Abstract
Thymoquinone (TQ), an active component of Nigella sativa L., is known to have anti-cancer and anti-inflammatory effects; however, no studies on its analytical detection in serum and its protein binding have been published. Using high performance liquid chromatography analysis, we show that the average recovery of TQ from serum is 2.5% at 10 μg/ml of TQ and 72% at 100 μg/ml. The low recovery of TQ from serum is due to its extensive binding to plasma proteins, as more than 99% of TQ was bound within 30 min of incubation. The binding of TQ to the major plasma proteins, bovine serum albumin (BSA) and alpha −1 acid glycoprotein (AGP), was studied and found to be 94.5 ± 1.7% for BSA and 99.1 ± 0.1% for AGP. Mass spectrometric analysis revealed that TQ was bound covalently to BSA, specifically on Cyst-34. Using WST-1 proliferation assay, we showed that BSA plays a protective role against TQ-induced cell death; pre-incubation with BSA prevented TQ from exerting its anti-proliferative effects against DLD-1 and HCT-116 human colon cancer cells. On the other hand, binding of TQ to AGP did not alter its anti-proliferative activity against both cell lines. When TQ was pre-incubated with AGP prior to the addition of BSA, the activity of TQ against DLD-1 was maintained, suggesting that AGP prevented the binding of TQ to BSA. This is the first time the covalent binding and inhibitory effect of BSA on TQ is documented. These data offer new grounds for TQ future pharmacokinetic analysis in vivo.
Electronic supplementary material
The online version of this article (doi:10.1007/s12154-010-0052-4) contains supplementary material, which is available to authorized users.
Keywords: Thymoquinone, Mass spectrometry, Serum, Protein binding, Anticancer activity
Introduction
Nigella sativa L. (Ranunculaceae) is an annual plant that grows in countries bordering the Mediterranean area, Pakistan, and India. This plant has been used for centuries in many Middle Eastern and Indian countries for culinary and medicinal purposes [1–4]. The activities exerted by N. sativa have been attributed to quinones, specifically to thymoquinone (TQ; Fig. 1a) [4]. TQ has been shown to exert anti-inflammatory [5], analgesic [1], hypoglycemic [6], anti-oxidant [7, 8], and oxidant [9, 10] activities. TQ has also shown antihypertensive, antimicrobial, and mast cell stabilizing effects [11], as well as a protective role against in vitro-induced ischemia [12]. In addition, the therapeutic potential of TQ has been confirmed in cancer research. In vitro, the ability of TQ to inhibit the proliferation of colon cancer cells [9, 13, 14], lymphoblastic leukemia cells [10], laryngeal carcinoma cells [15, 16], pancreatic cells [17], and prostate cancer cells [16, 18] is well established. Regardless of the cancer cell type, it appears that TQ inhibits the proliferation of cancer cells by inducing apoptotic cell death [9, 10, 13, 14]. A review of the literature showed that TQ induces apoptotic cell death via p53-dependent [19] and p53-independent [14, 20] pathways; a recent report has shown that, in the p53 mutant leukemic Jurkat cells, TQ induces apoptotic cell death via a p73-dependent pathways [10]. In vivo, TQ has been found to reduce colon cancer tumor growth using 1,2-dimethyl hydrazine and xenograft models [21], prevents tumor angiogenesis in a xenograft model of human prostate cancer [18], inhibits the incidence and multiplicity of benzo(a)pyrene-induced fore-stomach tumors [22], and 20-methylcholanthrene-induced fibrosarcoma tumors [23]. All of these findings make TQ an interesting compound that merits further clinical evaluation. Although more than 150 studies have been published on the pharmacology of TQ during the last 10 years, no methods dealing with its extraction and analysis from blood/serum have been published so far. Therefore, pharmacokinetics of TQ has not been explored. This is probably due to the absence of analytical methods for the isolation and analysis of TQ from serum. In fact, only two high-performance liquid chromatography (HPLC) methods have been reported for TQ quantification in black seed oil [24, 25]. In order to define the pharmacokinetic profile for a compound, it is necessary to optimize a method for its detection from blood/serum and to determine its protein binding to plasma components.
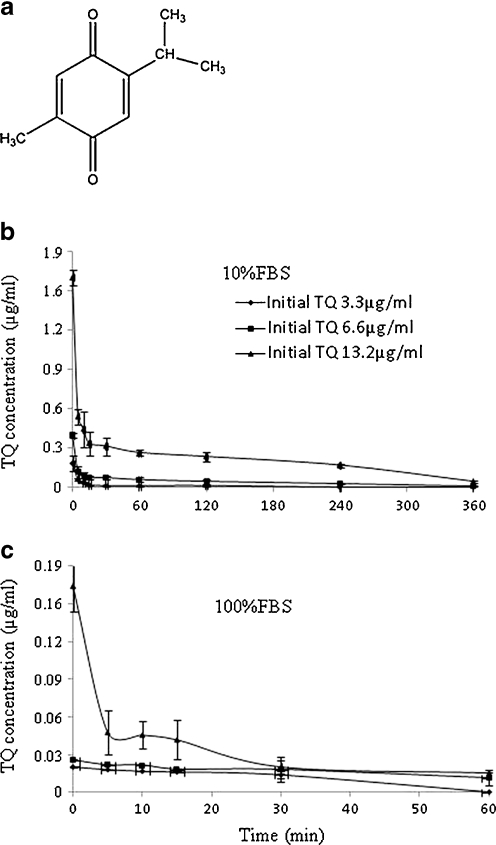
Fig. 1.
