Abstract
The anterior cruciate ligament (ACL) is the most commonly-injured knee ligament during sporting activities. After injury, most individuals experience episodes of the knee giving way during daily activities (non-copers). Non-copers demonstrate asymmetrical quadriceps strength and movement patterns which could have long-term deleterious effects on the integrity of the knee joint. The purpose of this study was to determine if non-copers resolve their strength and movement asymmetries within two years after surgery. 26 non-copers were recruited to undergo pre-operative quadriceps strength testing and 3-dimensional gait analysis. Subjects underwent surgery to reconstruct the ligament followed by physical therapy focused on restoring normal range of motion, quadriceps strength, and function. Subjects returned for quadriceps strength testing and gait analysis six months and two years after surgery. Acutely after injury, quadriceps strength was asymmetric between limbs, but resolved six months after surgery. Asymmetric knee angles, knee moments, and knee and hip power profiles were also observed acutely after injury and persisted six months after surgery despite subjects achieving symmetrical quadriceps strength. Two years after surgery, quadriceps strength in the involved limb continued to improve and most kinematic and kinetic asymmetries resolved. These findings suggest that adequate quadriceps strength does not immediately resolve gait asymmetries in non-copers. They also suggest that non-copers have the capacity to improve their quadriceps strength and gait symmetry long after ACL reconstruction.
Keywords: ACL, gait, non-coper, knee, rehabilitation
1. Introduction
Successful rehabilitation following anterior cruciate ligament (ACL) reconstruction is often measured by the achievement of symmetrical limb movement, both in clinical testing and during gait. Non-copers comprise the most common and the poorest functioning subset of all ACL-deficient individuals (Rudolph et al., 2001). Non-copers commonly experience episodes of knee instability and limited daily function early after injury (Eastlack et al., 1999). The aberrant involved-limb movement patterns characteristic of this group may be ineffective and potentially harmful means by which their neuromuscular systems attempt to compensate for knee instability (Rudolph et al., 1998). Repeated episodes of the knee giving way could expose non-copers to increased risk of meniscal and articular surface damage (Noyes et al., 1983; Shelton et al., 1997); therefore, surgical reconstruction is customarily recommended (Barrack et al., 1990; Marx et al., 2003).
Joint effusion, limited range of motion, and quadriceps weakness are common impairments associated with acute ACL injury (Noyes et al., 1983), and have a detrimental effect on daily function. Even after these initial deficits resolve, non-copers attempt to mitigate forces in the injured knee. Specifically, they achieve smaller peak knee flexion angles (Rudolph et al., 1998) and smaller internal knee extensor moments at peak knee flexion (Berchuck et al., 1990; Rudolph et al., 1998) during normal walking. Early in stance, non-copers absorb less power with the involved knee and compensate by absorbing more power with the ipsilateral hip (Rudolph et al., 2001).
Quadriceps weakness is ubiquitous following ACL reconstruction (ACLR). Restoring quadriceps strength is imperative to successful recovery following surgery because significant quadriceps weakness is associated with both poor functional performance (de Jong et al., 2007; Eitzen et al., 2009; Keays et al., 2001) and aberrant gait mechanics (Lewek et al., 2002). Six months after ACLR is a common time for athletes to return to sports participation because it is hypothesized that adequate functional recovery (Kvist, 2004; Shelbourne et al., 1992) and graft ligamentization and revascularization (Falconiero et al., 1998; Scranton et al., 1998) have occurred. Non-copers, however, experience persistent gait deviations (Hartigan et al., 2009) and have difficulty successfully returning to sport six months after surgery despite having adequate quadriceps strength (Hartigan et al., 2010). They are more successful at returning to sport from six months to one year after surgery, suggesting that additional recovery occurs following discharge from supervised physical therapy (Hartigan et al., 2010).
The functional outcomes and gait patterns of non-copers have been studied extensively immediately after injury (Devita et al., 1997; Hartigan et al., 2009; Hurd and Snyder-Mackler, 2007; Lewek et al., 2003; Rudolph et al., 2001; Rudolph et al., 1998) and within six months after surgery (Devita et al., 1997; Hartigan et al., 2010; Hartigan et al., 2009; Lewek et al., 2002), but little is known about their movement patterns long after ACLR. Aberrant joint kinematics may detrimentally affect knee joint health following ACL injury (Andriacchi et al., 2004); therefore, if movement asymmetries persist long after surgery non-copers may be at increased risk of developing osteoarthritis in the injured knee (Andriacchi and Dyrby, 2005; Andriacchi and Mundermann, 2006; Andriacchi et al., 2004), and plausibly retaining poorer function. The aim of this study was to evaluate a cohort of non-copers up to two years after ACLR to see if their quadriceps strength and weight acceptance strategies continue to improve after completing a formal physical therapy program. Our hypothesis was that non-copers would improve their quadriceps strength symmetry over time and achieve weight acceptance symmetry within two years of ACL reconstruction.
2. Methods
Individuals with a complete isolated rupture of the ACL were recruited from the University of Delaware Physical Therapy clinic. All subjects gave informed consent which was approved by the Institutional Review Board. ACL rupture was diagnosed by clinical examination and confirmed by magnetic resonance imaging (MRI). Subjects were between 13 and 50 years old at the time of screening and participated in level I or II (Daniel et al., 1994; Hefti et al., 1993) activities for at least 50 hours per week prior to injury (Hefti et al., 1993). A screening examination (Fitzgerald et al., 2000; Hurd et al., 2008) was performed in order to identify non-copers among the eligible ACL-deficient subjects. Non-copers were excluded from the study if they had articular cartilage damage (chondral defects >1 cm), meniscal tears, or grade III concomitant ligament injury in the involved knee.
