Abstract
In the past two decades neuroimaging research has substantiated the important role of the prefrontal cortex (PFC) in decision-making. In the current study, we use the complementary lesion based approach to deepen our knowledge concerning the specific cognitive mechanisms modulated by prefrontal activity. Specifically, we assessed the brain substrates implicated in two decision making dimensions in a sample of prefrontal cortex patients: (a) the tendency to differently weigh recent compared to past experience; and (b) the tendency to differently weigh gains compared to losses. The participants performed the Iowa Gambling Task, a complex experience-based decision-making task [3], which was analyzed with a formal cognitive model (the Expectancy-Valance model; [12]). The results indicated that decisions become influenced by more recent, as opposed to older, events when the damage reaches the posterior sectors of the ventromedial prefrontal cortex (VMPC). Furthermore, the degree of this recency deficit was related to the size of the lesion. These results suggest that the posterior area of the prefrontal cortex directly modulates the capacity to use time-delayed information. In contrast, we did not find similar modulation for the sensitivity to gains versus losses.
Keywords: Cognitive models, Reinforcement learning, Risk taking, Recency
1. Introduction
Several studies in the past two decades have demonstrated the important role of the human prefrontal cortex in decision making [3,18,6,45,58,44,48,16,15,34,37,28]. Using functional imaging approaches, neuroscientists have been able to address the question of how the brain encodes the value of various options on a common scale [38], thus suggesting that there may be a common neural “currency” that represents the value of different options. In other experiments, brain regions such as the parietal cortex and anterior cingulate cortex have been shown to be involved in computing the probability or certainty of outcomes predicted by available options [46,25]. Also, some studies have been directed toward understanding how the perceived delay in receiving a reward modulates activity in reward-related brain areas [36], while others have addressed how differences between expected and actual reward contribute to learning [40]. In essence, the field of functional neuroimaging continues to blossom and addresses many of the factors that modulate the “utility” of rewards, and in doing so, affect how these rewards influence decision making.
Although much of this work is well represented in the functional neuroimaging literature, very little work in this area has used the lesion approach. This is historically important since research on the differences between lesion patients and controls has been highly successful in revealing brain functions that support decision making [18]. Therefore, the primary aim of this study is to evaluate in brain damaged patients whether damage to the anterior ventromedial prefrontal cortex (VMPC) shifts decision-making from long-term to short-term outcomes, and whether the degree of this shortsightedness increases as the damage extends more posteriorly to include the more posterior sectors of the VMPC. A further support of this hypothesis would be that the degree of this deficit would depend also on the size of the lesion in the target region. An additional aim of the study is to assess whether the weighting of gains versus losses, which mirrors the difference in response to rewards and penalties, is supported by separate neural subsystems.
The ideas that patients with VMPC lesions have shortened time horizons, and that some of these patients with posterior damage (e.g., including basal forebrain) have some sort of working memory impairment are not new [3,60]. However, the current study is novel in its analysis of behavior and lesion location and size to determine how recency deficits are mapped within the VMPC region on the anterior to posterior axis. Furthermore, the use of these behavioral and anatomical analyses to determine whether gains and losses (reward and punishment) are neurally dissociable within the prefrontal cortex has not been attempted before in human lesion studies (for a similar approach in another domain of investigation, see [47]).
The major advancement in the size, complexity, and connectivity of the frontal lobes in humans has occurred in relation to Brodmann area 10, i.e., the frontal pole [49], and not so much to the more posterior areas of the VMPC [50]. Anatomically, the more posterior areas of the VMPC (e.g., Brodmann area 25) are directly connected to brain structures involved in triggering (autonomic, neurotransmitter nuclei) or representing (sensory nuclei in the brainstem, insular, and somatosensory cortices) affective states, while access of more anterior areas is polysynaptic and indirect [43].
Under Bechara and Damasio’s [5] framework, the intactness of both posterior and anterior VMPC cortices is important in order for a non-immediate outcome to exert an influence on behavior. If the anterior regions (frontal pole) are damaged, decision-making shifts towards shorter time horizons (i.e., more recent outcomes affect behavior). However, as the damage extends to the more posterior VMPC regions (including the anterior cingulate cortex, basal forebrain, and nucleus accumbens), the shortening of this time horizon (or high recency) becomes more severe [5]. The reason is that damage to anterior sectors shortens the horizon for time somewhat, but more posterior sectors (which are still intact) can still integrate the value of non-immediate outcomes to a certain degree. In contrast, damage to posterior regions isolates the anterior sectors as well and impairs their capacity to function and integrate more distant time. Damage to the posterior VMPC impairs the connectivity of the anterior VMPC to the limbic system, rendering the anterior VMPC disconnected and dysfunctional. As a result the time shortening becomes more extreme, and decisions shifts towards attending only to immediately recent or future events.
Empirical evidence for this theoretical framework remains lacking, although there are several clinical observations and studies that are consistent with this notion. The first experimental evidence in support of this notion was found by Fellows and Farah [26], who showed that patients with damage to the VMPC, but not dorsolateral prefrontal cortex (DLPC), demonstrated a severe short-sightedness in their self-defined future. Other studies have shown that these patients also have severe impairments in their prospective feeling-of-knowing judgments [52]. Although time processing has been studied extensively in animal experiments [39], only recently neuroscientists have begun to address this issue in functional neuroimaging [36] and human lesion studies [26,27]. Using a complex decision-making task, such as the Iowa Gambling Task (IGT; [3]), which relies on information of outcomes that occur more recently, or in the distant past, VMPC lesion patients are especially impaired in the “recency” parameter of a cognitive model described below, in that they base their next choice on the most recent outcomes, while neglecting outcomes from earlier trials [62,32]. However, the knowledge so far in this regard has not been sufficient. Thus, the main goal of the current study is to examine how temporal events may be processed across the anterior–posterior axis of the VMPC. While several papers have specifically addressed the cognitive bases of decision-making in the prefrontal cortex [6,27], the current study is a first attempt to evaluate the degree of recency as a function of location (i.e., anterior vs. posterior) and lesion size in the PFC.