Effect of 10 and 100% FBS on TQ concentration. Chemical structure of TQ (a), TQ was incubated with 10% FBS (b) and 100% FBS (c) and aliquots collected over 6 h. Samples were processed as described in the Materials and methods. Each value represents the mean±SE of two separate experiments (n = 3)
Human serum albumin (HSA) is the most abundant protein in plasma as it represents more than 60% of all plasma proteins. HSA has 35 cysteine residues 34 of which are engaged in disulfide bridges. HSA has also one free cysteine (Cys-34) that is redox-active capable of thiolation and nitrosylation and accounts for more than 80% of thiols in plasma. HSA binds many endogenous and exogenous compounds and plays a role in different processes such as drug delivery, detoxification, and antioxidant protection [26]. Alpha-1 acid glycoprotein (AGP), the second major protein in plasma, is considered as one of the acute phase proteins and has very high carbohydrate content of 45%. AGP is encoded by two genes, which differ by 22 base substitutions, resulting in two different polypeptides [27]. Both isoforms have two disulfide bridges, but the major difference resides in the presence of one additional free cysteine in position 147 of the amino acid sequence in one of the isoforms [28]. Although its exact function is not yet fully elucidated, many activities have been described for AGP, such as the ability to bind basic drugs and small molecules such as steroid hormones. AGP exists in plasma at a concentration that is normally 100 times lower than that of albumin [29]. AGP is considered as a low-capacity high-affinity protein while albumin is a high-capacity low-affinity protein [29]. Protein binding is generally referred to the reversible binding of drugs to plasma components. Once a drug reaches the blood circulation after intravenous injection or absorption, it interacts with plasma proteins via two different ways: either adsorption to the protein surface or, more rarely, by covalent binding [29]. Binding of drugs to proteins has serious implications since it can affect the therapeutic, pharmacodynamic, as well as the toxicologic actions of the drug [30]. Therefore, the determination of the unbound concentrations of the drug is essential for the determination of its pharmacokinetic and pharmacodynamic properties [30].
In this study, the aim was to optimize an analytical method for the detection of TQ from serum. However, a thorough investigation showed that the detection of free TQ is problematic. To understand the low recovery of TQ from serum, further analysis on its protein binding was performed. The binding studies were carried using fetal bovine serum (FBS), bovine serum albumin (BSA), and AGP, and the results were discussed with respect to analytical detection of TQ and its anticancer activity.
Materials and methods
Reagents
RPMI 1640, Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/l glucose, penicillin–streptomycin (100 U/ml), non-essential amino acids, and phosphate-buffered saline (PBS) were from Cambrex Bio Science (Verviers, Belgium). FBS was from Invitrogen (Carlsbad, California, US). Acetonitrile, methanol, and ethyl acetate were HPLC-grade from Rathburn (Walkerburn, UK). Dimethyl sulfoxide (DMSO) and BSA were from Merck (Darmstadt, Germany). TQ was from MP Biomedical (Strasbourg, France). AGP was from Sigma-Aldrich (Steinheim, Germany). Amicon Ultra centrifugal filters with 3 K cut off were from Millipore (Carrigtwohill, Co. Cork, Ireland). C18 PrepSep solid-phase extraction columns were from Fisher Scientific (Pittsburgh, PN, US). All coupling reagents, amino acid derivatives, and resins were from GL Biochem (Shanghai) Ltd.
Cell culture and treatment
DLD-1 human adenocarcinoma cells were kindly provided by Prof. M. Ocker (Institute for Surgical Research, Philipps University Marburg, Marburg, Germany). HCT-116 cells were kindly provided by Prof. H Gali-Mohtaseb (Biology Department, American University of Beirut, Beirut, Lebanon). Cells were grown in DMEM with 4.5 g/l glucose (DLD-1 cells) or in RPMI 1640 (HCT-116 cells) supplemented with 1% penicillin–streptomycin (100 U/ml), 1% non-essential amino acids, and 10% FBS. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, and seeded at 105 cells/ml. They were exposed at 40–50% confluency to 6.6 μg/ml TQ. TQ stock was prepared in DMSO, and the final DMSO concentration on cells was less than 0.5%.
Protein binding experiments
TQ (6.6 μg/ml) was prepared in different concentrations of FBS (5%, 10%, 20%, and 40%), BSA (30, 60, 120, and 240 μM), and AGP (1.25, 2.5, 5, and 10 μM). The different combinations were incubated for 30 min at 37 °C prior to treatment. Cells were washed with 1× PBS, treated, and proliferation assay was performed 6 h post-treatment.
Cell proliferation assay
Cells were plated in 96-well plates and treated at 50% confluency with TQ with or without pre-incubation with FBS, BSA, and/or AGP. Inhibition of cell proliferation by TQ was evaluated 6 h post-treatment using a colorimetric WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2 H-5-tetrazolio]-1,3-benzenedisulfonate) cell proliferation assay (Roche Diagnostics GmbH, Mannheim, Germany). The assay measures the ability of viable cells to cleave, by mitochondrial dehydrogenases, the WST-1 tetrazolium salt to formazan. The absorbance was measured at 440 nm using Varioscan scanning spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Chromatographic conditions
The analysis was conducted on an Agilent Technologies (Waldbronn, Germany) 1100 series instrument, comprising a vacuum degasser, an autosampler, a binary pump, and a diode array detector. Chromatographic separation was performed on a Phenomenex (Torrance, California, USA) C18 column (25 × 4.6 mm ID) with 5 μm packing material. TQ was detected using the method described by Crooks et al. with minor modification. The samples were eluted using an isocratic mobile phase of water: ACN (45:55% v/v) at a flow rate of 1 ml/min. The diode array detector signal was recorded at 254 nm, and the injection volume was 20 μl. The chromatographic data were acquired and analyzed using the Chemstation software package (Agilent). Since TQ is light and heat-sensitive, all the samples were protected from light using aluminum foil and processed on ice, unless otherwise indicated.
