Abstract
The relationship between myocardial blood flow (MBF) and stenosis severity has been determined previously using cyclotron-produced radiotracers such as 15O-H2O and 13N-ammonia. An attractive alternative to overcome the limitations related to the use of cyclotron might be to use the generator-produced Rubidium-82 as a flow tracer. The current study was undertaken to investigate the relationship between MBF and coronary vasodilator reserve (CVR) as measured by Rubidium-82 positron emission tomography (PET) and the percent diameter stenosis as defined by quantitative coronary arteriography.
Methods
We prospectively evaluated 22 individuals: 15 patients (60±11 years of age) with angiographically documented coronary artery disease (CAD) and seven age-matched (56±9 years) asymptomatic individuals without risk factors for CAD. Dynamic Rubidium-82 PET was performed at rest and after dipyridamole vasodilation. MBF, CVR and an index of “minimal coronary resistance” (MCR) were assessed in each of the three main coronary territories.
Results
Rest and stress MBF in regions subtended by vessels with <50% diameter stenosis was similar to that of the individuals with no risk factors for CAD. As a result, CVR was also similar in the two groups (1.9, interquartile [IQ] range from 1.7 to 2.7 vs. 2.2, IQ range from 2 to 3.4 respectively, p=0.09)). CVR successfully differentiated coronary lesions with stenosis severity 70% to 89% from those with 50% to 69% stenosis (1, IQ range from 1 to 1.3 vs. 1.7, IQ range from 1.4 to 2), respectively, p=0.001. In addition, hyperaemic MBF (r2=.74, p<0.001), CVR (r2=.69, p<0.001), and MCR (r2=.78, p<0.001) measurements were inversely and non-linearly correlated to the percent diameter stenosis on angiography.
Conclusion
MBF and CVR are inversely and non-linearly correlated to stenosis severity. Quantitative Rubidium-82 PET can be a clinically useful tool for an accurate functional assessment of CAD.
Keywords: Myocardial blood flow, Positron Emission Tomography, Rubidium-82
INTRODUCTION
Although myocardial perfusion is a sensitive means for diagnosing or ruling out the presence of obstructive coronary artery disease (CAD) in individual patients, it often uncovers only the coronary territory supplied by the most severe stenosis and, thus, underestimates the extent of anatomic disease. This is because in patients with CAD, coronary vasodilator reserve (CVR) is often abnormal even in territories supplied by non-critical angiographic stenoses (1, 2), thereby reducing the heterogeneity of flow between “normal” and “abnormal” zones and limiting the ability to delineate the presence of multi-vessel CAD.
Measurements of MBF (in mL/min/g of myocardium) and CVR may help overcome the limitations of relative perfusion assessments to uncover the presence of multi-vessel CAD. Positron emission tomography (PET) measures MBF and CVR with high precision and reproducibility. The relation of these parameters to coronary stenosis has been validated with the use of Oxygen-15-water (15O-H2O) and N-13 Ammonia (13N-ammonia) as perfusion tracers (3–5). However, these radiopharmaceuticals are cyclotron products with a very short physical half-life necessitating access to a medical cyclotron.
Rubidium-82 is a generator-product and the most widely used radiopharmaceutical to evaluate myocardial perfusion with PET clinically. Although absolute quantification of MBF with Rubidium-82 is feasible (6–10), it is challenging and not widely used in clinical practice. We have developed a simplified (11) and reproducible (12) approach to quantification of MBF with Rubidium-82 PET. Our objective was to quantify MBF at rest and during vasodilator stress using Rubidium-82 PET dynamic imaging in individuals with clinical atherosclerosis and to examine the relationship between blood flow changes and quantitative coronary angiography results across a wide spectrum of severity.
MATERIALS AND METHODS
Study group
We prospectively evaluated 15 patients (seven male, 60±11 years) with angiographically documented CAD undergoing Rubidium-82 PET imaging, and seven age-matched healthy volunteers with a low likelihood of CAD based on the absence of symptoms and risk factors. Patients with a history of prior CABG or valvular heart disease were excluded. The study complies with the Declaration of Helsinki and it was approved by the Institution Review Board of the Brigham and Women’s Hospital, Boston, Massachusetts; written informed consent was obtained from all individuals who have undergone a PET study for research purposes. Six patients had a previous remote myocardial infarction, based on clinical history or the presence of pathological Q waves on the resting ECG. Infarct-related coronary arteries and those inadequately visualised in coronary angiography were excluded from analysis. In vessels showing multiple stenoses in series, only the most severe stenosis was considered for analysis. All patients were clinically stable between the PET study and the angiographic procedure. The mean time interval between the PET scan and coronary angiography was 22 (±20) days.
