Abstract
The current investigation evaluates the expression of phosphatidylinositol kinase (PIK) genes in the parasitic protozoan, Giardia lamblia. The G. lamblia Genome Database revealed the presence of two putative phosphatidylinositol-3-kinase (gPI3K) and one phosphatidylinositol-4-kinase (gPI4K) genes resembling the catalytic subunit of eukaryotic PIKs. Primers, designed to amplify mRNA of these three genes, were used to measure transcription by quantitative reverse-transcriptase polymerase chain reactions. Results suggest that all three PIK genes are expressed in non-encysting and encysting trophozoites. The relative levels of the mRNA were highest in parasites cultured in pre-encysting medium that contained no bile. Two inhibitors of PIK, LY 294002 and wortmannin were found to inhibit the growth of the trophozoite in culture. However, wortmannin was more effective than LY294002. Altogether, the present study indicates that Giardia is capable of expressing PIKs that are necessary for the growth and differentiation of this pathogen.
While enormous advancements have been made in delineating the importance of phosphatidylinositol kinases (PIKs) in transducing extracellular signals and vesicular trafficking in higher eukaryotes, the role of PI3Ks, PI4Ks or other lipid kinases in growth and differentiation of intestinal protozoan cells remains poorly defined. Several investigators in recent years have reported that intracellular and other obligate parasites can modulate PI3K functions in host cells during host-parasite interactions. For example, Trypanosoma cruzi infection is responsible for the elevation of inositol phosphate levels in mouse peritoneal macrophages (Todorov et al. 2000). Cryptosporidium parvum was shown to trigger the synthesis of PI3K activity and cytoskeletal-remodeling in cultured epithelial cell lines (Forney et al. 1999). Theileria parva stimulates the constitutive expression of PI3K activity in infected B-lymphocytes (Baumgartner et al. 2000). Of further interest, wortmannin, a fungal inhibitor of PI3K, inhibited the phagocytosis of bacteria, red blood cells, and mucin-coated beads by Entamoeba histolytica, suggesting that protozoan PI3K is involved in phagocytic processes (Ghosh and Samuelson 1997). Recently, Makioka et al. (2001) documented that wortmannin inhibited the in vitro encystation of Entamoeba invadens; indicating the possible key roles in protozoan differentiation intracellular PI3K may play.
Here, we asked if PIK genes are present and expressed in G. lamblia trophozoites. Analysis of the Giardia lamblia genome database (McArthur et al. 2000; www.mbl.edu/Giardia) revealed the presence of three potential PIK catalytic subunit genes i.e., giardial phosphatidylinositol 3 kinase-1 (gPI3K-1), giardial phosphatidylinositol 3 kinase-2 (gPI3K-2), and giardial phosphatidylinositol 4 kinase (gPI4K). We also demonstrate that all three are transcribed, and that the PIK inhibitors, LY 294002 and wortmannin, inhibit growth of Giardia in culture.
Materials and Methods
Materials
TRIzol® reagent for RNA isolation and purification kit was purchased from Invitrogen (Carlsbad, CA). The cDNA synthesis kit was obtained from Super Array Bioscience Corporation (Frederick, MD). PI3-kinase inhibitors, LY-294002 and wortmannin, were purchased from Biomol (Plymouth, PA) and Alexis Biochemicals (San Diego, CA), respectively.
Organisms and cell culture
Giardia lamblia trophozoites, strain WB (ATCC 30957), clone C6, were cultivated following the method established by Diamond et al. (1978), using TYI-S-33 medium supplemented with adult bovine serum (10%, v/v) and bovine bile (0.5 mg/ml) in the presence of piperacillin (Keister 1983; Gillin et al. 1988). Cells were grown in tissue culture flasks at 37 °C to late log phase and harvested by chilling flasks in ice-cold water and centrifuging (1500 × g for 6 min at 4 °C). For encystation, the confluent monolayer of trophozoites was bovine-bile starved for 24 h prior to culturing in encystation medium (TYI-S-33 medium-pH 7.8, supplemented with 10% (v/v) bovine serum, 0.25 mg/ml porcine bile, and 1 mM lactic acid) for 6 and 12 h (Boucher and Gillin 1990; Das and Gillin 1996) before harvesting and the extracting the total RNA as described below.