Sample preparation
Rat serum (Sprague–Dawley) was diluted (1:1, v/v) with PBS and spiked with TQ to yield a final concentration of 10 μg/ml. Several extraction methods (a–c) were performed as follows: (a) 500 μl of rat serum or PBS spiked with TQ, as described above, were purified using a C18 PrepSep solid phase extraction (SPE) column prior to HPLC analysis. Prior to extraction, the C18 cartridges were conditioned with 2 ml methanol and equilibrated with 2 ml of distilled water. After sample addition, the cartridge was washed with 2.5 ml of water and TQ was subsequently eluted with methanol (MeOH), acetonitrile (ACN), isopropanol, or acetone. The fractions eluted were dried under a stream of nitrogen and reconstituted in methanol prior to HPLC analysis; (b) 100 μl of spiked samples were subjected to a simple liquid–liquid extraction (LLE) step with different ratios of ethylacetate (1:1, 1:1.5, 1:2, and 1:3, v/v) followed by centrifugation (14,000×g, 4 °C, 10 min). The organic layer was transferred into another tube, and the volume was adjusted to 400 μl with MeOH to improve the shape of the peak in HPLC analysis; (c) spiked samples were subjected to protein precipitation with MeOH and ACN at different ratios (1:1, 1:2, and 1:4, v/v) followed by centrifugation at 14,000×g, for 10 min. After phase separation, the supernatant was transferred into an auto-sampler vial for HPLC analysis. For quantification, stock solutions of TQ were prepared in ACN, and standard calibration solutions of the stock were generated by serial dilution using ACN or PBS. A linear regression analysis of the area of the peak versus the concentration showed r2 > 0.999 over the range of 0.032–320 μg/ml. The limit of detection was 0.003 μg/ml.
TQ-protein binding studies
To determine the percentage of TQ-protein binding, 100% FBS was used. FBS and 10% FBS (diluted with DMEM high glucose used in cell culture experiments) were spiked with TQ to yield a final concentration of 3.3, 6.6, and 13.2 μg/ml. Spiked FBS was incubated at 37°C, and 400 μl were collected at 0, 5, 10, 15, 30, 60, 120, 240, and 360 min. The samples were stored at −80 °C until analysis. On the day of analysis, the samples were allowed to thaw on ice and were filtered using Amicon Ultra with 3 K cut-off by centrifugation at 14,000×g for 30 min. The filtrates were then analyzed for their TQ content by HPLC and the percentage of binding was calculated using the following equation: [% binding = (TotalTQ − freeTQ)/TotalTQ *100].
Further binding experiments were performed using BSA and AGP. The concentrations of BSA and AGP in serum are approximately 40 mg/ml (600 μM) and 0.97 mg/ml (25μM), respectively. Therefore, BSA and AGP at concentrations corresponding to 10% FBS were incubated at 37 °C with TQ at 6.6 μg/ml for 30 min. Samples were stored and processed as described above. The recovery of TQ from standard solutions subjected to the same procedure was 91.3 ± 0.6%; therefore, no correction was made while calculating the percentage of binding.
Amino acid sequence synthesis and purification
Short amino acid sequences from the region containing free cysteine of BSA from 29 to 38 and AGP from 145 to 149 have been selected using protein data bank. One sequence of BSA (YLQQCPFED) and two sequences of AGP (CLCIP and CLAIP) have been synthesized. The two sequences of AGP have been selected based on the presence of two isoforms of this protein. The first cysteine from each AGP sequence is alkylated since in the original sequences the first cysteine is engaged in a disulfide bridge. The selected peptides were synthesized on ACT-396 peptide synthesizer at 0.2 mmol scale, using Rink amide resin, double coupling standard fluorenylmethyloxycarbonyl FMOC-chemistry protocols (sixfold excesses of protected amino acids), TBTU/HOBT as coupling reagent, 20% piperidine in NMP for Fmoc deprotection and mixture of phenol/H2O/EDT/thioanisol/TFA = 1.5:1:1:1:10 for 2 h at room temperature final cleavage/deprotection. After cleavage/deprotection, crude peptides were precipitated with ice-cold diethylether, filtered, dried, and analyzed by RP HPLC (HP 1050 series chromatography, Supelco Discovery C18 column (15 cm × 4.6 mm, 5 μm), λ = 220 nm, linear gradient from 0 to 70% B in 40 min; A, 0.1% TFA; B, 0.1% TFA in 80% ACN). To study TQ/thiol adduction, TQ was incubated with each of the synthesized sequences for 30 min at 37 °C prior to the analysis with mass spectrometry.
Mass spectrometric analysis
Mass spectrometric (MS) analysis of 10 μM (∼1.6 μg/ml) of TQ incubated with the synthesized peptides sample prepared in 50/50 H2O/MeOH containing 0.1% formic acid was conducted on a Waters (Micromass) Q-TOF micro mass spectrometer (Manchester, UK). An electrospray ion source was used in a positive ion mode. The samples were infused into the MS at a flow rate of 10 μL/min using a syringe pump (KDS 100, KD Scientific, Holliston, MA, USA). The desolvation temperature was 75 °C, and the source temperature was set to 150 °C. Capillary voltage was 3 kV; sample cone was 15.0 V, and extraction cone was 2.0 V. The flow rate of cone gas and desolvation gas (nitrogen), produced by N2 generator (Peak Scientific, NM 15 L, Inchinnan, UK), was set to 200 L/h. Thymoquinone and the synthesized peptides were used as internal calibrators with a mass accuracy of ± 4.9 mDa. MS analyses of BSA incubated with TQ were performed on a 4.7-T hybrid quadrupole Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometer (APEX-Qe; Bruker Daltonics, Billerica, MA, USA), equipped with an Apollo-II electrospray ion source. The instrument was operated in a positive ion mode. BSA was mixed with TQ in different molar ratios and incubated for 30 min at 37 °C in 10 mM ammonium acetate (pH 6.9) buffer. The samples were then diluted with 50/50 H2O/ACN containing 1% acetic acid to the final BSA concentration of ∼10 μM and directly infused at a flow rate of 1.5 μL/min. Ions were externally accumulated for 1 s in a hexapole collision cell and subsequently transferred to the ICR cell for “RF-chirp” excitation and broadband detection. Each spectrum consists of 500 co-added (128-kWord) time-domain transients that were zero-filled once prior to magnitude calculation and fast Fourier transformation. Mass calibration was done externally with respect to the ions of an ES Tuning Mix (Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
Results are expressed as means±standard errors (SE). Statistical analysis was performed using SYSTAT VERSION 12 Software Package. Comparisons between different treatments were evaluated using a one-tailed Student’s t test. The level of significance was set at 0.05: asterisk indicating significance with respect to control group and double dagger indicating significant with respect to TQ or as indicated in the figure legend.
