent with findings in the adult literature (8, 9) demonstrating gray matter volume reductions. In our adolescent sample these decreases were most marked for the orbitofrontal cortex, a brain region important in impulse control, but also showed a weak trend for whole frontal lobe. We speculate that the more subtle volume reductions that exist in other brain regions among obese adolescents may actually reach statistical significance in an expanded sample.
Importantly for this report, we found the group with excess weight to not only have higher disinhibition scores on the TFEQ, but lower performance on cognitive tests reflecting brain functions thought to be central to behavioral inhibition, even when controlling for IQ. Out of the frontal lobe regions and functions we measured, we were particularly interested in ascertaining the relationship between the disinhibition factor of the TFEQ and the OFC, a brain region that is very important for behavioral inhibition (impulse control). We selected the Stroop because it is the only one of our frontal lobe tasks (including those that tap executive functions) that specifically tests the ability to inhibit automated responses. This is the direct cognitive parallel of the behavioral (disinhibition factor of the TFEQ) and brain region (OFC) also involved in inhibition of automatic responses. Our interest was to ascertain the functional (Stroop vs. other frontal tasks that do not measure response inhibition) and anatomic (OFC) specificity of our findings and their association to the disinhibition factor of the TFEQ.
We also found significant associations between disinhibition factor scores and both BMI and OFC volume. When the relationship between disinhibition and OFC volume was examined separately in lean and obese participants, we found a strong negative association only for the lean group. It is possible that obese individuals have already experienced a critical level of disinhibition-(which as we demonstrated is associated with BMI), whereby additional disinhibition is not as clearly reflected in further changes in OFC, but perhaps in different brain regions or networks not assessed as part of this study. Another possibility for these different findings for each of the two weight groups is that given that the obese groups has higher degree of item endorsement, they may be more susceptible to issues of social desirability and therefore they may be less likely to fully report the extent of their behavioral disinhibition in eating, dampening the association in this group. Lastly, it is also possible that range restriction, namely the phenomena of correlations decreasing when the variance is decreased as occurs when we divide our sample in two could be affecting our results.
While our study finds that disinhibition in feeding behavior is associated with reductions in executive functioning and frontal gray matter volumes, the cross-sectional nature of our design does not allow us to address the issue of directionality or causation. With that being said, there are several plausible theories regarding the direction of these associations.
One possibility is that primary structural or functional brain deficits lead to disinhibited eating and reductions in neurocognitive function. This line of reasoning is partially supported by work showing disinhibtion in eating behavior to presage increased caloric intake (21) and obesity (22). It is also consistent with functional imaging work demonstrating that individuals, who in response to visualized intake of palatable foods show weaker activation of brain reward circuits, are at elevated risk for future weight gain (23); perhaps they need a larger stimulus (more food) to derive the same reward response.
Another possible explanation is that brain structural deficits like those demonstrated in this study result from obesity and its associated insulin resistance. This possibility is supported by a 24-year longitudinal study showing increased BMI beginning in middle age correlated with decreased temporal lobe volume in later life (24). Also supporting this effect order is our own work in adults where we find that hippocampal volumes were associated with impairments in glucose tolerance (25) as well as that in adolescents with T2DM, where we find cognitive impairments and reductions in frontal lobe volumes and in white matter microstructural integrity (26). We posit that the obesity-associated insulin resistance exhibited by our group of adolescents with excess weight may contribute to decreased executive function and structural deficits. We have described a possible model for these effects (27) in which we hypothesize that insulin resistance is associated with decreased brain vascular reactivity related to endothelial dysfunction. We know that during brain activation, such as occurs when performing a cognitive task, there is an increase in synaptic activity in the brain region involved. In the normal brain this results in regional vasodilation and thus an increase in glucose availability to that region to support the increased cognitive demand (28). Therefore, vascular reactivity, which is integral to well-regulated cerebral blood flow, is key for maintaining an optimal neuronal environment during brain activation (29). Research showing endothelial dysfunction in obese children, even before the development of diabetes (30), further supports this premise. In addition, the inflammatory marker C-reactive protein (CRP) was elevated in our obese adolescents. In studies examining large cohorts of adults, investigators have found increased levels of inflammatory cytokines as putative mediators of cognitive decline among individuals with metabolic syndrome (31-34). A possible mechanism for these cognitive effects is provided by animal data demonstrating that excess inflammatory cytokines can decrease long term potentiation (LTP), a process understood as essential in consolidation of memory in the hippocampus. Inflammatory cytokines may also cause impairment in neurogenesis and neuroplasticity, processes vital to the formation of memories and the maintenance of structural neural integrity.
