Abstract
We tested the hypothesis that obese individuals experience greater activation of the gustatory and somatosensory cortex, but weaker activation of the striatum, in response to intake and anticipated intake of high-fat chocolate milkshake versus an isocaloric milkshake labeled low-fat and a tasteless solution using functional magnetic resonance imaging (fMRI) with 17 obese and 17 lean young women. Obese relative to lean women showed greater activation in somatosensory (Rolandic operculum), gustatory (frontal operculum), and reward valuation regions (amgydala, ventralmedial prefrontal cortex (vmPFC) in response to intake and anticipated intake of milkshake versus tasteless solution, though there was little evidence of reduced striatal activation. Obese relative to lean women also showed greater activation in the Rolandic operculum, frontal operculum, and vmPFC in response to isocaloric milkshakes labeled regular versus lowfat. Results suggest that hyper-responsivity of somatosensory, gustatory, and reward valuation regions may be related to overeating and that top-down processing influence reward encoding, which could further contribute to weight gain.
Keywords: obesity, fMRI, low-fat labeling, top-down processing, reward circuitry
The incidence of obesity has reached epidemic proportions, which is concerning because obesity increases the risk for high blood pressure, adverse lipoprotein profiles, diabetes mellitus, atherosclerotic cerebrovascular disease, coronary heart disease, colorectal cancer, and death from all causes (Calle, Thun, Petrelli, Rodriguez, & Heath, 1999) and results in over 111,000 premature deaths annually in the US alone (Flegal, Graubard, Williamson, & Gail, 2005). Theorists have proposed that individuals who experience hyper-responsivity of the mesolimbic reward system in response to anticipated and actual receipt of food are at increased risk for overeating (Davis, Strachan, & Berkson, 2004; Dawe & Loxton, 2004; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008b). In contrast, others have hypothesized that obese individuals have hypofunctioning dopamine-based reward circuitry, which leads them to overeat to compensate for this deficiency (Comings & Blum, 2000; Wang, Volkow, & Fowler, 2002).
Although data from self-report, observational, and operant studies suggest that obese relative to lean individuals rate high-fat, high sugar foods as more pleasant and work harder for such palatable foods on computer tasks (Drewnowski, Kurth, HOlden-Wiltse, & Saari, 1992; Epstein et al., 2007; Saelens & Epstein, 1996; White, Whisenhunt, Williamson, Greenway, & Netemeyer, 2002), few studies have used brain imaging to examine whether there is hyper-responsivity of the reward circuitry in obese relative to lean adults. Studies have found that obese versus lean individuals show greater activation in the lateral OFC, amygdala, insula, nucleus accumbens, ventral striatum, pallidum, caudate, somatosensory cortex and hippocampus in response to pictures of high-calorie versus low-calorie foods (Rothemund et al., 2007; Stice, Yokum, Bohon, Marti, & Smolen, 2010; Stoeckel et al., 2008). Weaker activation in the frontal operculum, lateral OFC and striatum in response to images of high-fat food predicted future weight gain for individuals at genetic risk for reduced dopaminergic signaling, whereas elevated activation in these same regions predicted future weight gain for individuals not at-risk (Stice et al., 2010).
Only two studies have examined the relation of body mass index (BMI) scores to activation in response to actual food intake; activation in gustatory and somatosensory regions to intake and anticipated intake of chocolate milkshake correlated positively with BMI, but activation in the caudate nucleus in response to milkshake taste correlated negatively with BMI (Stice, Spoor, Bohon, & Small, 2008a; Stice et al., 2008b). Gustatory regions play a role in encoding food taste, somatosensory regions in encoding food texture, and the striatum in encoding reward value of stimuli. Thus, these findings suggest that individuals who show greater activation in somatosensory regions and the gustatory cortex in response to anticipation and consumption of food, but weaker activation in the striatum during food intake, may be at risk for overeating. To date, however, research has not expressly tested whether obese individuals show hyper-responsivity in somatosensory and gustatory regions in response to food intake and anticipated intake and hypo-responsivity in the striatum in response to food intake relative to lean individuals, which should provide a more sensitive test of relations. Thus, the first aim of the present study was to test the hypothesis that obese relative to lean individuals would show greater activation in somatosensory and gustatory regions in response to food intake and anticipated intake, and hypo-responsivity in the striatum.
The second aim was to examine the effects of perceived caloric content of food on reward circuitry responsivity to food. Given that people typically rate low-fat foods as less pleasant tasting and rewarding than high-fat foods (Yeomans, Lartamo, Procter, Lee, & Gray, 2001), we suspected that foods labeled as low-fat would activate reward circuitry to a lesser degree than foods labeled as high-fat. This is an important question because of the preponderance of low-fat foods in our current environment. If low-fat foods are in fact less able to activate reward circuitry, people may overeat these same foods, thwarting the weight control benefits of purchasing such foods. Societal pressure to eat low-fat foods can also influence food choice, even if such foods are unappealing (Nestle et al., 1998). Behavioral studies show that adults concerned with their weight consume more of a labeled fat-free food compared to a regular fat food in ad libitum taste tests (Miller, Castellanos, Shide, Peters, & Rolls, 1998). Critically, obese adults consume more of an isocaloric food that is labeled low-fat versus regular (Wansink & Chandon, 2006). No studies have used objective brain imaging to investigate the ability of foods labeled as low-fat versus high-fat to activate reward circuitry. Thus, we included a milkshake labeled as “low-fat” which was isocaloric to a milkshake labeled as “regular” so that we could investigate the effects of the perceived caloric content of the food independent of any difference in the actual taste of the food. We hypothesized that the “high-fat” milkshake would result in greater activation than the “low-fat” milkshake to food intake and anticipated food intake for obese versus lean individuals.