All subjects received ten sessions of preoperative physical therapy aimed at increasing quadriceps strength in the involved limb (Hartigan et al., 2009). 11 of the 26 subjects were randomly assigned to receive specialized perturbation training designed to facilitate coordinated muscle activation around the knee joint (Fitzgerald et al., 2000) as part of a larger randomized clinical trial. Surgery was subsequently performed by one local orthopedic surgeon to repair the ruptured ligament using either a semitendinosis-gracilis autograft or a soft-tissue allograft. After ACLR, subjects participated in the University of Delaware postoperative ACL rehabilitation protocol which included progressive quadriceps strength training, effusion management, range of motion restoration, and functional training. A licensed physical therapist advanced each subject through post-operative physical therapy using a criterion-based decision making scheme described previously (Manal and Snyder-Mackler, 1996).
2.1 Data collection
Subjects participated in quadriceps strength testing and 3-dimensional gait analysis acutely after injury (screening), six months after ACLR, and two years after ACLR. Isometric quadriceps strength was tested bilaterally on a dynamometer (KIN-COM; Chattanooga Corp., Chattanooga, TN). Subjects were seated in an upright position with straps fixing the patient’s hip and knee joints at 90 degrees of flexion (Snyder-Mackler et al., 1993). Three trials were conducted during which each subject attempted to extend their leg by maximally contracting their quadriceps. The trial with the highest force output for each limb was selected and normalized to body mass index (BMI).
3-dimensional gait analysis was performed overground along a 13m walkway which contained an embedded 6-component force plate (Bertec Corp., Worthington, OH) sampling at 1080 Hz. Retroreflective markers were placed on bony landmarks of the lower extremities and pelvis to determine joint centers and segment positions, and rigid shells were used to track segment motion. An 8-camera motion analysis system (VICON, Oxford Metrics Ltd., London, UK) captured marker positions at 120Hz. Preferred walking speed was determined overground. Subjects were instructed to walk along the 13m walkway through the capture volume at their preferred, community walking speed. Two infrared photos cells placed 2.865 m apart along the walkway measured walking speed. All subsequent motion analysis trials were acceptable only if subjects made isolated foot contact with the force plate and did not vary more than 5% from their measured preferred walking speed.
2.2 Data management and analysis
Kinematic and kinetic data were low-pass filtered using a bidirectional 4th order bidirectional Butterworth filter with 6Hz and 40Hz cutoff frequencies respectively. Data from five successful walking trials were post-processed and averaged using custom LabVIEW coding (National Instruments, Austin, TX) and Visual 3D (C-motion Inc., Germantown, MD) to compute joint angles, moments, and powers. All data were time normalized to 101 points of stance for each limb.
A mixed-model analysis of variance (ANOVA) with repeated measures was used to evaluate time × limb × group interactions across the three testing sessions, between limbs, and between the groups of subjects who either received or did not receive perturbation training. All comparisons were performed using a statistical software package (SPSS v18, Chicago, IL). Comparisons were performed on quadriceps strength, peak knee flexion angle and knee joint excursion during weight acceptance, internal knee and hip extensor moments at peak knee flexion, and peak knee power absorption. Post-hoc paired t-tests were performed when a significant main effect or interaction was detected. Statistical significance was set at p < 0.05. We also defined minimal clinically important differences for quadriceps strength (3 N/BMI), knee flexion angle (3 degrees), knee moment (0.04 Nm/kg*m), and hip moment (0.06 Nm/kg*m) to determine whether the results of our statistical comparisons were clinically meaningful (Copay et al., 2007). Finally, we used descriptive statistics to describe differences in hip power profiles because of the lack of clear peaks at which to perform comparisons.
3. Results
26 subjects (M = 18; age = 29.6 ± 10.7 years; walking speed = 1.56 ± 0.11 m/s) were recruited for the study. The results of our ANOVA testing revealed no statistically significant differences between the strength and perturbation groups; therefore, data from the two groups were collapsed and tested for time × limb interactions.