Identification of putative homologues of giardial PIKs through bioinformatic analysis
To identify putative giardial homologues of PIKs, all predicted open reading frames of the Giardia genome were compared with the Pfam HMM for PI3/PI4 kinase, PF00454 (Bateman et al. 2004, http://pfam.wustl.edu). The five ORFs with significant matches to the models were further characterized by both BLAST (Altschul et al. 1990) and the Pfam database. The catalytic domains of all five sequences were aligned using CLUSTALW (Thompson et al. 1997).
Determination of mRNA levels of putative PIKs through quantitative RT-PCR
To determine whether gPI3K-1, gPI3K-2, and gPI4K were expressed in non-encysting and encysting trophozoites, cells were cultivated in growth medium and subjected to pre-encystation and then encystation for 6 and 12 h as described above. Total RNA (1-2 μg) was reverse transcribed following the standard protocol of the Reaction Ready First Strand cDNA synthesis kit (Superarray Biosciences, Frederick, MD). Primers for PCR were designed using the Primer 3 software (primer3_www.cgi v 0.2; Rozen and Skaletsky 2000) and were synthesized by Sigma Genosys. The sequence of each primer pair is listed below: gPI3K-1 (forward 5′-TTGCCCTTAGTCCCCTTCTT-3′ and reverse 3′GAACGCGTTGCAGAACACTA-5′); gPI3K-2 (forward 5′-AAAGGCGGGGATACTGAGTT-3′ and reverse 3′-CTCCGAAACCCAGTGAATGT-5′); gPI4K (forward 5′-TCGACACGTCCATCATTCAT-3′ and reverse 3′-GAGATCACGATAGCCGAAGC-5′). cDNA samples were diluted 1:10 and 1 μl of each cDNA was used as a template in PCR reactions. The PCR products were run on a 2% agarose gel. For quantitative real time PCR, cDNA samples were diluted 1:25 and 2 μl of each sample were used in a 20-μl PCR reaction with 10 μl of 2 × SYBRgreen PCR master mix (Superarray Biosciences, Frederick, MD). The qRT-PCR was performed using MYiQ version 1.0 thermal cycler (BIORAD, Hercules CA). The relative standard curve method was used to quantify transcript levels.
Treatment with inhibitors
Stock solutions of wortmannin and LY 294002 were prepared in methanol following the manufacturer's recommendations. The appropriate inhibitor was diluted to the respective concentrations (between 0--15 μ1) and was added to 5-ml tubes before drying under N2. Dried inhibitors were then dissolved in 10 μ1 ethanol before adding culture media. Equal amounts of ethanol (∼10 μ1) were added to control tubes. Approximately 1 × 106Giardia trophozoites were added to each tube (control and treatment) and incubated overnight at 37 °C. The media were decanted and the tubes were filled with cold phosphate buffered saline (PBS). Adherent trophozoites were detached by chilling the tubes in ice-cold water for ∼30 min. Detached cells (viable) were counted with the help of a hemocytometer.