In addition to time processing, numerous studies have argued that the right hemisphere plays a dominant role in avoidance behavior, punishment, and negative emotions (which includes loss), whereas the left hemisphere is more important for approach behavior, reward, and positive emotions (which includes gain) [22]. A competing hypothesis has been that punishment and loss (and by inference, avoidance behaviors) is dependent on the lateral areas of the orbitofrontal cortex, including the inferior part of the inferior frontal gyrus, whereas reward and gain (and by inference, approach behaviors) is dependent on the mesial areas of the orbitofrontal cortex [41,34]. Contradictory functional neuroimaging evidence, however, suggests that losses and gains are processed by the same neural system [57]. Using the cognitive model described below, we examine whether the parameter of gain versus loss is affected by one particular lesion, and thus support any of the currently held views just outlined.
Given the assumption that different prefrontal cortex regions may mediate different sub-mechanisms of the overall decision-making process [2,17], and given that lesions studies can establish which specific region may be linked to a specific cognitive mechanism [2], we examined the effects of lesions within the PFC on several parameters of decision-making, namely the weighting of recent compared to past experiences and sensitivity to gains versus losses. In this study, we divided the prefrontal cortex into four sub-regions (bilaterally), and the percentage of each region that was occupied by lesions was calculated for each patient using Brainvox [19,31]. To pinpoint specific cognitive mechanisms which may be related to each region, we used cognitive modeling (e.g., [12,62,32]). The cognitive modeling analysis decomposes the overt behavior in a decision task to the underlying covert cognitive mechanisms.
While several cognitive models exist, we employed the Expectancy-Valance model (EV; [12]), an adaptive learning model which decomposes the behavior in the IGT into three basic mechanisms: (i) a motivational mechanism which captures the tendency to weigh gains and losses differently; (ii) a learning-rate mechanism which captures the tendency to focus on recent outcomes and to ignore past experiences; and (iii) a response mechanism which captures the tendency to respond consistently or in an erratic manner. The model produces quantitative estimations that provide continuous mapping of the decision-makers along the three mechanisms. For example, the recency parameter, which is the most important parameter for examining time discounting, assumes that decision makers form expectancies about the consequences of their choices. When a choice is being made, this expectancy is adjusted, as a function of its previous value and the value of the newly experienced outcome. However, the amount of the adjustment is dependent on the value of the recency parameter, which ranges from 0 to 1. Small parameter values represent less discounting of past experiences, while large values represent strong recency, that is, rapid discounting of past experiences. The model equations appear in the Appendix (for more information about the model, see [12,55,62]). The EV model was used because research shows it captures the essential attention and information-processing mechanisms which underlie experience-based decision tasks such as the IGT [24,61] and because it was found to have better explanatory power than several alternative models [63].
2. Materials and method
2.1. Participants
Fourteen patients with different prefrontal cortex lesions from the Patient Registry of the University of Iowa’s Division of Behavioral Neurology & Cognitive Neuroscience participated in the experiment. Nine patients were males (mean age = 49.33, SD = 17.97, range = 21–81) and five females (mean age = 58.6, SD = 11.72, range = 40–70 years old). The participants had, on average, 12 years of education (mean males = 12, SD = 2.34, range = 8–14 years; mean females = 12.1, SD = 3.22, range = 8–18 years), and they were all right-handed. The participants constitute a sub-sample of the patients studied elsewhere [3,6] for whom the extent of the damage to different areas of the prefrontal cortex was mapped and their neuropsychological data was previously collected.1 The behavioral analyses (EV model) are new for this sub-sample of patients, and the anatomical analyses (which involve measuring size of lesions within divided regions of the PFC) are new, and none has been published before. We note that throughout the years these patients have received testing, including the IGT, on multiple occasions. In order to be consistent, we selected the data collected during the very first time these patients took the IGT. In the studies originally conducted to collect the data, all subjects provided informed consents that were approved by the appropriate human subject committees at the University of Iowa. The details of the inclusion and exclusion criteria and the neuropsychological and neuroanatomical profiles of these patients have been published elsewhere [3,6]. In brief, the selection criteria for VMPC patients were absence of history of mental retardation, learning disability, psychiatric disorder, substance abuse, or any systemic disease capable of causing degeneration within the central nervous system. Each VMPC patient also had to have the following additional criteria: (1) a stable and chronic lesion (at least 3 months post onset); and (2) bilateral involvement of the VMPC region. A summary of the neuropsychological scores of these patients is presented in Table 1.
Table 1.
