Abstract
Background
Extra cardiac comorbidities are common in patients with heart failure and a preserved ejection fraction (HFPEF). We sought to evaluate the relationship between comorbidities and ventricular structure and function in patients with HFPEF through evaluation of pressure-volume analysis.
Methods and Results
Two hundred twenty Chinese patients with a preserved ejection fraction who were either healthy (n=75), hypertensive without heart failure (HTN, n=89), or hypertensive with HFPEF (HFPEF, n=56) were studied. Using echocardiographic measures, estimated endsystolic and end-diastolic pressure-volume relationships and the area between them as a function of EDP, the isovolumic pressure-volume area (PVAiso), were calculated. Ventricular capacitance, as measured by V30, was larger in patients with HFPEF compared to normal controls and tended to be larger compared to hypertensive controls. The presence of diabetes and renal insufficiency was independently associated with greater ventricular capacitance in patients with HFPEF. The PVAiso was increased in patients with HFPEF compared to HTN and normal controls, and in particular, was increased in HFPEF patients with multiple comorbidities.
Conclusion
The presence of co-morbid conditions is associated with altered PV relations and enhanced pump function in subjects with HFPEF supporting an important role for extra-cardiac comorbidities in the pathophysiology of patients with this condition.
Keywords: Heart Failure, Ejection Fraction, Co-Morbidities
INTRODUCTION
At least half of all patients with heart failure have preserved ejection fraction (HFPEF).1 These patients are often older adults with hypertension and several extra cardiac comorbidities including diabetes, obesity, anemia and chronic kidney disease (CKD) among others.1–4 These co-morbid conditions have been associated with adverse prognostic outcomes in patients with HFPEF.1, 4, 5 Concomitant medical conditions can impair exercise capacity and mimic symptoms of heart failure. Additionally, the presence of such comorbidities in patients with a phenotype compatible with HFPEF could confound the results of clinical trials which may be affected by competing risks from the co-morbid conditions.6 Given the high prevalence of important comorbidities, and because these comorbidities strongly influence outcomes, it has been suggested that identification and aggressive treatment of these conditions should be instituted currently rather than waiting for new HFPEF-specific treatments to emerge.7
While comorbidities could adversely affect the systolic and diastolic properties of the heart, their impact on ventricular structure and function in patients with hypertensive HFPEF has not been adequately addressed. The role of extra-cardiac comorbidities may be particularly important as multiple mechanisms, both cardiac and non-cardiac, have been proposed to explain the pathophysiology of this syndrome.8, 9 Accordingly, we sought to characterize the impact of co-morbid condition on ventricular structure and function through the use of non-invasive pressure volume indices. Specifically, we hypothesized that the presence of co-morbidities would be associated with alterations in ventricular structure and function, and therefore in the HFPEF phenotype, as determined through evaluation of parameters that characterize systolic and diastolic ventricular properties.
METHODS
Study Subjects
Two hundred twenty study subjects who were treated as inpatients or outpatients at the People's Liberation Army General Hospital (Beijing, China) from September 2005 to February 2008 were studied. These subjects included 56 patients with hypertensive HFPEF (EF>50%) and two control groups of 75 healthy controls and 89 patients with hypertension but without heart failure (HTN).
Normal control subjects were identified after a detailed health investigation including history, physical examination, blood tests, chest x-ray, electrocardiogram, and echocardiogram did not demonstrate any abnormality. Specific exclusion criteria for the normal control group included hypertension, coronary heart disease, diabetes, renal insufficiency, cardiomyopathy, congenital heart disease, arrhythmias and chronic obstructive pulmonary diseases. Subjects with hypertension (defined as a systolic blood pressure (SBP) > 140 or diastolic blood pressure (DBP) > 90 mm Hg or a clinical history of hypertension) but without concomitant heart failure constituted the HTN cohort. The presence of heart failure was based on the criteria developed by Rich et al10 and verified by two independent cardiologists (DB and MSM). The protocol was reviewed and approved by the institutional review board of the Chinese People's Liberation Army General Hospital and all study subjects provided written informed consent.
Definition of Co-Morbid Conditions
Extra-cardiac comorbidities within the HTN and HFPEF populations were identified, including obesity, anemia, chronic renal insufficiency and diabetes. Anemia was defined according to the WHO criteria11 as a hemoglobin (Hg) <13 mg/dl in men and <12 mg/dl in women. Diabetes was defined based on a clinical history of diabetes, use of oral hypoglycemic or insulin or a fasting blood glucose of ≥126 mg/dl or random blood glucose of >200 mg/dl.12 Renal insufficiency was defined as glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease (MDRD) formula of less than 60 ml/min/1.73m2. Overweight patients were defined as having a body mass index (BMI) of >25kg/m2.13 Coronary heart disease (CHD) was defined by clinical history of myocardial infarction (as evidenced by Q waves on an electocardigram, segmental wall motion abnormality on echocardiography), previous percutaneous intervention or coronary artery bypass grafting or a coronary stenosis >70% on cardiac catheterization.