Method
Participants
Participants were 38 female college students recruited from introductory social sciences courses and fliers posted on campus. Participants were selected if they reported that their body mass index (BMI) was between 20.0 and 25.0 or between 30.0 and 40.0, based on National Institutes of Health guidelines (1998). Exclusion criteria were past or current treatment for a psychiatric or neurological illness, current diagnosis of an eating disorder, ferromagnetic devices in or on the body that would be a danger to the participant or would cause artifacts in the images (e.g. braces, tattoos, pacemaker), and allergies to chocolate or lactose. Data from two participants were excluded due to excessive head movement greater than 1 mm. Two other participants were excluded because their BMI when directly measured did not fall within the range for lean or obese. The final sample of 34 women was 11% Asian, 77% Caucasian, 5% Hispanic, 5% American Indian or Alaskan Native and 5% who indicated more than one race. The mean age of the sample was 20.1 years old (SD=1.4; range=18-23). Paternal education, a proxy for socioeconomic status, was 28% high school graduate or less, 44% some college, 22% college graduate and 5% advanced degree in this sample. Informed consent was obtained from all participants under the IRB-approved protocol. Participants received $100 or research participation credit for completing the study.
Procedure
Participants were asked to refrain from eating and drinking (except water) for 4-6 hours before the imaging session. They reported a mean of 5.6 hours (SD = 1.19) since their last meal. We selected this deprivation period to capture the hunger state that most individuals experience as they approach their next meal, which is a time when individual differences in food reward may impact caloric intake. Hunger ratings were collected in a subset of participants (n=10), which showed no difference in hunger between obese (M=.56, SD=.24) and lean groups (M=.41, SD=.18; t[10]=-1.07, p=.32, Cohen's d=.71). Participants also completed a semi-structured interview assessing diagnostic criteria for eating disorders and a questionnaire about eating behaviors and attitudes, and height and weight were measured.
fMRI Paradigm
In this event-related paradigm, colored pictures of glasses of water, regular chocolate milkshake, low-fat chocolate milkshake or an empty glass were presented to participants. The pictures were labeled correspondingly (e.g. the empty glass was labeled, “nothing”). The pictures signaled the delivery of 0.5mL of the same chocolate milkshake for the regular and low-fat chocolate milkshake conditions, a tasteless solution for water, or nothing at all. The chocolate milkshake consisted of 1 cup of vanilla ice cream, 1.5 cups of 2% milk and 2 tablespoons of chocolate syrup. The tasteless solution, which was designed to mimic the natural taste of saliva, consisted of 25mM KCl and 2.5 mM NaHCO3. The tasteless solution was used because water has a taste that activates the gustatory cortex (Zald & Parvo, 2000).
The pictures were presented using MATLAB with a digital projector/reverse screen display system to a screen at the back end of the MRI scanner bore. Participants viewed the screen via a mirror attached to the birdcage head coil. Tastes were delivered using programmable syringe pumps (Braintree Scientific BS-8000) controlled by MATLAB to ensure consistent volume, rate and timing of taste delivery (0.5 mL over 5 s). Sixty-milliliter syringes filled with milkshake and tasteless solution were connected via Tygon tubing through a wave guide to a manifold attached to the birdcage head coil in the MRI scanner. The manifold fit into the participants’ mouths and delivered the taste to a consistent segment of the tongue. This procedure has successfully delivered liquids in the scanner and has been described in detail elsewhere (Stice et al., 2008b).
The taste was delivered 4 to 11 seconds (M = 7.5) after the onset of the picture. The picture remained on the screen for 6 to 11 seconds (M = 8.5) after the taste was delivered and participants were instructed to swallow when the picture went off. The next picture appeared 1 to 5 seconds (M = 3) after the prior picture went off. As a result, each event lasted 11-27 seconds and each run consisted of 38 events. Duration of stimulus presentation and order of presentation were randomized across participants. Each picture corresponded with the delivery of a particular taste or no taste. On 40% of the milkshake and tasteless solution trials, the taste was not delivered in order to examine the neural response to anticipatory reward that was not confounded by the receipt of the taste (unpaired trials). Versions of this milkshake paradigm have been found to validly measure individual differences in neural activation in response to receipt and anticipated receipt of palatable food (Stice et al., 2008b).
Data acquisition and statistical analysis
MRI scanning was performed on a 3.0 Tesla Siemens Allegra head-only scanner. A thermofoam vacuum pillow and padding were used to restrict head motion. Blood oxygen-level dependent, echo-planar images (BOLD-EPI) were acquired with T2*-weighted gradient single-shot echo sequence (TE = 30 ms, TR = 2000 ms, flip angle = 80°) with an in-plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 contiguous slices with 4 mm thickness (interleaved acquisition, no skip) were acquired along the AC-PC transverse oblique plane as determined by the midsagittal section. A total of 300 scans were collected during each of five functional runs. High-resolution structural scans were acquired using an inversion recovery T1-weighted sequence (MP-RAGE) in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans (FOV = 256 × 256 mm2, thickness = 1.0 mm, slice number ≈ 160).