PET Imaging Protocol
All patients were studied using a whole body PET-CT scanner (Discovery ST Lightspeed 16, GE Healthcare, Milwaukee, WI). Patients were studied following an overnight fast and 24-hour cessation of all caffeine- or methylxanthine-containing substances. All antinaginal medications were held on the morning of the test. Following a scout CT acquisition (120 kVp, 10 mA) used for proper patient positioning, a CT transmission scan was acquired (140 kVp, 20–30 mA) for subsequent attenuation correction. Beginning with an i.v. bolus administration of 40–60 mCi of Rubidium-82 (1,480–2,220 MBq), twenty six dynamic frames were acquired over six minutes as follows: eighteen 5-sec acquisitions were first performed, followed by six 15-sec acquisitions, one 120-sec acquisition and finally, one 60-sec acquisition. Immediately after completion of the rest images, a standard i.v. dipyridamole (0.142 mg/kg/min) was infused for 4 minutes. Three minutes after termination of the dipyridamole infusion, a second dose of 40–60 mCi of Rubidium-82 was injected and images were recorded in the same acquisition sequence. A second CT transmission scan (140 kVp, 20–30 mA) was then acquired for attenuation correction of the stress images. The heart rate, systemic blood pressure, and 12-lead ECG were recorded at baseline and throughout the infusion of dipyridamole. The rate pressure product was calculated as heart rate multiplied by systolic blood pressure.
Quantification of MBF
The method used for quantification of regional MBF has been described in detail elsewhere (11). Left (LV) and right (RV) ventricle input functions were estimated automatically using our generalized factor analysis of dynamic sequences (GFADS) approach. After fitting the time-varying factor model to the dynamic data using a least-squares objective function, GFADS penalized spatial overlap between factor images while preserving the least-squares fit and non-negativity constraints on the factors and factor images. Next GFADS input functions were used in a two-compartment kinetic model, that modeled Rb-82 extraction fraction, to estimate MBF flow (K1, ml/g/min) and egress (k2, min-1) in the LAD, LCX and RCA territories. The two compartments modelled were the free Rb-82 in the extracellular space and the trapped Rb-82 in the intra-cellular space. The extraction fraction model used was that reported by Marshall et al.: E= −0.085 F + 0.0841 (12). Parametric polar maps of regional myocardial blood flow were then generated using the conventional 17-segment model. The size of each sectorial region of interest was adjusted according to the individual coronary anatomy, e.g., the size of the stenosed vessel, the anatomical location of the stenosis, and the dominance as defined on the coronary angiograms. Within each vascular territory, only segments showing reduced Rubidium-82 concentration on visual inspection of the dipyridamole-stress images were used for assessment of MBF and CVR. The severity of reduction was evaluated using a five point scoring system (from 0=normal to 4=absent uptake), according to the standard practise at the Department of Nuclear Medicine of Brigham and Women’s Hospital. A segmental score of at least 1 was taken as a threshold for characterisation of abnormal uptake. In coronary artery territories showing normal Rubidium-82 uptake on the stress images, all the segments assigned to this territory were used in the analysis. Because MBF at rest is related to the rate-pressure product, an index of cardiac work, resting MBF values were normalized for their respective rate-pressure product by dividing the resting blood flow value by the rate-pressure product, multiplied by a linear factor of 10,000 in each individual patient (4). From sthe stress and the uncorrected resting MBF measurements, coronary vasodilator reserve (CVR) defined as the ratio of hyperaemic to resting MBF was assessed in patients and normal volunteers. In addition, and in order to normalize the hyperaemic response to the coronary perfusion pressure, we estimated the “minimal coronary resistance” by dividing the mean arterial pressure in each patient by the flow value at maximal vasodilation.
The quantification method used in the current study yields reproducible measurements of blood flow, as demonstrated previously in 13 normal volunteers studied twice at an average time interval of 15 days. Flow values at rest and during hyperaemia in each major vascular territory differed randomly between the two measurements by an average of 10% to 15% (13).