Neuropsychological data for VMPC patients. WAIS-III, Wechsler Adult Intelligence Scale-III score (VIQ, verbal IQ; PIQ, performance IQ; FSIQ, full-scale IQ; WMI, working memory index). AVLT Recall, z-score on the 30-min delayed recall trial of the Auditory Verbal Learning Test. BDAE CIM, T-score of the Boston Diagnostic Aphasia Examination Complex Ideational Material subtest, a measure of auditory comprehension. BNT, Boston Naming Test (#correct/60; scores > 54 are normal). TMT B-A, z-score of the Trail-Making Test Part B minus Part A times to completion.
| WAIS-III | AVLT BDAE | TMT | ||||||
|---|---|---|---|---|---|---|---|---|
| VIQ | PIQ | FSIQ | WMI | Recall | CIM | BNT | B-A | |
| Mean | 102 | 99 | 101 | 105 | .7 | 57 | 58 | .5 |
| S.D. | 16 | 16 | 17 | 12 | 1.7 | 2.9 | 1.9 | 1.1 |
2.2. Parcellation of the prefrontal cortex
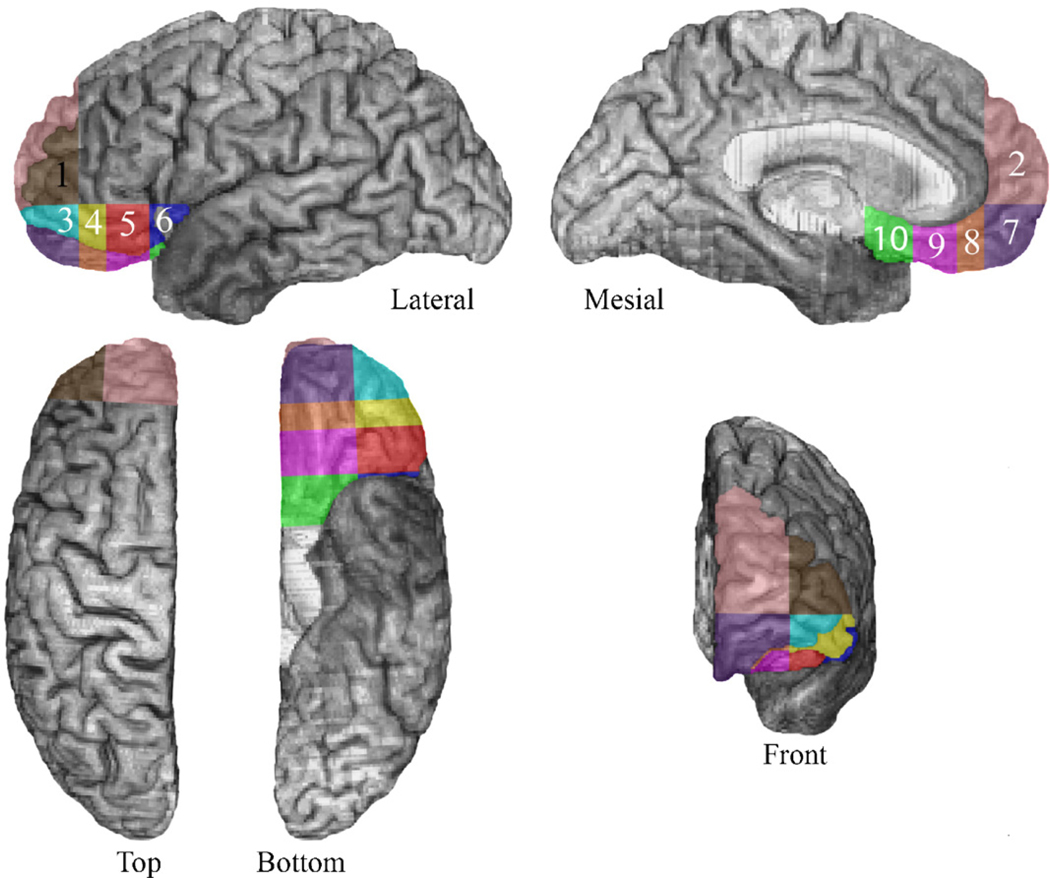
The neuroanatomical analysis was based on computerized axial tomography (CT) data in 6 of the 14 cases, because of the nature of the neurological damage, specifically, ruptured aneurysms that were subsequently clipped. The scans were obtained in a Picker 1200 scanner, or in a Toshiba Express SX scanner with various tilts (so as to avoid as much as possible the artifact produced by the aneurysm clips), with a zoom of 2.4, field of 51 cm, a fovea of 212.5 mm, and slice thickness that varied between 2 and 4 mm. In eight of the subjects, magnetic resonance (MR) scans were possible. These were obtained in a 1.5 T General Electric scanner with an SPg sequence, in thin (1.5 mm) contiguous T1 weighted coronal slices. All lesions were manually transferred into a normal reference brain using the MAP-3 technique [20]. We quantified the amount of brain damage in different regions of the VMPC by adding a new segmentation method to the original MAP-3 technique [20]. The prefrontal region was subdivided into 10 “cells” numbered 1 through 10 (see Fig. 1). The division was based on anatomical landmarks and lines drawn between them, as outlined below.
Fig. 1.
The sub-regions of the prefrontal cortex that were used to calculate the percent of cell occupied by lesion. Sub-regions 2 and 7 correspond to the anterior VMPC, while sub-regions 8, 9, and 10 correspond to the posterior VMPC. Similarly, sub-regions 1 and 3 correspond to the anterior lateral prefrontal cortex, while sub-regions 4, 5, and 6 correspond to the posterior lateral prefrontal cortex.