Diagnostic Evaluation
All patients underwent standardized clinical examination and research echocardiography, which were performed without interruption of a subject's medical therapies. Blood pressure was measured by standard cuff sphygmomanometer in the supine position after a subject rested comfortably for 5 minutes immediately before the performance of echocardiography. Echocardiography was performed by a professional technician with the use of a Sequoia 512 ultrasound instrument with a 3.5- to 4.5-MHz sector scanner (Siemens, Munich, Germany). Two-dimensional guided M-mode measurements of chamber dimensions and wall thickness were obtained according to recommendations of the American Society of Echocardiography14 and left ventricular mass (LVM) was derived from a formula described by Devereux and Reichek15 and indexed to body surface area (BSA). The presence of left ventricular hypertrophy (LVH) was defined based on the recommendations of the American Society of Echocardiography as posterior wall thickness greater than 1.0 cm for women and 1.1 cm for men.16 Left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes and left ventricular ejection fraction (EF) were calculated with the Simpson biplane method. Transmitral and tissue Doppler were used to analyze diastolic filling indexes and myocardial velocities, as previously described.17 These included the measure of transmiral early diastolic filling velocity (E) and average of the tissue Doppler measures of early diastolic tissue velocity at the septal, lateral and inferior walls (e'). Left ventricular end-diastolic pressure was estimated by the formula end-diastolic pressure = 11.96 + 0.596 × E/e'.17 Blood samples for natriuretic peptide assay were obtained within 12 hours (after or before) of echocardiographic examination. B-type natriuretic peptide (BNP) was measured by sandwich immunoassay using commercially available kits (Abbott Laboratories, Abbott Park, Illinois). The staff performing the measurements was blinded to the clinical data.
Non-Invasive Pressure-Volume Indices
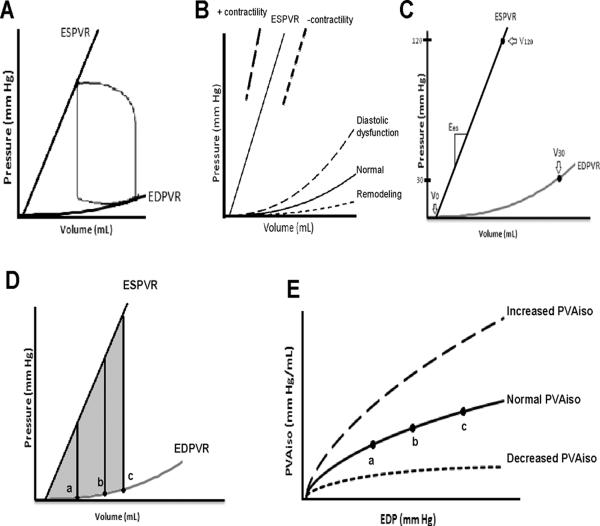
Parameters of end-systolic (ESPVR) and end-diastolic pressure–volume relations (EDPVR) were estimated using validated single-beat techniques (see Figure 1). The ESPVR, a reflection of chamber stiffness at the point of maximal myofilament activation18 indicative of chamber contractility was quantified by measuring both the slope (Ees) and volume axis intercept(V0) according to previously published methods.19 To account for changes in both the slope (Ees) and volume axis intercept (V0) of the ESPVR which collectively represent chamber contractility, we calculated the volume of the left ventricle required to generate an end systolic pressure of 120 mm Hg (V120, = 120/slope of ESPVR + volume axis intercept of the ESPVR). The EDPVR, a reflection of the passive mechanical properties of the myocardium during complete myofilament inactivation18, is non-linear and is represented by the formula EDP= αEDVβ, where α and β are constants which specify the curvature of the line and are determined by the mechanical properties of the muscle as well as the structural features of the ventricle.20, 21 While usually measured invasively, a method developed by Klotz et al,20, 21 allows non-invasive estimates of the EDPVR from the measured EDV and an estimate of left ventricular filling pressures derived from Doppler echocardiography. Since both constants α and β affects the shape and position of the EDPVR, we integrated these measures by calculating the EDV at a common end-diastolic pressure of 30 mm Hg (V30 = [30/α]1/β) which yields an index of ventricular capacitance.17 Accordingly, the larger the V30 (increased capacitance) indicates a rightward/downward shift of the EDPVR (e.g. remodeling) and the smaller the V30 (decreased capacitance) indicates a leftward/upward shift of the EDVPR (e.g. diastolic dysfunction) (figure 1, panel B).
Figure 1.
(Upper Figures) Pressure-volume analyses demonstrating the normal PV loop and the determinants of ventricular function, including the ESPVR and the EDPVR (Panel A). Shifts in the ESPVR are often equated with changes in inotropic state (Panel B), while shifts in the EDPVR toward smaller volumes or reduced capacitance (diastolic dysfunction) or toward larger volumes or increased capacitance (remodeling). The ESPVR is characterized by the slope [Ees] and the volume axis intercept [V0], which can be described collectively by the V120, the volume of the left ventricle required to generate an end systolic pressure of 120 mm Hg (Panel C). Shifts in the EDPVR can be characterized by V30, the ventricular volume at a pressure of 30 mm Hg, (Panel C). See Methods section for additional details.