Quantitative Coronary Angiograpy
Quantitative angiographic analysis was performed by use of a validated automated edge-detection algorithm (14). A range of coronary stenoses (from 20% to 100%) involving major coronary arteries was identified from selected images by use of angiographic projections minimizing the degree of vessel overlap. Both the percentage of luminal narrowing and the minimal luminal diameter (MLD) of the stenosed artery along with the adjacent reference segments were measured at end-diastole. Coronary vessels were grouped according to their degree of stenosis: <50%, 50% to 69%, 70% to 89%, and >90% of the vessel diameter.
Statistical analysis
Data analysis was performed using Stata 9.2 (StataCorp, Texas). With the exception of left ventricular ejection fraction (LVEF) and age, which are expressed as mean ±SD, all other continuous variables are presented as median and interquartile (IQ) range. The relationship between stenosis severity and hyperaemic response, CVR and MCR was assessed using mixed-model linear regression and Lowess smoothers (the quoted r2 values correspond to the former). Mixed models were employed since the values were taken from a maximum of three vessels within the same patients, and so the hierarchical relationship between vessels and patients needed to be accounted for. In the mixed-model analysis, patients were declared as a random effect. Stenosis was also categorised as being less than or greater than 50% and the hyperaemic response, CVR and MCR values in these vessels were also compared using a mixed model. The presence of risk factors on the outcome variables was also assessed using a mixed model. Kruskal-Wallis analysis was used to compare the MBF, CVR and MCR values with the level of stenosis in a vessel split into one of four categories: <50%, 50% to 70%, 70% to 90%, and >90%; for further comparison of the individual categories the Mann-Whitney test was employed. An identical approach was used for comparisons of resting perfusion in the corresponding segments. A two-sided probability value of <0.05 was used to define statistical significance.
RESULTS
Patient population
The baseline characteristics of the patients are summarized in table 1.
TABLE 1.
Patients (n=15) characteristics
| Characteristic | Value |
|---|---|
| Age (years) | 60±11 |
| Male gender | 7 (43%) |
| Previous myocardial infarction | 6 (40%) |
| LVEF | 57±10% |
| Dyslipidaemia | 9 (60%) |
| Diabetes | 6 (40%) |
| Family history of CAD | 4 (27%) |
| Smoking | 6 (40%) |
| Obesity | 3 (20%) |
| Hypertension | 7 (43%) |
| Typical angina | 8 (53%) |
| Atypical angina | 3 (20%) |
| Shortness of breath | 5 (33%) |
Values expressed as n (%), except left ventricular ejection fraction (LVEF) and age, which are expressed as mean ±SD. CAD: Coronary Artery Disease
Haemodynamic measurements
Comparisons of haemodynamic changes between patients and healthy volunteers and also from rest to dipyridamole stress are shown in table 2. There were no significant differences between patients and healthy volunteers in the increase in heart rate from rest to dipyridamole vasodilatation, although the change itself was significant (p=0.001 for patients and 0.018 for healthy volunteers). Similarly there were no significant differences between the two groups in blood pressure at rest and stress. Accordingly, the rate-pressure product in the patients was similar to that in the healthy volunteers both at base line and during dipyridamole vasodilatation but there were both increased compared to baseline in patients and healthy volunteers (p=0.002 for patients and 0.028 for healthy volunteers). Diastolic blood pressure was similar in both groups at rest and during dipyridamole vasodilatation. Likewise, the mean arterial pressure was similar in both groups at base line and during dipyridamole vasodilatation.
TABLE 2.
