Abstract
Rrp1 is the sole c-di-GMP producing protein (diguanylate cyclase) of Borrelia burgdorferi. To test the hypothesis that Rrp1 regulates critical processes involved in the transmission of spirochetes between ticks and mammals, an rrp1 deletion mutant (B31-Δrrp1) and a strain that constitutively produces elevated levels of Rrp1 (B31-OV) were constructed. The strains were assessed for progression through the enzootic cycle using an Ixodes tick/C3H-HeJ mouse model and tick immersion feeding methods. B31-Δrrp1 infected mice as efficiently as wild type but had altered motility, decreased chemotactic responses to N-acetylglucosamine (NAG) and attenuated ability to disseminate or colonize distal organs. While this strain infected mice, it was not able to survive in ticks. In contrast, the B31-OV displayed normal motility patterns and chemotactic responses but was non-infectious in mice. Using immersion feeding techniques we demonstrate that B31-OV can establish a population in ticks and survive exposure to a natural bloodmeal. The results presented here indicate Rrp1, and by extension, c-di-GMP, are not required for murine infection, but are required for the successful establishment of a productive population of B. burgdorferi in ticks. These analyses provide significant new insight into the genetic regulatory mechanisms
Keywords: Borrelia, Lyme disease, Rrp1, cyclic-di-GMP, diguanylate cyclase
INTRODUCTION
Lyme disease is a tick borne infection caused by B. burgdorferi, B. garinii, and B. afzelii (Benach et al., 1983; Burgdorfer et al., 1982). The Lyme disease spirochetes must adapt to different environmental conditions as they cycle between mammals and Ixodes ticks (Barbour and Hayes, 1986). Recent studies suggest that c-di-GMP, a ubiquitous bacterial nucleotide secondary messenger molecule (Cotter and Stibitz, 2007; Galperin, 2006; Hengge, 2009; Romling and Amikam, 2006), is a key regulator of B. burgdorferi adaptive responses and may play an important role in the enzootic cycle (Freedman et al., 2009; Pitzer et al., 2011; Rogers et al., 2009b; Ryjenkov et al., 2005; Sultan et al., 2010). In B. burgdorferi, c-di-GMP is produced by a single diguanylate cyclase, designated as Rrp1 (Galperin et al., 2001; Ryjenkov et al., 2005). The global regulatory ability of Rrp1, and by extension c-di-GMP, was revealed through microarray analysis of an rrp1 deletion mutant (Rogers et al., 2009b). The Rrp1 regulon, which consists of ~10% of the genome, includes genes that encode proteins spanning a wide range of functional categories with a concentration on motility, chemotaxis, and metabolism. Several of the Rrp1 regulated genes encode proteins that are likely to be in involved in the enzootic cycle.
As in other bacterial systems, c-di-GMP levels in B. burgdorferi are thought to be controlled by the opposing activities of diguanylate cyclases and phosphodiesterases. Borrelia strains encode a diguanylate cyclase (Rrp1), EAL domain phosphodiesterase (PdeA) and HD-GYP domain phosphodiesterase (PdeB) (Rogers et al., 2009b; Ryjenkov et al., 2005; Sultan et al., 2010). Little is known regarding the effector mechanisms of c-di-GMP in the Lyme disease spirochetes and arthropod-borne pathogens in general. In other bacteria, the regulatory effects of c-di-GMP have been linked to interactions with GEMM riboswitches (Smith et al., 2009; Sudarsan et al., 2008), ribonucleoprotein complexes (Tuckerman et al., 2011), transcriptional regulators (Hickman and Harwood, 2008), PilZ domain-containing proteins (Amikam and Galperin, 2006; Ryjenkov et al., 2006), and proteins with cyclic nucleotide monophosphate domains (Tao et al., 2011). Two PilZ domain containing proteins (PlzA and PlzB) have been identified in Borrelia strains and demonstrated to bind c-di-GMP in a highly specific manner (Freedman et al., 2009). All strains produce the chromosomally encoded PlzA protein. Subsets of strains carry a second gene encoding a c-di-GMP binding protein designated as PlzB. PlzB is encoded by a variably size linear plasmid (Freedman et al., 2009). The effector functions of these proteins have not been defined.
In this study, we test the hypothesis that Rrp1 and, by extension, c-di-GMP, regulate critical steps in the enzootic cycle of the Lyme disease spirochetes. B. burgdorferi strains that lack Rrp1 or that constitutively produced elevated levels of Rrp1 were generated and analyzed for their ability to infect and transit between mice and Ixodes ticks. Motility patterns, chemotactic responses to N-acetylglucosamine (NAG), and the expression of genes involved in NAG metabolism were also assessed. The data presented within demonstrate that Rrp1, presumably through its production of c-di-GMP, regulates critical processes required for completion of specific stages of the enzootic cycle. These analyses provide unique insight into a relatively unexplored area of the role of diguanylate cyclases in genetic regulation in B. burgdorferi and arthropod borne pathogens in general.
RESULTS
Generation and analysis of an rrp1 deletion mutant, rrp1 over-expressing strain and cis-complemented strain
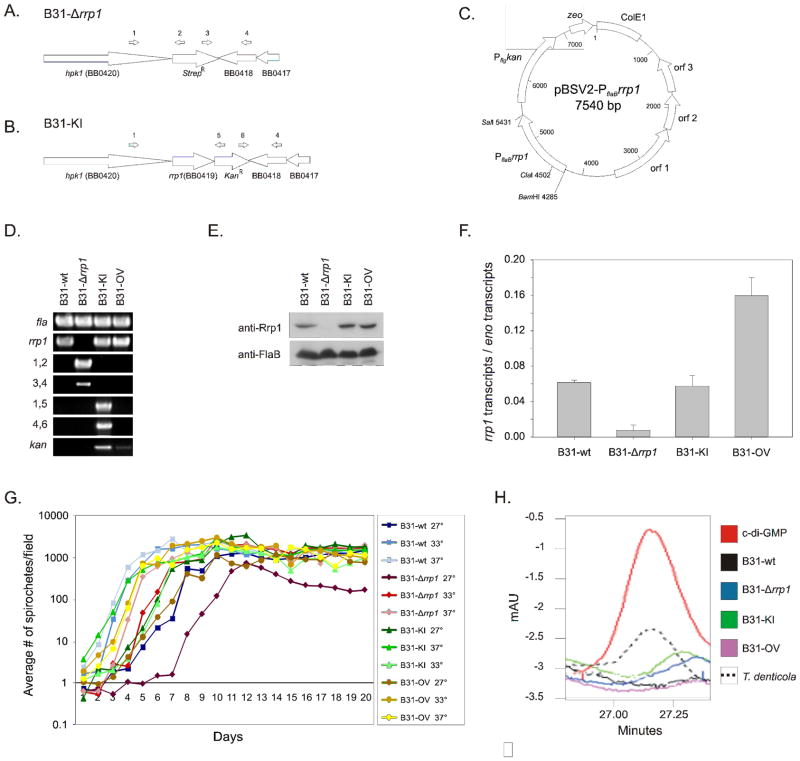
To determine if Rrp1 regulates cellular processes required for completion of the enzootic cycle, rrp1 of B. burgdorferi strain B31 clone 5A4 (an infectious clone) was replaced by a spectinomycin/streptomycin resistance cassette (strepR) to yield strain B31-Δrrp1 (Figure 1A). A functional cis-complemented strain, B31-KI (“knock-in”), was generated by replacing the strepR cassette of B31-Δrrp1 with wild-type rrp1 and a downstream kanamycin resistance cassette (Figure 1B). To generate a strain that constitutively produces elevated levels of Rrp1, B31-wt was transformed with pBSV2-PflaBrrp1 (Figure 1C) to yield a strain carrying a plasmid and chromosomal copy of rrp1. Clonal populations of each strain were assessed for insertion of the cassette or for the presence of the pBSV2-PflaBrrp1 plasmid using PCR (Figure 1D & Table 1). The Borrelia genome is segmented and consists of a linear chromosome and 20 or more linear and circular plasmid (Barbour and Garon, 1987). Genetic manipulation can result in the loss of plasmids required for survival in mammals (Labandeira-Rey and Skare, 2001; Purser et al., 2003). To assess plasmid content, PCR was performed with plasmid specific primer sets (data not shown) (McDowell et al., 2001). Only clones harboring the full set of plasmids were selected for further analyses. To evaluate Rrp1 protein and transcript levels, immunoblot and qRT-PCR analyses were conducted (Figure 1E &F, respectively). rrp1 transcript and protein was detected in the B31-wt, B31-KI, and B31-OV strains, but not in B31-Δrrp1. In vitro, B31-OV produced 2-fold more rrp1 mRNA than B31-wt or B31-KI (Figure 1F).
