Introduction
Childhood-onset schizophrenia (COS), defined by onset of psychosis before age 13, is a rare and severe form of the disorder 1 that can be diagnosed reliably in children, is neurobiologically and physiologically continuous with adult onset schizophrenia, and usually resembles chronic poor outcome adult patients 1. There is general consensus about a neurodevelopmental model for schizophrenia with COS showing more salient genetic and neuroanatomical underpinnings compared to the adult illness 2.
While auditory hallucinations are reported for the large majority of adult and childhood onset patients, visual hallucinations are seen less commonly. Studies in adult onset disorders from western cultures report visual hallucinations with rates ranging from 72% 3 to 12% 4 with most ratings under 35% 5–8 (see Table S1, available online).
A handful of studies of COS suggest higher rates of visual hallucinations than observed in the adult onset cases 9–12, with some studies reporting rates as high as 69% from chart reviews 13 or 48% using standardized rating scales 14.
During the course of the NIMH COS project, there has been a strikingly high rate of non-auditory hallucinations. In this study we report the rates of hallucinations for our COS sample in order to better estimate hallucination rates in comparison with other published studies. Our study also included a drug-free observation period, and thus provided a unique opportunity to evaluate severity in absence of treatment effect. We explored where interruption of other sensory systems (including olfactory and somatic/tactile) would reflect even greater impairment within our patient group.
Based on the severity of illness for this inpatient study, we expected that COS patients would show higher rates of visual (and other non-auditory) hallucinations over the course of their participation in the study. Similarly, based on clinical impressions, we hypothesized that within our sample, patients with both visual and auditory hallucinations would be more severely ill than those with auditory hallucinations alone.
Method
Subjects
The National Institute of Mental Health (NIMH) COS study has been ongoing since 1990. To date, our branch has received approximately 3,000 case submissions for the COS screening protocol, of which over 300 were invited to the NIH campus for outpatient screening. Following screening, over 200 were admitted for in-patient observation which included for most a 2–3 week drug-free observation period. (Those without a drug free period were either drug-free on admission or on very low doses of drug, and the drug-free period was not considered necessary). Inclusion criteria for the study were: onset of psychosis before the 13th birthday, premorbid IQ of 70 or above, and absence of significant neurological illness. To date, 117 patients aged 6.5–17 years (mean age 13.6 years, SD 2.6 years, 67 male), received a COS diagnosis.
Clinical Ratings
Details of the study’s clinical ratings are provided elsewhere 15. The Scale for the Assessment of Positive Symptoms (SAPS) 16 and the Scale for the Assessment of Negative Symptoms (SANS) 17 provide itemized scales for individual positive and negative symptoms, including hallucination intensity for each modality. Children’s Global Assessment Scale (CGAS) 18 is a rating scale from 0–100 with descriptive anchor points for overall level of functioning at home, school, and with peers. Hallucinations are not mentioned, and the stress is on overall behavior. These clinical ratings were obtained at screening, and during admission, and at follow-up. CGAS Inter-rater reliability agreement on final ratings was satisfactory (kappa = 0.8).
Hallucination Groups
To explore the relationship of severity measures to hallucination ratings, we classified probands within two time-based groups: at screening/admission and at drug-free. The overall presence/absence of hallucinations (during NIH screening, admission or follow-up) was also considered. Hallucinations were scored using the SAPS item 1 (auditory) item 6 (visual), item 4 (somatic/tactile), and item 5 (olfactory). A score of 2 or higher was considered “present” for that particular modality. If the score was a 1 (“questionable”) or 0 (“none”), the proband was placed in the ‘No’ group. The author of this scale has described the possibility for differences in base rates depending on the cutoff point used 19, and our selection of “mild” was based on previous work in COS hallucinations describing rates with this SAPS criteria 20. Other COS studies of hallucination rates have relied on retrospective chart reviews of patient admissions or single clinical interviews. Scoring method and other details for these studies are given in Table S1, available online.