Results
Low concentrations of TQ cannot be detected using conventional analytical methods
SPE, LLE, and protein precipitation prior to HPLC analysis were used to evaluate the recovery of TQ (10 μg/ml) from spiked samples. TQ recovery from control PBS, subjected to SPE followed by drying under nitrogen and HPLC analysis, was less than 15%, while no TQ peak was seen in spiked serum. Using the LLE-HPLC protocol that involved a simple extraction step with ethyl acetate improved the recovery of TQ from control PBS samples to 85–90% but did not ameliorate the recovery from spiked serum samples (1–2%). Furthermore, spiked serum subjected to protein precipitation with different ratios of MeOH or ACN resulted in very low recovery of 1.8–5%. The use of larger volumes (1–4 ml) of the organic solvents used for extraction did not improve TQ recovery. Spiking serum with high TQ concentrations (100 μg/ml) improved its recovery to 65–80% when the samples were subjected to SPE and LLE and to 30–40% when protein precipitation using MeOH and ACN were used (Supplemental data 1A). Therefore, fresh serum has the capacity to trap low concentrations of TQ, thus preventing its recovery and detection.
Detection of low concentrations of TQ alone (1.6 μg/ml ∼ 10 μM) was not possible with Electrospray Ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), or Atmospheric Pressure Photon Ionization (APPI) (Supplemental data 1B).
Protein binding prevents the analytical detection of free TQ
To understand the reasons behind the low recovery of TQ from spiked serum, the binding of TQ to serum proteins was further investigated. FBS (10% and 100%) was spiked with TQ (3.3, 6.6, and 13.2 μg/ml), incubated at 37 °C, collected at different time intervals over 6 h, and filtered with 3 K filter cut off prior to HPLC analysis. The concentration of free TQ decreased at all the concentrations used. The calculated concentrations after incubating 3.3 μg/ml TQ in 10% and 100% FBS at 0 min of incubation were 0.2 and 0.022 μg/ml in 10% and 100% FBS, respectively (Fig. 1b, c). In 10% FBS, 86–93% of TQ was bound at 0 min and >96% at 30 min. In 100% FBS, more than 98% of TQ was bound at 0 min, and this value increased to more than 99% within 1 h (Table 1).
Table 1.
TQ percentage of binding to 10% and 100% FBS
| 10% FBS | 100% FBS | 10% FBS | 100% FBS | 10% FBS | 100% FBS | |
|---|---|---|---|---|---|---|
| Time, min | 3.3 μg/ml | 3.3 μg/ml | 6.6 μg/ml | 6.6 μg/ml | 13.2 μg/ml | 13.2 μg/ml |
| 0 | 93.88 ± 0.38 | 99.33 ± 0.01 | 93.35 ± 0.11 | 99.57 ± 0.02 | 86.63 ± 0.38 | 98.63 ± 0.13 |
| 5 | 98.06 ± 0.05 | 99.40 ± 0.10 | 98.04 ± 0.31 | 99.64 ± 0.10 | 95.50 ± 0.41 | 99.60 ± 0.12 |
| 10 | 98.91 ± 0.01 | 99.45 ± 0.13 | 98.66 ± 0.24 | 99.64 ± 0.01 | 96.29 ± 0.93 | 99.62 ± 0.07 |
| 15 | 99.48 ± 0.01 | 99.46 ± 0.03 | 98.78 ± 0.09 | 99.70 ± 0.03 | 97.20 ± 0.60 | 99.65 ± 0.10 |
| 30 | 99.62 ± 0.01 | 99.54 ± 0.01 | 98.77 ± 0.10 | 99.70 ± 0.06 | 97.37 ± 0.45 | 99.83 ± 0.03 |
| 60 | 99.65 ± 0.01 | ND | 99.00 ± 0.11 | 99.81 ± 0.04 | 97.79 ± 0.14 | 99.87 ± 0.01 |
| 120 | 99.61 ± 0.01 | ND | 99.22 ± 0.12 | ND | 98.05 ± 0.23 | ND |
| 240 | ND | ND | 99.51 ± 0.02 | ND | 98.60 ± 0.08 | ND |
| 360 | ND | ND | 99.82 ± 0.01 | ND | 99.64 ± 0.07 | ND |
Values are results of three independent experiments each done in duplicate
ND not detected
FBS protects against TQ-induced cell death
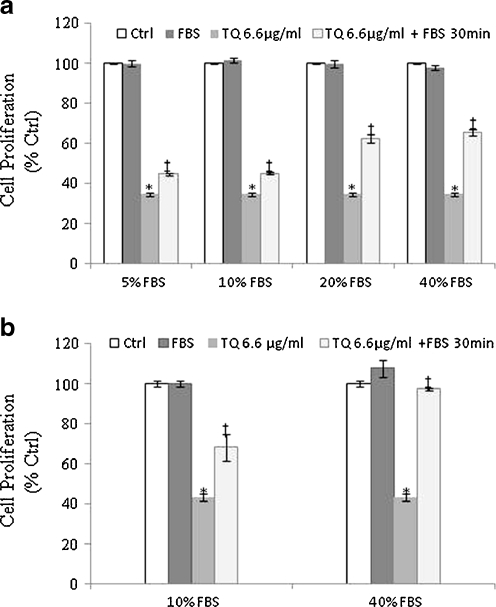
To test the effect of protein binding on TQ’s anticancer activities, DLD-1 and HCT-116 cells were treated with IC50 concentrations of TQ (6.6 μg/ml) prepared in different concentrations of FBS, and the inhibition of cell proliferation was determined by WST-1 assay 6 h post-treatment. As shown in Fig. 2a, incubation of TQ with different FBS concentrations at 37 °C for 30 min prior to addition to DLD-1 cells resulted in a significant decrease (p < 0.05) in TQ’s anti-proliferative activities. For instance, the percent of inhibition decreased from 55% in TQ + 10% FBS to 35% in TQ + 40% FBS. The addition of serum to TQ containing media decreased TQ-induced cell death from 65% to 55% (p < 0.05; Fig. 2a). The same protective effect was observed when TQ was incubated with 10% and 40% FBS and tested against HCT-116 colon cancer cells (Fig. 2b). The degree of inhibition observed with TQ in 10% FBS decreased significantly (p < 0.05) when compared with TQ in 40% FBS. The inhibition decreased from 31.6% to 3% when TQ was prepared in 10% and 40% FBS, respectively (Fig. 2b). Since TQ was incubated with FBS prior to treatment, it suggests that the observed loss of TQ’s activity comes from the interaction between FBS component(s) with TQ rather than from the action of FBS on the cells.