Figure 1. Generation and verification of rrp1 deletion, complementation, and overexpression strains and comparative analyses of their properties.
Schematics in panel A and B represent the chromosome of the B31-Δrrp1 and B31-KI complement mutants after allelic replacement, respectively. Schematic C depicts the pBSV2-PflaBrrp1 plasmid construct utilized in this study for the B31-OV mutant. Successful allelic exchange mutagenesis deleting and reinserting rrp1 was confirmed by PCR (Panel D), western blot (Panel E), and qRT-PCR analysis (Panel F). Verification of pBSV2-based overexpression of Rrp1 was likewise performed by PCR (Panel D), western blot (Panel E), and qRT-PCR (Panel F). Primers used for validation of proper integration are indicated by numbers above the schematic arrows and are listed to the left of the respective PCR panels. All primers used are listed in Table 1. The error bars in panel F indicate the standard deviation. Growth of each strain was assessed at 27, 33 and 37° over 20 days in BSK-H complete media. In panel H, HPLC chromatograms of nucleotide extracts from each strain are presented. The elution time of purified c-di-GMP is shown for reference. T. denticola nucleotide extracts were assessed as a positive control. All methods were as described in the text.
Table 1.
Oligonucleotides used in this study.
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| General primers | |
| KOverifyUp F (1) | CTTTAATGGGGCTTGGTATATGC |
| KOverifyDwn R (2) | GTTGATGCATCTGTTAAAATTG |
| aad1-5′-R (3) | TCCTTGAAGCTCGGGTATTA |
| pKFSS1-3′F (4) | GGCGAGATCACCAAGGTAGTC |
| kan-5′-R (5) | CAGCATCCATGTTGGAATTTAATCGC |
| kan-3′-F (6) | GATATGAATAAATTGCAGTTTCATTTG |
| kan pBSV2 F (7) | GCGATTAAATTCCAACATGGATGCTG |
| kan pBSV2 R (8) | ACTCATCGAGCATCAAATGAAACTGC |
| flaB F | CAGGTAACGGCACATATTCAGATGC |
| flaB R | CTTGGTTTGCTCCAACATGAACTC |
| BB0419F_LIC | GACGACGACAAGATGGAAATGATAATTAAAGATAAAGC |
| BB0419R_LIC | GAGGAGAAGCCCGGTTTAATATCTAAACTGATTTCTTCCAG |
| rrp1 KI construction | |
| Rev_419+AatII+AscI | GGCGCGCCTAGGACGTCTTAATATCTAAACTGATTTCTTCCAGAAAC |
| Fwd_KanR+1kbDNST419 | GAGTTTTTCTAAATTTATATTTAATAGACTTTAGTATTTATAAGTTATAGACATTCC |
| Rev_1kbDNST419+AscI | GGCGCGCCCTTTCCCAAGATCAACAGAACTTAG |
| Fwd_KanR+AatII | GACGTCCTTTAATAAAACAATATGTTGCGATGATTAAGG |
| Rev_KanR+1kbDNST419 | CTTATAAATACTAAAGTCTATTAAATATAAATTTAGAAAAACTCATCGAGCATCAAATG |
| rrp1 OV construction | |
| pFlaB+BamHI F | GTGGATCCTGAACTTAATACCTTGG |
| pFlaB+ClaI+SalI R | GTCGACATAATCGATTCCTCCATGATAAAATTTAAATTTC |
| 419+ClaI F | ATCGATAATGATAATTAAAGATAAAGCTTTTG |
| 419+SalI R | GTCGACCGCTTAATATCTAAACTGATTTCTTCC |
| qPCR primers | |
| FlaB BB0147F-RT | CAGGTAACGGCACATATTCAGATGC |
| FlaB BB0147R-RT | CTTGGTTTGCTCCAACATGAACTC |
| BB0419F-RT | TTGAGGTTGCAACAAATGGA |
| BB0419R-RT | CGGGATCGCTTTTTAGCTTT |
| eno BB0337F-RT | GCTTGAACTTGATGGCACCCCTAC |
| eno BB0337R-RT | GTACGCTCCAAGATATTGATAAGG |
| tick RIB-3 (ITS2) | CGGGATCCTTC(A,G)CTCGCCG(C,T)TACT |
| tick RIB-4 (ITS2) | CCATCGATGTGAA(C,T)TGCAGGACA |
| BB0002F-RT | GCGGAGCAGACAAAGGGATTGATT |
| BB0002R-RT | ACATGCTCCATGGCCGGAAA |
| NagA BB0151F-RT | GCAGCTGGTGGAGTATTTACAGGA |
| NagA BB0151R-RT | GTGTGTCCCGCTTGAAGGTTTATG |
| NagB BB0152F-RT | GGGCGGCTAATCATGTAGCACAAA |
| NagB BB0152R-RT | CAATCGGAGAGCTTCCTGTTGGA |
| PtsG BB0645F-RT | AGAACTTGCTGCCCAAGGTACAGA |
| PtsG BB0645R-RT | CCGGGCAAACCAAACATCATGGTA |
In nature B. burgdorferi encounters radically different environment conditions as it cycles between ticks and mammals. One environmental variable that it must adapt as it cycles between ticks and mammals is temperature. Cultivation in the laboratory, at 25 to 27°C, is thought to mimic at least in part the tick environment. Hence, we determined growth rates at 27, 33, and 37°C (Figure 1G). B31-Δrrp1 displayed an extended lag phase at 27°C (7 days) and did not enter stationary phase for 13 days. All remaining strains entered log phase within 2 days and stationary phase by day 7. It can be concluded that Rrp1 is not required for growth in vitro, but its absence influences growth at lower temperatures. This suggests that Rrp1 may play an important role in the tick environment. Consistent with a potential role for Rrp1 and c-di-GMP in the tick, rrp1 transcription has been demonstrated to be dramatically upregulated in ticks after ingestion of a bloodmeal (Rogers et al., 2009b).