The VMPC sector corresponds to cells 2, and 7–10, numbered in anterior-to-posterior sequence; cells 1, and 3–6 correspond to the lateral orbital sector, again in an anterior-to-posterior sequence. For each subject, the percent involvement (in terms of lesion) in each of these cells can be calculated using Brainvox capabilities [19,31]. Thus, lesion data providing quantities of VMPC damage in posterior-to-anterior sequence can be obtained with this technique [59]. We note that all parcellation results derive from a single normal reference brain onto which individual lesions from individual patients are transferred through the MAP-3 technique that has been used in our lab for many years [20]. Therefore, lesions scanned with MR, and the few ones scanned with CT, end up on the same reference brain and are analyzed in a similar manner. Also, segmenting the brain into the 10 different cells is not required for each brain. The segmentation is done only on the reference brain, and all lesions are analyzed according to how they fall within the different segments (cells) of the reference brain. For the anterior border of cell 10, we chose a vertical line descending from the tip of the rostrum of the corpus callosum; the anterior border of cell 9 is a vertical line from the tip of the genu of the corpus callosum; the anterior border of cell 8 is a vertical line from the most anterior edge of the cingulated sulcus. In the horizontal plane, the division between cell 7 and cell 2 is a straight horizontal line passing through the lowest tip of the genu of the corpus callosum. In the medial to lateral plane, the division between the cells on the medial side and those falling on the lateral side is a straight line passing through the lateral border of the medial orbital gyrus.
While this segmentation is different from the Brodmann’s area division, it can be roughly mapped to it. Regions 1 and 2 include the frontal pole, regions 3, 4, 5, and 6 includes the lateral orbitofrontal cortex, and regions 7, 8, 9, and 10 includes the mesial orbitofrontal cortex (on the anterior to posterior axis). The anterior parts (i.e., 2 and 7 in the ventromedial region and 1 and 3 in the lateral region of the PFC) correspond to Brodmann areas 10, most anterior and mesial parts of area 12, and the pregenual part of 32. The more posterior parts (i.e., 8–10 and 4–6) correspond to Brodmann areas 25, mesial 11, posterior part of mesial 12, and the subgenual parts of 24 and 32.
2.3. Measures
2.3.1. Iowa Gambling Task (IGT)
In this task, the participants are told that on each trial they are required to pick one card (without replacement), with their goal being to maximize their total earning. The task involves 100 trials, but participants are not informed beforehand how many cards they have to pick. When a given deck runs out of cards, participants are instructed to continue picking cards only from the remaining three decks. A selection from Deck A or B yields a constant gain of $100, and from Deck C or D, $50. However, occasionally, a selection also leads to a simultaneous loss. In Decks A and B the occasional losses are large, and the net amounts average to a loss of $25 per selection. Accordingly, these decks are considered disadvantageous. The losses in Decks C and D are lower in comparison, and the net amounts are positive, averaging $25 per selection. Thus, these decks are considered advantageous. The details of this task have been described elsewhere [3,6].2
2.3.2. Expectancy-Valence (EV) model parameter estimation
The model equations are briefly described in Appendix A. The parameters of the Expectancy-Valence (EV) model were optimized separately for each patient by maximizing the likelihood of the 100 actual choices of the patient. In the optimization process, the fit of the model is compared with the fit of a baseline model, which has three parameters based on the average choice proportions of Decks A, B, and C (the average choice proportions from Deck D are calculated accordingly).
The improvement in the fit of the EV model over the fit of the baseline model, in log likelihood, is G2, a model-fit statistic analogous to the chi-square. Positive G2 values indicate that the model outperforms the baseline model, whereas negative G2 values indicate the opposite. In addition to the model’s fit index, estimations of the three parameters of the model were calculated for each patient. The three estimated parameters were: W, which evaluates the weight of gains versus losses; ϕ, which evaluates the weight of recent outcomes; and c, which measures the consistency of choice behavior, that is whether choices are based consistently on the accumulated experiences.
3. Results
3.1. Mapping of brain lesions
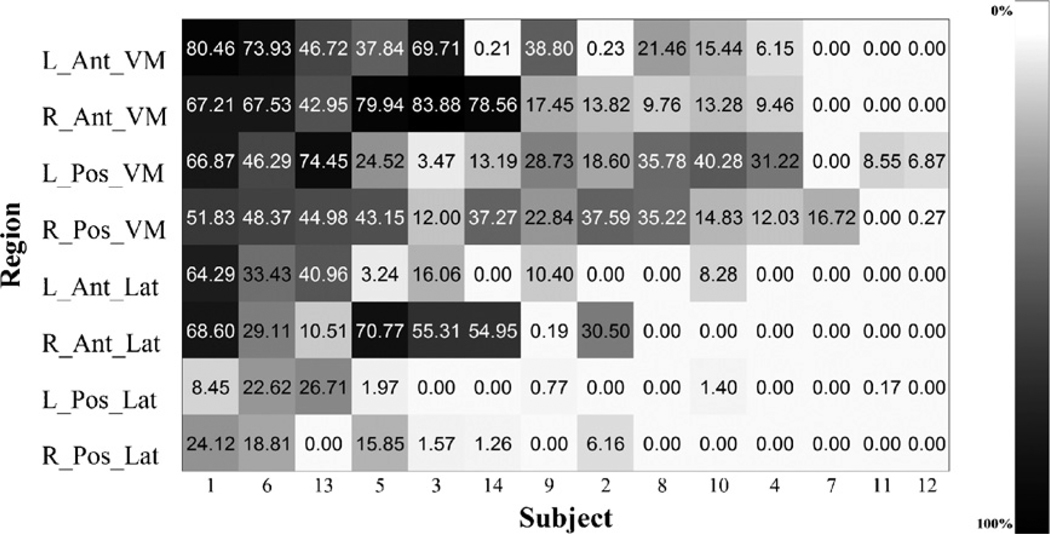
The extent of the lesions occupying each of the PFC regions (in %) is presented in Fig. 2. Brain damage could extend over several separate sectors identified in the analyses, but the degree of that damage within each sector varied considerably. The extent of the damage was calculated as a percentage range. The most extensive damage for the current sample was in right anterior ventromedial region, where the damage was approximately 34.56 ± 8.98%, and in the left anterior ventromedial, where it was about 27.9 ± 8.02%. In the right posterior ventromedial region the percent damage was about 26.9 ± 4.76%, while in the left posterior ventromedial region it reached 28.5 ± 6.09%. On the lateral side of the PFC, in the right anterior lateral region the damage was about 22.85 ± 2.22%, and in the left anterior lateral region it was 12.6 ± 5.32%. Finally, in the left and the right posterior lateral regions, the damage reached about 4.4 ± 2.35% and 4.5 ± 8.3%, in accordance.