3.1 Quadriceps strength
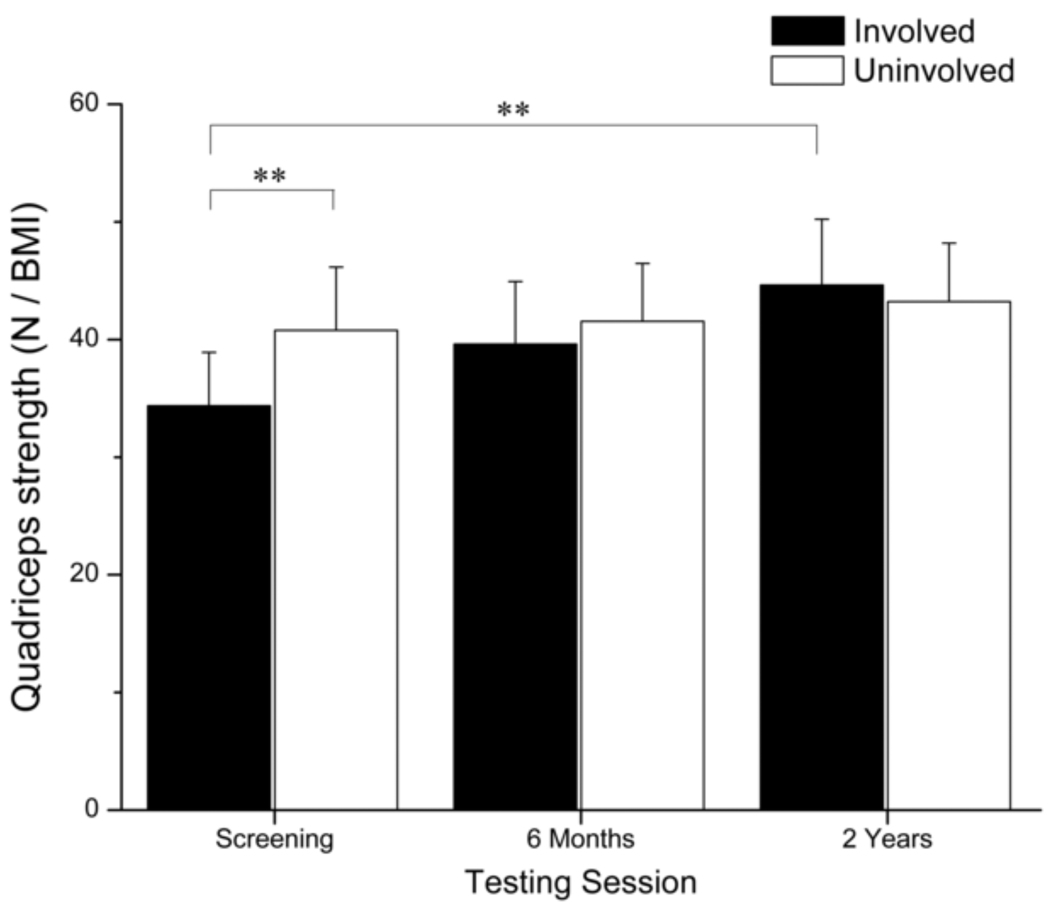
Quadriceps strength data for only 24 subjects could be included in the time × limb analysis because two subjects could not complete testing two years after surgery. One subject had patellofemoral pain and another subject tore his graft. A significant time × limb interaction (p < 0.001) was detected for quadriceps strength (Fig. 1). The involved limb (34.4 ± 10.7 N/BMI) was weaker than the uninvolved limb at screening (40.8 ± 12.7 N/BMI) (p < 0.001). Strength in the involved limb significantly increased six months (p < 0.001) after surgery at which point quadriceps strength was not different between limbs (Involved = 39.6 ± 12.6 N/BMI; Uninvolved = 41.6 ± 11.6 N/BMI) (p = 0.169). Quadriceps strength in the involved limb continued to improve two years after surgery (p = 0.001) and remained similar to quadriceps strength in the uninvolved limb (Involved = 44.6 ± 13.5 N/BMI; Uninvolved = 43.2 ± 11.8 N/BMI) (p = 0.182). Strength gains in the involved limb were also clinically meaningful between each testing session. Strength in the uninvolved limb did not change over time.
Fig. 1.
Average quadriceps strength is presented for the 24 subjects who completed quadriceps strength testing. Error bars represent 95% confidence intervals. At screening, the involved limb was significantly weaker than the uninvolved limb. Quadriceps strength in the involved limb increased over time. Six months after surgery no differences in quadriceps strength were detected between limbs. (** p < 0.001).
3.2 Knee
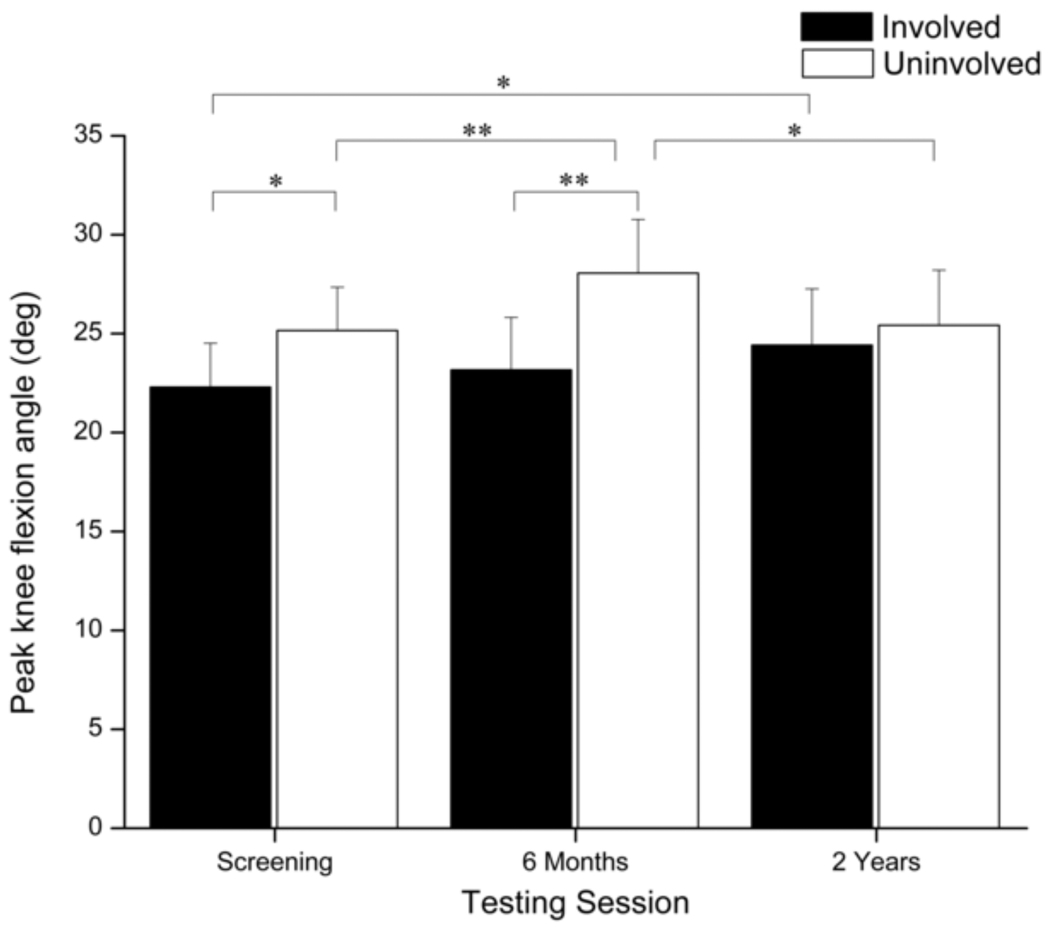