Analysis of c-di-GMP levels
C-di-GMP production by each strain was assessed through HPLC analysis of nucleotides extracted from in vitro cultivated bacteria. This approach has been successfully applied to measure c-di-GMP in E. coli (Antoniani et al., 2010) and it has a sensitivity of 0.3 pmol nucleotide/mg cells (data not shown). B. hermsii DAH (tick-borne relapsing fever) and Treponema denticola (periodontal disease) were included as controls. B. hermsii harbors orthologs of each of the proteins that have been demonstrated to participate in the synthesis and breakdown of c-di-GMP in B. burgdorferi and hence we reasoned that it would behave similarly to B. burgdorferi. T. denticola was included because it actively produces several diguanylate cyclases during in vitro cultivation (R.T. Marconi, unpublished data), thereby making it a good positive control for c-di-GMP detection. In addition, because B. hermsii and T. denticola have a similar membrane composition to B. burgdorferi (no lipopolysaccharide with a lipoprotein rich outer membrane) we reasoned that they could serve as controls for the nucleotide extraction process. C-di-GMP was readily detected in T. denticola but not in B. hermsii (data not shown), B. burgdorferi B31-wt, B31-Δrrp1 B31-KI or B31-OV (Figure 1H) demonstrating that c-di-GMP concentrations are inherently low in cultivated Borrelia. This is consistent with the low level expression of rrp1 during in vitro cultivation (Rogers et al., 2009b).
Demonstration that Rrp1 directly or indirectly influences infectivity and dissemination and or secondary colonization of B. burgdorferi in mice
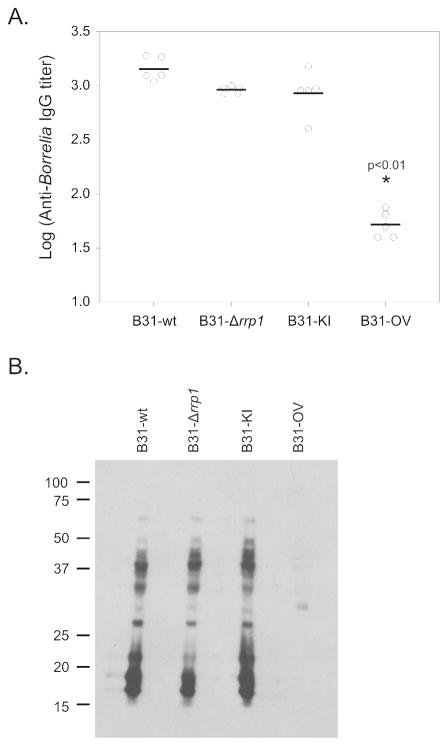
Mice were subcutaneously needle inoculated with each strain and infection assessed by seroconversion (ELISA and immunoblotting) 4 weeks post-inoculation (Figure 2A). All mice inoculated with B31-wt, B31-Δrrp1, and B31-KI were seropositive with equivalent IgG titers. The B31-OV strain had a significantly lower IgG titer that all other strains (p<0.01). The low level IgG titer elicited by the B31-OV strain is similar to that observed for other non-infectious isolates and it presumably results an antibody response to the inoculum itself (Earnhart et al., 2010). To compare antibody responses evoked by each strain, the overall pattern of immunoreactive proteins detected was determined by immunoblotting (Figure 2B). Consistent with the ELISA data, serum from mice inoculated with B31-OV did not recognize any proteins in the immunoblot analyses. No significant differences in immunoreactive profiles were observed with the serum collected from mice infected with B31-wt, B31-Δrrp1, and B31-KI.
Figure 2. Rrp1 is not essential to mammalian infection, but overproduction inhibits disease establishment.
C3H-HeJ mice were needle inoculated subcutaneously with 104 spirochetes (5 mice per strain). Four weeks post-infection, mice were bled and sera collected. Seroconversion was evaluated using whole-cell ELISA (Panel A) and immunoblot (Panel B).
To further assess infectivity, dissemination, and or secondary colonization, biopsies from each mouse were collected from organs and tissues distal to the inoculation site and placed in BSK-H media for spirochete cultivation. Cultures were obtained from all clinical specimens recovered from B31-wt and B31-KI inoculated mice (Table 2). None of the heart biopsies and only a subset of the bladder and skin samples harvested from B31-Δrrp1 inoculated mice yielded positive cultures. Positive cultures were not obtained from mice inoculated with B31-OV. This observation is consistent with the immunological data described above. It can be concluded that Rrp1 is not required for infectivity but is required for maximal dissemination and or secondary colonization.
Table 2.
Murine infection study
| Strain | # spirochete positive cultures from each tissue or organ biopsy | |||
|---|---|---|---|---|
| bladder | heart | skin | # of culture positive/# tested | |
| B31-wt | 5/5 | 5/5 | 5/5 | 5/5 |
| B31-Δrrp1 | 3/5 (mice 1 and 3 were negative | 0/5 | 3/5 (mice 2 and 5 were negative) | 5/5 |
| B31-KI | 5/5 | 5/5 | 5/5 | 5/5 |
| B31-OV | 0/5 | 0/5 | 0/5 | 0/5 |
Rrp1 is essential for spirochete acquisition by Ixodes scapularis
To assess the ability of each strain to transit from infected mammals to feeding ticks, larval stage Ixodes scapularis ticks were fed to repletion on mice (4 weeks post-inoculation), collected, DNA extracted, and qPCR was performed (Figure 3A & B). Ticks fed on B31-wt and B31-KI infected mice had an average of ~2.0 and ~2.4 flaB copies per 1000 tick genome equivalents indicating establishment of a productive population in ticks. Spirochetes were not detected in ticks fed on mice inoculated with B31-OV or B31-Δrrp1. The results with the B31-OV strain are as expected since this strain did not establish a detectable infection in mice. To determine if the B31-OV and B31-Δrrp1 strains can survive in ticks, a mouse independent route of infecting ticks was employed. Larval ticks were submerged in cultures of each strain and then fed on mice to provide bloodmeal derived nutrients (Figure 3B). The efficiency of infecting ticks by this approach was 20 to 40% with each strain except B31-Δrrp1 (0%). These data suggest that Rrp1 directly or indirectly regulates processes that are required for survival in ticks.
Figure 3. The diguanylate cyclase, Rrp1, is necessary for Ixodes infection.