(Lower Figures) Demonstration of how PVAiso is calculated from the PV diagram (Panels D and E). One value for PVAiso (shaded area) can be obtained for each end-diastolic PV point shown by the black circles along the EDPVR. The points of a, b, and c in Panel D correspond to the solid line PVAiso curve in Panel E. With shifts of the ESPVR and EDPVR (not shown), the PVAiso curve can show increased or decreased cardiac function (dashed PVAiso curves), Panel E.
Since overall ventricular pump function is determined by both systolic and diastolic properties of the ventricle, the area between the EDPVR and the ESPVR measured as a function of EDP was calculated to index overall pump function.22 (See Figure 1, panel D and E) This specific area, called the isovolumic pressure-volume area (PVAiso), is independent of afterload and can be calculated analytically as a function of left ventricular end diastolic pressure following curve fitting of the EDPVR and the ESPVR: PVAiso(V)=∫[Pes(V)-Ped(V)]dV = 0.5Ees(sb)(VVo)2–Vm(β/α) eα*(V/Vm), where Pes(V) and Ped(V) are the end-systolic and end-diastolic pressures, respectively, as a function of volume. Upward shifts of the PVAiso -EDP relation indicate enhanced overall pump function at that time point; specifically, this means that, at any given filling pressure, the heart is capable of generating more pressure and more work. On the contrary downward shifts of the PVAiso -EDP relation indicated reduced overall pump function at that time point, indicating that at any given filling pressure, the heart generated less pressure and less work.
Effective arterial elastance (Ea), an index of vascular hemodynamic load, was estimated by Pes/stroke volume, where Pes is left ventricular end-systolic pressure estimated by 0.9 × systolic blood pressure.17
Statistical Analysis
Data are expressed as mean ± one standard deviation (SD). Ventricular volumes were indexed to body surface area (BSA). Data were compared between normal control patients, hypertensive control patients without HF, and patients with HFPEF by analysis of variance with Bonferroni post-hoc correction for continuous variables or chi-square for dichotomous variables. Patients with HFPEF were further characterized based on the presence of comorbidities and analysis of variance was used to evaluate for trends associated with increasing comorbidity burden. Where values were not normally distributed, the non-parametric Kruskal-Wallis test was used. To evaluate the impact of comorbidities on the observed differences in pressure volume relations between cohorts studied, we performed multivariate linear regression analysis with group (HFPEF, HTN and controls) and comorbidities (presence or absence) including diabetes, elevated BMI, renal insufficiency, anemia, coronary heart disease, and left ventricular hypertrophy entered into a full model. While coronary heart disease and left ventricular hypertrophy are not extra-cardiac comorbidities, they were included in the model because of the potential effects on ventricular remodeling. All statistical analyses were performed with SAS 9.1 (SAS Institute, Cary, North Carolina). A p value <0.05 was considered statistically significant.
RESULTS
The baseline characteristics of the study population are shown in Table I. Subjects with HFPEF were older and more often women than HTN controls, and had higher blood pressures than normal control patients. Blood pressure did not differ between HFPEF and HTN controls. HFPEF patients had significantly elevated B-type natriuretic peptide (BNP) and E/e' ratio compared to both hypertensive and normal controls, consistent with the diagnosis of heart failure. Hypertensive controls and patients with HFPEF had higher prevalence of elevated BMI, anemia, diabetes, renal insufficiency, and coronary heart disease than healthy controls (Table 1). Patients with HFPEF compared with HTN controls in an age-adjusted model had higher prevalence of renal insufficiency (21% vs. 9%, p=0.03) and tended to have higher prevalence of anemia (27% vs. 10%, p=0.07), but did not differ in the prevalence of diabetes (30% vs. 21%, p=0.24)
Table I.
Demographic, Echocardiogaphic, and Pressure-Volume measures among Preserved controls, Hypertensive controls, and subjects with HFPEF
| Preserved | HTN without HF | HFPEF | |
|---|---|---|---|
| No. of Subjects | 75 | 89 | 56 |
| Demographics | |||
| Age (years) | 67±7 | 65±12 | 71±9^ |
| Gender (% Women) | 45 | 18* | 30 |
| SBP (mmHg) | 122±10 | 141±22* | 144±26* |
| DBP (mmHg) | 73±8 | 81±13* | 77±16 |
| HR (bpm) | 68±9 | 68±10 | 69±10 |
| Comorbidities | |||
| Hg (mg/dl) | 15.0±1.3 | 14.5±1.7 | 13.8±2.3^ |
| Diabetes (%) | 0 | 21* | 30* |
| eGFR (ml/min) | 96±21 | 94±28 | 79±26*^ |
| CHD (%) | 0 | 40* | 64* |
| BMI (kg/m2) | 24±4 | 26±3* | 26±4* |
| BSA(m2) | 1.71±0.17 | 1.82±0.15* | 1.82±0.18* |
| Biomarkers | |||
| BNP (pg/ml) | 89±98 | 80±59 | 561±701*^ |
| Echo Parameters | |||
| LVEDV/BSA (ml/m2) | 48±10 | 49±14 | 55±17*^ |
| LVESV/BSA(ml/m2) | 17±4 | 18=1=6 | 21±9*^ |
| SV (ml) | 52±12 | 56±17 | 61±19* |
| EF (%) | 65±3 | 64±4 | 63±6* |
| E/E' | 7.1±1.6 | 7.8±2.4 | 10.0±2.8*^ |
| LVM/BSA (gm/m2) | 86±18 | 113±25* | 125±43*^ |
| LVH (%) | 12 | 44* | 63* |
| P-V Indices | |||
| Ea (mm Hg/ml) | 2.2±0.5 | 2.4±0.8 | 2.3±0.8 |
| Ees (mmHg/ml) | 2.1±0.5 | 2.1±0.7 | 1.9±0.8 |
| Ea/Ees | 1.1±0.2 | 1.3±0.3* | 1.3±0.3* |
| V0 (ml) | −27±9 | −38±22* | -42±26* |
| V30 (ml) | 90±21 | 98±29 | 109±37 |
| V30/BSA(ml/m2) | 53±11 | 54±15 | 60±19* |
| V120 (ml) | 34±12 | 30±14 | 32±19 |
P<0.05 vs control,
P<0.05 between HTN with and without HF by Bonferroni post hoc test.