A significant time × limb interaction was detected for peak knee flexion angle (p = 0.003) (Fig. 2). A significant difference was detected between limbs at screening (Involved = 22.3 ± 5.5°; Uninvolved = 25.2 ± 5.4x°) (p = 0.009), which was nearly clinically meaningful (Δ = 2.9°). Six months after surgery, the peak knee flexion angle increased in the uninvolved limb (p < 0.001) resulting in a significant difference between limbs (Involved = 23.2 ± 6.6°; Uninvolved = 28.1 ± 6.7°) (p < 0.001). This difference was clinically meaningful. Two years after surgery, the peak knee flexion angle in the uninvolved limb decreased (p = 0.002) to its pre-surgical values while no changes were detected in the involved limb (p = 0.122). No differences in peak knee flexion angle were detected between limbs at this time (Involved = 24.4 ± 7.0°; Uninvolved = 25.4 ± 6.9°) (p = 0.236). The increase in peak knee flexion angle in the involved limb from screening to two years after surgery was statistically significant (p = 0.034), but it was not clinically meaningful (Δ = 2.1°).
Fig. 2.
Comparison of peak knee flexion angle during weight acceptance between limbs and across testing sessions. Error bars represent 95% confidence intervals. Peak knee flexion angle was significantly smaller in the involved limb at screening and six months after surgery. Peak knee flexion angle increased in the uninvolved limb six months after surgery, but returned to its presurgical amount two years after surgery. Peak knee flexion angle increased in the involved limb over time until two years after surgery when no differences were detected between limbs. (* p < 0.05)(** p < 0.001).
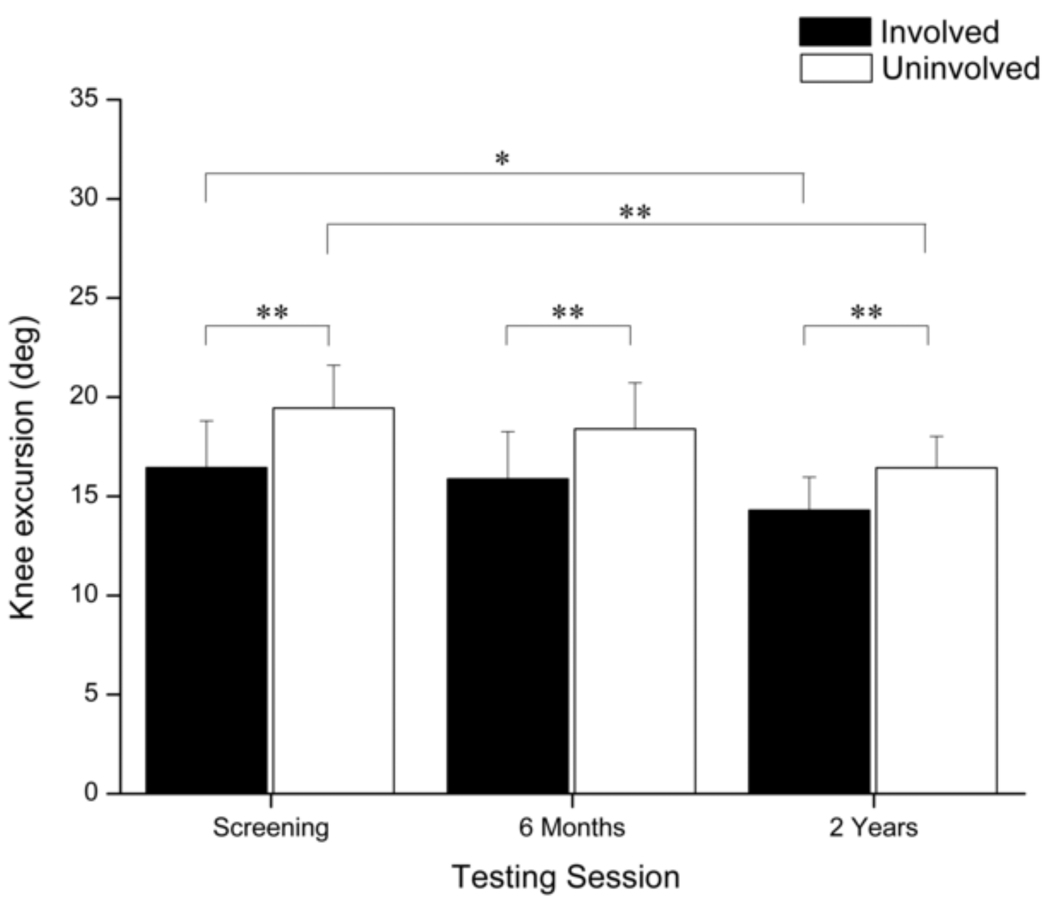
Main effects of time (p = 0.002) and limb (p < 0.001) were detected for knee joint excursions during weight acceptance (Fig. 3). Knee excursions were smaller in the involved limb at each testing session (p < 0.001) and decreased over time (Involved, p = 0.010; Uninvolved, p < 0.001). A clinically meaningful difference between limbs was identified at screening, but was not evident six months or two years after surgery.