The number of patients in analyses varies somewhat due to missing data. IQs (post-onset) were obtained either pre-admission or during admission to NIH. The Institutional Review Board of the National Institutes of Health approved the protocol for this study. Adult subjects received informed consents and minor children received assents alongside parental permission.
Statistical analysis
We used t-tests to explore group differences in continuous demographic and clinical measures. We assessed violations of homogeneity of variance with a Modified-Levene test, and assessed the normality assumption using the D’Agostino omnibus test. If a violation of either the homogeneity of variance assumption or the normality assumption was noted, we examined the Aspin-Welch t and p values and the Mann-Whitney U and p values, respectively. These results were consistently comparable (relative to rejecting or failing to reject the null) to the t-test for independent groups, and as a result, for the sake of simplicity, we only report the t and corresponding p values. For group associations with categorical measures, we used chi square tests of independence. Statistical analyses were performed using R 21, SPSS version 16.0 and NCSS Statistical Software 2007. We also examined the frequency of combinations of visual, auditory, olfactory and/or somatic/tactile symptoms and present the results with a Venn diagram: checks for the number assignment for placement within the 4 set Venn were performed using Venny 22.
Results
As expected, most COS (95%) patients reported auditory hallucinations, which are often part of the basis for referral. Supporting our clinical impressions, 94 (80.3%) of patients also reported visual hallucinations, 71 (60.7%) reported somatic/tactile hallucinations and 35 (30%) had olfactory hallucinations. These are generally higher rates of hallucinations compared to their adult schizophrenia counterparts across all hallucination subtypes and higher than most previous reports for childhood onset patients (see Supplementary Table 1). We searched the literature for large samples (n = 50 or more for adults) describing schizophrenia hallucination rates; methods varied and only Andreasen’s 1987 adult study 8, met this criteria, and no other large COS studies employed these instruments. We located 20 English language reports, 18 of which drew from populations of Western societies, and all reports of COS that measured hallucinations were included.
Chi square comparison’s with Andreasen’s adult schizophrenia study 8 (which used the same SAPS items) showed our sample to have higher auditory [p < 0.01], visual [p < 0.01], somatic/tactile [p < 0.01], and olfactory [p < 0.01] hallucination rates in our COS cohort.
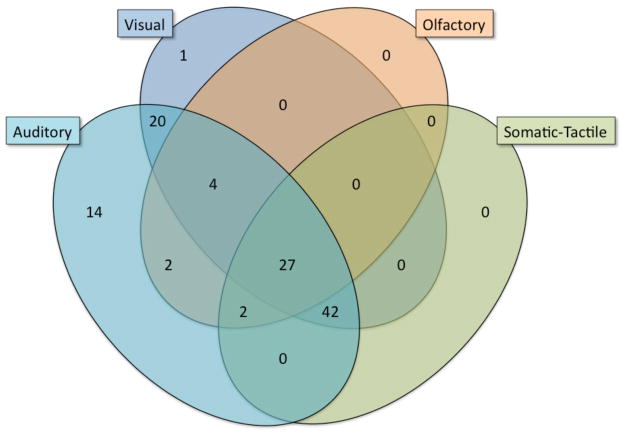
The overall overlap between groups was considerable, with auditory, visual and other modalities (i.e. no subjects only had olfactory or somatic/tactile hallucinations). As seen in Figure 1, within the COS subjects, there was significant overlap between visual and auditory hallucinations; only one subject had visual without also having auditory hallucinations. Somatic/tactile and olfactory hallucinations were absent in cases without both visual and auditory hallucinations being present. Thus there appears an additive progression of hallucination categories in order of descending frequency. Qualitative nature of the psychotic phenomena did not indicate a predominance of overlapping experiences on a unitary theme. For example, a typical patient reported demonic voices, saw magical fairies, and felt spiders crawling on her skin. Another patient explained: “You’re not getting it, it’s not just about hearing the demons, it’s about seeing other stuff too. Like the aliens in their spaceships.”
Figure 1.