Fig. 2.
Individual level percent of region occupied by lesion in the prefrontal cortex. Patient are displayed according to overall severity of damage. R, right; L, left; Pos, posterior; Ant, anterior; VM, ventromedial; Lat, lateral.
3.2. Modeling results
The average G2 of the EV model was 5.6 ± 3.64. Since positive values of the G2 statistic indicate that the cognitive model performs better than the statistical baseline model [12], this result supports the use of the learning model for predicting the participants’ trial to trial choices. Pearson product-moment correlations coefficients were calculated for each sub-region to determine the impact of the extent of lesions on each of the EV model parameters (Fig. 3A). In this analysis, IQ was used as a covariant to eliminate any unique effect of this variable.3
Fig. 3.
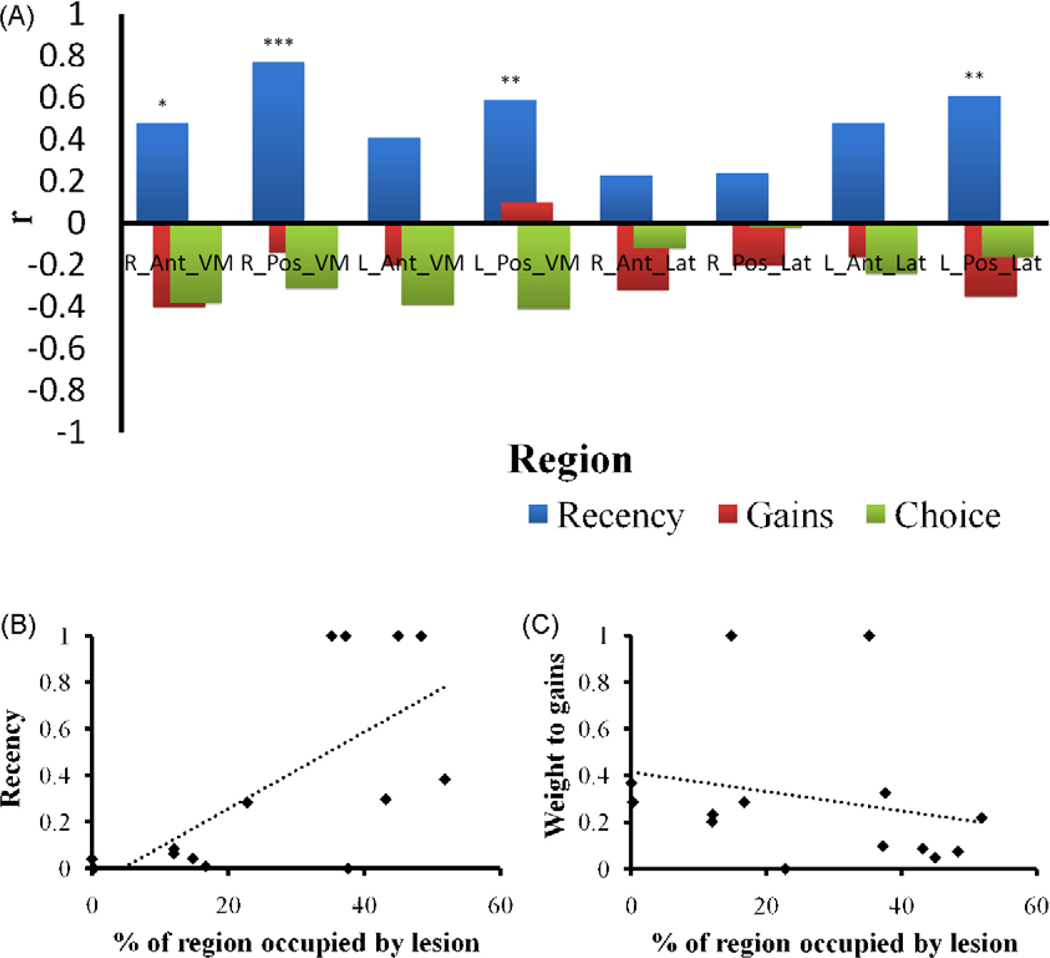
(A) Pearson product-moment correlation coefficients between the damage to prefrontal regions and the EV model parameter: Sensitivity to recent outcomes (recency), sensitivity to gains versus losses (gains), and choice consistency (choice). R, right; L, left; Pos, posterior; Ant, anterior; VM, ventromedial; Lat, lateral. Asterisks represent significance levels (*p < .1, **p < .05, ***p < .005). (B and C) Estimations of the recency and weight to gains parameters as a function the damage (% occupied by lesion) to the right posterior VMPC. The dotted lines represent the linear regression curve.