Gender and percent with diabetes are analyzed via Chi-Square. SBP-systolic blood pressure, DBP-diastolic blood pressure, BMI-body mass index, Hg-hemoglobin, eGFR-estimated glomerular filtration rate, BMI-body mass index, BNP- B-type natriuretic peptide, EDVI-end diastolic volume index, ESVI-end systolic volume index, SV-stroke volume, EF-ejection fraction, LVM-left ventricular mass, LVH-left ventricular hypertrophy, BSA-body surface area, LVEDP-left ventricular end-diastolic pressure, PV-pressure-volume, Ea-arterial elastance, Ees –End systolic elastance, V0-estimated ventricular volume at an end-systolic pressure of 0, V30- estimated ventricular volume at an end-systolic pressure of 30, V120- estimated ventricular volume at an end-systolic pressure of 120
While end-systolic elastance, arterial elastance, and ventricular vascular coupling (Ea/Ees ratio) did not differ between patients with HFPEF and HTN controls, both of these cohorts had a higher Ea/Ees ratio than controls. Among patients with HFPEF, female sex, but not age, was associated with higher Ea (2.6±0.7 vs. 2.1±0.7 mmHg/ml, p=0.02) and a trend toward higher Ees (2.3±0.8 vs. 1.8±0.8 mmHg/ml, p=0.057). Among patients with HFPEF, but not among the whole cohort or the whole hypertensive (HTN and HFPEF) cohort, the presence of diabetes was associated with lower Ea (1.9±0.5 vs. 2.5±0.8 mmHg/ml, p=0.003) and a trend toward a lower Ees (1.6±0.8 vs. 2.1±0.8 mmHg/ml, p=0.08). Among patients with HFPEF, differences in Ea remained significant, and differences in Ees became significant, when adjusted for the presence of age and sex, and for the presence of age, sex, and other comorbidities. No other comorbidities in patients with HFPEF were associated with significant changes in Ea, Ees, or the Ea/Ees ratio.
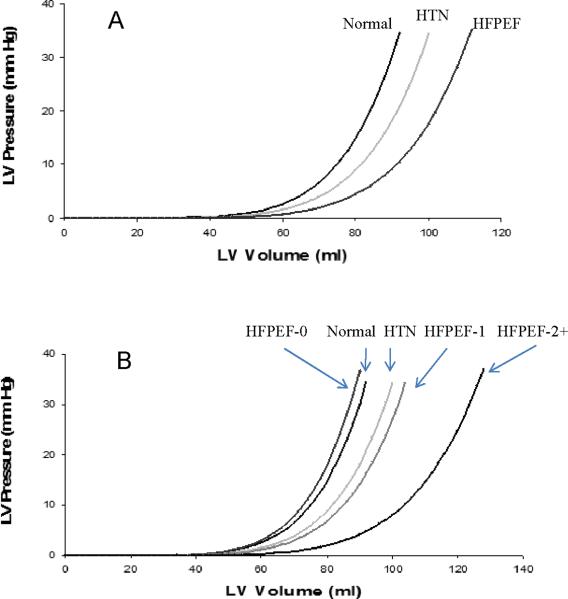
Patients with HFPEF had larger ventricular capacitance compared to normal controls and trended to have larger capacitance compared to HTN control patients (p=0.054) in unadjusted analyses (Figure 2, panel A). Capacitance was larger in HFPEF patients compared to HTN controls when adjusted for age, sex, and BMI (p=0.003) and when adjusted for age, sex, and the presence of anemia, diabetes, elevated BMI and renal insufficiency (p=0.038). The rightward shifted EDPVR in the HFPEF cohort was most prominent in HFPEF patients with 2 or more comorbidities (Figure 2, panel B). Table II describes ventricular parameters in HFPEF patients stratified by the presence of none, 1, and 2 or more comorbidities. Patients with no comorbidities had lower systolic (SBP) and diastolic blood pressures (DBP) compared with patients with 1 or more comorbidities (p=0.036 and 0.009 for SBP and DBP, respectively by t-test).
Figure 2.
Estimated graphical representation of the end-diastolic pressure-volume relationships among the studied populations. Hypertensive without heart failure (HTN), heart failure with preserved ejection fraction and 0,1, and 2+ comorbidities, HFPEF-0, HFPEF-1, HFPEF-2, respectively
Table II.