Data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). EPI images were corrected for head movement using Prospective Acquisition CorrEction (PACE), which adjusts slice position, orientation and regrids the residual volume-to-volume motion during data acquisition. In vivo experiments demonstrate significant reduction of variance in pre- and post-motion volumes (Thesen, Heid, Mueller, & Schad, 2000). Functional images were then realigned to the mean. Anatomical and functional images were normalized to the standard MNI template brain implemented in SPM5 (ICBM152, based on an average of 152 normal MRI scans). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel. For time series analysis of all participants, a high-pass filter was included in the filtering matrix (per convention in SPM5) in order to remove low frequency noise and slow drifts in the signal, which could bias the estimates of the error.
Condition-specific effects were estimated for each participant at each voxel using the general linear model. Vectors of the onsets for each event of interest were compiled and entered into the design matrix so that event-related responses could be modeled by the canonical hemodynamic response function (HRF), as implemented in SPM5, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 seconds and the subsequent undershoot. To account for the variance induced by swallowing the tastes, the time of disappearance of the cue (when participants were instructed to swallow) was included as a control variable. Temporal derivatives of the HRF were included to obtain a better model of the data (Henson, Price, Rugg, Turner, & Friston, 2002). Accounting for latencies in the HRF by including temporal derivatives has been shown to be a more sensitive method of modeling the data for event-related designs (Hopfinger, Buchel, Holmes, & Friston, 2000).
Individual SPM contrast maps were constructed to compare the activations within each participant for the specified contrasts (detailed below) in SPM5. Random effects ANOVA models tested whether obese relative to lean participants show differential activation patterns. For analysis of food receipt, parameter estimate images comparing activation in the obese group to the lean group were entered into three, second-level 2×2 ANOVA models using the following contrasts: 1) regular milkshake vs. tasteless; 2) low-fat milkshake vs. tasteless; and 3) regular milkshake vs. low-fat milkshake. For analysis of anticipated receipt of food we used a set of second-level 2×2 ANOVA models to compare relative activation of the obese and lean groups in response to pictures signaling impending receipt of: 1) regular milkshake vs. tasteless, 2) low-fat milkshake vs. tasteless, and 3) regular milkshake vs. low-fat milkshake. Based on evidence that reward-related neural function is heightened in women during the mid-follicular phase (Dreher et al., 2007), a dichotomous variable was created that reflected whether participants completed scanning during their mid-follicular phase (4-8 days after onset of menses (n = 4) or not (n = 30)). When controlled for this variable, the activation patterns reported in the tables remained significant.
We performed small volume correction (SVC) analyses with a radius of 10 mm based on peaks from prior studies examining food reward processing (Grabenhorst & Rolls, 2009; Plassman, O'Doherty, Shiv, & Rangel, 2008; Rothemund et al., 2007; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001; Stice et al., 2008b) and we tested for bilateral activation. SVC is a widely used ROI technique in neuroimaging studies (e.g., (Batterink, Yokum, & Stice, 2010; Small et al., 2003; Small, Veldhuizen, Felsted, Mak, & Mcglone, 2008; Stice et al., 2010). Significance for these a priori SVC was assessed at a statistical threshold of P < 0.005 corrected across the small volume with a cluster extent ≥ 3 voxels. Correction for multiple comparisons were made using a false discovery rate (FDR) at P < 0.05 across voxels within the prior defined small volume.
Measures
Body Mass Index
BMI (kg/m2) was used to reflect adiposity. Height was measured to the nearest millimeter with a portable direct-reading stadiometer. Participants were measured without shoes and with the body positioned such that the heels and buttocks were against the vertical support of the stadiometer. The head was aligned so that the auditory canal and lower rim of the orbit were in a horizontal plane. We assessed weight to the nearest 0.1 kg using digital scales, with participants wearing light clothing without shoes or coats. Two measures of height and weight were obtained and averaged. BMI correlates with direct measures of total body fat, such as dual energy X-ray absorptiometry (r=.80-.90), and with health measures, including blood pressure, abdominal fat distribution, adverse lipoprotein profiles and diabetes mellitus in adults (Ledoux, Lambert, Reeder, & Despres, 1997).
Results
Participant Characteristics
Participants in the obese group (n = 17) had a mean BMI of 36.3 (SD = 3.39) and those in the non-obese group (n = 17) had a mean BMI of 22.1 (SD = 1.04). There were no differences between the two groups on demographic factors. Obese (M=8.53, SD=3.68) also did not differ on restraint (M=6.71, SD=3.37, t(32)=-2.40, p=.14, Cohen's d=.91). Hunger ratings were collected in a subset of the sample (n=10). These scores also did not differ between obese (M=.56, SD=.24; n=3) and lean (M=.41, SD=.18; n=7, t((8)=-1.07, p=.74, Cohen's d =.71),
Obese versus lean response to anticipated receipt of high-fat milkshake (high-fat milkshake cue versus tasteless cue)
Obese participants showed greater relative activation in the right posterior cingulate gyrus (η2 = .14) and right caudate (η2= .13; Table 1, Figure 1).
Table 1.