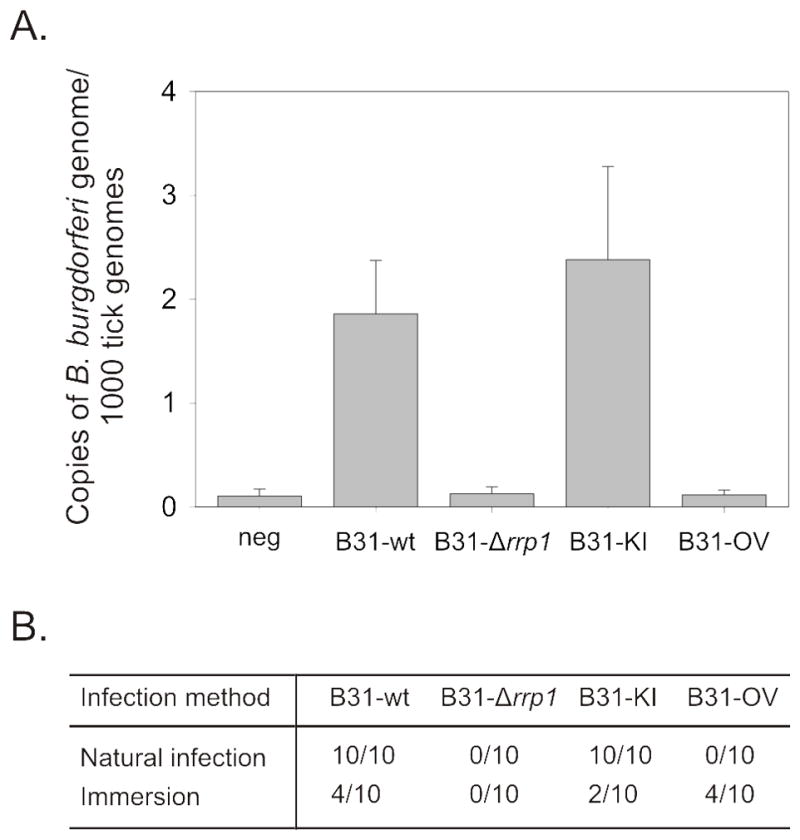
Evaluation of spirochete acquisition by Ixodes ticks was assessed by qPCR. DNA was isolated from naive ticks fed on infected mice till repletion (Panel A & B). Larval ticks were also immersed into spirochete culture, washed, fed, and assessed for spirochete uptake (Panel B).
Aberrant motility and chemotaxis patterns of the B31-Δrrp1 and B31-OV strains
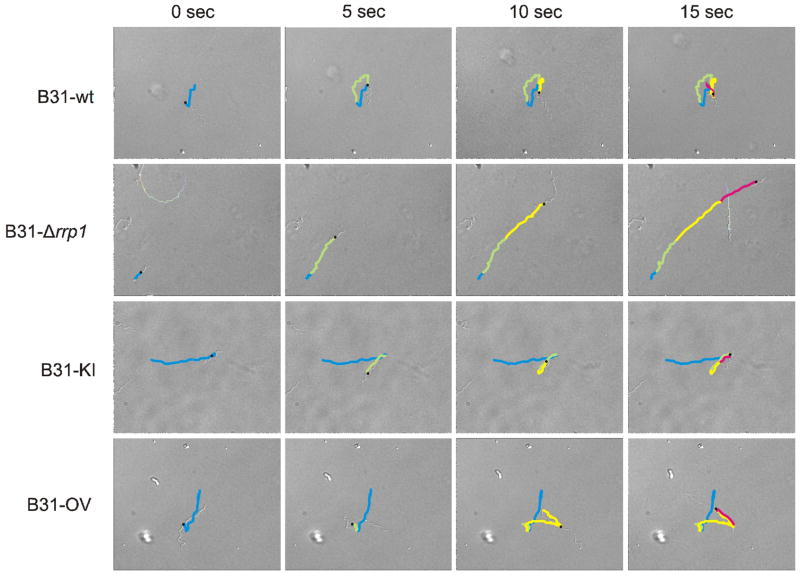
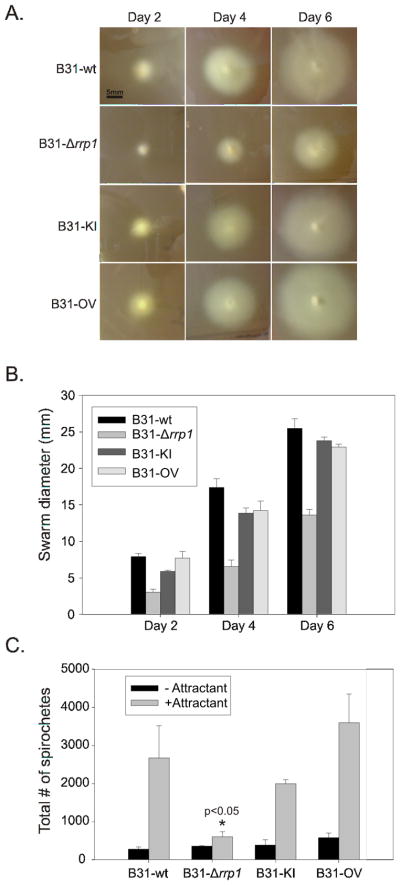
Several approaches were employed to to determine if rrp1 deletion or elevated production of Rrp1 regulates B. burgdorferi motility and or chemotaxis. General motility patterns were assessed for spirochetes in BSK-H media with and without 1% methylcellulose using dark-field and differential interference contrast (DIC) microscopy (with video tracking). Methylcellulose increases media viscosity in a matrix dependent manner (Ruby and Charon, 1998). All strains displayed wave propagation but their translational motion (directional forward movement) patterns and flex frequency differed (Figure 4). Note that all figures and supplemental videos of motility depict cells that are representative of the population as a whole. Consistent with earlier analyses of B. burgdorferi motility, B31-wt moves short distances (run speed of 4.14 μm sec−1), flexes (0.1738 flexes sec−1), and reverses direction (Figure S1 & Table 3) (Li et al., 2002; Sultan et al., 2010). Motion-tracking revealed that B31-Δrrp1 had a faster run speed (5.58 μm sec−1) and a significantly decreased flex frequency (0.015 flexes sec−1). Hence it was locked in a constant straight run (Figure S2 & Table 3). In contrast, elevated production of Rrp1 as in the B31-OV strain had no effect on motility patterns (Figure S4). As expected, the B31-KI strain (Figure S3) displayed motility patterns consistent with B31-wt. Motility was also assessed in semi-solid media using swarm assays. The B31-Δrrp1 swarm diameter was found to be reduced by 47% relative to B31-wt (p<0.01). The swarm diameters of B31-KI and B31-OV were similar to B31-wt (Figure 5A and 5B). The results demonstrate that Rrp1, presumably through its production of c-di-GMP, plays an important role in regulating flagellar motor function.
Figure 4. Translational motion patterns requires Rrp1.
Movement patterns of the B31-wt, B31-Δrrp1, B31-KI, and B31-OV strains were visualized using DIC microscopy. Images were captured at 5 sec intervals are shown. Motion tracks were manually recorded and overlaid on the images using motion tracking software. The tracks are colored according to motion achieved during each timelapse (blue - 0 sec, green - 5 sec, yellow - 10 sec, pink - 15 sec). Note that the patterns shown are representative of the entire population of cells.