The results showed significant correlations between the recency parameter of the EV model and lesions to the right and left posterior ventromedial regions (r = .77, p < .005 and r = .59, p < .05 in accordance; see e.g., Fig. 3B) as well as lesions to the left posterior lateral region (r = .61, p < .05). In addition, a marginally significant correlation was found between recency and damage to the right anterior ventromedial region (r = .47, p = .09). Finally, although not significant (p = .16), we also found a relatively strong correlation between recency and left anterior ventromedial damage (r = .41).4
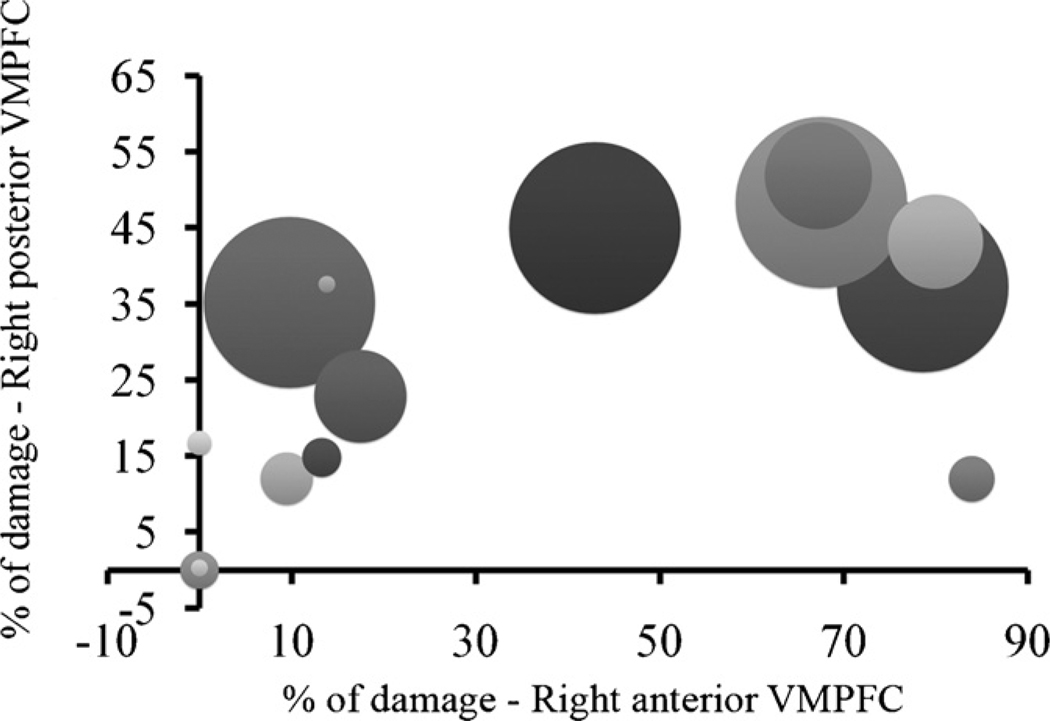
These results suggest that the greater the damage to these regions, the greater the sensitivity to recent outcomes. However, this was not true to any of the other (lateral) regions. In addition, the results suggest that more weight is assigned to recent outcomes as the lesion is shifted from anterior to posterior regions, and that this tendency is skewed to the right. To demonstrate this, Fig. 4 depicts the values of the recency parameter at the individual level as a function of lesion size to the right posterior and anterior ventromedial regions. As can be seen, recency tends to increase as the lesion size in the posterior region increases; however this tendency is less prominent for the anterior region.
Fig. 4.
Recency parameter (size of bubble, smallest bubble = 0) at the individual level as a function of damage size in right posterior and anterior ventromedial prefrontal cortices. This figure illustrates the relationship between recency and the anterior–posterior axis. As can be seen, the recency parameter tends to increase as the damage to the posterior cortex increases (as the size of the bubble increases, the percent of lesion increases). However, this tendency is less prominent for the anterior cortex.
In addition, we found no significant correlations between the weight to gains versus losses parameter and the percent of lesion occupying any of the prefrontal regions (see, for example, Fig. 3C). These correlations were also of relatively low magnitudes (average r = −.20). The results thus indicate that the tendency to differently weigh gains and losses is not associated with the extent of damage to any of the PFC regions. Similarly, the same pattern of results was observed for the choice consistency parameter.
4. Discussion
Using a cognitive model to dissect the behavioral decisions made on the IGT into more specific processes (recency effects, and attention to gain vs. loss), the results supported our hypothesis that damage to the more anterior regions of the VMPC (and specifically regions corresponding to Brodmann’s area 10, most anterior and mesial parts of area 12, and the preigenual part of area 32) increases the sensitivity to recent outcomes, but the degree of this sensitivity increases significantly as the damage extends more posteriorly to include the more posterior sectors of the VMPC (i.e., Brodmann’s areas 25, mesial 11, posterior part of mesial 12, and the subgenual parts of 24 and 32). These effects were stronger for right side of the brain as opposed to the left side. Furthermore, the relative size of lesions in the posterior and anterior ventromedial cortices was highly associated with an increased sensitivity to recent outcomes. In relation to the neurology of rewards versus penalties, no association was found between damage to any of the PFC regions and increased sensitivity to gains versus losses.
The current findings are highly consistent with an influential animal study demonstrating that damage to the shell of the nucleus accumbens (a part of the basal forebrain region which is included in the posterior VMPC areas defined in this study) precipitates impulsivity [14], where choice becomes dominated by immediate contingencies. In this study we add that this immediacy is hierarchically organized, and that the degree of shortsightedness increases as the damage moves from more anterior to more posterior location to include the more posterior sectors of the VMPC. The strong correlations we found between lesions to the right ventromedial cortex and the degree of recency is also consistent with previous views arguing that the neural mechanisms subserving decision-making are tied more to the right side areas of the prefrontal cortex [18], as well as previous findings showing that certain executive functions of the PFC are more linked to the right hemisphere [2,54]. However, it is quite possible that this effect may be due to the fact that our sample of lesion patients contained almost twice as many males relative to females. Although no statistically significant differences emerged in connection with gender in the current study, the possibility remains that due to the small number of females in the current sample this right-sided effect may be more prominent in males. Indeed, in a functional imaging study, it was found that men generated more right-sided prefrontal activation during a decision-making task (the IGT), whereas women generated more left-sided prefrontal activation [10], and see also [59]. Overall, these results provide complementary information to several lines of research showing that the PFC is critical for advanced, goal-directed forms of representing environmental stimuli [11,2,9,7,30,35].