The cohort of patients with HFPEF grouped by the number of extra cardiac comorbidities
| HFPEF | |||||
|---|---|---|---|---|---|
| Total | 0 Comorbidities | 1 Comorbidity | 2+ Comorbidities | P Value | |
| No. of Subjects | 56 | 9 | 22 | 25 | |
| Demographics | |||||
| Age (years) | 71±9 | 74±5 | 71±8 | 70±11 | 0.47 |
| SBP (mmHg) | 144±26 | 130±18 | 144±23 | 149±30 | 0.20 |
| DBP (mmHg)# | 77±16 | 68±8 | 79±16 | 78±18 | 0.20 |
| Biomarkers | |||||
| BNP (pg/ml)# | 561±701 | 246±111 | 721±924 | 570±577 | 0.84 |
| Echo Parameters | |||||
| LVEDV (ml) | 100±34 | 80±23 | 93±30 | 114±36 | 0.013 |
| LVESV (ml) | 38±17 | 29±12 | 34±14 | 45±18 | 0.016 |
| LVEDV/BSA(ml/m2) | 55±17 | 47±12 | 53±18 | 60±17 | 0.14 |
| LVESV/BSA(ml/m2) | 21±9 | 18±7 | 19±8 | 24±9 | 0.11 |
| SV (ml) | 61±19 | 50±14 | 58±17 | 69±20 | 0.022 |
| EF (%) | 63±6 | 63±6 | 64±5 | 61±5 | 0.31 |
| LVM/BSA(ml/m2) | 12543 | 103±19 | 117±31 | 140±52 | 0.036 |
| Estimated PV Indices | |||||
| Ea (mm Hg/ml) | 2.3±0.8 | 2.6±0.9 | 2.4±0.8 | 2.1±0.6 | 0.17 |
| Ees (mmHg/ml) # | 1.9±0.8 | 2.1±07 | 2.0±0.9 | 1.8±0.8 | 0.13 |
| Ea/Ees # | 1.3±0.3 | 1.1±0.2 | 1.3±0.3 | 1.3±0.4 | 0.15 |
| V0(ml) # | −42±26 | −30±18 | −45±31 | −43±25 | 0.34 |
| V30 (ml) | 109±37 | 87±26 | 102±33 | 124±38 | 0.0145 |
| V30/BSA(ml/m2) | 60±19 | 52±14 | 57±20 | 65±18 | 0.15 |
| V120 (ml) | 32±19 | 28±20 | 28±19 | 36±18 | 0.30 |
P values via ANOVA unless variable non-parametric (#) in which case Kruskal-Wallis test used. SBP-systolic blood pressure, DBP-diastolic blood pressure, BNP- B-type natriuretic peptide, LVEDV/BSA-left ventricular end diastolic volume index, LVESV/BSA-end systolic volume index, SV-stroke volume, EF-ejection fraction, LVM-left ventricular mass, LVEDP-left ventricular end-diastolic pressure, PV-pressure-volume, Ea-arterial elastance, Ees –End systolic elastance, V0-estimated ventricular volume at an end-systolic pressure of 0, V30- estimated ventricular volume at an end-systolic pressure of 30, V120- estimated ventricular volume at an end-systolic pressure of 120.
Among HFPEF patients, both diabetes and renal insufficiency were associated with larger ventricular capacitance independent of age, gender and other comorbidities (Table III). The strength of the association of both comorbidities (diabetes and renal insufficiency) in HFPEF patients is similar based on the regression coefficient (Table III).
Table III.
Effects of specific comorbidities on Ventricular Capacitance V30 in multivariate analysis
| NL and HTN and HFPEF | HTN and HFPEF | HFPEF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | p value | Coefficient | SE | p value | Coefficient | SE | p value | |
| Age | −0.60 | 0.21 | 0.004 | −0.56 | 0.27 | 0.035 | - | - | 0.44 |
| Sex (female) | −11.6 | 4.3 | 0.007 | −14.9 | 5.7 | 0.017 | −21.0 | 8.8 | 0.021 |
| Comorbidities | |||||||||
| Anemia | - | - | 0.33 | - | - | 0.36 | - | - | 0.40 |
| Overweight | - | - | 0.57 | - | - | 0.33 | - | - | 0.78 |
| Diabetes | - | - | 0.22 | - | - | 0.13 | 18.4 | 9.1 | 0.049 |
| CKD | 13.0 | 6.9 | 0.059 | 14.4 | 3.5 | 0.063 | 24.7 | 10.2 | 0.019 |
| CHD | 8.6 | 4.5 | 0.057 | 11.9 | 5.4 | 0.03 | - | - | 0.29 |
| LVH | 7.6 | 4.3 | 0.08 | 9.1 | 5.4 | 0.09 | 28.8 | 8.8 | 0.002 |
NL-normal controls, HTN-hypertensive controls without heart failure, HFPEF-heart failure and a preserved ejection fraction, CKD-chronic kidney disease, CHD-coronary heart disease, LVH-left ventricular hypertrophy
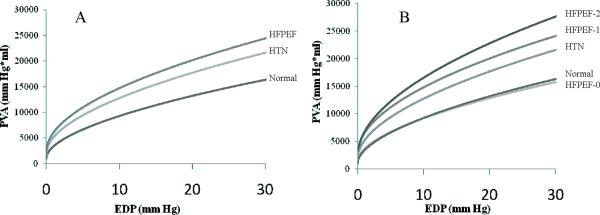
The rightward shift in the EDPVR resulted in an increase in overall pump function, as measured by the PVAiso-EDP relationship among HTN and HFPEF subjects compared to normal controls (Figure 3, Panel A). Additionally, as shown in Figure 3, Panel B, subjects with HFPEF without comoribidites had overall pump function that was similar when compared to healthy controls and below HTN controls, while those with 2 or more comorbidities demonstrated enhanced pump function.