Hallucination Modalities in the National Institute of Mental Health (NIMH) Childhood Onset Schizophrenia (COS) Cohort
Ethnicity, gender, and SES were not significantly different in patients with visual hallucinations compared to those without. Duration of illness, as measured from the time of psychosis onset to the time of rating at the NIMH (min = 0.75, max = 11.9, mean = 3.75 years) was not found to be significant, while duration of illness, as measured from time of first symptom onset (min = 0.24 years, max = 14.92, mean = 6.97) was found to be significant [p < 0.01], for patients with visual hallucinations compared to those without.
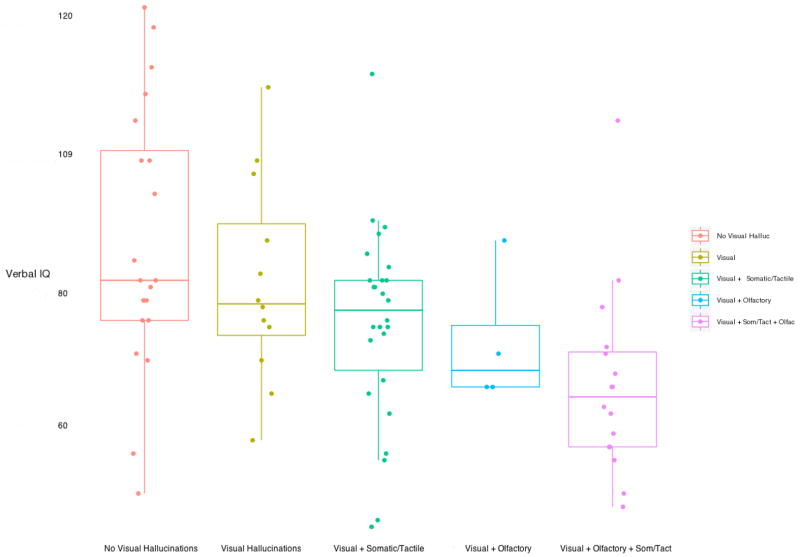
Table 1 shows clinical measures in relation to presence/absence of visual hallucinations. As shown in Table 1, the presence of visual hallucinations indicated greater severity as evidenced by earlier age of psychosis onset [p < 0.05], younger age at assessment [p<0.01], lower FSIQ scores [p < 0.01], lower CGAS scores at admission [p < 0.01], and at drug-free [p < 0.01], and a shorter duration of illness from age of first symptoms onset [p<0.01]. Also, girls were more likely to experience olfactory hallucinations [X2 = 6.1, df = 1, p = .016]. Most survived the False Discovery Rate procedure (Benjamini and Hochberg 1995): visual hallucinations (ever) and IQ, VIQ, CGAS (from drug-free and admission), age at assessment, duration of illness from age of first symptoms, and SANS SUM (from drug-free), as well as visual hallucinations (at drug free) and VIQ. When accounting for the additive nature of hallucination modalities in the COS cohort, a step-wise decline in VIQ was observed (Figure 2). VIQ Distribution of hallucinations (by modality) between the two individual admission/screening and drug-free time points appeared similar, with a mean time between admission/screening and drug-free assessments of 38.1 days (SD = 17.1). Not surprisingly, the screening/admission clinical measures did not relate as consistently to hallucination status, presumably due to variability in treatment availability and response.
Table 1.