Fig. 3.
Comparison of knee excursions during weight acceptance between limbs and across testing sessions. Error bars represent 95% confidence intervals. Knee excursions decreased in both limbs over time; however, excursions were significantly smaller in the involved limb during each testing session. (* p < 0.05)(** p < 0.001).
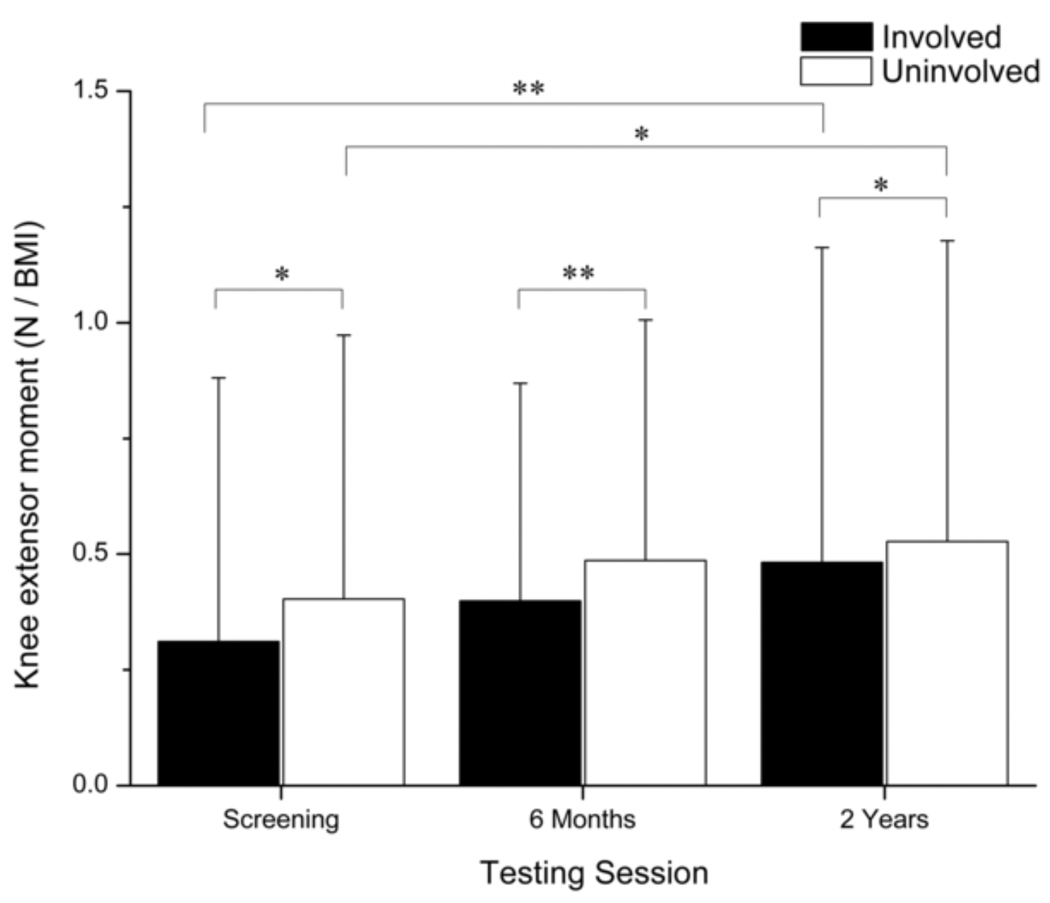
Main effects for time (p < 0.001) and limb (p < 0.001) were detected for knee extensor moment (Fig. 4). Knee extensor moment was smaller in the involved limb at each testing session and increased in both limbs over time (Involved, p < 0.001; Uninvolved, p = 0.002). Clinically meaningful differences were detected between limbs at each testing session.
Fig. 4.
Comparison of the internal knee extensor moment during weight acceptance between limbs and across testing sessions. Error bars represent 95% confidence intervals. Knee extensor moments increased in both limbs over time; however, the extensor moment was significantly smaller in the involved limb during each testing session. (* p < 0.05)(** p < 0.001).
