Abstract
Objective
Recent studies identify aberrant Wnt signaling in scleroderma and pulmonary fibrosis. The aim of present study was to investigate the effect of ectopic Wnt10b expression on skin homeostasis and differentiation in transgenic mice with FABP4-directed Wnt10b expression.
Methods
The expression of Wnt10b was analyzed by real-time qPCR in bleomycin-induced fibrosis. Dermal thickness and the myofibroblasts were determined in skin sections from FABP4-Wnt10b transgenic and wildtype mice. The expression of collagen was analyzed by Sircol assays, immunoblot and real-time qPCR. The effects of Wnt10b were examined in explanted fibroblasts and in adenovirus infected preadipocytes or fibroblasts.
Results
FABP4-Wnt10b transgenic mice showed progressive loss of adipose tissue in the skin. The dermis was fibrotic and showed increased collagen deposition, fibroblast activation and myofibroblast accumulation. Increased canonical Wnt signaling correlated with elevated collagen gene expression in these biopsies. Explanted fibroblasts from transgenic mice showed increased canonical Wnt signaling and elevated Type I collagen and α-smooth muscle actin gene expression in vitro. Infection of normal preadipocytes with Wnt10b resulted in blockade of adipogenesis, whereas in fibroblasts Wnt10b induced marked up-regulation of Type I collagen.
Conclusion
We demonstrate that ectopic Wnt10b expression in vivo causes progressive loss of adipose tissue accompanied by the spontaneous development of dermal. These findings suggest that ectopic Wnt10b drives a switch in mesenchymal cell fate toward myofibroblasts by induction of a fibrogenic program while suppressing adipogenesis. Therefore FABP4-Wnt10b transgenic mice represent a novel animal model to study the role and mechanisms of Wnt signaling in fibrosis.
INTRODUCTION
Systemic sclerosis (SSc) is a connective tissue disease of unknown etiology associated with autoimmunity, small vessel vasculopathy and prominent fibrosis in the skin, lungs and multiple target organs (1). Fibrosis is due to excessive synthesis and deposition of Types I and VI collagens, fibronectin and other extracellular matrix (ECM) molecules, accompanied by accumulation of myofibroblasts positive for α-smooth muscle actin (α-SMA) in the lesional tissue (2). It is currently believed that fibroblasts and myofibroblasts are responsible for the overproduction of ECM in fibrosis. The source of ECM-producing fibroblasts is controversial (3, 4). In situ transition of fibroblasts into myofibroblasts, tissue accumulation of bone marrow-derived fibrocytes trafficking from the circulation, and transdifferentiation of epithelial and endothelial cells into myofibroblasts-all might contribute to expansion of the pool of biosynthetic mesenchymal cells (1), and these processes are potently induced by transforming growth factor-β (TGF-β) (5). It is becoming recognized, that the pathogenesis of fibrosis in SSc is complex and multi-factorial, and in addition to TGF-β, other extracellular cues appear to be important for the initiation or propagation of fibrosis. The list of candidates includes connective tissue growth factor (CTGF), interleukin (IL)-13, endothelin-1, bioactive lipids and the Wnt ligands (6).
Recent observations provide evidence for the potential importance of Wnts in the development of fibrosis. The Wnt family comprises 19 cysteine-rich glycoproteins that serve dual roles in ligand-dependent transcriptional regulation and cell-cell adhesion (7). The paracrine effects of Wnts are mediated via canonical and non-canonical intracellular signaling pathways. β-catenin plays a fundamental role in mediating canonical signaling (8). Wnt ligands bind to the cell surface receptors FZD and low-density lipoprotein receptor-related proteins LRP5 and LRP6. In the presence of Wnt ligand, the LRP receptors are phosphorylated, resulting in inhibition of GSK-3β activity, with consequent stabilization and accumulation of hypophosphorylated β-catenin. After translocation into the nucleus, the hypophosphorylated β-catenin regulates target gene expression via the T cell factor (TCF) family of DNA-binding transcription factors (9). Wnts via β-catenin regulate expression of a large number of genes with diverse biological functions and in a highly cell context-specific manner (10). Transcriptional Wnt targets include DKKs and Axin2, respectively extracellular and intracellular inhibitors of Wnt signaling, permitting tight regulation of β-catenin signaling via negative feedback.
The Wnts are known as developmental regulators that are essential for normal embryonic morphogenesis and cell fate determination. Wnt signaling is required for specification of dermal cell identity in the mouse embryo (11). In the adult, Wnts regulate diverse physiological processes including pluripotent cell differentiation, tissue repair and regeneration. Acquired alterations in Wnt expression or function are associated with cancer and aging, along with diverse diseases, including diabetes, pulmonary arterial hypertension, rheumatoid arthritis, osteosclerosis and osteoporosis (12–14). A potential role for Wnts in regulation of tissue remodeling in the adult is now emerging. Moreover, Wnts appear to be involved in aberrant tissue repair and pathological fibrosis. Several genes involved in tissue remodeling and fibrogenesis are known to be directly regulated by Wnts, and pathological fibrosis in various organs has been linked to aberrant local expression and/or activation of Wnt signaling (15, 16). Wnt10b is a canonical Wnt with potent effects on the regulation of adipogenesis and osteoblastogenesis (17, 18). Transgenic mice in which Wnt10b is expressed from the fatty acid binding protein-4 (FABP4) promoter showed greatly reduced adipose tissues, resistance to diet-induced obesity and cold intolerance (17). It was incidentally observed that the transgenic mice have an increase in dermal thickness with a reduced number of adipocytes in the skin (17). Additional observations provide further evidence linking altered Wnt signaling and cutaneous fibrosis. For instance, Tsk1 mice with progressive skin fibrosis showed aberrant expression of selected Wnts and their endogenous inhibitors in the skin (19). Moreover, analysis of skin biopsies from patients with SSc revealed altered expression of Wnts and their regulators (20). Of note, skin and lung, the most prominent targets for fibrosis in SSc, express all components of the Wnt pathway, including ligands, receptors, inhibitors and intracellular signal transducers. Together, these observations suggest a plausible role for altered Wnt signaling in the pathogenesis of fibrosis in SSc.