Table 3.
Strain swimming patterns and observations.
| Strain | BSK-H | 1% methylcellulose |
|---|---|---|
| B31-wt | Motile | Translational motion: runs, stops/flexes, reverses |
| Avg run velocity: 4.1426 ± 0.9442 μm/sec Flexes/sec: 0.1738 ± 0.0319 |
||
|
|
||
| B31-Δrrp1 | Motile | Translational motion: runs with very infrequent stops/flexes |
| Avg run velocity: 5.5815 ± 1.3920 μm/sec Flexes/sec: 0.0150 ± 0.0430 |
||
|
|
||
| B31-KI | Motile | Translational motion; runs, stops stops/flexes, reverses |
| Avg run velocity: 3.9116 ± 0.7613 μm/sec Flexes/sec: 0.1429 ± 0.0679 |
||
|
|
||
| B31-OV | Motile | Translational motion; runs, stops stops/flexes, reverses |
| Avg run velocity: 4.0359 ± 0.7859 μm/sec Flexes/sec: 0.1593 ± 0.0340 |
||
Figure 5. Rrp1 positively regulates motility and chemotaxis.
The B31-wt, B31-Δrrp1, B31-KI, and B31-OV strain motility and chemotaxis abilities were assessed by swarming assay and capillary chemotaxis assay. Borrelia were spotted into punched wells and diameters were measured at day 2, 4 and 6 (Panel B) from the respective swarms (Panel A). Mutant movement toward N-acetyl-D-glucosamine was analyzed by capillary assay. Spirochetes in capillary tubes with and without chemoattractant were counted after a 2h incubation at 33° (Panel C). The error bars indicate the standard deviation.
C-di-GMP has been demonstrated play a role in regulating chemotaxis in several gram negative enteric bacteria (Paul et al., 2010). To determine if the presence or absence of Rrp1 directly or indirectly influences chemotaxis, capillary assays were performed using NAG (Figure 5C). NAG is a known chemoattractant of B. burgdorferi (Bakker et al., 2007; Shi et al., 1998). The B31-Δrrp1 strain was highly attenuated in its NAG driven chemotactic response (p<0.05) while the response of B31-OV was not statistically different from that of the B31-wt strain. Complementation of rrp1 (B31-KI) restored wild type chemotactic responses to B31-Δrrp1. Collectively, the data indicate Rrp1 is required for regulation of flagella motor activity and NAG driven chemotactic responses.
Transcriptional analysis of genes involved in NAG metabolism
NAG, which is abundant in ticks and in glycosaminoglycans of mammalian tissue, is required for in vitro growth of Borrelia (Barbour, 1984). To determine if Rrp1 regulates the transcription of genes involved in NAG metabolism, qRT-PCR analyses were performed. The expression levels of nagA (NAG-6-phosphate deaminase), nagB (glucosamine-6-phsophate-isomerase), ptsG (PTS system, glucose-specific IIBC component), and ORF BB0002 (β-N-acetylhexosaminidase; a putative chitobiase) were examined (Table 4). Deletion of rrp1 resulted in a significant decrease in the amount of nagA and nagB mRNA but had no affect on BB0002 and ptsG (BB0645) transcription. B31-OV also had decreased nagB expression but transcription of the other 3 genes were not significantly affected. These results indicate that Rrp1 directly or indirectly regulates the transcription of important genes involved in the utilization of NAG. The decreased transcription of these genes could influence the ability of B. burgdorferi to survive in tissues rich in NAG and in the tick.
Table 4.
Glucosamine metabolism pathway qRT-PCR analysis
| Description | Functional Categorya | Δrrp1/WT | p-value | OV/WT | p-value | |
|---|---|---|---|---|---|---|
| BB0002 | glycosyl hydrolase family 3 N domain protein (β- – acetylhexosaminidase) | EM | 0.2941 | 0.0536 | 0.4964 | 0.0996 |
| BB0151 | N-acetylglucosamine-6-phosphate deaminase (nagA) | IM | 0.4611 | 0.0029 | 0.8787 | 0.2872 |
| BB0152 | glucosamine-6-phosphate isomerase (nagB) | IM | 0.3320 | 0.0176 | 0.4331 | 0.0319 |
| BB0645 | PTS system, glucose- specific IIBC component (ptsG) | TP | 0.4444 | 0.0921 | 0.5196 | 0.1137 |
As published in TIGR B. burgdorferi B31MI genome database.
DISCUSSION
In the Lyme disease spirochete, B. burgdorferi, c-di-GMP has been postulated to regulate cellular processes required for completion of the enzootic cycle (Freedman et al., 2009; Pitzer et al., 2011; Rogers et al., 2009b; Sultan et al., 2010). Rrp1 is the sole diguanylate cyclase encoded by the Borrelia genome and deletion of rrp1 results in pronounced changes in its transcriptome (Rogers et al., 2009b). Since Rrp1 lacks a DNA binding domain or other known functional domains, its regulatory capability is thought to be linked specifically to its diguanylate cyclase activity. Until this study, the potential requirement for c-di-GMP over the course of the enzootic cycle of a tick-borne bacterial pathogen has not been assessed. Recent analyses of B. burgdorferi pdeA (EAL domain phosphodiesterase) and plzA (PilZ domain c-di-GMP binding protein) mutants, which display attenuated virulence, have provided indirect evidence that c-di-GMP is an important regulatory molecule in vivo (Pitzer et al., 2011; Sultan et al., 2010). The Lyme disease spirochetes offer an excellent model system for studying diguanylate cyclase mediated regulation over the course of the enzootic cycle because B. burgdorferi can be genetically manipulated and there is a well characterized and biologically relevant mouse-tick enzootic model.
In this study, we test the hypothesis that high c-di-GMP levels interfere with passage of spirochetes from ticks to mammals (transmission stage) while the absence of c-di-GMP attenuates the ability of B. burgdorferi to transit from mammals to ticks (acquisition phase). To assess these possibilities, strains that lack rrp1 (B31-Δrrp1) or produce it at elevated levels (B31-OV) were generated. While B31-Δrrp1 retained its ability to infect mice the B31-OV strain was not infectious. B31-Δrrp1 was also found be less efficient at dissemination and/or secondary colonization as it was not cultivated from heart biopsies and was inconsistently cultured from biopsies collected from sites distal to the inoculation site. In addition, while B31-Δrrp1 readily infected mice, natural tick feeding experiments revealed that this strain is not able to transit into ticks and establish a productive population. Because B31-OV cannot infect mice we were not able to assess its potential to transit from mammals to ticks via natural tick feeding. To allow us to assess the ability of this strain to survive in ticks an immersion feeding approach was employed (Policastro and Schwan, 2003). B31-OV established a productive population in ticks while B31-Δrrp1 did not. The data presented here and in earlier studies suggest that c-di-GMP levels regulate processes involved in transmission, acquisition and survival of B. burgdorferi in ticks (Rogers et al., 2009b; Sultan et al., 2010).