Haemodynamic changes
| Patients | Rest | Stress | P value |
|---|---|---|---|
| SBP | 134 (125,146) | 124 (114,146) | 0.146 |
| DBP | 69 (63, 77) | 70 (58, 76) | 0.955 |
| HR | 64 (55,75) | 79 (70,90) | 0.001 |
| RPP | 9375 (7370, 9792) | 10440 (8775, 12045) | 0.002 |
| MAP | 94 (86, 99) | 87 (78, 98) | 0.842 |
| HV | Rest | Stress | P value |
| SBP | 133 (123,156) | 150 (124,171) | 0.398 |
| DBP | 69 (61, 83) | 69 (67, 79) | 0.553 |
| HR | 62 (59, 78) | 90 (77, 91) | 0.018 |
| RPP | 9204 (7714, 11544) | 12425 (11284, 15390) | 0.028 |
| MAP | 95 (85,110) | 99 (66,106) | 0.753 |
SBP=systolic blood pressure; DBP=diastolic blood pressure; HV=healthy volunteers; HR=heart rate; RPP=rate pressure product; MAP=mean arterial pressure
Relationship between regional MBF and stenosis severity
Seven coronary vessels (LAD: 3, LCX: 3, RCA: 1) were excluded from the analysis due to poor visualization on coronary angiography, of which 6 were infarct related arteries
In the 38 vessels that were analysed, coronary lesions were identified in 12 left anterior descending arteries (LADs) (5 proximal, 6 middle, 1 distal), 12 left circumflex (LCxs) (3 proximal, 9 distal) and 14 right coronary arteries (RCAs) (3 proximal, 5 middle, 6 distal). Sixteen arteries had <50%, stenosis, 9 had 50%–69% stenosis, 6 arteries had 70%–89% stenosis and 7 more than 90% stenosis. At rest, MBF in regions supplied by vessels with ≥70% stenosis (0.9, IQ range 0.8, to 1.1 mL/g/min,) was comparable to that in regions supplied by vessels with <50% stenosis (0.9, IQ range 0.8 to 0.9, mL/g/min, p=0.9, Table 3). Resting blood flow was similar to that in the healthy volunteers (0.9, IQ range 0.8 to 0.9 mL/g/min, p=0.46).
Table 3.
MBF grouped according to Percent Diameter Stenosis
| Percent Diameter Stenosis | |||||
|---|---|---|---|---|---|
| <50% n=16 |
50–69% n=9 |
70–89% n=6 |
>90% n=7 |
p-val | |
| MBF (ml/g /min) | |||||
| Rest | 0.9, IQ: 0.8–1.1 | 0.9, IQ: 0.7–1.1 | 0.9, IQ: 0.7–0.9 | 0.9, IQ: 0.8–1.1 | 0.9 |
| Corrected | 1.1, IQ: 0.9–1.3 | 0.9 IQ: 0.9–1.1 | 0.8, IQ: 0.8–1.0 | 0.9, IQ: 0.7–1.1 | 0.08 |
| Dypiridamole | 1.8, IQ: 1.7–2.2 | 1.4, IQ: 1.2–1.7 | 0.9, IQ: 0.8–1.2 | 0.7, IQ: 0.6–0.8 | <0.00 |
| CVR | 1.9, IQ: 1.7–2.7 | 1.7, IQ: 1.4–2.0 | 1.0, IQ: 1.0–1.3 | 0.8, IQ: 0.7–0.9 | <0.00 |
| MCR (mmHg/mL/g/min) | |||||
| Dipyridamole | 50, IQ: 42–58 | 64, IQ: 54–69 | 81, IQ: 72–118 | 132, IQ: 121–161 | <0.00 |
MBF: Myocardial Blood Flow, CVR: Coronary vasodilator reserve, MCR: Minimal Coronary resistance.
All pairwise group comparisons were significant at p<0.03.
All pairwise group comparisons were significant at p<0.02.