In the present studies, we used transgenic mice to examine effects of Wnt10b on cutaneous fibrogenesis. The results demonstrate that Wnt10b overexpression in transgenic mice resulted in potent fibrogenesis and replacement of subcutaneous adipose tissue with fibrosis. Elevated Wnt10b signaling in the skin correlated with increased expression of collagen. Fibroblasts explanted from transgenic skin showed sustained expression of Wnt10b and activation of canonical β-catenin signaling that was associated with elevated expression of fibrotic genes and concurrent suppression of adipogenic genes. Ectopic Wnt10b abrogated adipogenesis in preadipocytes, and promoted increased collagen gene expression in fibroblasts. Lesional skin from mice with bleomycin-induced scleroderma showed markedly elevated levels of Wnt10b and Axin2, indicating locally enhanced β-catenin activity. Taken together, these results provide compelling evidence for the fibrogenic potential of Wnt10b via β-catenin, and suggest that targeting aberrant Wnt10b expression or activity might be a novel therapeutic strategy for the control of pathological fibrosis.
MATERIALS AND METHODS
Animals and Experimental Protocols
All animal protocols were institutionally approved by the Northwestern University Animal Care and Use Committee. FABP4-Wnt10b transgenic mice harbor the Wnt10b gene under the control of a 7.6-kb fragment of the FABP4 promoter/enhancer (17). FABP4-Wnt10b mice were crossed to FVB background, and heterozygous female were studied at six months of age. Each experimental group consisted of 4–6 mice.
The association of Wnt10b expression with fibrosis was investigated in a mouse model of scleroderma induced by bleomycin. 6–8 week-old female C57BL/6J mice (Jackson Laboratory) were given daily subcutaneous injections of bleomycin for 14 days, and lesional skin was harvested at 28 day and analyzed as previously described (21).
Histochemical Analysis
Consecutive 4-μm serial sections of paraffin-embedded tissue from the interscapular skin were stained with hematoxylin and eosin (H&E). To evaluate collagen content and organization, deparaffinized sections of skin were stained with Mason’s trichrome or Picrosirius Red (22). Dermal thickness was determined at five different locations per slide for each mouse (21). After identification by Astra blue staining (23), mast cells in the dermis were quantified in six random fields per mouse.
Immunohistochemistry
Sections from skin were treated with target retrieval solution (DAKO). Primary antibodies at 1:100 against α-SMA (Sigma), phospho-Smad2 (Cell Signaling) were used. Bound antibodies were detected using secondary antibodies from the Histomouse-Max kit (Zymed) and the DakoCytomation Envision+System-HRP according to the manufacturers’ instructions. Substitution of primary antibody with isotype-matched IgG served as a negative control. Sections were counterstained with hematoxylin, and viewed under an Axioskop microscope (Zeiss). Images were captured by Nuance Multiple Spectra CCD with Nuance 2.10 software.
Quantification of Tissue Collagen
Collagen content was quantified by colorimetric assays from 8 mm punch skin biopsies. The tissues were diced in 0.5 M glacial acetic acid and centrifuged at 10,000 rpm for 15 min, and collagen content was determined in supernatants by Sircol-colorimetric assays (Biocolor).
Serum Adiponectin
Serum adiponectin levels were determined by enzyme-linked immunosorbent assay (ELISA) (R&D).
RNA Isolation and real-time quantitative Polymerase Chain Reaction (qPCR)
Total RNA was isolated from skin tissue or explanted fibroblasts using Trizol reagent (Invitrogen). Reverse transcription was performed using qScript™ cDNA SuperMix (Quanta Biosciences). Real-time qPCR was performed in triplicates using SYBR Green Master Mix (ABI) in an ABI Prism 7900 sequence detection system as described (24). The following mouse primer pairs were used for the analyses: Wnt10b, 5′-TGGGACGCCAGGTGGTAA-3′(forward) and 5′-CTGACGTTCCATGGCATTTG-3′(reverse); Axin2, 5′-GAGGCAGAAGCCACACAGAGA-3′(forward) and 5′-CTGGCCGACAGTGCAAGAC-3′(reverse); COL1A1, 5′-CCTGAGTCAGCAGATTGAGAA-3′(forward) and 5′-ACTGAACTTGACCGTACACCAGTACTCTCCGCTCTTCAA-3′(reverse); 36B4, 5′-AGATGCAGCAGATCCGCAT-3′ (forward) and 5′-GTTCTTGCCCATCAGCACC-3′(reverse). The results are expressed as fold-change in mRNA levels relative to 36B4 in each sample.
Cell cultures and differentiation assays
Primary fibroblast cultures were established by explantation from skin biopsies from the interscapular region of transgenic mice and wildtype littermates. Cultures were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% vitamins, 100 U/ml penicillin/ streptomycin and L-glutamine (BioWhittaker) at 37°C in 5% CO2 atmosphere. Cultures of low-passage skin fibroblasts were studied at early confluence (23). Mouse 3T3-L1 preadipocytes from ATCC were seeded in 24-well plates. At early confluence, cultures were infected with Wnt10b adenovirus (Ad-Wnt10b) or control virus (Ad-GFP) and incubated in differentiation media (DM2-L, Zen-Bio) for 48 h, followed by incubation with adipocyte media (AM, Zen-Bio) media for up to 7 d. At the end of the incubation, cells were stained with Oil Red O (25). Experiments were repeated at least three times. Cellular Oil Red O uptake was quantified by dissolving Oil Red O in 2-propanol and measuring optical density of supernatant at λ510 nm (26). In other experiments, cultures were stained with primary antibodies specific for perilipin (R&D) and Alexa® 594-conjugated chicken-anti-rabbit IgG as secondary antibody. Nuclei were identified with DAPI.