We did not find an effect of lesions in any area of the PFC on the modulation of gains and losses. Although null results in correlation analyses must be interpreted with caution, our results are more consistent with the functional neuroimaging findings shedding doubts on possible dissociations between specific prefrontal mechanisms in the weighting of gains and losses [57,13]. Furthermore, our results support the assertion that the PFC is involved not in the simple modulation of gains and losses but rather in the control of mixed responses to gains and losses based on experiences that are more recent or more distant [30].
The current results also have some general implications concerning the functional significance of the ventromedial regions. First, the results suggest that time may be processed within the PFC in relative, as opposed to absolute, terms. In other words, damage to the VMPC does not necessarily shorten time in days, months, or years. Rather, such damage shortens the time distance between a more immediate and a delayed event. For instance, in real-life, the VMPC patients are myopic to consequences that may happen a few days, months, or years down the line. However, in the IGT, the time delays are in seconds or minutes at the most. Since these delays impose a distance between one event and another, lesions of the VMPC seem to interfere with the adherence to a delayed event, once another event is more immediately available. The current results reveal that the shortening of this time distance increases as damage encroaches on the more posterior regions. A second implication of the current results is that prospective time and past time seem to have overlapping neural substrates. Clinical evidence as well as experimental studies [26] have found shortening of prospective or future time in patients with VMPC lesions. The current study demonstrates shortening of past time inpatients with similar VMPC lesions. Such findings are highly consistent with the literature on prospective memory suggesting that the memory for the past and for the future may have overlapping neural substrates [42,1,51,56,21].
Other implications of the current results include applications to a variety of social behaviors and clinical disorders that are not overtly associated with PFC damage, but have been implicated in PFC dysfunction. For instance, research shows that prefrontal lesioned patients exhibit promiscuous, antisocial and aggressive behavior (see, for example, [18,8]), and fail to accurately distinguish between right and wrong [33]. In addition, animal research shows that 5-HT receptors in the ventromedial and orbitofrontal cortex play a major role in the inhibition of aggressive behavior in male mice [23]. Impulsive violent behavior may be the result of extreme recency, as it can be described as the result of over-weighting recent experiences that lead to highly negative feeling towards another person [64]. In support for this claim, the authors’ own research shows that criminals convicted with violent crimes, especially assault, murder and robbery, have high recency [64]. In contrast, those convicted with crimes involving lower or no levels of violence such as theft, drug crimes, and OWI, were found to exhibit normal levels of recency and increased sensitivity to gains.
Similarly, studies using imaging methods show that neuronal activity in the orbitofrontal cortex is altered in drug addicts (see review in [53]). As previously noted [7], sensitivity to recent experiences may account for the inability to resist drugs, as these produce strong, mostly attractive, experiences. This does not rule out other pathways to drug abuse. For example, in many studies of drug abusers, we find increased attention to gains compared to losses in drug abusers [29,55,62]. The possible effect of both mechanisms is consistent with the suggestion that mere increased sensitivity to gains is only one condition implicated in the sensitivity to drug abuse, and that a weakness in control processes implicated in working memory is a second important contributing factor [29].
Finally, the notion of recency is important for understanding the decision problems associated with prefrontal impairments. Such understanding may contribute to ways of supporting and possibly enhancing the decision skills of brain injured patients and drug abusers. The current findings suggest that emphasis should be put not so much on illuminating the negative future consequences of the person’s actions but rather on increasing one’s ability to utilize experiences from the more distant past in order to resist temptations that lead to immediate positive results.
Limitations of the current study include the small number of participants and the use of correlation analysis which can be sensitive to extreme values. Nevertheless, the major statistical trends are replicated using Spearman rank-order correlation. Moreover, the current findings are consistent with those of relevant animal studies. Taken together, the findings point out to the important role of the posterior part of the VMPC for integrating non-recent events in humans’ decisions.
Acknowledgments
The research described in this study was supported by a Program Project Grant from the National Institute of Neurological Disorders and Strokes (NINDS) P01 NS019632 and by a research grant from the National Institute on Drug Abuse (NIDA) R01 DA023051.
Appendix A
The Expectancy-Valence model
The Expectancy-Valence model [12] is a computational model analyzing trial-by-trial choice-behavior. It distills the performance in the IGT (and similar tasks) into three underlying components characterizing the cognitive-motivational profile of the decision-maker.
- Weight of gains vs. losses: The evaluation of wins and losses experienced after making a choice is called a valence, denoted u(t), and is calculated as a weighted average of gains and losses from the chosen option in trial t.
The parameter w determines the subjective weight to gains versus losses (0 ≤ w ≤ 1).(1) - Weight of recent vs. past outcomes: The valences produced by deck j are summarized by an accumulated subjective value for each deck, called an expectancy, and denoted Ej(t). A Delta learning rule updates the expectancy after each choice from deck j:
The recency parameter, ϕ, describes the degree to which the expectancies reflect the influence of the most recent outcomes or more distant past experience (0 ≤ ϕ ≤ 1).(2) - Consistency of choice behavior: The predicted probability that deck j will be selected on trial t, Pr[Gj(t)], is calculated using a ratio of strengths rule:
(3)
The term θ(t) controls the consistency of choice probabilities and expectancies, where: θ(t) = (t/10)c and c is the choice consistency parameter. The parameter c is constrained between 5 and −5, covering the range between expectancy-based and nearly random choices, respectively.