Results and Discussion
Phosphatidylinositol kinases (PIKs), in general, catalyze the phosphorylation of phosphoinositides to generate various phosphatidylinositol phosphates. These inositol-based phospholipids have been implicated in signaling roles (Carpenter and Cantley 1996), phagocytosis (Marshall et al. 2001), leukocyte function (Wymann et al. 2000), cytoskeletal remodeling (Forney et al. 1999), and protein trafficking (Sato et al. 2001) in mammalian cells. Automatic annotation of preliminary Giardia genome sequence data has suggested that there are at least three PIK genes in Giardia (McArthur et al. 2000). To confirm these results and to be sure that all potential PIK genes were accounted for, we compared the predicted open reading frames (gORFs) from the Giardia genome project to the pfam model PF00454, PI3/PI4 kinase (Bateman et al. 2004). Only five gORFs contained significant hits to this signature motif (Table 1). Because this motif can be found in other kinases as well, we further characterized each of the ORFs by BLASTp sequence comparisons (Altschul et al. 1990) and more extensive motif identification. The best match from the initial screening had been previously identified as a homologue of the mammalian target of rapamycin gene, gTOR (Morrison et al. 2002). Two of the ORFs demonstrated pfam and BLAST matches consistent with PI3K from Dictyostelium discoideum and Nicotinica tabacum (Table 1) and were designated gPI3K-1, gPI3K-2, while the third strongly correlated with PI4K of Saccharomyces pombe and was called gPI4K. The final gORF presented with only a weak correlation to a serine-threonine protein kinase in the PI3K family and was designated PIK-like. The gORF PI3/PI4K catalytic domains (Table 1) were combined with PI3/PI4K motif seed alignment sequences from Pfam and a multiple sequence alignment compiled using CLUSTALW. All gORF catalytic motifs aligned well in the multiple sequence alignment with the exception that gPI3K-2 had a 207 amino acid insertion approximately 80 amino acids into the conserved region, almost doubling the size of the motif (data not shown). It should be noted that all of the identified, potential homologues were for the catalytic subunit only. We were unable to identify the presence of the regulatory subunit (p85) of PIK. Since Zhou et al. (1995) reported that p110 catalytic domains of Dictyostelium PI3Ks are not associated with p85, and in fact no putative homologue of the p85 gene is present in D. discoideum, the absence of p85 in Giardia supports the notion that PIKs in this early-diverging eukaryote may not require the regulatory domain for their function.
TABLE-1.
Perdicted Giardia lamblia ORFs Containing PI3/PI4 Kinase Catalytic Motif.
| Designation (gORF) | GenBank Accession | Match to Pfam PI3/PI4 Kinase (motif location) | Other Pfam matchesd | Best BLASTp match species (Expectation Value) |
|---|---|---|---|---|
| gTOR (35280a) | AY095369.1 | 9e-64 (aa2883-3134) | Mammalian TOR H. sapien (8e-148) | |
| gPI3K-1 (14855) | XP771557.1 | 5.1e-47 (aa1776-2096) | PI3Kab | PI3 Kinase D. discoideum (2e-36) |
| gPI3K-2 | XP771095.1 | 6.7e-33 (aa1133-1590) | PI3Kac PI3KC2 | PI3 Kinase N. tabacum (5e-29) |
| gPI4K (16558) | XP768275.1 | 1.9e-24 | PI4 Kinase S. pombe (1e-45) | |
| PIK-like | XP779251.1 | 3.3e-6 (aa 2352-2590) | FATC | Ser/Thr prot kinase |
PI3Ka is an accessory domain believed to be involved in substrate presentation (Flanagan et al. 1993); PI3KC2 interacts with structural components of the PIK protein (Djordjevic and Driscoll, 2002); FATC domain is found at the C-terminal end of some PIK proteins and may act as an intracellular sensor (Bosotti et al. 2000.)
gORF 35280 uses an alternative start site for N-terminus from genbank reference.
HMMTOP (Tusnády and Simon, 2001) predicts two transmembrane helices, 1650-1667 and 1676-1693.
HMMTOP predicts three transmembrane helices, 1171-1189, 1564-1582, and 1605-1623.
gPI3K-1, giardial phosphatidylinositol 3 kinase; gPI3K-2; giardial phosphatidylinositol 3 kinase-2; gPI4K, giardial phosphatidylinositol 4 kinase; PIK, phosphatidylinositol kinase; D. discoideum, Dictostelium discoideum; N. tabacum, Nicotinica tabacum; H. sapiens; Homo sapiens; S. pombe, Saccahromyces pombe.