Regions showing increased activation during anticipated food intake in obese women (N=17) compared to lean women (N=17).
| Reference coordinate | MNI x | MNI y | MNI z | Max Z | PFDR-CORR | Effect size (η2) | Brain region |
|---|---|---|---|---|---|---|---|
| High-fat chocolate milkshake vs. tasteless solution | |||||||
| 9, -48, 12a | 3 | -45 | 6 | 3.44 | .025 | .14 | Posterior cingulate gyrus |
| -6, 12, 6a | 3 | 18 | 9 | 3.08 | .04 | .12 | Caudate |
| Low-fat chocolate milkshake vs. tasteless solution | |||||||
| 54, -6, 27b | -48 | -6 | 36 | 3.83 | .005 | .07 | Rolandic operculum |
| 9, 54, -3a | -9 | 57 | 0 | 3.37 | .04 | .10 | vmPFC |
| -27, -28, -7c | -24 | -24 | -3 | 3.21 | .049 | .19 | Hippocampus |
| -27, -28, -7c | -21 | -27 | -9 | 2.76 | .049 | .12 | Parahippocampal gyrus |
| High-fat chocolate milkshake vs. low-fat chocolate milkshake | |||||||
| -48, -12, 33b | -48 | -6 | 36 | 3.46 | .016 | .22 | Rolandic operculum |
| 57, 12, 24a | 57 | 12 | 18 | 3.53 | .01 | .14 | Inferior frontal gyrus |
| 51, 3, 18b | 54 | 12 | 18 | 3.34 | .04 | .08 | Frontal operculum |
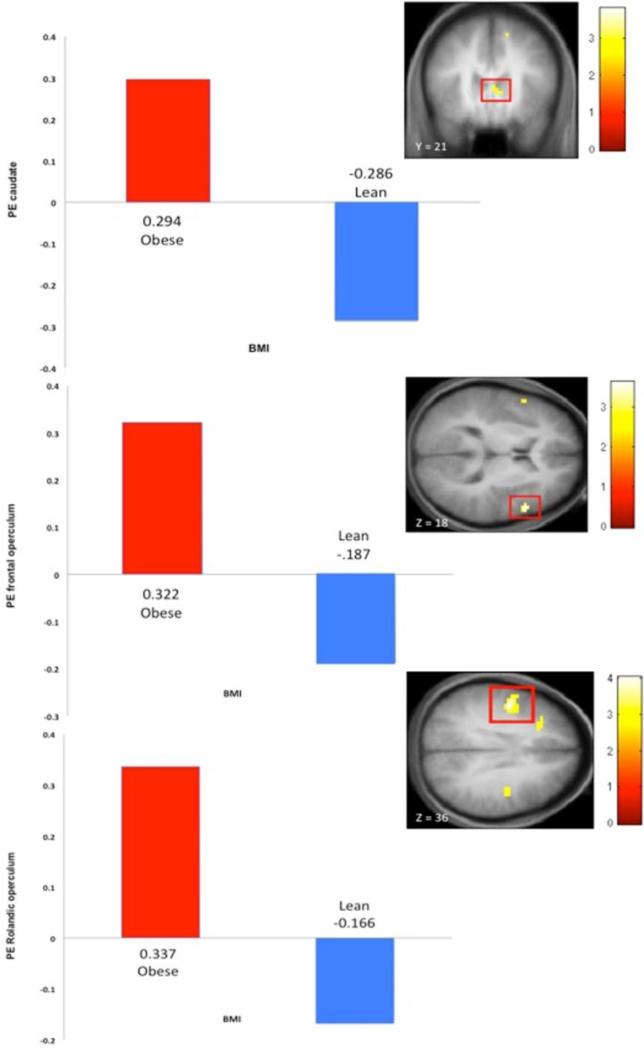
Figure 1.
Images and parameter estimates of activation in response to intake and anticipated intake of a palatable taste in obese versus lean women. Elevated activation in the caudate (3, 18, 9, z = 3.08, pFDR = .04) to anticipated intake of high-fat milkshake versus tasteless solution(A) and in the frontal operculum (54, 12, 18, z = 3.34, pFDR = .04) to anticipated intake of high-fat versus low-fat milkshake (B) and in the Rolandic operculum (-48, -6, 36, z = 3.84, pFDR = .006) to receipt of high-fat versus low-fat milkshake (C).
Obese versus lean response to anticipated receipt of low-fat milkshake (low-fat milkshake cue versus tasteless cue)
Obese participants showed greater activation in the left Rolandic operculum (η2 = ..17), left vmPFC (η2 = .10), left hippocampus (η2 = .19) and left paraphippocampal gyurs (η2 = .12; Table 1).
Obese versus lean response to anticipated receipt of high-fat versus low-fat milkshake (high-fat milkshake cue versus low-fat milkshake cue)
Obese participants showed greater activation in the left Rolandic operculum(η2 = .22), right inferior frontal gyrus (η2 = .14), and right frontal operculum (η2 = .08; Table 1, Figure 1).
Obese versus lean response to intake of high-fat milkshake (receipt of high-fat milkshake versus receipt of tasteless)
Obese participants showed greater activation in the left Rolandic operculum (η2 = .13) and right amygdala (η2 = .12) compared to lean participants (Table 2).
Table 2.