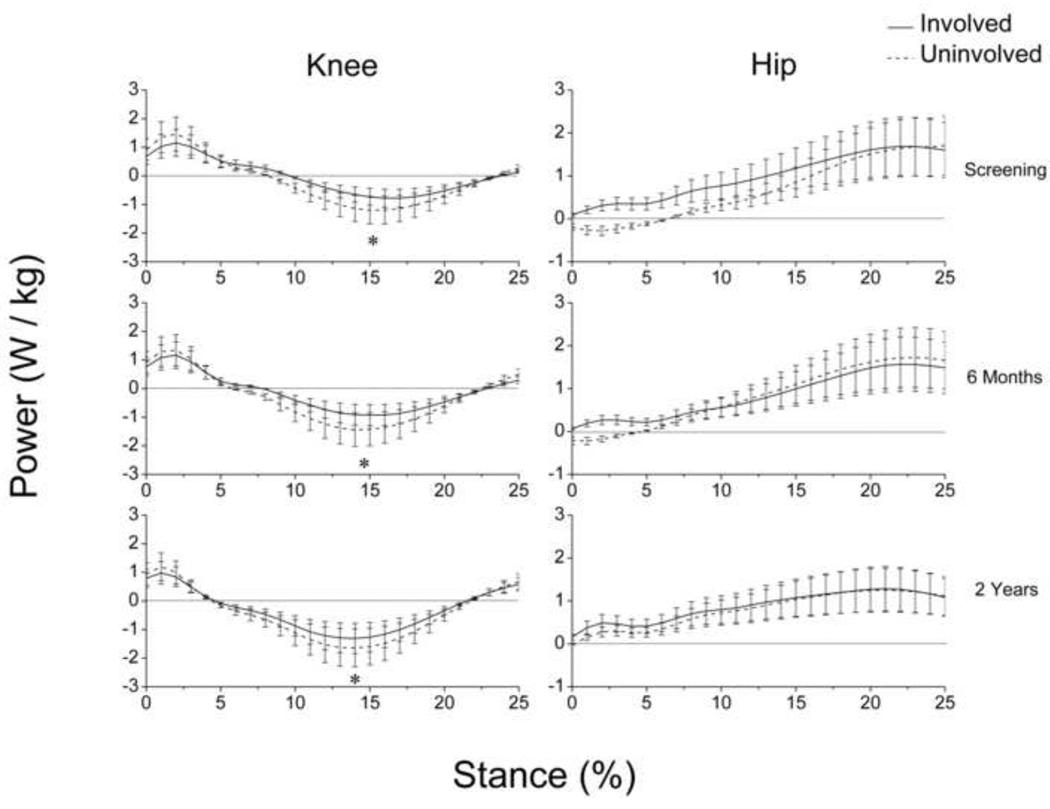
Main effects for time (p = 0.001) and limb (p < 0.001) were detected for peak knee power absorption (Fig. 5). Peak knee power absorption was lesser in the involved limb at each testing session and increased in both limbs over time (Involved, p = 0.002; Uninvolved, p = 0.006).
Fig. 5.
Knee and hip power profiles during weight acceptance (i.e. the first 25% of stance) for each testing session. Positive values indicate power generation and negative values indicate power absorption. Error bars represent 95% confidence intervals. Peak knee power absorption increased over time, but remained lesser in the involved limb. At screening, the uninvolved hip (dashed line) absorbed power immediately after heel strike (~0–5% stance). The power profile of the uninvolved hip changed over time, and two years after surgery both limbs appeared to generate power similarly throughout the entirety of weight acceptance.(* p < 0.05).
3.3 Hip
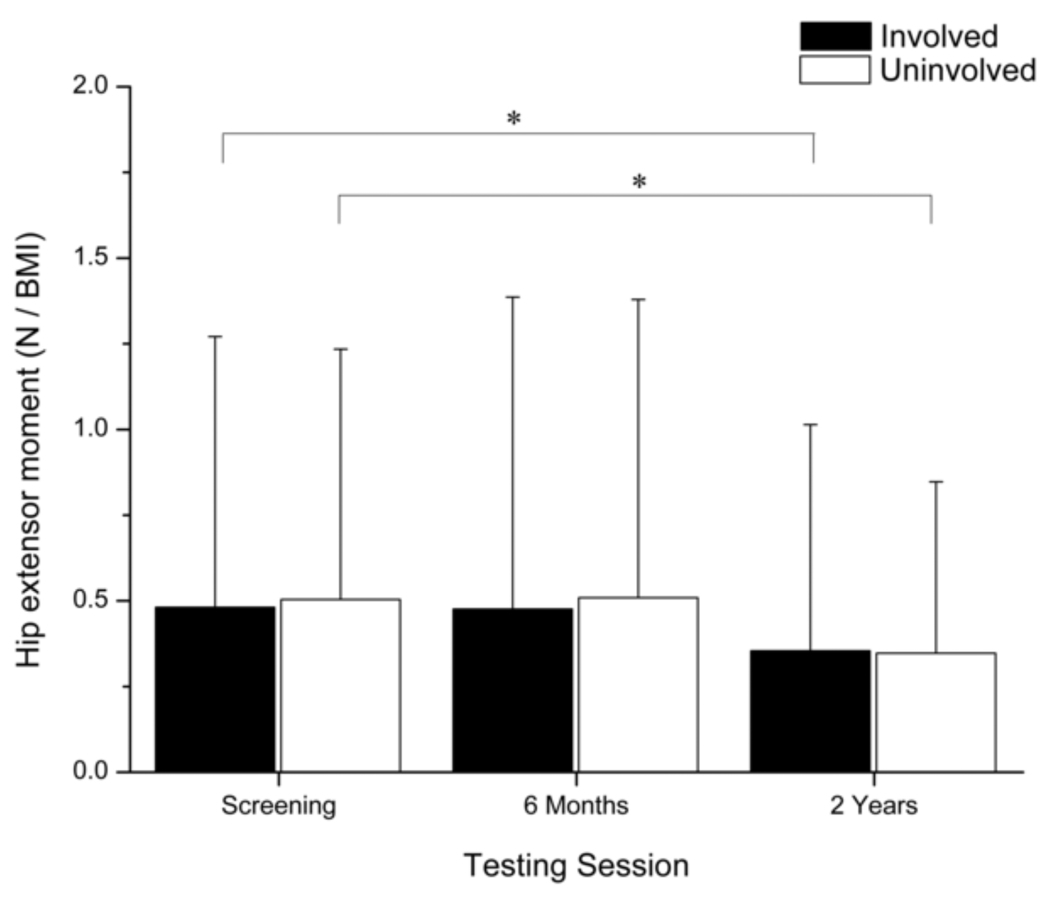
A main effect for time (p < 0.001) was detected for the hip extensor moment at peak knee flexion (Fig. 6). The hip extensor moment did not change from screening to six months after surgery, but it showed a statistically (involved: p = 0.005; uninvolved: p < 0.001) and clinically meaningful decrease from six months to two years after surgery.
Fig. 6.