Fig. 2.
Concentration-dependent protective effect of FBS on TQ-induced cell death in DLD-1(a) and HCT-116 (b) cells. Cells were treated with 6.6 μg/ml TQ pre-incubated with different percentages (5–40%) of FBS. Cell proliferation was determined 6 h post-treatment by the WST-1 assay as described in the Materials and methods. Results are expressed as percent control. Each value represents the mean±SE of three separate experiments (n = 4). Statistical significance: *P < 0.05 with respect to control, ‡P < 0.05 with respect to TQ
BSA and not AGP protects colon cancer cells from TQ-induced cell death
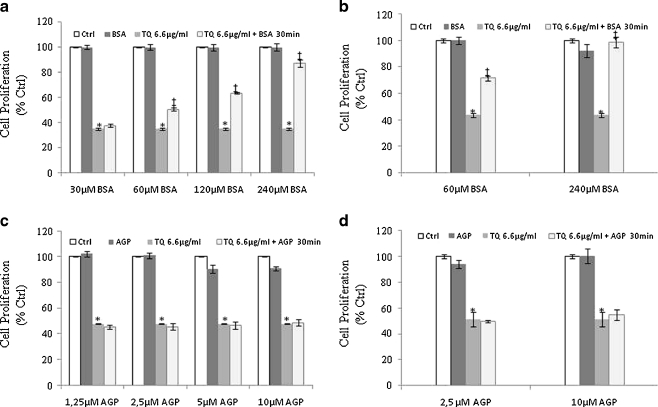
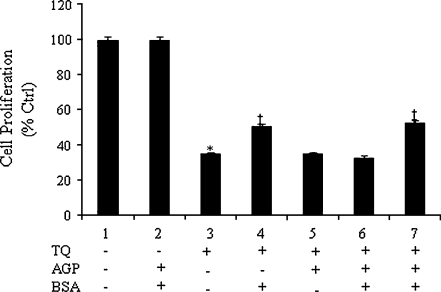
Since FBS, a universal serum used in cell culture, showed an inhibitory effect on TQ’s anti-neoplastic activity at high concentrations, we investigated the effect of two major plasma proteins, BSA and AGP, on activity of TQ. The concentrations of BSA and AGP in serum are around 40 mg/ml (600 μM) and 0.97 mg/ml (25 μM), respectively [29]. Therefore, to determine the involvement of the aforementioned proteins on TQ’s binding and loss of activity, binding and proliferation assays were performed. Binding assays showed that, in spiked samples, TQ was found to bind BSA (94.5 ± 1.7%) and AGP (99.1 ± 0.1%). To study the effect of this binding on TQ’s activity, DLD-1 cells were treated with TQ alone or with TQ pre-incubated for 30 min with BSA and AGP at concentrations representing their concentrations at 5%, 10%, 20%, and 40% FBS. As seen in Fig. 3a, TQ incubated with increasing concentrations of BSA resulted in a dose-dependent loss of activity. The percent of inhibition decreased from 50% to 12.8% in cells treated with TQ + 60 μM and 240 μM BSA, respectively (Fig. 3a). The same effect was observed in HCT-116 colon cancer treated with TQ pre-incubated with 60 and 240 μM (Fig. 3b). The degree of inhibition decreased from 28.4% to 1% in cells treated TQ + 60 μM and 240 μM BSA, respectively (Fig. 3b). Interestingly, in both colon cancer cells used, pre-incubation of TQ with AGP prior to treatment did not alter its growth inhibitory effects at almost all the concentrations used (Fig. 3c, d). The loss of TQ activity was more pronounced in DLD-1 cells treated with TQ + 240 μM BSA (Fig. 3a) than with those treated with TQ + 40% FBS (Fig. 2a). These results imply that TQ binds to proteins in serum where they may play a protective role and allow TQ to exert its effect while other (s) inactivate TQ. To prove this hypothesis, further experiments were conducted in which TQ was pre-incubated with BSA prior to the addition of AGP and vice versa. As seen earlier, the activity of TQ was significantly lower (p < 0.05) when TQ was pre-incubated with BSA than with TQ alone while no such effect was observed when the cells were treated with TQ + AGP (Fig. 4). Interestingly, when TQ was pre-incubated with AGP prior the addition of BSA, AGP protected TQ from binding to BSA and TQ’s effect was retained (Fig. 4). The contrary was observed when the incubation order was reversed, whereby pre-incubation of TQ with BSA prior to AGP resulted in a significant loss of TQ’s activity as compared with TQ alone (Fig. 4).
Fig. 3.
Effect of BSA and AGP binding on TQ-induced cell death. 6.6 μg/ml TQ was incubated for 30 min at 37 °C with different concentrations of BSA (a, b) and AGP (c, d) prior to treatment. Cell proliferation was determined 6 h post-treatment by the WST-1 assay as described in the Materials and methods. Each value represents the mean±SE of three separate experiments (n = 4). Statistical significance: *P < 0.05 with respect to control, ‡P < 0.05 with respect to TQ
Fig. 4.