Regions showing increased activation during food intake in obese women (N = 17) compared to lean women (N = 17).
| Reference coordinate | MNI x | MNI y | MNI z | Max Z | PFDR-CORR | Effect size (η2) | Brain region |
|---|---|---|---|---|---|---|---|
| High-fat chocolate milkshake vs. tasteless solution | |||||||
| -48, -12, 33a | -45 | -9 | 36 | 3.59 | .007 | .13 | Rolandic operculum |
| 21, 0, -18b | 30 | 0 | -15 | 3.01 | .048 | .12 | amgydala |
| Low-fat chocolate milkshake vs. tasteless solution | |||||||
| 57, 12, 24b | 57 | 12 | 15 | 3.46 | .009 | .18 | Inferior frontal gyrus |
| 51, 3, 18a | 54 | 12 | 18 | 3.41 | .034 | .20 | Frontal operculum |
| -48, -12, 33a | -48 | -6 | 39 | 3.40 | .029 | .13 | Rolandic operculum |
| High-fat chocolate milkshake vs. low-fat chocolate milkshake | |||||||
| -48, -12, 33a | -48 | -6 | 36 | 3.84 | .006 | .14 | Rolandic operculum |
| -18, 42, 6b | -21 | 36 | 0 | 3.33 | .046 | .17 | vmPFC |
Obese versus lean response to intake of low-fat milkshake (receipt of low-fat milkshake versus receipt of tasteless)
Obese participants showed greater activation in the right inferior frontal gyrus (η2 = .18), right frontal operculum (η2 = .20) and left Rolandic operculum (η2 = .13; Table 2).
Obese versus lean response to receipt of high-fat versus low-fat milkshake (receipt of high-fat milkshake versus low-fat milkshake)
Obese participants showed greater activation in the left Rolandic operculum (η2 = .14) and left vmPFC (η2 = .17; Table 2, Figure 1).
Discussion
As hypothesized, obese compared to lean women showed greater activation in gustatory, somatosensory and reward processing regions in response to receipt and anticipated receipt of palatable food. In response to anticipated intake of palatable food, obese women showed greater activation in the Rolandic operculum (gustatory cortex) and caudate. Increased activation in the gustatory cortex has been reported in one of our previous studies of receipt and anticipated receipt of palatable food (Stice et al., 2008b) and increased striatal activity (putamen) to imagined food intake has also been reported in a more recent study (Stice et al., 2010). Further, obese versus lean adults also showed increased activation in the posterior cingulate gyrus and hippocampus, parrahippocampal gyrus and the vmPFC, areas that have been implicated in encoding reward value (Plassman et al., 2008; Stoeckel et al., 2008) and in determining subsequent behavioral decisions when presented with a rewarding stimulus (O'Doherty, Critchley, Deichmann, & Dolan, 2003). In particular, activation in the vmPFC positively correlates with reported pleasantness of taste (Plassman et al., 2008). Heightened activation in the vmPFC, inferior frontal gyrus, posterior cingulate and the hippocampus/parrahippocampal gyrus in response to anticipated food intake were not found in our previous study (Stice et al., 2008b). One possibility for this new finding is that this current study has greater sensitivity to detect these effects because the sample was restricted to obese and lean women. Additionally, the paradigm in this study used pictures of milkshake and water that were labeled accordingly as cues signaling impending receipt of the taste whereas our previous study used geometric shapes as cues; the former may have made the relation between the image and the taste more apparent. Although prior studies show that the operculum responds strongly to taste during taste detection tasks (Bender, Veldhuizen, Meltzer, Gitelman, & Small, 2009; Veldhuizen, Bender, Constable, & Small, 2007), it should be noted that brain regions are implicated in a variety of functions. For instance, the Rolandic operculum is also involved in speech processing (Indefrey et al., 2001) and the hippocampus is also implicated in memory retrieval (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000). While we focused on regions previously implicated in taste processing and reward valuation, it is possible that other processes occurred concurrently to food anticipation and receipt.
In response to intake of palatable food, obese women showed greater activation in the Rolandic operculum (somatosensory cortex), frontal operculum, inferior frontal gyrus and amygdala. Heightened activation in the Rolandic and frontal opercular regions replicates previous findings of increased activation in the gustatory and somatosensory cortices in obese versus lean adolescent girls (Stice et al., 2008b) and converges with prior findings of greater resting metabolism in the somatosensory cortex in obese relative to lean adults (Wang et al., 2002). Moreover, the amygdala has been shown to be involved in implicit taste reward encoding (Bender et al., 2009) and scales with incentive value (Arana et al., 2003).
The present study did not find evidence of hypo-activation in the striatum, unlike two prior studies (Stice et al., 2008a; Stice et al., 2008b.) Reward sensitivity assessed by self-report has been found to relate to BMI non-linearly (Davis & Fox, 2008) and it could be that striatal response to food intake relates in the same manner. We tested for a non-linear relation between BMI and caudate response in each of our two previous studies and in the current study and found that a linear relation was a better fit for the relation than a quadratic function.1 This suggests that there should have been a pronounced effect of hypo-responsivity in obese versus lean groups. This null finding could be due to the nature of the obese participants in the current study. There was a larger range in the BMI of obese participants compared to those in prior studies, which included mostly overweight participants. Striatal response for those in a higher obese BMI range (BMI>35) may differ from those in the lower obese BMI range (BMI=30-35), and this difference may have washed out any other differences that might have been observed between the obese and lean groups. Another possibility is that hypo-responsivity in the caudate may be moderated by genetic vulnerabilities, as prior studies have shown that carriers of the TaqlA A1 allele versus non-carriers show blunted responses in the striatum to food intake (Stice et al., 2008a; Felsted, Ren, Chouinard-Decorte, & Small, 2010), which this study did not test.