Primary cultures of human dermal fibroblasts were established by explantation from neonatal foreskins (27). Tissues were obtained in compliance with the Institutional Review Board for Human Studies. Fibroblasts were maintained at 37°C in an atmosphere of 5% CO2 in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS, 1% vitamins, 1% penicillin/streptomycin, and 2 mM L-glutamine (BioWhittaker), and studied between passages 4 and 8. Wnt10b retrovirus infections of confluent fibroblasts were performed as described (18).
Western analysis
Total protein was extracted from skin tissue or explanted fibroblasts. Aliquots containing equal amounts of proteins (10–15 μg) were subjected to Western analysis using primary antibodies to Type I collagen (1:400; Southern Biotechnology, Birmingham, AL), α-SMA (1:5000, Sigma), FABP4 (1:1000, BD), active β-catenin (1:1000, Millipore) or GAPDH (1:200, Santa Cruz) (28). Membranes were incubated with appropriate secondary antibodies, and antigen–antibody complexes were visualized by chemiluminescence according to the manufacturer’s instructions (Pierce). The autoradiographs were scanned and the band intensities were determined by ImageJ software (http://rsb.info.nih.gov/ij/index.html). Results were normalized against the intensity of the GAPDH in each sample.
Statistical Analysis
Results are expressed as the means ± SD. Student’s t-test was used for comparison between two groups. p values <0.05 were considered statistically significant.
RESULTS
Elevated Wnt10b expression and activity in skin fibrosis
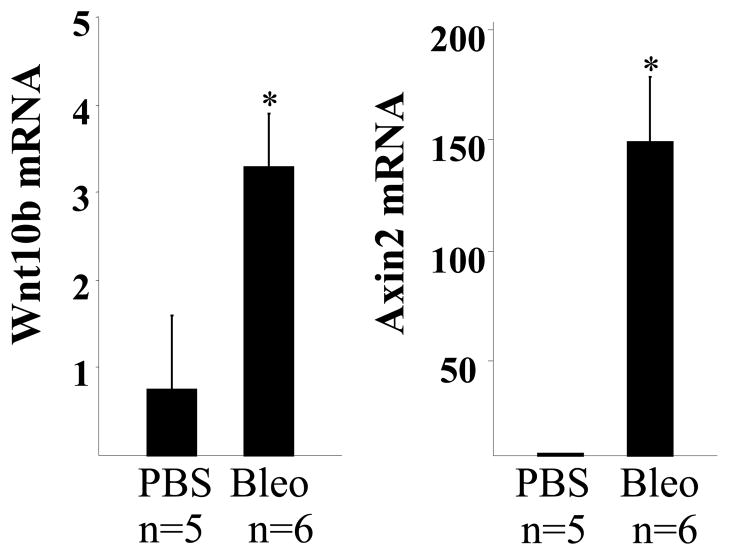
To evaluate Wnt10b expression in skin fibrosis, we investigated bleomycin-induced mouse scleroderma (29). C57BL/6J mice were given daily injections of subcutaneous PBS or bleomycin for 14 days, lesional skin was harvested on day 28 and examined by H&E stains. A > 70% increase in dermal thickness was evident in the lesional skin from bleomycin-treated mice, while the subcutaneous adipose layer showed marked atrophy (data not shown). Real-time qPCR analysis showed that Wnt10b mRNA was increased 4-fold from bleomycin-injected mice compare to PBS-treated controls (Fig. 1). The mRNA levels of Axin2, a well-known canonical Wnt target gene, showed a ~100 fold increase (Fig. 1). These results demonstrate elevated Wnt10b expression and activity associated with experimentally-induced skin fibrosis.
Figure 1. Increased Wnt10b expression and activity in skin biopsies from mice with bleomycin- induced fibrosism.
Total RNA was isolated form lesional skin from bleomycin-injected mice (n=6) and control mice (n=5). Gene expression levels were examined by real-time qPCR. Results were normalized with 36B4 and represent the means ±SD of triplicate determinations from 5–6 mice in each experimental group. *, p<0.05.
Spontaneous dermal fibrosis and adipose tissue loss in Wnt10b transgenic mice
FABP4-Wnt10b transgenic mice were used to investigate the effect of Wnt10b in the skin. There was no significant difference in body weight between FABP4-Wnt10b transgenic mice and wildtype littermates fed normal chow diet for up to six months (data not shown). However, striking loss of visceral fat was evident in the FABP4-Wnt10b transgenic mice, with markedly reduced epididymal and perirenal adipose tissue (data not shown). Serum levels of adiponectin, an adipokine produced exclusively by adipose tissue, showed a >80% reduction compared to wildtype mice (0.7 μg/ml in transgenic mice vs 5.6 μg/ml in wildtype mice, p<0.005).
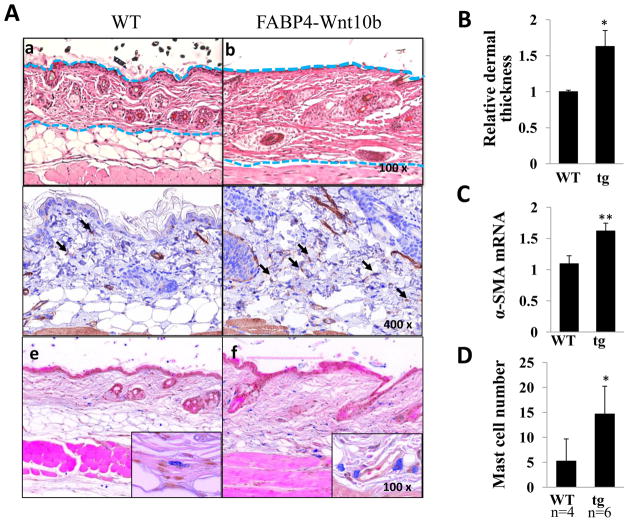
Examination of the skin revealed marked differences. Abundant subcutaneous adipose tissue consisting of 2–3 layers of large adipocytes was seen in wildtype mice, whereas in Wnt10b transgenic mice adipocytes were virtually absent in the hypodermis (Fig. 2A). The adipose tissue was largely replaced by fibrous tissue. Although the transgenic epidermis appeared to be similar to wildtype, the dermis showed > 60% increase in thickness (Fig. 2B). Because myofibroblast differentiation plays a central role in the development and progression of tissue fibrosis, we examined the expression of myofibroblast marker α-SMA. Immunohistochemistry showed a significant increase in the number of α-SMA-positive cells in the dermis of transgenic mice (Fig. 2A, middle panels). Moreover, qPCR demonstrate a 50% increase in α-SMA mRNA level (Fig. 2C). While the thickened dermis from transgenic mice showed a general paucity of infiltrating cells, cells with prominent granules were noted in the reticular dermis, generally in close proximity to blood vessels. These cells, identified as mast cells by Astra blue staining (Fig. 2A, lower panels) showed > 3-fold increase in numbers in Wnt10b transgenic mice compared to wildtype controls (Fig. 2D).