The inability of the B31-Δrrp1 to transit from ticks to mammals and or survive in ticks could be due to impaired chemotactic responses or the inability to efficiently utilize tick derived nutrients. Earlier transcriptional analyses demonstrated that genes that encode proteins involved in chemotaxis (che and mcp) and NAG utilization (nagA and nagB) are positively regulated by Rrp1 (Rogers et al., 2009b). Consistent with the transcriptional data, a 75% reduction in the chemotactic response of B31-Δrrp1 to NAG was observed. Complementation restored wild type chemotactic responses to NAG. Wild type levels of nagA and nagB transcription were also restored by complementation. NAG, which is a major constituent of chitin and chitobiose, is highly abundant in the peritrophic membrane of the tick midgut (Rudzinska et al., 1982; Zhu et al., 1991). The inability of B31-Δrrp1 to pass from infected animals into ticks could be due to the reduced ability of this strain to sense tick derived NAG and migrate to the tick bite site. It stands to reason that efficient acquisition would be dependent on such responses and that the ability of B. burgdorferi to survive in ticks would require efficient utilization of NAG.
Studies of other bacteria indicate an inverse correlation between c-di-GMP levels and bacterial motility (Jonas et al., 2009; Kim and McCarter, 2007; Martinez-Wilson et al., 2008; Paul et al., 2010; Simm et al., 2004; Wolfe and Visick, 2008). The influence of Rrp1 on motility of B. burgdorferi was first assessed by evaluating basic flagella motor function with wave propagation as a read out. All strains displayed wild type wave propagation patterns. To assess translational motion (i.e., directional movement away from the point of origin) methylcellulose was added to the media (Ruby and Charon, 1998). In standard BSK-H media, which lacks methylcellulose, spirochetes display limited translational motion due to low viscosity and the absence of an inherent matrix. For spirochetes to undergo translational motion, the flagella motors located at each end of the cell must rotate in opposite directions (i.e., clockwise and counterclockwise).
When the flagella motors rotate in the same direction, the cells flex and when both flagella motors reverse direction, a change in direction occurs (Charon et al., 2009). All strains had translation motion but B31-Δrrp1 was drastically reduced in flex frequency and was locked in constant one-directional motion (see supplementary movie files and Table 3 for rates). In E. coli, c-di-GMP has been demonstrated to influence protein-protein interactions that are control flagella motor rotation (Fang and Gomelsky, 2010; Paul et al., 2010). The motility phenotype of B31-Δrrp1 suggests that c-di-GMP may contribute to flagella motor activity in B. burgdorferi as well. However, the wild type motility phenotype of the B31-OV strain, which would be predicted to have elevated levels of c-di-GMP, seems at odds with the paradigm that high levels of c-di-GMP inhibit motility and promote a sessile life style (Hengge, 2009). Because the diguanylate cyclase activity of Rrp1 is strictly dependent on its phosphorylation state (Freedman et al., 2009; Ryjenkov et al., 2005) elevated production of Rrp1 may not necessarily lead to an increase in c-di-GMP. Rrp1 is cotranscribed with the histidine kinase, Hpk1, which is thought to serve as its phosphate donor (Rogers et al., 2009b). Increased production of c-di-GMP may require elevated production and autophosphorylation of Hpk1. The series of events required for this to occur may be dependent on environmental stimuli that are unique to the tick-mammal interface (Rogers et al., 2009b). These stimuli may not be encountered during in vitro cultivation.
To determine if Rrp1 production levels correlate with c-di-GMP concentration, we sought to measure the relative intracellular c-di-GMP concentration for each strain. Nucleotide extracts were assayed using reverse phase-high performance liquid chromatography. Precedent for the application of this highly sensitive approach comes from studies of c-di-GMP levels in E. coli (Antoniani et al., 2010). However, c-di-GMP levels in in vitro cultivated B. burgdorferi proved to be below the detection threshold. This raises the question: if the concentration of c-di-GMP is so low that it can’t be detected during in vitro cultivation, is it even produced in vitro and is it plausible that the unique properties of the B31-Δrrp1 strain are in fact due to the loss of diguanylate cyclase activity? The recent demonstration that c-di-GMP pools are asymmetrical and localized within a cell offers a possible explanation (Christen et al., 2010). It is possible that B. burgdorferi flagellar motor function is controlled by a concentrated local pool of c-di-GMP that represents a small fraction of the total cellular nucleotide pool. The deletion of rrp1 would eliminate this pool and thus result in aberrant motility and chemotactic responses. Additional evidence for c-di-GMP production by the Borrelia during in vitro growth has come from the study of a B. hermsii pdeA deletion mutant. Deletion of pdeA, the primary phosphodiesterase involved in c-di-GMP breakdown in the Borrelia, leads to the accumulation of c-di-GMP thus allowing it to be detected by HPLC (R.T. Marconi, unpublished results). While it would be informative to measure c-di-GMP levels in spirochetes in vivo, such analyses are technically challenging due to the low numbers of spirochetes in mammals and ticks. It may be possible in future analyses to employ FRET based c-di-GMP biosensor approaches to assess changes in c-di-GMP levels in response to environmental stimuli (Christen et al., 2010).
In summary, this study demonstrates that Rrp1 regulates stage specific steps in the enzootic cycle of the Lyme disease spirochetes. Deletion of rrp1 prevented the establishment of a productive population in ticks, while elevated production blocked infectivity in mice. The presence or absence of Rrp1 was found to be intricately linked to flagellar motor regulation and chemotactic responses, processes which are likely to be critical for completion of the enzootic cycle. Since diguanylate cyclase activity is the only known function of Rrp1, it is highly probable that c-di-GMP levels are responsible for the phenotypes observed for the strains generated in this report. In constructing future models for Borrelia pathogenesis, genetic regulation and motility, it will be important to consider the far reaching regulatory potential of c-di-GMP.
EXPERIMENTAL PROCEDURES
Bacterial strains and cultivation conditions
Borrelia strains were cultivated in BSK-H media with 5% CO2 at 25, 33 or 37° C. BSK-H media was prepared using bovine serum albumin from Gemini Bio-Products, Inc (lot #C54) as previously described (Samuels et al., 1994). Spirochetes were harvested by centrifugation and washed with phosphate buffered saline (PBS).
Allelic exchange mutagenesis
Allelic exchange replacement of rrp1 with an antibiotic resistance cassette (Frank et al., 2003) was performed as previously described using plasmid pΔBB0419 (Rogers et al., 2009b). Prior to introduction of DNA into B. burgdorferi by electroporation, the suicide plasmids were linearized with NcoI and ScaI. This was done to increase transformation efficiency (Samuels et al., 1994) and to inactivate the kanamycin and ampicillin resistance cassettes. Electroporation was done using previously described conditions (Earnhart et al., 2010). After allowing the cells to recover, the strains were grown in BSK-H media containing 75 μg ml−1 streptomycin and clonal populations obtained by sub-surface plating.