Reported Visual Hallucinations and Clinical Ratings for Childhood Onset Schizophrenia Patients (n =117)
| Measure | No Visual Hallucinations (mean ± SD) (n = 24) | Visual Hallucinations (mean ± SD) (n = 94) | T (df) | P value |
|---|---|---|---|---|
| Age at Assessment | 15.1 (2.62) | 13.23 (2.79) | 2.9 (115) | 0.003 |
| IQ | 83.3 (21.0) | 71.2 (17.4) | 2.7 (93) | 0.007 |
| VIQ | 87.8 (20.17) | 74.1 (15.1) | 3.2 (76) | 0.002 |
| Age of Psychosis Onset | 10.7 (1.59) | 9.7 (2.1) | 2.1 (115) | 0.036a |
| CGAS Score (Admission) | 38.7 (8.19) | 31.8 (11.3) | 2.4 (104) | 0.016 |
| CGAS Score (Med-Free) | 33.1 (14.79) | 22.7 (12.2) | 2.9 (93) | 0.003 |
| SANS Sum (Med-Free) | 31.2 (16.94) | 53.1 (21.2) | −3.4 (88) | 0.001 |
| Duration of Illness from Psychosis Onset | 4.4 (2.30) | 3.5 (2.16) | 1.5 (115) | 0.130a |
| Duration of Illness from Age of 1st Symptoms | 9.2 (3.61) | 6.4 (3.67) | 3.2 (115) | 0.002 |
Note:
Did not survive False Discovery Rate (FDR) procedure. CGAS = Children’s Global Assessment Scale; IQ = Intelligence Quotient; SANS = Scale for the Assessment of Negative Symptoms; VIQ = Verbal Intelligence Quotient.
Figure 2.
Verbal IQ in relation to Visual and additional modalities for the National Institute of Mental Health (NIMH) Childhood Onset Schizophrenia (COS) Cohort
Discussion
The NIMH childhood onset patients show high rates across all hallucination modalities. This may reflect severity of our inpatient population. As expected, almost all had auditory hallucinations, since auditory hallucinations are important diagnostic criteria for these pediatric patients, and were often the symptom that led to referral to our study. The relationship between non-auditory hallucinations to severity, however, is strongly supported by analyses within our sample. Visual hallucinations showed a significant relationship to lower IQ and earlier age of onset. Drug-free observation permitted further within-sample observation of the significant relationship between visual hallucinations and overall clinical severity. Both the duration of psychotic illness as well as duration of ‘psychiatric or other problems requiring attention’ were shorter in children who had visual hallucinations although only the latter reached significance.
There were surprisingly high rates for tactile and olfactory hallucinations in this group, and they occurred exclusively in patients who had visual hallucinations. Visual, olfactory and tactile hallucinations thus appear to be a general marker of increased severity of psychosis. In addition, verbal IQ, in particular, demonstrated a consistent relationship with the presence of visual, somatic/tactile and olfactory hallucinations (see Figure 2), even when each modality was considered independently, although the numbers of subjects remain too small to compare statistically. It is important to note, however, that COS is extremely rare and the majority of children experiencing hallucinations do not progress to COS 23.
Nonetheless, this is unique data demonstrating an additive nature of hallucinations by the pattern of overlap, and the additive pattern’s relationship to IQ. We do not believe that the finding of greater severity rated by CGAS score with two or more modalities of hallucination to be tautological. The CGAS is an overall global rating without reference to hallucinations. In addition, the finding of greater severity was held with respect to IQ, VIQ, and earlier ages of onset of non-psychotic symptoms. The use of the SAPS rating scale provided more reliable information in contrast to several previous reports, some of which relied upon chart review or clinical interviews (see Table S1, available online). Due to the lack of other studies with equal numbers of subjects and inconsistent methodology, difficulty of inter-study comparison is noted. Despite methodological differences between our study’s data collection and previous smaller reports, the similar distributions of hallucination modality membership in probands at both the admission/screening and drug-free time points indicates that treatment status does not affect lifetime reporting of these events. We also found that those studies that assessed hallucination rates from a more comprehensive timeframe 13, instead of a one-time clinical interview, also reported higher hallucination rates more in line with our findings.
A limitation of this study was that although this is the largest reported study of COS, the sub-group of patients without visual hallucinations is small. In addition, national recruiting of a rare patient population has referral biases that are not easily identified. An impaired connectivity model of schizophrenia is consistent with the broader impairment of sensory domains seen in our severely ill chronic subjects. Future research may link these four hallucination modalities to other indicators of brain disconnectivity. Functional neuroimaging of these COS patients with consideration to visual hallucinations is currently underway.