Figure 2. Spontaneous dermal fibrosis and adipose tissue loss in Wnt10b transgenic mice.
A. Skin biopsies were taken from the interscapular region of wildtype mice (a, c, e) and FABP4- Wnt10b transgenic mice (b, d, f) at 6 months of age. a, b, H&E staining; c, d, IHC of α-SMA; e, f, atra blue staining. Dashed lines indicate the extent of the dermis. Arrows indicate α-SMA positive fibroblasts. B. The shortest distance from epidermis to top of the subcutaneous adipose tissue was measured at 5 separate sites for each biopsy. The results, normalized with mean dermal thickness in wildtype mice, represent the means ± SD of five determinations from 4–6 mice in each experimental group. C. RNA was isolated from the skin and examined by real-time qPCR. The results, normalized with 36B4, represent the means ± SD of triplicate determinations from 3–5 mice in each experimental group. D. Numbers of Astra blue-positive cells in dermis were counted in 6 random field for each mouse. Results present the means ± SD in four wildtype and six transgenic mice. *, p<0.05; **, p<0.005.
Increased collagen accumulation in Wnt10b transgenic mice skin
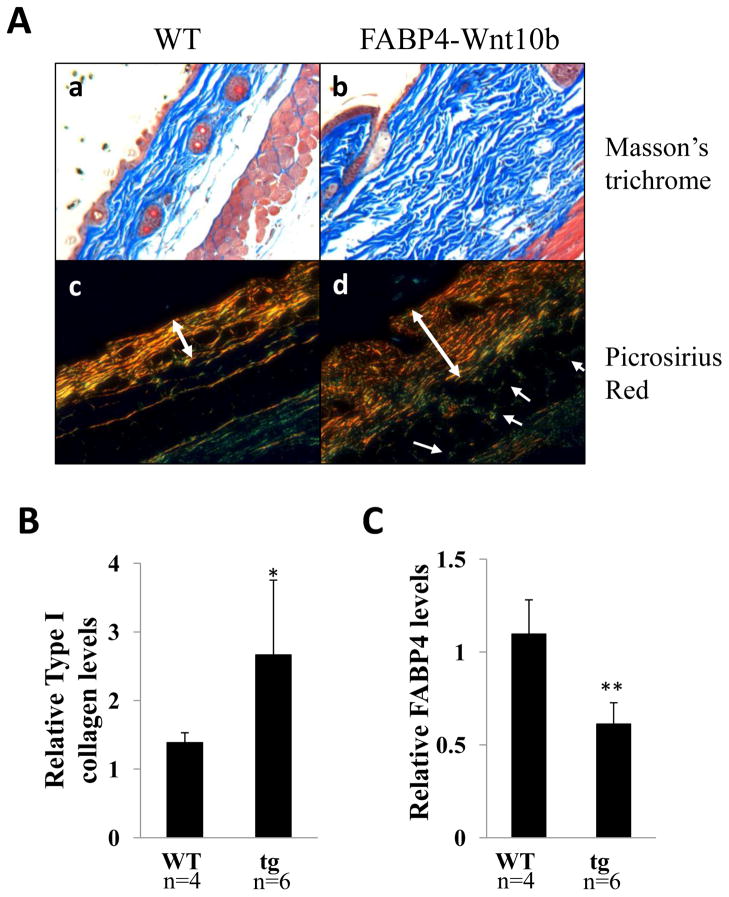
Masson’s trichrome and Picrosirius Red stains were performed on skin sections. The result showed markedly increased collagen deposition in the dermis from FABP4-Wnt10b transgenic mice (Fig. 3A). Collagen bundles were thicker and more compact. Moreover, Picrosirius Red stains showed that collagen fibers in transgenic mice had a woven architecture distinct from the nearly parallel pattern seen in wildtype mice, and displayed enhanced refraction under polarized light, indicating more mature collagen (30). Collagen deposition was seen between muscle bundles in transgenic but not wildtype mice. The accumulation of collagen in the skin was further evaluated by immunoblot analysis. The results showed a 95% increase in dermal Type I collagen levels in transgenic mice compared to wildtype controls (Fig. 3B). The increase in collagen was confirmed by Sircol assays (data not shown). In contrast, levels of FABP4, a marker of adipocytes, were significantly reduced in the skin from Wnt10b transgenic mice (Fig. 3C).
Figure 3. Increased cutaneous collagen accumulation in Wnt10b transgenic mice.
A. Skin biopsies were obtained from the back of wildtype (a, c) and FABP4-Wnt10b transgenic mice at 6 months of age and stained for. Masson’s trichrome (upper panels) or Picrosirius Red (lower panels). Representative images. Arrows indicate collagen bundles within muscle layer. Double arrows indicate dermal borders. B. Total protein lysates were extracted from interscapular skin were examined by immunoblot analysis. Relative levels of Type I collagen and FABP4 were determined by densitometry. The results, normalized to GAPDH, represent the means ± SD of triplicate determinations of 4–6 mice in each group. *, p<0.05; **, p<0.005.