Figure 3.
PVAiso relationships, demonstrating cardiac function, in patients with HTN, in normal controls, and in HFPEF with varied comorbidity burden (0,1, or 2+ comorbidities)
DISCUSSION
The results of this investigation demonstrate that differences in ventricular structure and function as assessed by non-invasive pressure-volume analyses between subjects with HFPEF, HTN, and healthy controls are influenced by the presence of extra-cardiac comorbidities. Among patients with HFPEF, greater comorbidity burden, particularly of diabetes and CKD, is associated with greater ventricular capacitance, and diabetes is associated with lowered Ea and Ees. These changes in ESPVR and EDPVR in HFPEF patients resulted in enhanced pump function that was associated with the greater burden of comorbidities. These data provide insight into the pathophysiology of HFPEF in patients with and without multiple comorbidities by highlighting the association of comorbidities with cardiac structure and function in patients with HFPEF in comparison to normal controls and HTN subjects without HF.
Prevalence of Co-morbidities and Impact on Prognosis
Comorbidities are known to play an important role in patients with HFPEF and are associated with significant morbidity and mortality. Among extra-cardiac comorbidities, patients with HFPEF have high rates of obesity, anemia, diabetes, and renal insufficiency,2 with the latter three serving as independent risk factors for mortality in multivariate analyses.4 Other studies further highlight the impact of comorbidities on outcomes in this population.5, 6, 23 Since data from large randomized clinical trials24–26 has not demonstrated significant benefits of cardiovascular therapies on outcomes in subjects with HFPEF, some have suggested that the focus shift to treating comorbidities that are highly prevalent in this condition.7, 27 In this regard, a further understanding of the impact of the most common comorbid conditions on the physiology of HFPEF is warranted.
Role of Comorbidities on Physiology of HFPEF
In addition to directly contributing to morbidity in HFPEF, current data suggest that comorbidities, particularly diabetes and renal insufficiency, are associated with changes in ventricular structure and function. Though the results of the current investigation do not establish a causal association between comorbidities and ventricular structure or function, it is possible to postulate that extra-cardiac comorbidities may affect ventricular structure and function through various mechanisms. For example, diabetes leads to glycosylated end-product formation, impaired endothelial function, sympathetic nervous system activation and derangements in myocardial metabolism, leading to so-called “diabetic cardiomyopathy.”28, 29 Likewise, in obese individuals, accumulation of nonesterified fatty acids and metabolic dysregulation may result in the accumulation of toxic lipid byproducts and subsequent contractile dysfunction.30 The systemic inflammation associated with obesity, diabetes, and other comorbidities may further contribute to cardiac dysfunction.31 Cardiorenal syndrome also plays a prominent role in cardiac structure and function, whether originating as HF leading to chronic kidney disease or chronic kidney disease leading to worsening of HF symptoms.32 Numerous pathophysiologic mechanisms are thought to underlie the cardiorenal syndrome, including ventricular hypertrophy, neurohormonal activation, cardiac remodeling, systemic inflammation, and venous congestion.32, 33 The addition of anemia to this syndrome, termed cardio-renal-anemia syndrome, can further affect cardiac structure and function through inflammation, neurohormonal activation, and cardiac remodeling.34 In addition to the direct effects of diabetes, anemia, and kidney disease on cardiac structure and function, these extra-cardiac comorbidities may alter such parameters through extra cardiac effects including expansion of plasma volume. Volume overload is an important mechanism in the pathophysiology of patients with HFPEF,35 and anemia,36, 37 diabetes,38 obesity39 and renal insufficiency have all been associated with volume derangements. As not all patients with HFPEF have significant extra-cardiac comorbidities, stratification based on the presence of comorbidities may be important in explaining disease occurrence and progression. Those without extra-cardiac comorbidities, who in our population had smaller ventricular capacitance and lower blood pressure than those with co-morbid conditions, may have a pathophysiology similar to patients with diastolic heart failure as described by others,40 and may exhibit fundamentally different disease than those with multiple comorbidities, though both are currently labeled as having HFPEF.
Stratifying or subgrouping individuals with HFPEF by comorbidities may be important to consider in future studies of therapeutic interventions for patients with HFPEF. Additionally, specifically targeting comorbidities in this population may not only reduce the burden of the comorbidities themselves but may alter ventricular structure and function and thus affect the pathophysiology of HFPEF. Ventricular capacitance, as a marker of HFPEF pathophysiology through volume overload, may therefore be a potential target for treatment through management of extra-cardiac comorbidities. Additionally, trials to evaluate the benefits of more aggressively targeting volume status as a principal goal of therapy in this population should be explored.