Supplementary Material
Acknowledgments
This research was funded through the Intramural Program at the National Institute of Mental Health in Bethesda, MD.
Footnotes
The work described in the above manuscript was conducted as part of employment with the federal government at the National Institute of Mental Health (NIMH).
Disclosure: Drs. Greenstein, Clasen, Miller, Tossell, Mattai, Gogtay, and Rapoport, and Mr. David, and Mr. Gochman report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999 Nov 15;46(10):1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 2.Addington AM, Rapoport JL. The genetics of childhood-onset schizophrenia: when madness strikes the prepubescent. Curr Psychiatry Rep. 2009 Apr;11(2):156–161. doi: 10.1007/s11920-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin DW, Alderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders. A study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971 Jan;24(1):76–80. doi: 10.1001/archpsyc.1971.01750070078011. [DOI] [PubMed] [Google Scholar]
- 4.Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990 Jul;82(1):26–29. doi: 10.1111/j.1600-0447.1990.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewandowski KE, DePaola J, Camsari GB, Cohen BM, Ongur D. Tactile, olfactory, and gustatory hallucinations in psychotic disorders: a descriptive study. Ann Acad Med Singapore. 2009 May;38(5):383–385. [PubMed] [Google Scholar]
- 6.Bracha HS, Wolkowitz OM, Lohr JB, Karson CN, Bigelow LB. High prevalence of visual hallucinations in research subjects with chronic schizophrenia. Am J Psychiatry. 1989 Apr;146(4):526–528. doi: 10.1176/ajp.146.4.526. [DOI] [PubMed] [Google Scholar]
- 7.Small IF, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966 May;27(5):349–353. [PubMed] [Google Scholar]
- 8.Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9–22. doi: 10.1093/schbul/13.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Green WH, Campbell M, Hardesty AS, et al. A comparison of schizophrenic and autistic children. J Am Acad Child Psychiatry. 1984 Jul;23(4):399–409. doi: 10.1016/s0002-7138(09)60317-4. [DOI] [PubMed] [Google Scholar]
- 10.Russell AT, Bott L, Sammons C. The phenomenology of schizophrenia occurring in childhood. J Am Acad Child Adolesc Psychiatry. 1989 May;28(3):399–407. doi: 10.1097/00004583-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Green WH, Padron-Gayol M, Hardesty AS, Bassiri M. Schizophrenia with childhood onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry. 1992 Sep;31(5):968–976. doi: 10.1097/00004583-199209000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Egdell HG, Kolvin I. Childhood hallucinations. J Child Psychol Psychiatry. 1972 Dec;13(4):279–287. doi: 10.1111/j.1469-7610.1972.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 13.Spencer EK, Campbell M. Children with schizophrenia: diagnosis, phenomenology, and pharmacotherapy. Schizophr Bull. 1994;20(4):713–725. doi: 10.1093/schbul/20.4.713. [DOI] [PubMed] [Google Scholar]
- 14.Asarnow JR, Tompson MC, Goldstein MJ. Childhood-onset schizophrenia: a followup study. Schizophr Bull. 1994;20(4):599–617. doi: 10.1093/schbul/20.4.599. [DOI] [PubMed] [Google Scholar]
- 15.Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006 Jul;63(7):721–730. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen NC. The Scale for the Assessment of Postivie Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984b. [Google Scholar]
- 17.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1984a. [Google Scholar]
- 18.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983 Nov;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17(1):27–49. doi: 10.1093/schbul/17.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Russell AT. The clinical presentation of childhood-onset schizophrenia. Schizophr Bull. 1994;20(4):631–646. doi: 10.1093/schbul/20.4.631. [DOI] [PubMed] [Google Scholar]
- 21.R: A language and environment for statistcal computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 22.Oliveros JC. [Accessed 01/015/2011];An interactive tool for comparing lists with Venn Diagrams. 2007 http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- 23.Polanczyk G, Moffitt TE, Arseneault L, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. Apr;67(4):328–338. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.