In sum, our results replicate findings from three prior studies (Stice et al., 2008a; Stice et al., 2008b; Stice et al., 2010) that elevated responsivity of the somatosensory and gustatory cortices in response to anticipation of and actual receipt of a palatable food may play a role in overeating. In particular, heightened activity in the Rolandic operculum and frontal operculum was replicated several times across the contrasts of the current study as well as in previous studies, which suggests that the effect in this area may be particularly robust in response to receipt and anticipated receipt of palatable food in obese versus lean women. Collectively, the findings of the present study suggest that obese versus lean individuals engage more readily in evaluating a reward and may be better prepared to pair a stimulus with a positive experience when that reward is food.
Results of the present study also supported our hypothesis that perceived caloric density would impact activation of reward circuitry and taste processing in obese relative to lean women. In response to anticipated receipt of regular milkshake versus an isocaloric milkshake labeled “low-fat”, obese women showed greater activation in the Rolandic operculum, inferior frontal gyrus, and frontal operculum. In response to receipt of regular milkshake versus “low-fat” milkshake, obese women also showed greater activation in the Rolandic operculum and vmPFC. These results replicate prior findings that manipulating labels can modulate reward response to a food reward. Plassman et al. (Plassman et al., 2008) found that when participants tasted the same wine described as expensive or inexpensive, the former was rated as more pleasant and resulted in greater activation in the vmPFC. McClure et al. (McClure et al., 2004) also found that activation in the vmPFC scaled with soda preference when participants were blinded to the brand. This suggests that the vmPFC not only codes reward value, but might also act in a top-down processing manner that modulates reward processing.
Such findings indicate that obese individuals show elevated activation in somatosensory, gustatory, and reward evaluation regions in response to regular milkshake versus the labeled “low-fat” milkshake, even though both milkshakes were identical. This is the first study to date using neuroimaging to examine the effects of perceived fat content and reward processing in obesity. For obese individuals, a food that is higher in calories—even if based only on perception and not on actual caloric content—results in greater recruitment of regions involved in encoding a highly rewarding experience relative to food that is perceived as lower in calories.
These results indicate two possible mechanisms that could be involved in food reward processing that may contribute to overeating. One possibility is that high-calorie foods increase the hedonic reward experienced at the time of consumption and increases the expectation of reward, which consequently increases the risk of overeating high-calorie foods. When faced with eating a low-calorie food, adults may tend to overeat in order to compensate for the relative lack of pleasantness and reward experienced. This behavior may occur because of social pressures to eat foods that are perceived as healthy, yet be unrewarding (Nestle et al., 1998). Indeed, obese relative to lean adults consume more of an isocaloric food labeled as low-fat and feel less guilty when consuming a low-fat versus regular fat food (Wansink & Chandon, 2006). Alternatively, a top-down processing of attention might modulate response to a primary reward. When people are instructed to direct their attention to properties of a stimulus related to the affect (i.e. pleasantness) versus a physical property of the stimulus (i.e. intensity), different brain regions involved in processing sensory stimuli are engaged (Grabenhorst & Rolls, 2008; Rolls, Grabenhorst, & Parris, 2008).
Low-fat labels on food may draw selective attention to the physical properties of food and result in less subjective reward. Indeed, self-report data indicate that healthy adults expect to and do experience foods labeled low-fat as less palatable than an isocaloric food labeled regular (Yeomans et al., 2001). Further, studies have found that healthy adults more often select a low-fat over a high-fat food to consume in laboratory taste tests when warned of the fat content, even though they report wanting the high-fat food more (Bushman, 1998) and consume a greater volume of low-fat versus high-fat foods as assessed by a weighed food diary (Matthiessen, Fagt, Biltoft-Jensen, Beck, & Ovesen, 2002). Healthy adults also consume more low-fat food when they are primed with sentences linking health words (e.g. fit, vitamins, sports, etc.) to low-fat foods (e.g. apple, vegetables, fruit, etc.) prior to covert observation of ad libitum food consumption (Geyskens, Pandalaere, Dewitte, & Warlop, 2007) and they consume more at a subsequent meal when given a preload labeled low-fat versus regular (Shide & Rolls, 1995). Additionally, adults concerned with their weight also consume more of a labeled fat-free food compared to a regular fat food in ad libitum taste tests (Miller et al., 1998) and obese adults consume more of an isocaloric food that is labeled low-fat versus regular and report less guilt when consuming a low-fat food in naturalistic, observational studies (Wansink & Chandon, 2006).