To complement the rrp1 deletion, rrp1 was reintroduced back into the chromosome of B31-Δrrp1 using allelic exchange. To generate the construct for complementation, a PCR product extending from 1 kb upstream of rrp1 to the 3′ end of rrp1 (with 3′ flanking AatII and AscI sites) was cloned into pCR2.1 using TOPO-TA approaches (Invitrogen) to generate pBB0419-UP. A kanamycin resistance cassette, derived from pBSV2 (Stewart et al., 2001), with a 5′ AatII site, was fused by overlap extension to the downstream 1 kb sequence flanking rrp1 (3′ AscI site). The kanamycin-downstream fusion was cloned into pCR2.1, then digested and inserted into the pBB0419-UP to create pBB0419-kan. The resulting plasmid was purified, linearized with ScaI and NcoI, and electroporated into B31-Δrrp1 to produce the complemented strain, B31-KI. Selection was achieved with 75 μg ml−1 kanamycin and clonal populations were obtained by sub-surface plating.
Plasmid content analysis
The plasmid content of all clones selected for analysis was confirmed by PCR using plasmid specific primer sets exactly as previously described (Rogers et al., 2009b).
Generation of a strain that overproduces rrp1
Constitutive overexpression of rrp1 was accomplished by using a pBSV2 based plasmid (Stewart et al., 2001) with rrp1 expression under the control of the flaB promoter. The flaB promoter and complete rrp1 gene were PCR amplified from B31-5A4 genomic DNA in separate PCR reactions and cloned into pCR2.1-TOPO to yield pCR2.1-PflaB and pCR2.1-rrp1. The plasmids were digested with ClaI and SalI. The rrp1 containing fragment was excised from a gel and ligated into the pCR2.1-PflaB to yield pCR2.1-PflaB-rrp1. pBSV2 and pCR2.1-PflaB-rrp1 were linearized with BamHI and SalI. The PflaB-rrp1 fragment was ligated into the pBSV2 vector to generate pBSV2-PflaB-rrp1. The final plasmid was propagated and electroporated into B31-5A4 cells. Selection of the overexpression strain (B31-OV) was achieved using kanamycin (200 μg ml−1). Clones were selected by sub-surface plating and plasmid content determined by PCR.
Growth curve analysis
To determine growth rates for the B31-wt, B31-Δrrp1, B31-KI, and B31-OV, an equal number of actively growing spirochetes (33°C) were transferred into fresh BSK-H complete media and incubated at 25, 33, and 37°. Cells were counted every 24h for 20 days using dark field microscopy. The average number of spirochetes per field was determined by averaging the counts from 10 fields for each time point.
SDS PAGE and immunoblotting
Lysates were fractionated using 12.5% Criterion Precast Gels (BioRad) and transferred to PVDF membrane as previously described (Rogers et al., 2009a). The membranes were blocked (1xPBS, 0.2% tween, 5% non-fat dry milk) and screened with a variety of antisera including mouse anti-Rrp1 (1:1000), mouse anti-Borrelia infection sera (1:1,000), and mouse anti-FlaB (1:400,000). Antibody binding was detected using horseradish peroxidase-conjugated secondary antibody (Pierce −1:40,000) and SuperSignal West Pico chemiluminescence substrate (Pierce).
Determination of intracellular c-di-GMP concentration
C-di-GMP determination in B. burgdorferi acid soluble extracts was performed as previously described (Antoniani et al., 2010) with minor modifications. Briefly, bacterial cells were collected, resuspended in 0.4 M ice-cold HClO4, and lysed by sonication. After centrifugation (16,000 × g; 3 min; 4°C) to remove cell debris, supernatants were neutralized with 0.16 M K2CO3, kept on ice for 10 min, and centrifuged (16,000 × g; 3 min; 4°C). Neutralized samples were injected into an HPLC system equipped with a diode-array detector. Nucleotides separation was performed on a Sinergi 4.0 μ Fusion-RP 250 × 4.6 mm column (18°C; Phenomenex, Inc). Elution conditions were 12 min at 100% buffer A (100 mM potassium phosphate, pH 6.0), 8 min up to 12% buffer B (buffer A containing 20% methanol), 3 min up to 45% buffer B, and 4 min up to 100% buffer B, holding at 100% buffer B for 7 min and returning to 100% buffer A in 6 min. Flow rate was maintained at 1 ml min−1.
RNA isolation and real time reverse transcriptase polymerase chain reaction (qRT-PCR)
RNA was isolated from Borrelia cultures using the RNeasy Midi kit as described by the manufacturer (Qiagen) and treated with DNase I (Invitrogen). cDNA was generated using the Superscript III First Strand cDNA Synthesis kit (Invitrogen), 50 ng random hexamer primers, and 1 μg total RNA. RT-PCR was performed with SYBR green PCR Master Mix (Applied Biosystems) and the DNA Engine Opticon System (MJ Research) with primers listed in Table 1. The following cycle parameters were used: 1 cycle of 10 min at 95° followed by 40 cycles of 10 sec at 94°, 30 sec at 50°, and 30 sec at 72°. Melting curves were generated over the temperature range 45–95° to assess amplification specificity. All reactions were run in triplicate with three biological replicates and the data were normalized against enolase (eno; BB0377). Statistical analyses were performed using a paired, two-tailed t-test. Alterations of gene expression were considered significant if p<0.05.
Infectivity in mice: seroconversion and cultivation analyses
The potential ability of each strain to infect C3H-HeJ mice was assessed by subcutaneous needle inoculation of 104 spirochetes between the shoulder blades (in 100 μl BSK-H complete media). Four weeks post-inoculation, the mice were sacrificed and blood, organs, and tissues were harvested. Seroconversion was assessed by ELISA. The ELISA plates were coated with 0.1 OD B31-5A4 spirochetes ml−1 in 100 μl of carbonate buffer (pH 9.6). The plates were incubated for 2h at RT with shaking, blocked with 1% bovine serum albumin in PBS-T, and antiserum from each mouse was applied in serial 3-fold dilutions (1:50–1:109, 1:350). Then, the plates were washed three times and bound IgG detected with peroxidase conjugated goat-anti-mouse IgG antiserum (1:20,000) and ABTS chromagen. The plates were read at OD405. Statistical analysis was performed using a one-way ANOVA. Seroconversion was also assessed through immunoblotting (1:1000). To further confirm infectivity, tissue cultivation was performed. Tissues and organ biopsies were immediately inserted into complete BSK-H media containing Borrelia antibiotic cocktail (phosphomycin, rifampicin, and amphotericin B) and incubated at 33° for 2–3 weeks followed by dark-field microscopy.
Tick studies
Naïve larval stage Ixodes scapularis ticks (Oklahoma State University Tick Rearing Facility) were brushed onto mice 4 weeks after the mice had been inoculated with each of the strains described above. The ticks were fed to repletion, collected and DNA isolated using the DNeasy Blood and Tissue kit (Qiagen). qPCR was performed using a B. burgdorferi flaB primer set and the data were normalized against tick rDNA using the RIB-3 and RIB-4 primers (Zahler et al., 1995). Ticks were also infected using immersion methods (Policastro and Schwan, 2003). Larval ticks were submerged in B. burgdorferi cultures (108 cells/ml; 33°C; 2 hr), washed, dried, and then fed on naive mice. One week after drop off, DNA was isolated and analyzed by qPCR.