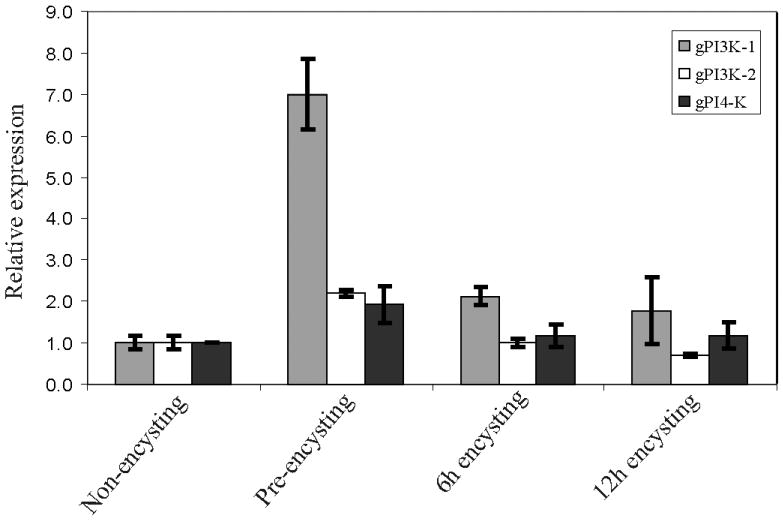
The qRT-PCR analysis demonstrated that these genes are transcribed in non-encysting, pre-encysting, and encysting trophozoites (Fig. 1). However, the dramatic increase in transcript levels of gPI3K-1 (∼7 fold) in pre-encysting cells indicates that Dictyosteluim-like gPI3K-1 is important and likely to be modulated by bile acid. Earlier, it was postulated that exogenous bile is important for giardial growth and involved in importing lipid molecules by forming mixed micelles (Farthing et al. 1985; Das et al. 1997). In the current investigation, the increased gPI3K-1, gPI3K-2, and gPI4K transcripts in bile-starved cells may encode respective enzymes that are responsible for the increased production of inositol phosphates, which are otherwise acquired by Giardia from bile acids during its colonization in the small intestine (Farthing et al. 1985).
Fig. 1.
The mRNA expression of giardial phosphatidylinositol 3 kinase-1 (gPI3K-1), giardial phosphatidylinositol 3 kinase-2 (gPI3K-2) and giardial phosphatidylinositol 4 kinase (gPI4K) transcripts of pre-encysting and encysting trophozoites of Giardia lamblia relative to non-encysting trophozoites. Results shown here are the, mean ± SD of three separate experiments where individual experiments were carried out in triplicate. The qRT-PCR analysis of the gPI3K-1, gPI3K-2 and gPI4K genes was carried out as described in Materials and Methods.