Additional insight into cardiac function in patients with HFPEF can be gained through the evaluation of the heart not as a systolic or diastolic organ but by integrating systolic and diastolic ventricular properties through the calculation of the isovolumetric pressure-volume to EDP relationship, an overall measure of pump function.22 The PVAiso-EDP relation describes the total mechanical energy that can be generated at a given ventricular preload22 and therefore expresses cardiac function in a manner similar to the Frank-Starling curve which typically demonstrates a measure of cardiac output at a given ventricular preload. The current cohort of patients with HFPEF exhibit increased cardiac function compared to HTN and normal controls. Furthermore, HFPEF patients with no comorbidities exhibit pump function similar to normal controls and reduced compared to hypertensive subjects without heart failure, while HFPEF patients with multiple comorbidities exhibited enhanced pump function. These findings are in accordance with animal data from salt fed hypertensive Dahl rats, who exhibited increased overall pump function as determined by PVAiso during the development of the heart failure state with a preserved ejection fraction.41
HFPEF has been associated with abnormalities in arterial-ventricular (AV) coupling, particularly with elevations in both Ea and Ees compared with normal or hypertensive controls.42 While AV mismatching explains some features of HFPEF, including altered blood pressure regulation and limited cardiovascular reserve,42 abnormal AV coupling has not been found in all patients with HFPEF.9 In the cohort described here, there were no significant alterations in AV coupling at rest between hypertensive controls without HF and patients with HFPEF, indicating other pathophysiologic mechanisms likely play a more prominent role in this population, or that it is necessary to extend the evaluation to characterization of these properties during exertion. Among the current cohort of patients with HFPEF, diabetes was an independent predictor of decreased Ea and Ees. While impaired fasting glucose and diabetes have been associated with increased arterial stiffness through a variety of experimental techniques,43–45 animal models of diabetic heart disease using measures of stiffness used in the current study suggest that diabetic cardiomyopathy may be associated with decreases in both Ea and Ees46, 47 which mimics the current findings. Decreases in Ea in these animal models may be associated with vascular smooth muscle dysfunction or alterations in heart rate,46 while decreases in Ees, a measure of contractility, may be associated with glycosylated end product deposition or microvascular disease.
LIMITATIONS
There are several limitations of the present study. The study focused on a homogeneous Chinese population with lower rates of obesity than those found in the United States and other countries with subjects of European descent. The average BMI in the Chinese population has been reported to be 18.5 to 23.9, with a BMI above 24 therefore considered overweight.48 The roles of ethnicity may additionally be an important factor in pathophysiology of patients with HFPEF, which may affect the applicability of these findings to other populations. The analysis of ventricular-vascular coupling and pressure-volume relationships presented here is derived from non-invasive estimates using a single loading condition at rest. While the gold standard for such analysis is invasive determination of pressure-volume loops and end-systolic and end-diastolic pressure-volume relations, invasive measures are impractical for large studies and the non-invasive estimates applied here have been well validated for studying populations of subject.20 Arterial stiffness was not measured directly. Data about the duration of co-morbidities, heart failure symptoms, and medication usage in this population was not available and the absence of such data has the potential to confound study results. All comorbidities were treated as binary variables in regression analysis even when continuous data was available because effects on ventricular structure and function may only become evident when weight, fasting glucose, hemoglobin, and renal function sufficiently deteriorate. Indeed, it is quite likely that a certain threshold of abnormality in a given co-morbidity is required to produce a change in ventricular pressure-volume relations. Future studies with longitudinal data will be required to address this. The study populations differed in age and gender as can be expected based on demographics of patients with and without heart failure, and the regression models used attempted to correct for such differences.
CONCLUSION
Extra cardiac comorbidities are prevalent in patients with HFPEF and the comorbidities of diabetes and renal insufficiency are associated with greater ventricular capacitance in the HFPEF population. Among subjects with HFPEF and multiple comorbidities, overall pump function is enhanced compared to healthy controls and hypertensive controls without heart failure, but among HFPEF subjects without co-morbidities, overall pump function is reduced compared to hypertensive controls. These data suggest that patients with HFPEF and extra-cardiac comorbidities may have different underlying pathophysiologies of their heart failure phenotype than patients with HFPEF without comorbidities and suggests that stratifying HFPEF patients based on comorbidities might provide insights into the pathophysiology and suggest pathways toward effective interventions for this growing population of older adults.