Protective effect of AGP on TQ/BSA binding in DLD-1 cells. TQ binding was determined after 30 min of incubation with 60 μM BSA and 2.5 μM AGP, at concentrations that correspond to their concentrations in 10% FBS. 6.6 μg/ml TQ was incubated at 37 °C with BSA prior to addition of AGP for another 30 min (column 6) or with AGP for 30 min prior to addition of BSA for another 30 min (column 7). Cell proliferation was determined 6 h post-treatment by the WST-1 assay as described in the Materials and methods. Each value represents the mean±SE of two separate experiments (n = 4). Statistical significance: *P < 0.05 with respect to control, ‡P < 0.05 with respect to TQ
Direct binding of TQ to BSA
TQ as a quinone can undergo facile adduction with electron-rich nucleophilic species such as activated amino, hydroxyl, and thiol groups [31, 32]. BSA has 35 cysteine residues, 34 of which are engaged in disulfide bridges and one free cysteine. The free cysteine is present on the position 34 of the amino acid sequence. AGP, on the other hand, has two isoforms. Each has two disulfide bridges, but the major difference is in the presence of free cysteine in position 147 in one isoform while the other has an arginine residue instead [30].
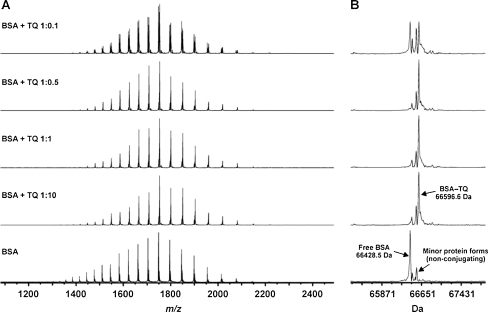
MS analysis of BSA incubated with TQ at different molar ratios (1:0.1, 1:0.5, 1:1, and 1:10) showed that TQ reacts with BSA to form a covalent 1:1 conjugate, most probably through the free thiol group of Cys-34 residue (Fig. 5). The reaction seems very specific whereby no other stoichiometries were present even at high molar excess of TQ (BSA, TQ = 1:10; Fig. 5). BSA appeared in three different protein forms, the average mass of the main form agrees with the mass calculated from the sequence (66428.68 Da). Two other minor protein forms did not conjugate with BSA as can be seen from the spectra (Fig. 5). The other form (+125 Da) is likely due to the N-ethylmaleimide (NEM)-conjugation to Cys-34. NEM is most probably a contamination from our instrument due to its extensive use in another project during the time of the analyses. It makes sense, then, that in this protein form, Cys-34 is unavailable for TQ conjugation. The third form (+39) is unknown but did not conjugate either. The observed mass of the BSA-TQ conjugate (66596.6 Da) suggests that upon conjugation, TQ has been twice reduced (+168 Da) in the reaction to form tetrahydrothymoquinone. The reason why even having a BSA, TQ = 1:0.1 molar ratio gives roughly 50% conjugate is that the concentration of BSA is likely to be overestimated by the method used. Native-MS measurements with BSA, to reveal if there were non-covalent adducts besides the covalent conjugate, failed since BSA is hard to properly desalt for these measurements.
Fig. 5.
TQ covalent binding to free thiol (-SH). TQ was incubated with BSA at different molar ratios (1:0.1, 1:0.5, 1:1, and 1:10 BSA/TQ). ESI/FT-ICR mass spectrometric analysis was carried out, 30 min post-incubation, using ESI in positive ion mode: a broadband mass spectra, b deconvoluted mass spectra. The data presented are representative of two independent runs
To mimic TQ binding to the free thiol group in BSA and AGP, short sequences (from four to nine amino acids) from regions surrounding the free thiol in BSA and AGP were synthesized. TQ was incubated with the sequences for 30 min after which TQ binding was detected by ESI/MS in the positive ion mode.
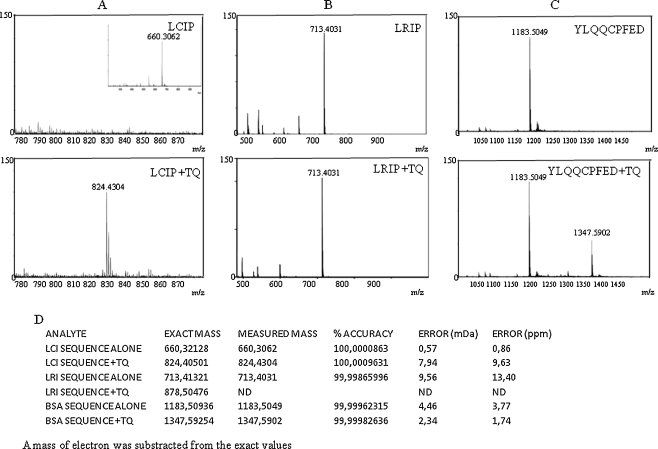
As seen in Fig. 6, when TQ was incubated with the AGP isoform that has a free cysteine (showing protonated molecule at m/z 660.3213), another ion appeared at m/z 824.4050 which corresponds to the covalent binding of TQ to the free thiol (with mass accuracy of 9.6 ppm). However, no binding was observed when TQ was incubated with the AGP isoform that has no free cysteine. The BSA sequence alone appeared at m/z 1183.5094, and the covalent complex of the sequence and TQ was observed at m/z 1347.5925 with a mass accuracy of 1.8 ppm. TQ + BSA complex was also detected in the mass spectrum as double charged ion at m/z 674.2962.
Fig. 6.
TQ covalent binding to free thiol (-SH). TQ was incubated with synthesized sequences from AGP (a, b) and BSA (c). d Results from accurate mass measurement of synthesized sequences as protonated molecules with and without TQ using 30 min post-incubation and ESI in positive ion mode. The data presented are representative of three independent runs
These data confirm that TQ can bind covalently to the free thiol in BSA and AGP. The fact that TQ did not react with the hydroxyl and amine groups in the sequences confirmed that TQ binds selectively to the free thiol.
Discussion
In the last 10 years, more than 150 studies were published on TQ’s potent activities but surprisingly none dealt with its pharmacokinetic profile. In addition, there are no analytical methods to detect TQ in blood/serum. The analysis of quinones is challenging due to their high reactivity as fast redox cycling molecules and their facile adduction with electron-rich nucleophilic species, such as activated amino, hydroxyl, and thiol groups [31, 32]. In biological systems, such nucleophiles are found as reactive side-groups of lysine, serine, and cysteine in proteins [33].