Comparison of the internal hip extensor moment during weight acceptance between limbs and across testing sessions. Error bars represent 95% confidence intervals. Hip extensor moment decreased in both limbs over time, and was not significantly different between limbs during any of the testing sessions. (* p < 0.05).
At screening, the involved hip generated power throughout the entirety of weight acceptance, whereas the uninvolved hip absorbed power during the first 6% of stance and generated power throughout the remainder of weight acceptance (Fig. 5). Additionally, there was no overlap in 95% confidence intervals during this period of stance indicating a significant difference in mean hip power behavior. Two years after surgery, these opposing hip strategies resolved and both hips generated power similarly throughout weight acceptance.
4. DISCUSSION
The aim of this study was to track a cohort of non-copers over time to determine if their ability to symmetrically load their knees improved over time. Six months after ACLR, subjects demonstrated asymmetrical gait patterns despite restoring symmetrical quadriceps strength. Two years after surgery, the majority of gait impairments had diminished, long after sufficient quadriceps strength had been achieved. These data suggest that neuromuscular impairments are common in non-copers even several months after surgery, and that abnormal movement strategies are not resolved solely by addressing their quadriceps strength deficits.
At screening, the non-copers presented with asymmetrical quadriceps strength, peak knee flexion angles, knee excursions, knee moments, peak knee power absorption, and hip power profiles, consistent with the findings of earlier investigations (Berchuck et al., 1990; Hartigan et al., 2009; Hurd and Snyder-Mackler, 2007; Rudolph et al., 2001; Rudolph et al., 1998). When compared to uninjured controls and to higher-functioning ACL-deficient athletes (potential copers), the non-copers demonstrated a reduced contribution from the injured knee and a increased contribution from the hip on the involved side (Hurd and Snyder-Mackler, 2007; Rudolph et al., 2001). This study also identified a unique compensation in the contralateral hip. At screening, the uninvolved hip unexpectedly absorbed power immediately after heel strike whereas the involved hip generated power throughout weight acceptance.
Six months after ACLR is a common time for athletes to begin reintegration into their sporting activities (Cascio et al., 2004; Kvist, 2004; Shelbourne and Nitz, 1992). Symmetrical quadriceps strength is not only used to decide when to allow athletes to return to sports after surgery (Hartigan et al., 2010; Myer et al., 2006), but it is also considered fundamental for restoring symmetrical movement patterns. Lewek et. al. reported that patients with inadequate quadriceps strength following ACLR produced knee kinematics and kinetics similar to acutely-injured non-copers (Lewek et al., 2002). Conversely, patients who obtained symmetrical quadriceps strength moved like uninjured controls. While there is a clear relationship between quadriceps weakness and abnormal gait patterns early after surgery, the effect of restoring adequate quadriceps strength on gait has not been clearly defined. The non-copers in the current study regained symmetrical quadriceps strength six months after surgery, yet they continued to demonstrate statistically and clinically meaningful asymmetries in their knee kinematics and kinetics. These results suggest that there is a neuromuscular component to recovery that cannot be resolved by simply restoring symmetrical quadriceps strength.
Two years after surgery, kinematic asymmetries in the knee resolved, but asymmetrical knee extensor moments and peak knee power absorption persisted despite continued increases in involved-limb quadriceps strength. This was not altogether surprising, as mitigated knee extensor moments in the reconstructed limb have been detected in patients up to one year after ACLR (Hooper et al., 2002; Timoney et al., 1993). As expected, hip moments in the involved limb decreased over time as knee moments increased. Interestingly, hip power profiles became more symmetrical over time because the behavior of the uninvolved hip appeared to change rather than behavior of the involved hip. These findings suggest that bilateral adaptations can result from a unilateral ACL injury (Hart et al., 2010). They also suggest that while non-copers may continue to improve their strength and gait symmetry after leaving supervised therapy, gait deficiencies may persist. Serial gait analyses may be warranted for these athletes to determine, in combination with clinical performance, when movement symmetry has been achieved and when return to sport can be advised.
The relationship between gait symmetry and functional performance in non-copers has not been established; however, the importance of movement symmetry on reinjury risk is emerging. Paterno and colleagues found that subjects who produced asymmetrical internal knee extensor moments during a drop vertical jump task were three times more likely to sustain a second ACL rupture than subjects who produced symmetrical knee extensor moments (Paterno et al., 2010). These findings are of particular importance to high-level athletes because most intend to return to their pre-injury activity levels immediately following supervised rehabilitation. Graft rupture rates range from 6.0% to 12.7% within five years of ACL reconstruction (Laboute et al., 2010; Salmon et al., 2005; Shelbourne et al., 2009). The greatest risk for re-rupture may occur within the first several months after reconstruction (Laboute et al., 2010; Salmon et al., 2005), a time when non-copers continue to demonstrate movement asymmetries. Identifying and intervening with athletes who have movement asymmetries after ACLR may help mitigate this risk.
Our data exclusively describe non-copers; therefore, the functional performance of this group after both injury and ACLR may not be generalizable to all ACL-deficient athletes (e.g. potential copers). Joint kinematics and kinetics can identify movement asymmetries in non-copers, but the addition of electromyographic data may help describe the specific neuromuscular control strategies that they adopt. Non-copers have the capacity to improve their strength and movement patterns long after leaving a formal rehabilitation setting and may simply need additional time to resolve all gait asymmetries. Future work should explore the effects of aberrant movement patterns on ACL reinjury, as well as neuromuscular retraining paradigms to help clinical scientists improve post-operative care following ACLR.