Acknowledgements
none
Funding: This work is supported by Grants 2007AA02Z4B7 and 2006DFB32210 from the Ministry of Science and Technology of China, Beijing, China and by Grant 7052063 from the Beijing Natural Science Foundation, Beijing, China. Dr. Maurer was supported by Grant R01AG027518-01A1 and K24AG036778-01AA from the National Institutes of Health/National Institute on Aging, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none declared
Reference List
- (1).Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- (2).Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- (3).Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, et al. Hospitalization for heart failure in the presence of a preserved left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- (4).Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- (5).Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–47. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- (6).Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in Heart Failure: a Community Perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–3. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- (8).Maeder MT, Kaye DM. Heart failure with preserved left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–18. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- (9).Maurer MS, King DL, El-Khoury RL, Packer M, Burkhoff D. Left heart failure with a preserved ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–87. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- (10).Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- (11).Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–61. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- (12).Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2000;23(Suppl 1):S4–19. [PubMed] [Google Scholar]
- (13).Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- (14).Schiller NB, Shah PM, Crawford M, Demaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantification of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- (15).Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- (16).Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- (17).He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, et al. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40% Am J Cardiol. 2009;103:845–51. doi: 10.1016/j.amjcard.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a preserved ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–81. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- (19).Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- (20).Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- (21).Ten Brinke EA, Burkhoff D, Klautz RJ, Tschope C, Schalij MJ, Bax JJ, et al. Single-beat estimation of the left ventricular end-diastolic pressure-volume relationship in heart failure patients. Heart. 2010;96:213–219. doi: 10.1136/hrt.2009.176248. [DOI] [PubMed] [Google Scholar]
- (22).Maurer MS, Kronzon I, Burkhoff D. Ventricular pump function in heart failure with preserved ejection fraction: insights from pressure-volume measurements. Prog Cardiovasc Dis. 2006;49:182–95. doi: 10.1016/j.pcad.2006.08.007. [DOI] [PubMed] [Google Scholar]
- (23).Philbin EF, Rocco TA, Jr., Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–13. doi: 10.1016/s0002-9343(00)00601-x. [DOI] [PubMed] [Google Scholar]
- (24).Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- (25).Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- (26).Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- (27).Maurer MS. Heart failure with a preserved ejection fraction (HFPEF): embracing complexity. J Card Fail. 2009;15:561–4. doi: 10.1016/j.cardfail.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Leung AA, Eurich DT, Lamb DA, Majumdar SR, Johnson JA, Blackburn DF, et al. Risk of heart failure in patients with recent-onset type 2 diabetes: population-based cohort study. J Card Fail. 2009;15:152–7. doi: 10.1016/j.cardfail.2008.10.004. [DOI] [PubMed] [Google Scholar]
- (29).van HL, Somsen A, Paulus WJ. The failing diabetic heart: focus on diastolic left ventricular dysfunction. Curr Diab Rep. 2009;9:79–86. doi: 10.1007/s11892-009-0014-9. [DOI] [PubMed] [Google Scholar]
- (30).Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;52:181–7. doi: 10.1161/HYPERTENSIONAHA.108.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, et al. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–29. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- (33).Damman K, van Deursen VM, Navis G, Voors AA, Van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- (34).Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38:295–310. doi: 10.1007/s11255-006-0064-8. [DOI] [PubMed] [Google Scholar]
- (35).Bench T, Burkhoff D, O'Connell JB, Costanzo MR, Abraham WT, St John SM, et al. Heart failure with preserved ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57–64. doi: 10.1007/s11897-009-0010-z. [DOI] [PubMed] [Google Scholar]
- (36).Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;(Suppl 3):14–8. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- (37).Westenbrink BD, Visser FW, Voors AA, Smilde TD, Lipsic E, Navis G. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur Heart J. 2007;28:166–71. doi: 10.1093/eurheartj/ehl419. [DOI] [PubMed] [Google Scholar]
- (38).Ubels FL, Muntinga JH, Links TP, Hoogenberg K, Dullaart RP, Reitsma WD, et al. Redistribution of blood volume in type I diabetes. Diabetologia. 2001 April;44:429–32. doi: 10.1007/s001250051639. [DOI] [PubMed] [Google Scholar]
- (39).Frohlich ED, Susic D. Mechanisms underlying obesity associated with systemic and renal hemodynamics in essential hypertension. Curr Hypertens Rep. 2008;10:151–5. doi: 10.1007/s11906-008-0028-8. [DOI] [PubMed] [Google Scholar]
- (40).Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- (41).Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–11. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- (42).Fox JM, Maurer MS. Ventriculovascular coupling in systolic and diastolic heart failure. Curr Heart Fail Rep. 2005;2:204–11. doi: 10.1007/BF02696651. [DOI] [PubMed] [Google Scholar]
- (43).Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107:2089–95. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- (44).Rerkpattanapipat P, D'Agostino RB, Jr., Link KM, Shahar E, Lima JA, Bluemke DA, et al. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: implications for left ventricular hypertrophy from the Multi-Ethnic Study of Atherosclerosis. Diabetes. 2009;58:946–53. doi: 10.2337/db08-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–81. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- (46).Peng YI, Chang KC. Acute effects of methoxamine on left ventricular-arterial coupling in streptozotocin-diabetic rats: a pressure-volume analysis. Can J Physiol Pharmacol. 2000;78:415–22. [PubMed] [Google Scholar]
- (47).Van den BA, Flameng W, Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail. 2006;8:777–83. doi: 10.1016/j.ejheart.2006.03.001. [DOI] [PubMed] [Google Scholar]
- (48).Cheng TO. Chinese body mass index is much lower as a risk factor for coronary artery disease. Circulation. 2004;109:e184. doi: 10.1161/01.CIR.0000124888.14804.D2. [DOI] [PubMed] [Google Scholar]