The results of the current study may explain why obese individuals prefer such high calorie foods and work harder to obtain them than lean individuals (Drewnowski et al., 1992; Epstein et al., 2004; Saelens & Epstein, 1996). This study replicated and extended previous findings examining neural response in obese and lean women and is the first to test whether perceptions of the caloric content of food affect food reward using imaging techniques. However, it is important to consider study limitations when interpreting the findings. First, we examined responses to only one high-fat, high-sugar food. Individuals who overeat and are obese consume a variety of high-fat and high-sugar foods. Second, receiving tastes during an fMRI scan may not be comparable to overeating experienced during a meal in the real world, which involves considerably more calories. Third, this study was cross-sectional, which precludes inferences regarding the direction of the effects. Fourth, as participants were female, results should be generalized to males with caution. Finally, although hunger ratings did not show differences between lean and obese participants, this data was collected in only a subset of participants. It is possible that differences in hunger might have emerged with a larger sample. Studies have shown that lean adults who are hungry versus full show increased activation in limbic regions (del Parigi et al., 2002; Goldstone et al., 2009).
In sum, this study makes two novel contributions to the neuroimaging literature on obesity. First, this study replicated previous findings of elevated activation in gustatory, and somatosensory regions to anticipated and actual palatable food intake in obese relative to lean women. These results support the theory that an abnormal reward response to food may be related to overeating. Second, this study is the first to examine the impact of manipulating perceived fat content on reward circuitry response. Although supermarkets abound with foods labeled as “low-fat” and “low-calorie” with the expectation that this will help people make healthier food choices, it may actually be a hindrance for those struggling with weight gain. Indeed, the number low-fat, low-calorie food products has substantially increased (Harris, 2000) and the number of people consuming such products has more than doubled over the past two decades, representing 86% of the population (Control, 2004). However, while the percentage of total fat intake in the American diet has decreased, total calorie intake has increased (CDC). Obese individuals may continue to consume high-calorie foods because they experience greater reward. Alternatively, obese individuals could respond to social pressures to eat low-fat foods, yet overeat these foods to compensate for the decrease in reward experienced during consumption. These results and future studies can inform the current public health obesity crisis and the environmental factors that can influence food choice and consequently, overeating and weight gain.
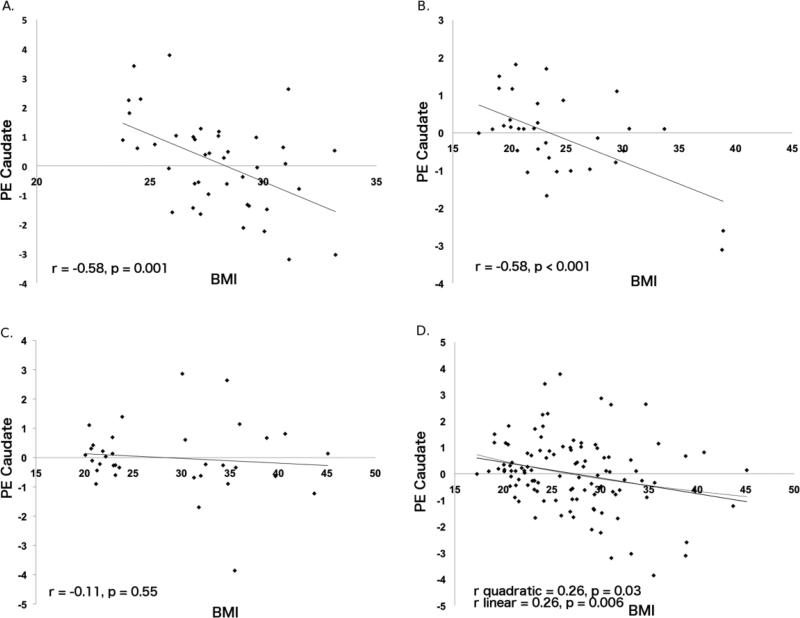
Figure 2.
Parameter estimates of caudate (-15, 18, 12) activation as a function of BMI from Stice et al. [14] (A; Study 1, N=43), Stice et al. [15] (B; Study 2, N=33), and the current study (C; Study 3, N=34) and all three studies (D, N=110).
Acknowledgments
This study was supported by research grants R01 DK072932 and R01 DK080760 from the National Institutes of Health and the Carolyn M. Stokes Memorial Scholarship from the University of Oregon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Figure 2 showing the linear relation (R2=.067, p=.006) and quadratic function (R2=.068, p=.03) between BMI and caudate response across all studies.
References
- Arana F, Parkinson J, Hinton E, Holland A, Owen A, A R. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. The Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage. 2010;52:1695–1793. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender G, Veldhuizen M, Meltzer J, Gitelman D, Small D. Neural correlates of evaluative compared with passive tasting. European Journal of Neuroscience. 2009;30:327–338. doi: 10.1111/j.1460-9568.2009.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman B. Effects of warning and information labels on consumption of full-fat, reduced-fat, and no-fat products. Journal of Applied Psychology. 1998;83:97–101. doi: 10.1037/0021-9010.83.1.97. [DOI] [PubMed] [Google Scholar]
- Calle E, Thun M, Petrelli J, Rodriguez C, Heath C. Body mass index and mortality in a prospective cohort of US adults. New England Journal of Medicine. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control [May 25, 2010];Obesity and Overweight. fromwww.cdc.gov/nccdphp/dnpa/obesity/index.htm.