Footnotes
Because the main hypothesis in this study involves the association between the location and extent of brain lesions and decision parameters (e.g., recency) we did not study a control group. An analysis of healthy adults’ performance in this task as well as the performance of other neuropsychological samples appears elsewhere [5–7].
Two participants were administered a variant of the original task (called the A′B′C′D′ version). The variant task was identical to the original task, with the exception that the disadvantageous decks become somewhat worse over the course of the task, and the advantageous decks become better (see description in [4]). Since no effect was found for the task version, we collapsed the data of all participants.
The behavioral data for this study was reported elsewhere [3,7], and the fact that PFC lesions patients perform poorly in the IGT had been well established. For conciseness, these results are not reported here.
No significant gender differences were found for any of the results. For conciseness the analysis covarying for gender effects was not included.
References
- 1.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- 6.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 7.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 8.Beer JS, John OP, Donatella S, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion–cognition interactions. J Cogn Neurosci. 2006;18:871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 9.Beer JS, Shimamura AP, Knight RT. Frontal lobe contributions to executive control of cognitive and social behavior. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 2004. pp. 1091–1106. [Google Scholar]
- 10.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- 11.Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. Psychol Res. 2000;63:279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- 12.Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assessment. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- 13.Camara E, Rodriguez-Fornells A, Münte TF. Functional connectivity of reward processing in the brain. Front Hum Neurosci. 2009;2:1–14. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 15.Clark L, Cools R, Robbins T. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 16.Clark L, Manes F. Social and emotional decision-making following frontal lobe injury. Neurocase. 2004;10:398–403. doi: 10.1080/13554790490882799. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MX, Ranganath C. Behavioral and neural predictors of upcoming decisions. Cogn Affec Behav Neurosci. 2005;5:117–126. doi: 10.3758/cabn.5.2.117. [DOI] [PubMed] [Google Scholar]
- 18.Damasio AR. Descartes’ error: emotion, reason, and the human brain. Putnam Publishing. 1994:336. [Google Scholar]
- 19.Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Arch Neurol. 1992;49:137–143. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- 20.Damasio H. The lesion method in cognitive neuroscience. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier; 2000. pp. 77–102. [Google Scholar]
- 21.D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 23.De Almeida RMM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology. 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- 24.Erev I, Roth AE. Predicting how people play games: reinforcement learning in experimental games with unique mixed strategy equilibria. Am Econ Rev. 1998;88:848–881. [Google Scholar]
- 25.Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiat. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Fellows LK, Farah MJ. Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005;43:1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Fellows LK, Farah MJ. Different underlying impairments in decision making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 28.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 29.Finn P. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcohol. Behav Cogni Neurosci Rev. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- 30.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 31.Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- 32.Kalidindi K, Bowman H. Using ε-greedy reinforcement learning methods to further understand ventromedial prefrontal patients’ deficits on the Iowa Gambling Task. Neural Networks. 2007;20:676–689. doi: 10.1016/j.neunet.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Krueger F, Moll J, Zahn R, Heinecke A, Grafman J. Event frequency modulates the processing of daily life activities in human mediakl preforntal cortex. Cereb Cortex. 2007;17:2346–2353. doi: 10.1093/cercor/bhl143. [DOI] [PubMed] [Google Scholar]
- 36.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 37.Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 39.Nichelli P. The processing of temporal information in the frontal lobe. In: Grafman J, editor. Handbook of neuropsychology: frontal lobes. Amsterdam: Elsevier; 2002. pp. 175–193. [Google Scholar]
- 40.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 42.Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and past: the role of the frontal pole and the medial remporal lobes. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 43.Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 44.Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Brain Imaging. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- 45.Rahman S, Sahakian BJ, Cardinal RN, Rogers RD, Robbins TW. Decision making and neuropsychiatry. Trends Cogn Sci. 2001;6:271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- 46.Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiat. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: application to category-related naming and recognition deficits. Neuroimage. 2008;41:907–984. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanfey A, Hastie R, Colvin M, Grafman J. Phineas Guaged: decision-making and the human prefrontal cortex. Neuropsychologia. 2003;41:1218–1229. doi: 10.1016/s0028-3932(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 49.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 50.Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 51.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for the right medial prefrontal cortex in accurate feeling-of-knowing judgments: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42:957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiat. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired ‘affective theory of mind’ is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- 55.Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon B Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- 56.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 58.Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:598–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- 59.Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- 60.Tranel D, Jones RD. Knowing what and knowing when. J Clin Exp Neuropsychol. 2006;28:43–66. doi: 10.1080/13803390490919344. [DOI] [PubMed] [Google Scholar]
- 61.Weber EU, Shafir S, Blais AR. Predicting risk sensitivity in humans and lower animals: risk as variance or coefficient of variation. Psychol Rev. 2004;111:430–445. doi: 10.1037/0033-295X.111.2.430. [DOI] [PubMed] [Google Scholar]
- 62.Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 63.Yechiam E, Busemeyer JR. Comparison of basic assumptions embedded in learning models for experience based decision-making. Psychon B Rev. 2005;12:387–402. doi: 10.3758/bf03193783. [DOI] [PubMed] [Google Scholar]
- 64.Yechiam E, Kanz J, Bechara A, Stout JC, Busemeyer JR, Altmaier EM, et al. Neurocognitive deficits related to poor decision-making in people behind bars. Psychon B Rev. 2008;15:44–51. doi: 10.3758/pbr.15.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]