Acknowledgements
We would like to acknowledge Drs. Daniel Ramsey, Wendy Hurd, and Erin Hartigan for their efforts with data collection and processing; Dr. Michael J. Axe for his patient referrals; Martha Callahan for her assistance with subject recruitment and retention; and the Physical Therapy Clinic at the University of Delaware for their excellence in caring for our patients. Support for this work was provided by the National Institutes of Health R01AR048212 and S10RR022396, and the Foundation for Physical Therapy (PODS I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We have no financial or personal conflicts of interest to disclose.
References
- Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38(2):293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18(5):514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH. The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clin Orthop Relat Res. 1990;(259):192–199. [PubMed] [Google Scholar]
- Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871–877. [PubMed] [Google Scholar]
- Cascio BM, Culp L, Cosgarea AJ. Return to play after anterior cruciate ligament reconstruction. Clin Sports Med. 2004;23(3):395–408. doi: 10.1016/j.csm.2004.03.004. ix. [DOI] [PubMed] [Google Scholar]
- Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC., Jr. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- de Jong SN, van Caspel DR, van Haeff MJ, Saris DB. Functional assessment and muscle strength before and after reconstruction of chronic anterior cruciate ligament lesions. Arthroscopy. 2007;23(1):21–28. doi: 10.1016/j.arthro.2006.08.024. 28 e1-3. [DOI] [PubMed] [Google Scholar]
- Devita P, Hortobagyi T, Barrier J, Torry M, Glover KL, Speroni DL, Money J, Mahar MT. Gait adaptations before and after anterior cruciate ligament reconstruction surgery. Med Sci Sports Exerc. 1997;29(7):853–859. doi: 10.1097/00005768-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc. 1999;31(2):210–215. doi: 10.1097/00005768-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43(5):371–376. doi: 10.1136/bjsm.2008.057059. [DOI] [PubMed] [Google Scholar]
- Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14(2):197–205. doi: 10.1016/s0749-8063(98)70041-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30(4):194–203. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon) 2010;25(4):277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Hartigan E, Axe M, Snyder-Mackler L. Timeline for non-copers to pass return to sports criteria after ACL reconstruction. J Ortho Sports Phys Ther. 2010 [Google Scholar]
- Hartigan E, Axe MJ, Snyder-Mackler L. Perturbation training prior to ACL reconstruction improves gait asymmetries in non-copers. J Orthop Res. 2009;27(6):724–729. doi: 10.1002/jor.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3–4):226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- Hooper DM, Morrissey MC, Drechsler WI, Clark NC, Coutts FJ, McAuliffe TB. Gait analysis 6 and 12 months after anterior cruciate ligament reconstruction surgery. Clin Orthop Relat Res. 2002;(403):168–178. doi: 10.1097/00003086-200210000-00025. [DOI] [PubMed] [Google Scholar]
- Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: Part 1, outcomes. Am J Sports Med. 2008;36(1):40–47. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd WJ, Snyder-Mackler L. Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007;25(10):1369–1377. doi: 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keays SL, Bullock-Saxton J, Keays AC, Newcombe P. Muscle strength and function before and after anterior cruciate ligament reconstruction using semitendonosus and gracilis. Knee. 2001;8(3):229–234. doi: 10.1016/s0968-0160(01)00099-0. [DOI] [PubMed] [Google Scholar]
- Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- Laboute E, Savalli L, Puig P, Trouve P, Sabot G, Monnier G, Dubroca B. Analysis of return to competition and repeat rupture for 298 anterior cruciate ligament reconstructions with patellar or hamstring tendon autograft in sportspeople. Ann Phys Rehabil Med. 2010;53(10):598–614. doi: 10.1016/j.rehab.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Chmielewski TL, Risberg MA, Snyder-Mackler L. Dynamic knee stability after anterior cruciate ligament rupture. Exerc Sport Sci Rev. 2003;31(4):195–200. doi: 10.1097/00003677-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Manal TJ, Snyder-Mackler L. Practice guidelines for anterior cruciate ligament rehabilitation: a criterion-based rehabilitation progression. Operative Techniques in Orthopaedics. 1996;6(3):190–196. [Google Scholar]
- Marx RG, Jones EC, Angel M, Wickiewicz TL, Warren RF. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy. 2003;19(7):762–770. doi: 10.1016/s0749-8063(03)00398-0. [DOI] [PubMed] [Google Scholar]
- Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154–162. doi: 10.2106/00004623-198365020-00003. [DOI] [PubMed] [Google Scholar]
- Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, Hewett TE. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper: Movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998;8(6):349–362. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. doi: 10.1016/j.arthro.2005.04.110. [DOI] [PubMed] [Google Scholar]
- Scranton PE, Jr., Lanzer WL, Ferguson MS, Kirkman TR, Pflaster DS. Mechanisms of anterior cruciate ligament neovascularization and ligamentization. Arthroscopy. 1998;14(7):702–716. doi: 10.1016/s0749-8063(98)70097-0. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Klootwyk TE, Decarlo MS. Update on accelerated rehabilitation after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1992;15(6):303–308. doi: 10.2519/jospt.1992.15.6.303. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1992;15(6):256–264. doi: 10.2519/jospt.1992.15.6.256. [DOI] [PubMed] [Google Scholar]
- Shelton WR, Barrett GR, Dukes A. Early season anterior cruciate ligament tears. A treatment dilemma. Am J Sports Med. 1997;25(5):656–658. doi: 10.1177/036354659702500511. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1993;25(7):783–789. doi: 10.1249/00005768-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Timoney JM, Inman WS, Quesada PM, Sharkey PF, Barrack RL, Skinner HB, Alexander AH. Return of normal gait patterns after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21(6):887–889. doi: 10.1177/036354659302100623. [DOI] [PubMed] [Google Scholar]