- Comings D, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Control C. f. D. Trends in intake of energy and macronutrients - United States, 1971-2000. Morbidity and Mortality Weekly Report. 2004;53:80–82. [PubMed] [Google Scholar]
- Davis C, Fox J. Sensitivity to reward and body mass index (BMI): Evidence for a non-linear relationship. Appetite. 2008;50:43–49. doi: 10.1016/j.appet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton N. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Review. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- del Parigi A, Gautier J, Chen K, Salbe A, Ravussin E, Reiman E, et al. Neuroimaging and obesity: Mapping the brain responses to hunger and satiation in humans using positron emission tomography. Annals of the New York Academy of Sciences. 2002;967:389–397. [PubMed] [Google Scholar]
- Dreher J, Schmidt P, Kohn P, Furman D, Rubinow D, Berman K. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, HOlden-Wiltse J, Saari J. Food preferences in human obesity: Carboyhydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Eldridge L, Knowlton B, Furmanski C, Bookheimer S, Engel S. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Epstein L, Temple J, Neaderhiser B, Salis R, Erbe R, Leddy J. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L, Wright S, Paluch R, Leddy J, Hawk L, Jaroni J, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology and Behavior. 2004;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with underweight, overweight, and obesity. The Journal of the American Medical Assocation. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Geyskens K, Pandalaere M, Dewitte S, Warlop L. The backdoor to overconsumption: The effect of associating “low-fat” food with health references. American Marketing Association. 2007;26:118–125. [Google Scholar]
- Goldstone A, Prechtl de Hernandez C, Beaver J, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems toward high-calorie foods. European Journal of Neuroscience. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls E. Different representations of relative and absolute subjective value in the human brain. NeuroImage. 2009;48:258–268. doi: 10.1016/j.neuroimage.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Harris J. Food product introductions continue to decline. FoodReview. 2000;25:24–27. [Google Scholar]
- Henson R, Price C, Rugg M, Turner R, Friston K. Detecting latency differences in event-related BOLD responses: Applicaiton to words versus nonwords in initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hopfinger J, Buchel C, Holmes A, Friston K. A study of naalysis parameters that influence the sensitivity of event-related fMRI analyses. NeuroImage. 2000;11:326–333. doi: 10.1006/nimg.2000.0549. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Brown C, Hellwig F, Amunts K, Herzog H, Seitz R, et al. A neural correlate of syntactic encoding during speech production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5933–5936. doi: 10.1073/pnas.101118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux M, Lambert J, Reeder B, Despres J. Correlation between cardiovascular disease risk factors and simple anthropometric measures. Canadian Heart Health Surveys Research Group. 1997;157:S46–53. [PubMed] [Google Scholar]
- Matthiessen J, Fagt S, Biltoft-Jensen A, Beck A, Ovesen L. Size makes a difference. Public Health Nutrition. 2002;6:65–72. doi: 10.1079/PHN2002361. [DOI] [PubMed] [Google Scholar]
- McClure S, Li J, Tomlin D, Cypert K, Montague L, Montague P. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Miller D, Castellanos V, Shide D, Peters J, Rolls B. Effect of fat-free potato chips with and without nutrition labels on fat and energy intakes. American Journal of Clinical Nutrition. 1998;68:282–290. doi: 10.1093/ajcn/68.2.282. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Practical guide to the identification, evaluation and treatment of overweight and obesity in adults. 1998.
- Nestle M, Wing R, Birch L, DiSogra L, Drewnowski A, Middleton S, et al. Behavioral and social influences on food choice. Nutrition Reviews. 1998;56:S50–S56. doi: 10.1111/j.1753-4887.1998.tb01732.x. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan R. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. The Journal of Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E, Grabenhorst F, Parris B. From affective value to decision-making in the prefrontal cortex. European Journal of Neuroscience. 2008;28:1930–1939. doi: 10.1111/j.1460-9568.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschof C, Bohner G, Bauknecht H, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Saelens B, Epstein L. The reinforcing value of food in obese adn non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Shide D, Rolls B. Information about the fat content of preloads influences energy intake in healthy women. Journal of the American Dietic Association. 1995;95:993–998. doi: 10.1016/S0002-8223(95)00273-1. [DOI] [PubMed] [Google Scholar]
- Small D, Gregory M, Mak E, Gitelman D, Marsel M, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Cell. 2003;39(701-711) doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small D, Veldhuizen M, Felsted J, Mak E, Mcglone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D, Zatorre R, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small D. Relation between obesity and blunted striatal response to food is moderated by the TaqlA1 gene. Science. 2008a;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008b;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:636–647. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L, Weller R, Cook E, Twieg D, Knowlton R, Cox J. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad L. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic REsonance in Medicine. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Veldhuizen M, Bender G, Constable R, Small D. Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chemical Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Fowler J. The role of dopamine in motivation for food in humans: Implications for obesity. Expert Opinion on Therapeutic Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- Wansink B, Chandon P. Can “low-fat” nutrition labels lead to obesity? Journal of Marketing Research. 2006;43:605–617. [Google Scholar]
- White M, Whisenhunt B, Williamson D, Greenway F, Netemeyer R. Development and validation of the food-craving inventory. Obesity Research. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Yeomans M, Lartamo S, Procter E, Lee M, Gray R. The actual, but not labeled, fat content of a soup preload laters short-term appetite in healthy men. Physiology & Behavior. 2001;73:533–540. doi: 10.1016/s0031-9384(01)00502-9. [DOI] [PubMed] [Google Scholar]
- Zald D, Parvo J. Cortical activation induced by intraoral stimulation with water in humans. Chemical Senses. 2000;25:267–277. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]