Motility analyses
Motility in BSK-H complete media with and without 1% methyl cellulose was assessed using dark-field and DIC microscopy. Timelapse movies were recorded and analyzed using Slidebook 5 (Intelligent Imaging Inovations) motion-tracking software. Velocity measurements were calculated from twenty tracks per strain. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility.
Swarm plate and capillary tube chemotaxis assays
Swarm assays were adapted from Li et al (Li et al., 2002) and Motaleb et al (Motaleb et al., 2000). Plates containing 0.35% (wt/vol) Seakem GTG agarose in BSK-H were punched 3 times with a 1 ml pipette tip. 5×105 spirochetes were resuspended in 5 μl of a 1:10 BSK-H dilution in dPBS, and then placed in the punched holes. Plates were incubated at 33°C and colony diameters assessed at 2, 4, and 6 days. Statistics were generated using a one-way ANOVA on the means from 3 independent experiments.
The capillary assay was performed using a 96 well format as previously described (Motaleb et al., 2005) with some modifications. All experiments were conducted in triplicate. In brief, cells were harvested from liquid cultures by centrifugation and suspended at 107 cells ml−1 in motility buffer containing 1% (wt/vol) BSA and 1% (wt/vol) methylcellulose (Shi et al., 1998). The cells were placed in the wells of a 96 well plate. Capillary tubes, filled with motility buffer plus or minus chemoattractant (0.1 M N-acetyl-D-glucosamine), were inserted into the wells and the open end was sealed with Critoseal (Leica). The plates were incubated at 33° for 2h and then the contents of the capillary tubes were expelled into microcentrifuge tubes. The average number of spirochetes per field using dark-field microscopy was determined by averaging 10 fields of view. One-way ANOVA statistical analysis was performed on the means from 3 separate experiments.
Supplementary Material
Acknowledgments
Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463–02).
References
- Amikam D, Galperin MY. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics. 2006;22:3–6. doi: 10.1093/bioinformatics/bti739. [DOI] [PubMed] [Google Scholar]
- Antoniani D, Bocci P, Maciag A, Raffaelli N, Landini P. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl Microbiol Biotechnol. 2010;85:1095–1104. doi: 10.1007/s00253-009-2199-x. [DOI] [PubMed] [Google Scholar]
- Bakker RG, Li C, Miller MR, Cunningham C, Charon NW. Identification of specific chemoattractants and genetic complementation of a Borrelia burgdorferi chemotaxis mutant: flow cytometry-based capillary tube chemotaxis assay. Appl Environ Microbiol. 2007;73:1180–1188. doi: 10.1128/AEM.01913-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Garon CF. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease--a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, Rowe N. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol. 2009;191:600–607. doi: 10.1128/JB.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science. 2010;328:1295–1297. doi: 10.1126/science.1188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Stibitz S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol. 2007;10:17–23. doi: 10.1016/j.mib.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, Marconi RT. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol. 2010;76:393–408. doi: 10.1111/j.1365-2958.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Gomelsky M. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol. 2010;76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, Marconi RT. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol Med Microbiol. 2009;58:285–294. doi: 10.1111/j.1574-695X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006;188:4169–4182. doi: 10.1128/JB.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas K, Edwards AN, Ahmad I, Romeo T, Romling U, Melefors O. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ Microbiol. 2009 doi: 10.1111/j.1462-2920.2009.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, McCarter LL. ScrG, a GGDEF-EAL protein, participates in regulating swarming and sticking in Vibrio parahaemolyticus. J Bacteriol. 2007;189:4094–4107. doi: 10.1128/JB.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgorferi strain B31 is associated with the loss of either linear plasmid 25 or 28–1. Infection and Immunity. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bakker RG, Motaleb MA, Sartakova ML, Cabello FC, Charon NW. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc Natl Acad Sci U S A. 2002;99:6169–6174. doi: 10.1073/pnas.092010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Wilson HF, Tamayo R, Tischler AD, Lazinski DW, Camilli A. The vibrio cholerae hybrid sensor kinase VieS contributes to motility and biofilm regulation by altering the cyclic diguanylate level. J Bacteriol. 2008;190:6439–6447. doi: 10.1128/JB.00541-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Sung SY, Labandeira-Rey M, Skare JT, Marconi RT. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun. 2001;69:3670–3677. doi: 10.1128/IAI.69.6.3670-3677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, Charon NW. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A. 2000;97:10899–10904. doi: 10.1073/pnas.200221797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Miller MR, Li C, Bakker RG, Goldstein SF, Silversmith RE, Bourret RB, Charon NW. CheX is a phosphorylated CheY phosphatase essential for Borrelia burgdorferi chemotaxis. J Bacteriol. 2005;187:7963–7969. doi: 10.1128/JB.187.23.7963-7969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell. 2010;38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. Analysis of the Borrelia burgdorferi cyclic-di-GMP binding protein PlzA reveals a role in motility and virulence. Infect Immun. 2011 doi: 10.1128/IAI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun. 2009a;77:4396–4405. doi: 10.1128/IAI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009b;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Amikam D. Cyclic di-GMP as a second messenger. Curr Opin Microbiol. 2006;9:218–228. doi: 10.1016/j.mib.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ruby JD, Charon NW. Effect of temperature and viscosity on the motility of the spirochete Treponema denticola. FEMS Microbiol Lett. 1998;169:251–254. doi: 10.1111/j.1574-6968.1998.tb13325.x. [DOI] [PubMed] [Google Scholar]
- Rudzinska MA, Spielman A, Lewengrub S, Piesman J, Karakashian S. Penetration of the peritrophic membrane of the tick by Babesia microti. Cell Tissue Res. 1982;221:471–481. doi: 10.1007/BF00215696. [DOI] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Simm R, Romling U, Gomelsky M. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem. 2006;281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- Samuels DS, Mach K, Garon CF. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. Journal of Bacteriology. 1994;176:6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Yang Z, Geng Y, Wolinsky LE, Lovett MA. Chemotaxis in Borrelia burgdorferi. J Bacteriol. 1998;180:231–235. doi: 10.1128/jb.180.2.231-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm R, Morr M, Kader A, Nimtz M, Romling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR, Strobel SA. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16:1218–1223. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Thalken R, Bono JL, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Molecular Microbiology. 2001;39:714–721. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Pitzer JE, Miller MR, Motaleb MA. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol Microbiol. 2010;77:128–142. doi: 10.1111/j.1365-2958.2010.07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, He YW, Wu DH, Swarup S, Zhang LH. The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors. J Bacteriol. 2011;192:1020–1029. doi: 10.1128/JB.01253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA. Cyclic di-GMP Activation of Signal-Dependent RNA Processing. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Wolfe AJ, Visick KL. Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol. 2008;190:463–475. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler M, Gothe R, Rinder H. Diagnostic DNA amplification from individual tick eggs, larvae and nymphs. Exp Appl Acarol. 1995;19:731–736. doi: 10.1007/BF00052084. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Gern L, Aeschlimann A. The peritrophic membrane of Ixodes ricinus. Parasitol Res. 1991;77:635–641. doi: 10.1007/BF00931028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.