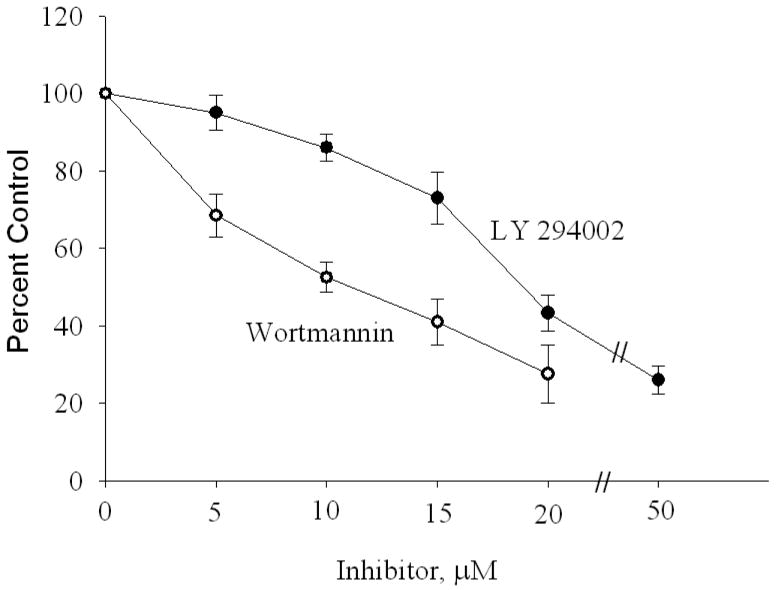
Trophozoites of Giardia in culture treated with LY 294002 and wortmannin and show reduced growth (Fig. 2). Wortmannin, a fungal metabolite, binds to the ATP pocket of the p110α catalytic subunit of the PI3K enzyme and covalently modifies the Lys-833 residue that is involved in transferring a phosphate group during ATP and PI reactions (Walker et al. 2000). LY 294002, on the other hand, a synthetic PI3K inhibitor (flavonoid quercetin-based), binds to the ATP pocket of p110 in an orientation distinct from that of wortmannin (Walker et al., 2000). Since wortmannin inhibited the growth slightly more effectively (IC50 ∼11 μM) than LY 294002 (IC50 ∼ 18 μM) (Fig. 2), it is likely that the ATP binding pockets of giardial PI3Ks are oriented in a manner that favors the binding of wortmannin over LY 294002. It is also possible that gPI3K-1, gPI3K-2 and gPI4K possess different binding affinities toward wortmannin and LY 294002.
Fig. 2.
Effects of PI3K inhibitors on giardial growth. Trophozoites were cultured in growth medium in the presence of LY 294002 or wortmannin as described in “Materials and Methods.” After overnight incubation at 37 °C, non-attached cells were decanted and the tubes were filled with cold PBS. Adherent trophozoites were detached by chilling the tubes in ice-cold water for ∼30 min and counted under phase-contrast microscopy. Data presented here are the mean ±SD of four separate experiments. Each experiment was carried out in duplicate. ● denotes LY 294002 and ○ indicates wortmannin.
During the review process for this manuscript, Cox et al. (2006) provided a detailed bioinformatic analysis of two of the gPI3Ks covered in the present report (i.e., gPI3K-1 and gPI3K-2). While many of their analyses are complementary to ours, there is a curious discrepancy in that Cox et al. (2006) observed no effects of wortmannin on the growth of Giardia trophozoites. Until details of their experiments are provided, it is difficult to explain this difference. Further studies of the PIK proteins are required to resolve this issue as well as elucidate their biological functions.
Acknowledgments
This work was supported by the National Institutes of Health Grants S06 GM 008012-34 (SD), and 5G112RR08124-09 (UTEP). Generous support from the Hazel-Harvey and Coldwell Foundations (Texas) is also acknowledged. Ms. E. Willams was supported by EPCC RISE Grant 2R25GM 06042.