In this study, TQ was extracted from spiked serum, and the samples were subjected to sample cleanup procedures (SPE, LLE, protein precipitation) prior to HPLC analysis. The recovery of TQ from spiked serum was less than 5% at low concentrations of TQ (10 μg/ml) but increased to 72% at high concentration (100 μg/ml) of TQ. This was hypothesized to be due to the TQ binding to serum components. At low concentration of TQ, many irreversible binding sites are available, and the concentration of free and reversibly bound TQ is below the detection limit of the analytical method thus explaining the low recovery. At high concentrations, the irreversible binding sites were occupied, and the free TQ was detectable. Our findings show extensive binding of TQ to serum proteins (Table 1). Mass spectrometric analysis confirmed that TQ binds covalently to the cysteine residue on the 34th position on the amino acid sequence of BSA, the most abundant protein in serum.These data confirmed the previous assumption of serum capacity and explained why HPLC-MS methods using non-labeled TQ cannot be used at low concentration for pharmacokinetic studies. Due to high percentage of binding, the concentration of free TQ might be below the detection limit and hence, cannot be monitored.
In addition, to further test the binding effect on TQ activity, DLD-1 and HCT-116 cells were treated with TQ (IC50) pre-incubated with increasing concentrations of FBS. Increasing the concentration of FBS resulted in a decrease in TQ’s inhibitory effect against colon cancer cells (Fig. 2a, b). This is probably due to the increase in serum proteins/molecules with free cysteine which result in covalent binding and consequently, in the loss of free TQ necessary to induce the antiproliferative effect against the cancer cells.
BSA/TQ binding prevents TQ from exerting its inhibitory effect against colon cancer cells (Fig. 3a, b). On the other hand, the extensive binding observed with AGP, the second major serum protein, did not affect TQ’s inhibitory activity. Interestingly, the inhibitory effect of TQ with BSA in DLD-1 cells was more pronounced than the inhibitory effect with FBS. This effect was probably due to a competition in the binding of TQ to different plasma components. The substantial binding at the start of incubation implies the high rate at which TQ binds to serum components. Serum contains many components that have free thiol groups to which TQ can be bound covalently. Furthermore, our data shows that the covalent binding of TQ to BSA is an inactivation process and that only the free and non-covalently bound TQ are responsible for inhibiting the proliferation of cancer cells. The loss of activity was not complete due to the fact that almost 50% of the free cysteine in the BSA molecule is occupied by circulating low molecular weight thiol containing molecules [26]. Our data on TQ covalent binding contradicts a recent study published by Lupidi et al., in which they showed that TQ binds to the site I of HSA by hydrophobic interaction [34]. The discrepancy between our results using BSA and the results reported by Lupidi et al. on TQ binding to HSA could be due to differences in protein species and analytical techniques used. In addition, it might be that TQ binds to BSA by non-covalent interactions, yet our attempt to reveal such binding besides the covalent conjugate using native-MS measurements with BSA failed, since BSA is hard to properly desalt for these measurements. In all cases, however, our data confirm the loss of TQ activity when bound to BSA.
The inhibitory effect of FBS components on TQ activity is similar to the effect of FBS components exerted on the activity of ET-743, a novel marine antitumor compound against soft tissue sarcomas and ovarian cancer in phase I and II clinical studies [35]. The in vitro inhibitory effect of ET-742 was lost when the percentage of FBS was increased. This effect, however, was restored when the compound was first prepared in HSA then diluted with FBS, confirming thus the protection effect of HSA on the drug against the inactivation exerted by FBS components [35]. This protection offered by HSA resembled the protective effect offered by AGP to TQ prior to the addition of BSA in our study (Fig. 4). Interestingly, a recent study by Ravindran et al. [36] showed that, in vitro, TQ encapsulated in nanoparticles (TQ-NP) inhibited more the proliferation of colon, breast, prostate, and myeloma cancer as compared with cells treated with the non-encapsulated TQ [36].
Using the same concentration (10 μM), the average inhibitory effect against all cancers tested increased from 32.5% with TQ to 82% with TQ-NP [36]. This data correlated well with our finding that the covalent binding of TQ to plasma components, such as BSA, results in its inactivation while its binding to other components such as AGP do not alter its activity. The nanoparticles protected TQ for such inhibitory effect and increased TQ potency against cancer cells. TQ can bind covalently and non-covalently to serum components, thus limiting the use of conventional analytical methods for its detection in plasma and determining its bioavailability. Similar challenges were reported for β-lapachone, an anticancer compound that belongs to the quinone family as well. While studying the in vitro metabolism of β-lapachone in plasma and whole blood, the compound could not be detected with ESI-LC/MS. The failure in the detection of β-lapachone in blood by conventional analytical methods led to the use of the radiolabeled 14C β-lapachone that was necessary in studying its metabolic profile [37].
Conclusions and future prospects
The reversible and irreversible binding of drugs to proteins has direct implication on drug distribution, elimination, and metabolism and can be associated with adverse side effect. The ability of TQ to bind covalently to free thiol raises concerns about its long-term use.
On one hand, the covalent binding might be responsible for the instant absence of toxicity due to the decrease in the available free drug; on the other hand, the consecutive administration of the drug could lead to free drug accumulation which could result in toxicity. While short-term in vivo toxicity studies has shown that TQ is not toxic [21], long-term studies experiments should be performed to confirm its safe use.
Since TQ belongs to the quinones’ family that represents a large group of anti-neoplastic drugs, the outcome of the present study could help in understanding the behavior of chemically similar compounds in the drug discovery and development process.
Even though many reports showed TQ being active in vivo against colon [21], prostate [18], stomach, and fibrosarcoma cancer [22, 23], the actual behavior of TQ in plasma remains an unanswered question that merits further investigation. Consequently, based on the reported data in this study, the use of radiolabeled or isotopically labeled TQ is important for clarifying its pharmacokinetic properties, required for its future clinical development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(JPEG 188 kb)
(JPEG 131 kb)
Acknowledgments
The Finnish Cultural Foundation (FCF) and the Centre for International Mobility (CIMO) are acknowledged for their financial support to Dr. Nahed El-Najjar.