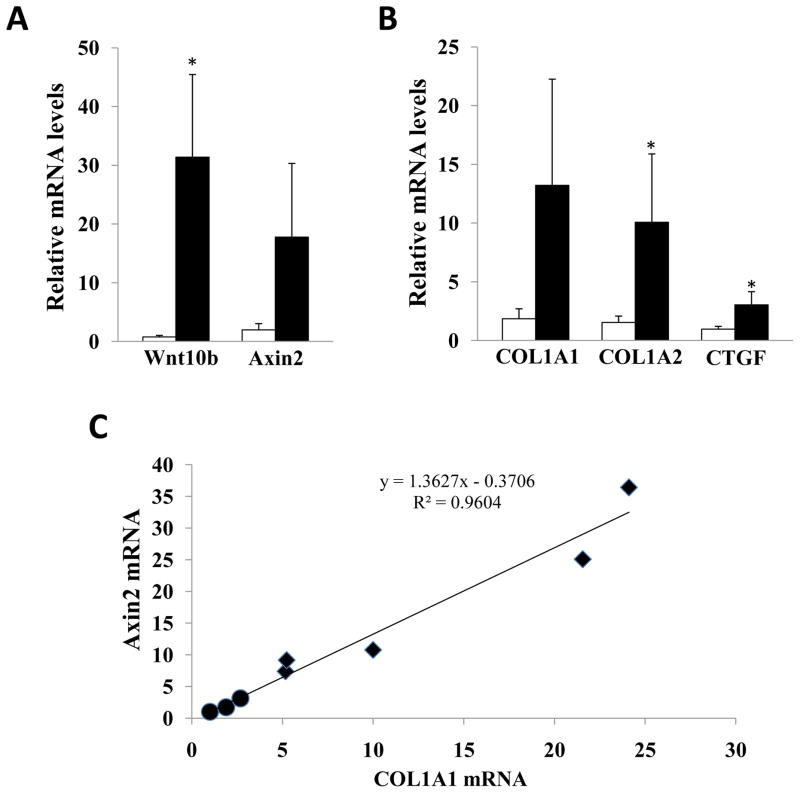
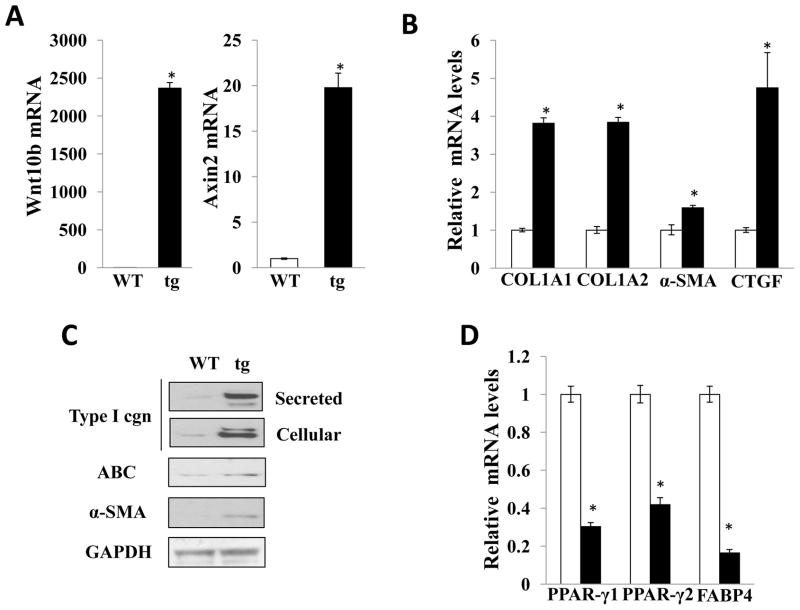
Next, profibrotic gene expression in the skin was evaluated by real-time qPCR. Skin levels of Wnt10b mRNA were 40-fold greater in transgenic mice than in wildtype littermates (Fig. 4A). To determine if the Wnt10b transgene was biologically active, the levels of Axin2 mRNA were examined. The results showed a 10-fold increase in Axin2 mRNA levels in Wnt10b transgenic mice. Expression of mRNA for COL1A1, COL1A2, and CTGF was 7.1-, 6.5-, and 3.2-fold higher, respectively, in skin from Wnt10b transgenic mice (Fig. 4B). Moreover, the increase in COL1A1 mRNA was strongly correlated with Axin2 mRNA levels in the same tissues (Fig. 4C). These results therefore indicate that transgenic mice display increased Wnt10b expression and activity in the skin, which correlated with increased collagen gene expression.
Figure 4. Cutaneous collagen mRNA expression positively correlates with Wnt10b expression and activity.
RNA isolated from the skin was examined by real-time qPCR. The results, normalized with 36B4, represent the means ± SD of triplicate determinations from 3–5 mice in each group (A, B). Open bars, wildtype mice; closed bars, transgenic mice. C. Type I collagen mRNA levels were correlated to Axin2 mRNA expression in the same tissues. ●, wildtype mice; ■, FABP4- Wnt10b transgenic mice.
TGF-β, a key mediator of fibroblast activation and implicated in a variety of fibrotic disorders, induces fibrotic responses in resident fibroblasts via both Smad-dependent and Smad-independent signaling. In order to determine whether upregulated Wnt leads to fibrosis by stimulating TGF-β, we assessed the in situ expression of phosphorylated-Smad2, a highly sensitive and specific marker of TGF-β activity, by IHC. No significant difference between transgenic and wildtype mice in the numbers of phospho-Smad2 positive fibroblasts was observed (data not shown).