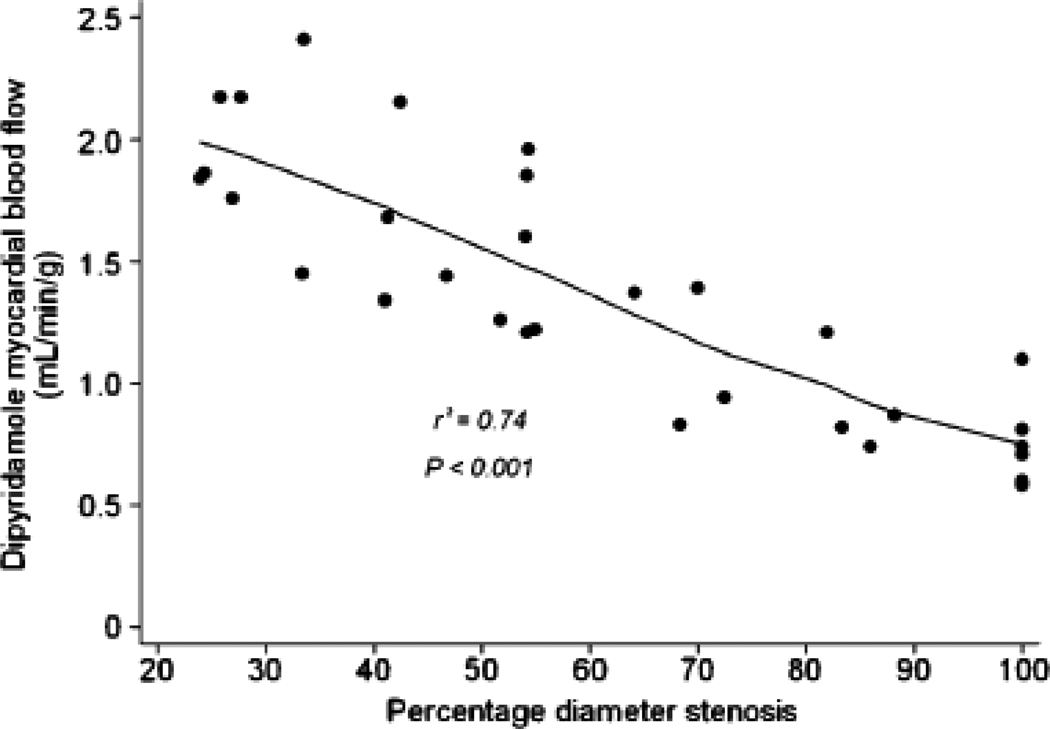
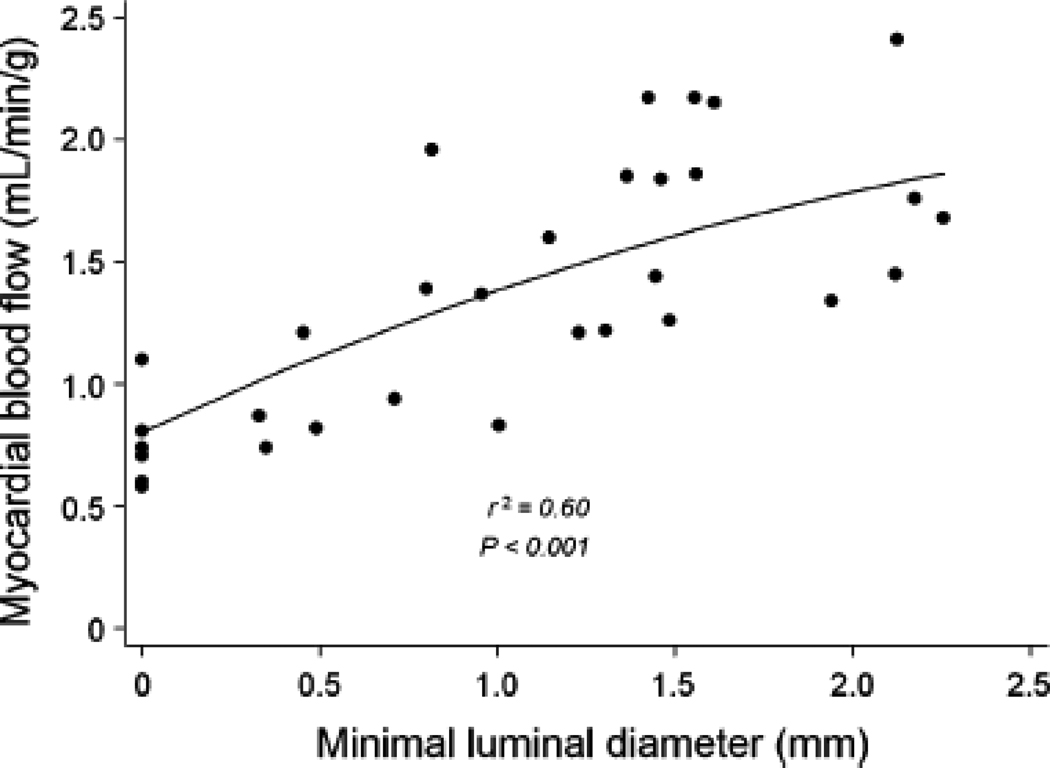
Dipyridamole-stimulated MBF showed a progressive reduction with increasing stenosis severity (Figures 1a and 1b) with the decline being more pronounced when an additional stenosis of at least 50% was also present in a different vessel (Table 4). In myocardial regions supplied by coronary arteries with <50% diameter stenosis, median MBF increased significantly to 1.8 (IQ range 1.7 to 2.2 mL/g/min, p<0.001). The increase in peak MBF progressively declined in regions supplied by vessels with 50–69% diameter stenosis (1.4, IQ range 1.2 to 1.7 mL/g/min), 70–89% (0.9, IQ range 0.8 to 1.2 mL/g/min), and those with >90% diameter stenosis (0.7, IQ range 0.6 to 0.8 mL/g/min). A similar relationship was observed between peak MBF and minimal luminal diameter (Figure 2).