References
- 1.Padhye S, Banerjee S, Ahmad A, et al. From here to eternity - the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 2.Norwood AA, Tan M, May M, et al. Comparison of potential chemotherapeutic agents, 5-fluoruracil, green tea, and thymoquinone on colon cancer cells. Biomed Sci Instrum. 2006;42:350–356. [PubMed] [Google Scholar]
- 3.Marsik P, Kokoska L, Landa P, et al. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005;71:739–742. doi: 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- 4.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 5.Xuan NT, Shumilina E, Qadri SM, et al. Effect of thymoquinone on mouse dendritic cells. Cell Physiol Biochem. 2010;25:307–314. doi: 10.1159/000276563. [DOI] [PubMed] [Google Scholar]
- 6.Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sci. 2009;85:830–834. doi: 10.1016/j.lfs.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Nader MA, el-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33:637–643. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 8.Alenzi FQ, El-Bolkiny Y, Salem ML. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci. 2010;67:20–28. doi: 10.1080/09674845.2010.11730285. [DOI] [PubMed] [Google Scholar]
- 9.El-Najjar N, Chatila M, Moukadem H, et al. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 10.Alhosin M, Abusnina A, Achour M, et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol. 2010;79:1251–1260. doi: 10.1016/j.bcp.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: a promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Mousavi SH, Tayarani-Najaran Z, Asghari M, et al. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30:591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gali-Muhtasib H, Kuester D, Mawrin C, et al. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609–5618. doi: 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- 14.El-Mahdy MA, Zhu Q, Wang QE, et al. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 15.Womack K, Anderson M, Tucci M, et al. Evaluation of bioflavonoids as potential chemotherapeutic agents. Biomed Sci Instrum. 2006;42:464–469. [PubMed] [Google Scholar]
- 16.Richards LR, Jones P, Hughes J, et al. The physiological effect of conventional treatment with epigallocatechin-3-gallate, thymoquinone, and tannic acid on the LNCaP cell line. Biomed Sci Instrum. 2006;42:357–362. [PubMed] [Google Scholar]
- 17.Chehl N, Chipitsyna G, Gong Q, et al. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11:373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards LR, Jones P, Benghuzzi H, et al. A comparison of the morphological changes associated with conventional and sustained treatment with pigallocatechin3gallate, thymoquinone, and tannic acid on lncap cells. Biomed Sci Instrum. 2008;44:465–470. [PubMed] [Google Scholar]
- 19.Gali-Muhtasib H, Diab-Assaf M, Boltze C, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 20.Roepke M, Diestel A, Bajbouj K, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 21.Gali-Muhtasib H, Ocker M, Kuester D, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badary OA, Al-Shabanah OA, Nagi MN, et al. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435–440. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- 24.Ghosheh OA, Houdi AA, Crooks PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.) J Pharm Biomed Anal. 1999;19:757–762. doi: 10.1016/S0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 25.Aboul-Enein HY, Abou-Basha LI. Simple HPLC method for the determination of thymoquinone in black seed oil (Nigella sativa Linn) J Liq Chromatogr Related Technol. 1995;18:895–902. doi: 10.1080/10826079508010400. [DOI] [Google Scholar]
- 26.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 27.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–171. doi: 10.1016/S0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 28.Schmid K, Kaufmann H, Isemura S, et al. Structure of 1 -acid glycoprotein. The complete amino acid sequence, multiple amino acid substitutions, and homology with the immunoglobulins. Biochemistry. 1973;12:2711–2724. doi: 10.1021/bi00738a026. [DOI] [PubMed] [Google Scholar]
- 29.Barnaby RJ, Bottacini M. Protein binding in plasma: a case history of a highly protein-bound drug. In: Evans G, editor. A HandBook of Bioanalysis and Drug Metabolism. Boca Raton, FL: CRC Press; 2004. pp. 156–175. [Google Scholar]
- 30.Vallner JJ. Binding of drugs by albumin and plasma protein. J Pharm Sci. 1977;66:447–465. doi: 10.1002/jps.2600660402. [DOI] [PubMed] [Google Scholar]
- 31.Land EJ, Ramsden CA, Riley PA. Quinone chemistry and melanogenesis. Methods Enzymol. 2004;378:88–109. doi: 10.1016/S0076-6879(04)78005-2. [DOI] [PubMed] [Google Scholar]
- 32.Li WW, Heinze J, Haehnel W. Site-specific binding of quinones to proteins through thiol addition and addition-elimination reactions. J Am Chem Soc. 2005;127:6140–6141. doi: 10.1021/ja050974x. [DOI] [PubMed] [Google Scholar]
- 33.Magee PS. Exploring the chemistry of quinones by computation. Quant Struc-Act Relat. 2000;19:22–28. doi: 10.1002/(SICI)1521-3838(200002)19:1<22::AID-QSAR22>3.0.CO;2-U. [DOI] [Google Scholar]
- 34.Lupidi G, Scire A, Camaioni E, et al. Thymoquinone, a potential therapeutic agent of Nigella sativa, binds to site I of human serum albumin. Phytomedicine. 2010;17:714–720. doi: 10.1016/j.phymed.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Tognon G, Frapolli R, Zaffaroni M, et al. Fetal bovine serum, but not human serum, inhibits the in vitro cytotoxicity of ET-743 (Yondelis, trabectedin), an example of potential problems for extrapolation of active drug concentrations from in vitro studies. Cancer Chemother Pharmacol. 2004;53:89–90. doi: 10.1007/s00280-003-0704-y. [DOI] [PubMed] [Google Scholar]
- 36.Ravindran J, Nair HB, Sung B, et al. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79:1640–1647. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Miao XS, Song P, Savage RE, et al. Identification of the in vitro metabolites of 3, 4-dihydro-2, 2-dimethyl-2 H-naphthol[1, 2-b]pyran-5, 6-dione (ARQ 501; beta-lapachone) in whole blood. Drug Metab Dispos. 2008;36:641–648. doi: 10.1124/dmd.107.018572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(JPEG 188 kb)
(JPEG 131 kb)