Elevated fibrotic gene expression in explanted transgenic fibroblasts
Fibroblasts are the major cell population responsible for ECM production in skin. Because Wnt10b transgenic mice showed dermal fibrosis, we wondered whether FABP4-driven expression of Wnt10b was associated with constitutive activation of dermal fibroblasts. To address this question, primary skin fibroblast cultures from Wnt10b transgenic mice and wildtype littermates were established by explantation. After 2–3 serial passages, Wnt10b transgenic fibroblasts and wildtype fibroblasts showed comparable morphologic characteristics and proliferation rates (data not shown). Confluent transgenic fibroblasts showed a marked and persistent elevation in Wnt10b mRNA levels (Fig. 5A,). Levels of the canonical Wnt target Axin2 showed a 20-fold increase in Wnt10b fibroblasts. Type I collagen, α-SMA and CTGF mRNA (Fig. 5B) and protein levels (Fig. 5C) were all elevated in explanted Wnt10b fibroblasts. Activation of canonical Wnt signaling was detected by immunoblotting using antibodies to the active β-catenin (Fig. 5C). The expression of adipogenic markers FABP4, PPAR-γ1 and PPAR-γ2 was diminished in transgenic fibroblasts (Fig. 5D). To investigate effects of activated Wnt signaling on adipogenesis, fibroblasts were incubated with differentiation medium DM2-L. Wildtype fibroblasts readily underwent adipogenesis in response to DM2-L. In contrast, fibroblasts explanted from Wnt10b transgenic mice were relatively resistant to the adipogenic effects of DM2. Real-time qPCR showed that induction of adipogenic markers, FABP4 and adiponectin, was reduced in transgenic fibroblasts (data not shown).
Figure 5. Elevated fibrotic gene expression in explanted transgenic fibroblasts.
Fibroblasts were explanted by explantation from skin biopsies of wildtype and transgenic littermates, and examined at early passage in parallel. RNA was isolated and subjected to real-time qPCR. The results, normalized with 36B4, represent the means ±SD (A, B) of triplicate determinations from a representative experiment. Open bars, wildtype fibroblasts; closed bars, transgenic fibroblasts. C. Whole cell lysates were examined by immunoblot analysis. Representative images. D. Relative mRNA levels of adipogenic genes. Real-time qPCR results, normalized with 36B4, represent the means ± SD of triplicate determinations from a representative experiment. Open bars, wildtype fibroblasts; closed bars, transgenic fibroblasts. *, p<0.05.
Ectopic Wnt10b inhibits adipogenesis and induces fibrotic responses in vitro
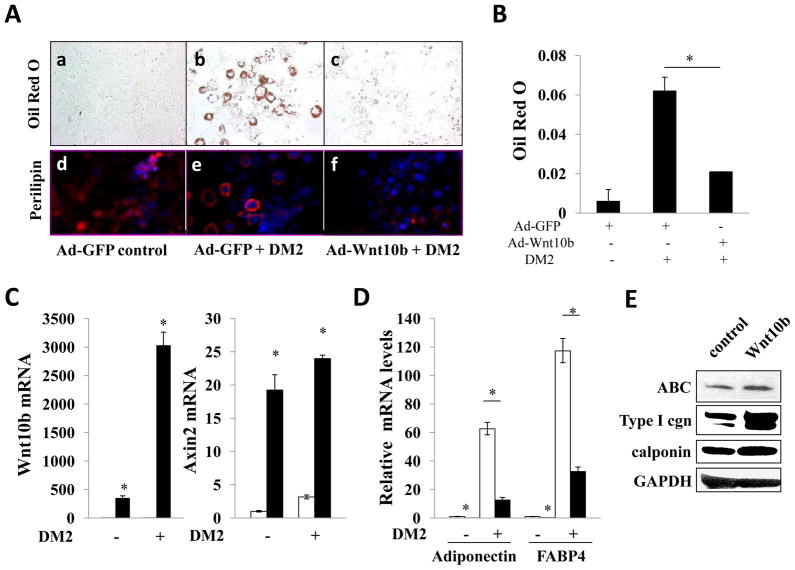
At six months of age, substantial dermal fibrosis in FABP4-Wnt10b transgenic mice was associated with a reciprocal reduction of subcutaneous adipose tissue. The role of Wnt10b in adipogenesis in vitro was investigated in 3T3-L1 cells. In the presence DM2-L differentiation media, 3T3-L1 cells acquired a rounded shape and accumulated prominent Oil Red O and perilipin-positive intracellular lipid droplets (Fig. 6A). Ectopic Wnt10b expression inhibited the induction of adipogenesis with a > 70% reduction in Oil Red O staining (Fig. 6B). Real-time qPCR confirmed overexpression of Wnt10b and elevated expression of Axin2 in Ad-Wnt10b infected cells (Fig. 6C). Wnt10b inhibited expression of the adipogenic markers, PPARγ, FABP4 and adiponectin, in both unstimulated and in DM2-stimulated preadipocytes (Fig. 6D and data not shown). The role of Wnt10b was further examined in normal fibroblasts. Confluent foreskin fibroblasts were infected with Wnt10b and following 5 day incubation, cultures were harvested and whole cell lysates were analyzed. Immunoblots showed a marked increase in Type I collagen levels in Wnt10b-expressing fibroblasts (Fig. 6E). Calponin, a novel myofibroblast marker, similarly showed a significant increase. These results therefore demonstrate that Wnt10b is sufficient by itself to cause a reciprocal stimulation of fibrogenic markers and repression of adipogenesis in mesenchymal cells.
Figure 6. Ectopic Wnt10b inhibits adipogenesis and induces fibrotic responses in vitro Confluent 3T3 L1 cells were infected with Ad-GFP or Ad-Wnt10b (30 MOI) for 48 h.
Adipogenic differentiation was induced as described in Materials and Methods. A. Cultures were fixed after 7 d, and stained with Oil Red O (upper panels) or anti-perilipin (lower panels). (Blue: DAPI; Red, perilipin). Original magnification, 200×. a,d, DMEM; b, c, e, f, adipogenic differentiation media. B. Cellular Oil Red O stain were extracted by isopropanol and examined at 510 nm. Results are means± SD of duplicate determinations from triplicate experiments. C. RNA was isolated and examined by real-time qPCR. The results, normalized with 36B4, represent the means ±SD of triplicate determinations from a representative experiment. D. Relative expression levels of adipogenic genes. Results represent the means ±SD of triplicate determinations from a representative experiment. Open bars, Ad-GFP; closed bars, Ad-Wnt10b. *, p<0.05. E. Foreskin fibroblasts were infected with Wnt10b retrovirus or empty retrovirus. Following incubation for 5 d, cultures were harvested and whole cell lysates were subjected to immunoblot analysis. Representative images.