Literature Cited
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bateman A, Coin D, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner M, Chaussepied M, Moreau MF, Werling D, Davis WC, Garcia A, Langsley G. Constitutive PI3-K activity is essential for proliferation, but not survival, of Theileria parva-transformed B cells. Cell Microbiol. 2000;4:329–339. doi: 10.1046/j.1462-5822.2000.00062.x. AND SO ON. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Gillin FD. Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun. 1990;58:3516–3522. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosotti R, Isacchi A, Sonnhammer EL. FAT: a novel domain in PIK related kinases. Trends Biochem Sci. 2000;25:225–227. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta. 1996;1:M11–16. doi: 10.1016/0304-419x(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Cox SSE, van der Giezen M, Tarr SJ, Crompton MR, Tovar J. Evidence from bioinformatics, expression and inhibition studies of phosphoinositide-3-kinase signaling in Giardia intestinalis. BMC Microbiology. 2006;18:6–45. doi: 10.1186/1471-2180-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Gillin FD. Giardia lamblia: Increased UDP-N-acetyl-D-glucosamine and N-acetyl-D-galactosamine activities during encystation. Exp Parasitol. 1996;84:18–31. doi: 10.1006/expr.1996.0045. [DOI] [PubMed] [Google Scholar]
- Das S, Schteingart CD, Hofmann AF, Reiner DS, Aley SB, Gillin FD. Giardia lamblia: evidence for carrier-mediated uptake and release of conjugated bile acids. Exp Parasitol. 1997;87:133–141. doi: 10.1006/expr.1997.4197. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic culitivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;27:487–488. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002;27:426–32. doi: 10.1016/s0968-0004(02)02136-9. [DOI] [PubMed] [Google Scholar]
- Farthing MJG, Keusch GT, Carey MC. Effects of bile and bile salts on growth and membrane lipid uptake by Giardia lamblia. J Clin Invest. 1985;76:1727–32. doi: 10.1172/JCI112162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan CA, Schnieders EA, Emerick AW, Kunisawa R, Admon A, Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993;262:1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Forney JR, DeWald DB, Yang S, Speer CA, Healey MC. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect Immun. 1999;67:844–852. doi: 10.1128/iai.67.2.844-852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Samuelson J. Inhibition of phagocytosis, cytokinesis, and capping by overexpression of mutated p21racA in cultured Entamoeba histolytica. Infect Immun. 1997;65:4243–4249. doi: 10.1128/iai.65.10.4243-4249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin FD, Reiner DS, Boucher SE. Small intestinal factors promote encystation of Giardia lamblia in vitro. Infect Immun. 1988;56:705–707. doi: 10.1128/iai.56.3.705-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Makioka A, Kumagai M, Ohtomo H, Kobayashi S, Takeuchi T. Inhibition of encystation of Entamoeba invadens by wortmannin. Parasitol Res. 2001;87:371–375. doi: 10.1007/s004360000339. [DOI] [PubMed] [Google Scholar]
- Marshall JG, Booth JW, Stambolic V, Mak T, Balla T, Schreiber AD, Meyer T, Grinstein S. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J Cell Biol. 2001;153:1369–1380. doi: 10.1083/jcb.153.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AG, Morrison HG, Nixon JE, Passamaneck NQ, Kim U, Hinkle G, Crocker MK, Holder ME, Farr R, Reich CI, Olsen GE, Aley SB, Adam RD, Gillin FD, Sogin ML. The Giardia genome database. FEMS Microbiol Lett. 2000;189:271–3. doi: 10.1111/j.1574-6968.2000.tb09242.x. [DOI] [PubMed] [Google Scholar]
- Morrison HG, Zamora G, Campbell RK, Sogin ML. Inferring protein function genomic sequence: Giardia lamblia expresses a phosphatidylinositol kinase-related kinase similar to yeast and mammalian TOR. Com Biochem Physiol B Biochem Mol Biol. 2002;4:477–491. doi: 10.1016/s1096-4959(02)00218-x. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Sato TK, Overduin M, Emr SD. Location, location, location: membrane targeting directed by PX domains. J Cell Biol. 2001;294:1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov AG, Einicker-Lamas M, de Castro SL, Oliveira MM, Guilherme A. Activation of host cell phosphatidylinositol 3-kinases by Trypanosoma cruzi infection. J Biol Chem. 2000;275:32182–32186. doi: 10.1074/jbc.M909440199. [DOI] [PubMed] [Google Scholar]
- Tusnády GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Sozzani S, Altruda F, Mantovani A, Hirsch E. Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol Today. 2000;21:260–264. doi: 10.1016/s0167-5699(00)01649-2. [DOI] [PubMed] [Google Scholar]
- Zhou K, Takegawa K, Emr SD, Firtel RA. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol Cell Biol. 1995;15:5645–56. doi: 10.1128/mcb.15.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]