A third possibility is that these effects are bidirectional whereby behavioral disinhibition predisposes to obesity, which may negatively impact brain areas responsible for executive function and inhibition of caloric intake, thus causing a vicious cycle of dysfunction. This third possibility could help explain why it so difficult for individuals to lose weight once it has been gained.
We are encouraged by the fact that among the few brain regions that we evaluated, the OFC, a brain region that has been demonstrated as important in behavioral inhibition in both animal and human studies, had the most significant volume reduction among obese adolescents. Our findings, including lower performance on cognitive tests thought to require intact OFC, coupled with volume reductions in this area associated to behavioral disinhibition point to its likely importance in weight gain.
This study has some clear limitations. First, it is a cross-sectional view that does not allow us to comment on clear causality. Second, given our relatively modest sample size we restricted our measurements to brain regions that in previous studies had either been found to have been associated to obesity or disinhibition, or those that we had good theoretical reasons to believe could be involved. Therefore, it is possible that there are other brain areas, which we did not evaluate, that may also be involved. A third limitation of our study is that we only have participants' current weight and we cannot comment on duration of obesity; the sample that we studied is likely to have considerable variability in duration of obesity and its associated insulin resistance. Nevertheless, our study has significant strengths, including the careful matching between groups, the multidimensional evaluations conducted, and the unbiased MRI methods utilized in the analyses of the MRI data.
To better understand the issues described here, future work should evaluate subjects longitudinally, tracking the development of obesity across time while concomitantly measuring cognitive, behavioral, and neurostructural changes. Alternatively, our understanding could also be improved through a study designed to examine the consequences of a successful obesity treatment (e.g. bariatric surgery), and thus ascertain whether some of these deficits are reversible. Furthermore, future work should evaluate other possible associated factors such as pro- and anti-inflammatory cytokines as well as utilize more sensitive MRI techniques such as diffusion tensor imaging (DTI).
Figure 1. Association between Body Mass Index and Disinhibition.
Shows the association between Body Mass Index (in kg/m2) and Three Factor Eating Questionnaire Disinhibition factor score. Obese individuals are noted by filled pyramids and lean individuals by filled circles.
Figure 2. Association between OFC Gray Matter Volume and Disinhibition in Adolescents (Lean and Obese).
Shows the association between Body Mass Index and ICV-residualized orbitofrontal cortex volume. Panel A shows all study participants, Panel B shows the significant association for lean youth, and Panel C, shows the lack of association for obese adolescents.
Acknowledgements
The study was supported by grants from the National Institutes of Health R21 DK070985 and RO1 DK083537 and, supported in part by grant1UL1RR029893 from the National Center for Research Resources. The authors wish to acknowledge the children and families who participated in this research as well as Po Lai Yau and Valentin Polyakov in the collection and processing of the data and the assistance of Allison Larr in the preparation of this manuscript.
Footnotes
Financial Disclosures:
None of the other authors have any financial/conflicting interests to disclose
References
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299:2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 4.Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. N Engl J Med. 2003;349:926–8. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 5.Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–60. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 6.Del Parigi A, Gautier JF, Chen K, et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–97. [PubMed] [Google Scholar]
- 7.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 8.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–64. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–24. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 10.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–5. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–8. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 13.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–7. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 15.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–52. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Lezak MD, Howleson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford University Press; New York: 2004. [Google Scholar]
- 17.Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 19.Convit A, Wolf OT, de Leon MJ, et al. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- 20.Westenhoefer J, Broeckmann P, Munch AK, Pudel V. Cognitive control of eating behaviour and the disinhibition effect. Appetite. 1994;23:27–41. doi: 10.1006/appe.1994.1032. [DOI] [PubMed] [Google Scholar]
- 21.Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–76. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55-65 y. Am J Clin Nutr. 2002;75:476–83. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- 23.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–81. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 25.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–22. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010 doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–5. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Benton D, Parker PY, Donohoe RT. The supply of glucose to the brain and cognitive functioning. J Biosoc Sci. 1996;28:463–79. doi: 10.1017/s0021932000022537. [DOI] [PubMed] [Google Scholar]
- 29.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–52. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Karpoff L, Vinet A, Schuster I, et al. Abnormal vascular reactivity at rest and exercise in obese boys. Eur J Clin Invest. 2009;39:94–102. doi: 10.1111/j.1365-2362.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 31.Dik MG, Jonker C, Comijs HC, et al. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30:2655–60. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 32.Roberts RO, Geda YE, Knopman DS, et al. Metabolic Syndrome, Inflammation, and Nonamnestic Mild Cognitive Impairment in Older Persons: A Population-based Study. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e3181a4485c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweat V, Starr V, Bruehl H, et al. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation. 2008;31:198–207. doi: 10.1007/s10753-008-9065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]