DISCUSSION
Fibrosis in skin and internal organs is a hallmark of SSc and is a major factor contributing to poor prognosis. The pathogenesis of fibrosis in SSc remains poorly understood, and effective therapies are lacking. Multiple extracellular cues in addition to TGF-β are aberrantly expressed in patients with SSc, and might play a role in triggering or sustaining pathological fibrogenesis. Our studies provide evidence that the canonical Wnt10b is sufficient to induce striking skin fibrosis in transgenic mice. This was associated with a virtual disappearance of the subcutaneous adipose layers, and its replacement by fibrous tissue. These pathological changes are strikingly similar to the pathological changes seen in SSc skin, where progressive diseases is associated with replacement of adipose tissue by fibrotic tissue and with characteristic tethering of the skin to underlying connective tissues. Expression of Type I collagen and CTGF was markedly elevated in the Wnt10b transgenic skin as seen in SSc skin, whereas expression of genes involved in regulating adipogenesis were suppressed. These observations point to a reciprocal relationship between adipogenic and fibrogenic gene expression in the skin, and a crucial role for Wnt10b/β-catenin signaling in regulating the developmental balance between adipogenesis and fibrogenesis. Skin fibroblasts explanted from transgenic mice displayed elevated Wnt10b expression and β-catenin signaling that was associated with increased expression of fibrogenic markers, and resistance to induction of adipogenesis. Ectopic Wnt10b induced collagen gene expression in skin fibroblasts while abrogating adipogenesis. These observations suggest that ectopic Wnt10b expression via autocrine or paracrine pathways induces β-catenin signaling in cutaneous mesenchymal cells, leading to their activation and the induction of sustained fibrotic responses.
The Wnt10b transgenic mice are viable and appear healthy. This implies that despite the inhibitory effects of Wnt10b on adipocyte differentiation (31), neonatal adipogenesis in these mice is unaffected. This may indicate that the FABP4 promoter driving Wnt10b expression gets turned on at a later stage during development. Moreover, no significant differences in weight were seen between wildtype and transgenic mice, despite the substantial reduction in both subcutaneous and visceral fat deposits seen at 6 months of age. This might reflect a concomitant increase in skin thickness and weight. Indeed, we observed a 33% increase in skin weight in the transgenic mice at 6 months. Transgenic mice spontaneously develop significant dermal thickening, with marked accumulation of collagen in the skin and increased expression of Type I collagen genes. No evidence of inflammation was apparent, indicating that the Wnt10b transgenic mouse is a novel model for inflammation-independent fibrosis. However, we noted an increase in Astra-blue staining mast cells, and observed evidence of mast cell degranulation in the deep dermis of transgenic mice. Thus, in Wnt10b transgenic mice, the effects of Wnt10b overexpression appears to most likely cause fibrosis by affecting mesenchymal progenitor differentiation rather than regulating inflammation. There was no significant difference in the levels of phospho-Smad2 expression in the skin from wildtype and transgenic mice, suggesting that fibrogenic effects of Wnt10b are not primarily mediated through induction of endogenous TGF-β. Fibroblasts explanted from the fibrotic skin showed sustained expression of Wnt10b gene, accompanied by increased Axin2 mRNA levels, indicating the persistence of active β-catenin signaling in these cells in vitro. Constitutive β-catenin signaling in explanted fibroblasts was associated with elevated expression of Type I collagen genes and a reciprocal decrease in expression of PPARγ and its target gene, FABP4. PPARγ is one of the master regulators for adipogenesis, and its expression is known to be suppressed by Wnt10b in a variety of cell types (18). Multipotent mesenchymal progenitor cells have the ability to differentiate into various cell types, including adipocytes and fibroblasts. PPARγ plays a crucial role in lineage specification of mesenchymal progenitor cells. Suppression of PPARγ by canonical Wnts inhibits adipogenesis in mesenchymal progenitors, and promotes their osteoblastic differentiation (31). We observed that the marked activation of β-catenin signaling in the skin, and in explanted fibroblasts from Wnt10b transgenic mice, was associated by substantial reduction of PPARγ levels. Moreover, infection of 3T3-L1 preadipocytes with Wnt10b adenovirus dramatically reduced their ability to differentiate into adipocytes in response to PPARγ ligands in vitro. In normal fibroblasts, ectopic Wnt10b induced collagen gene expression. Reduction of PPARγ expression and loss of subcutaneous adipose tissue observed in transgenic mice was associated with increasing dermal thickness and expression of fibrogenesis markers, such as Type I collagen. These observations suggest that ectopic overexpression of Wnt10b in cutaneous mesenchymal cells resulted in suppression of adipogenic programs and concomitant up-regulation of fibrogenic programs in these cells, and their differentiation into fibroblasts directly contributed to excessive cutaneous collagen deposition.
While Wnt signaling is essential for normal embryonic development, aberrant Wnt expression, regulation or activity in adults is implicated in cancer, aging and a variety of chronic diseases (12–14). The potential relevance of Wnt signaling to fibrogenesis has only recently begun to be appreciated. Evidence from both animal models and human fibrotic disorders links aberrant Wnt ligand expression or unregulated Wnt activity, due for example to loss of endogenous inhibitors, with pathological tissue remodeling and fibrosis. Aging-associated fibrosis of muscle can be attributed to upregulation of canonical Wnt signaling (32). In transgenic mice, activation of β-catenin by using a stabilized mutant form that resists ubiquitin-mediated degradation (Catnblox(ex3)) resulted in exuberant wound healing and increased local collagen synthesis (33). Genes important in tissue repair and fibrosis such as PAI-1, CTGF and fibronectin are known to be Wnt-β-catenin transcriptional targets, although the mechanism of their regulation by Wnts is not well-defined (34–37).
Lungs from patients with idiopathic pulmonary fibrosis (IPF) show increased nuclear β-catenin (15). Moreover, fibrotic foci showed elevated expression of WISP1 (a Wnt target gene), and increased phosphorylation of LRP6 and GSK-3β indicative of activated Wnt signaling at fibroblastic foci (38, 39). Genome-wide transcriptional profiling of IPF lungs revealed elevated expression of genes coding for Wnts, Wnt receptors, Wnt regulators, and Wnt targets such as osteopontin and WISP1 (39, 40). Moreover, explanted IPF fibroblasts maintain enhanced β-catenin activation in culture (37). Importantly, a recent study demonstrated that inhibition of Wnt/β-catenin/CREB binding protein signaling reverses bleomycin-induced pulmonary fibrosis (41). We have detected elevated β-catenin levels in fibrotic lungs from all three SSc patients with advanced lung disease that were examined (Lam et al, manuscript under revision). In these lungs, β-catenin nuclear accumulation was prominent in epithelial cells and fibroblastic cells in fibrotic foci. Several Wnt ligands abnormally expressed in TSK1 mouse, a scleroderma model associated with extensive subcutaneous fibrosis (20). Aberrant Wnt expression in these mice preceded the appearance of fibrosis, supporting the notion that altered Wnt expression can redirect mesenchymal cell differentiation toward profibrotic fibroblast phenotype in both cutaneous (shown here) and subcutaneous tissues (20). Moreover, in the present studies we also detected elevated Axin2 mRNA levels, indicative of active Wnt signaling, in mice treated with subcutaneous bleomycin, a model for scleroderma. Interrogating microarray datasets generated from expression profiling of SSc skin biopsies revealed increased mRNA levels of specific Wnt receptors (e.g. FZD2) and Wnt target genes (e.g. CCND, ASPM), accompanied by reduced expression of Wnt inhibitors (e.g. WIF1, DKK2) (Wei et al, unpublished,(42, 43)). These correlative observations suggest that deregulated Wnt signaling is associated with aberrant tissue repair in the skin and lungs, and implicate Wnts in the excessive matrix accumulation and myofibroblast differentiation that underlie fibrosis in SSc.
In summary, the present results demonstrate that FABP4-driven ectopic expression of Wnt10b results in cutaneous β-catenin signaling in transgenic mice that is accompanied by marked loss of subcutaneous adipose tissue. PPARγ expression and adipogenesis are reduced in skin, with reciprocal development of dermal fibrosis and the up-regulation of collagen and other markers of fibrogenesis in the absence of significant inflammation. In light of the potent inhibitory effects of canonical Wnt signaling on adipocyte differentiation mediated via the adipogenic master regulator PPARγ, our results suggest that excessive Wnt10b-β-catenin signaling in cutaneous mesenchymal cells causes suppression of adipogenesis and concomitant promotion of fibrogenesis. These results clearly indicate that canonical Wnt signaling is sufficient to induce skin fibrosis. Wnt signaling might therefore be an important novel target for the control of fibrosis in SSc and related fibrosing conditions.
Acknowledgments
We thank Drs Cara J. Gottardi, Anna Lam (Northwestern University) and members of the laboratory for helpful discussions. Supported by grants to O.A.M. from the National Institutes of Health (DK51563 and DK62876) and to J.V. from the National Institutes of Health (AR49025) and the Scleroderma Research Foundation.
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2(3):134–44. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 3.Krieg T, Abraham D, Lafyatis R. Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactions. Arthritis Res Ther. 2007;9 (Suppl 2):S4. doi: 10.1186/ar2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16(6):733–8. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- 5.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postlethwaite AE, Harris LJ, Raza SH, Kodura S, Akhigbe T. Pharmacotherapy of systemic sclerosis. Expert Opin Pharmacother. 11(5):789–806. doi: 10.1517/14656561003592177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–14. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 8.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 9.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20(11):1394–404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 10.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2(9):e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohtola J, Myers J, Akhtar-Zaidi B, Zuzindlak D, Sandesara P, Yeh K, et al. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135(13):2321–9. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien AJ, Moon RT. WNTS and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–57. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129(7):1614–27. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162(5):1495–502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5(3):e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–9. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayle J, Fitch J, Jacobsen K, Kumar R, Lafyatis R, Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128(4):871–81. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 20.Lemaire R, Farina G, Bayle J, Dimarzio M, Pendergrass SA, Milano A, et al. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J Invest Dermatol. 130(6):1514–23. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Melichian DS, Chang E, Warner-Blankenship M, Ghosh AK, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174(2):519–33. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, et al. Targeted disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;165(1):203–17. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagawa S, Lakos G, Mori Y, Yamamoto T, Nishioka K, Varga J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J Invest Dermatol. 2003;121(1):41–50. doi: 10.1046/j.1523-1747.2003.12308.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya S, Wei J, Melichian DS, Milbrandt J, Takehara K, Varga J. The transcriptional cofactor nab2 is induced by tgf-Beta and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS One. 2009;4(10):e7620. doi: 10.1371/journal.pone.0007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausman GJ. Techniques for studying adipocytes. Stain Technol. 1981;56(3):149–54. doi: 10.3109/10520298109067302. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–7. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 27.Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol. 1999;112(1):49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh AK, Bhattacharyya S, Lakos G, Chen SJ, Mori Y, Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50(4):1305–18. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Varga J. In perspective: murine models of scleroderma. Curr Rheumatol Rep. 2008;10(3):173–82. doi: 10.1007/s11926-008-0030-9. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, de Crombrugghe B, Davidson JM, Bhowmick NA. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 176(1):98–107. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19(6):612–7. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 33.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, et al. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99(10):6973–8. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 285(11):8196–206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal. 2007;1(3–4):175–83. doi: 10.1007/s12079-007-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafson B, Smith U. Activation of canonical wingless-type MMTV integration site family (Wnt) signaling in mature adipocytes increases beta-catenin levels and leads to cell dedifferentiation and insulin resistance. J Biol Chem. 285(18):14031–41. doi: 10.1074/jbc.M110.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He W, Tan R, Dai C, Li Y, Wang D, Hao S, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem. 285(32):24665–75. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173(2):188–98. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 107(32):14309–14. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi JT, Pergamenschikov A, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A. 2003;100(21):12319–24. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemaire R, Farina G, Bayle J, Dimarzio M, Pendergrass SA, Milano A, et al. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J Invest Dermatol. 130(6):1514–23. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]