FIGURE 1.
(A) Median values of myocardial blood flow after dipyridamole with IQ range (25–75percentiles) and with upper and lower adjacent values; *p value from Kruskal Wallis analysis for four groups (<50%, 50%–69%, 70%–89% and >90% diameter stenosis). (B) Scatter plot of relation of myocardial blood flow after dipyridamole and quantitative coronary angiography measurements of percent diameter stenosis.
Table 4.
Effect of additional remote coronary lesions on MBF and CVR of vessels with similar stenosis severity
| No additional vessel with stenosis > 50% | At least 1 additional vessel with stenosis >50% | |
|---|---|---|
| Stenosis > 50% | ||
| MBF(ml/g /min) | 1.8 (1.3 to 1.9) | 1.3 (1.2 to 1.4) * |
| CVR | 1.9 (1.7 to 2.0) | 1.5 (1.3 to 1.6) † |
| N | 4 | 5 |
N: number of vessels, MBF: Myocardial Blood Flow, CVR Coronary vasodilator reserve
Values are presented as medians (IQ range),
p=0.047 and
p=0.054 respectively
FIGURE 2.
Scatter plot of relation of myocardial blood flow after dipyridamole and quantitative coronary angiography measurements of minimal luminal diameter.
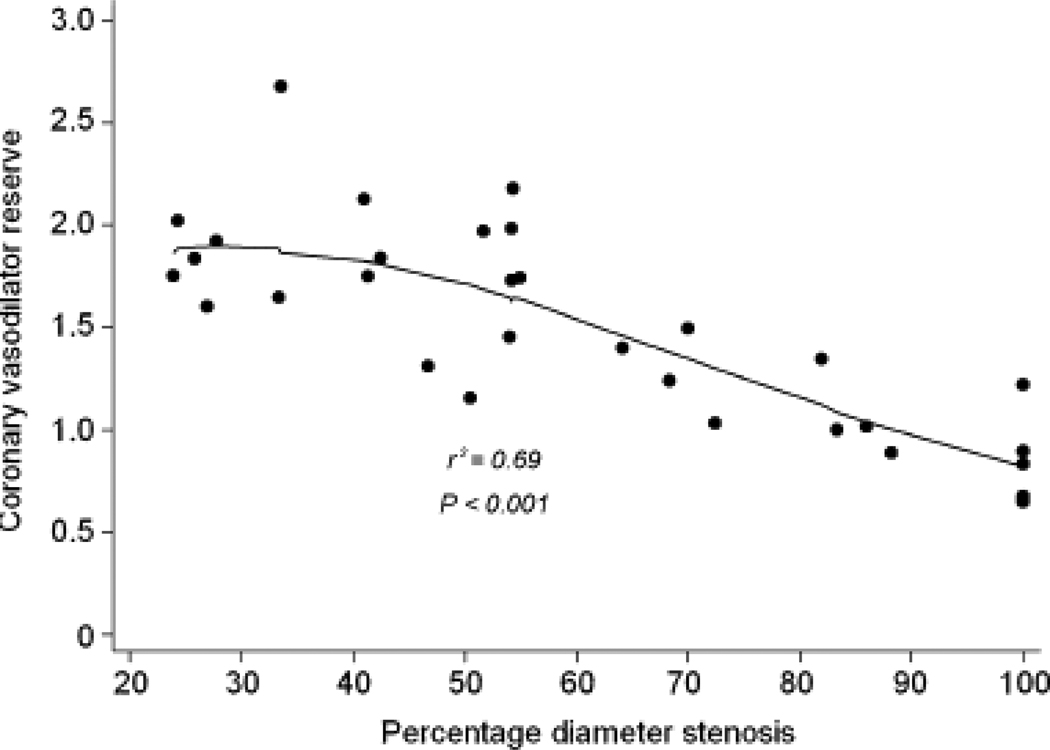
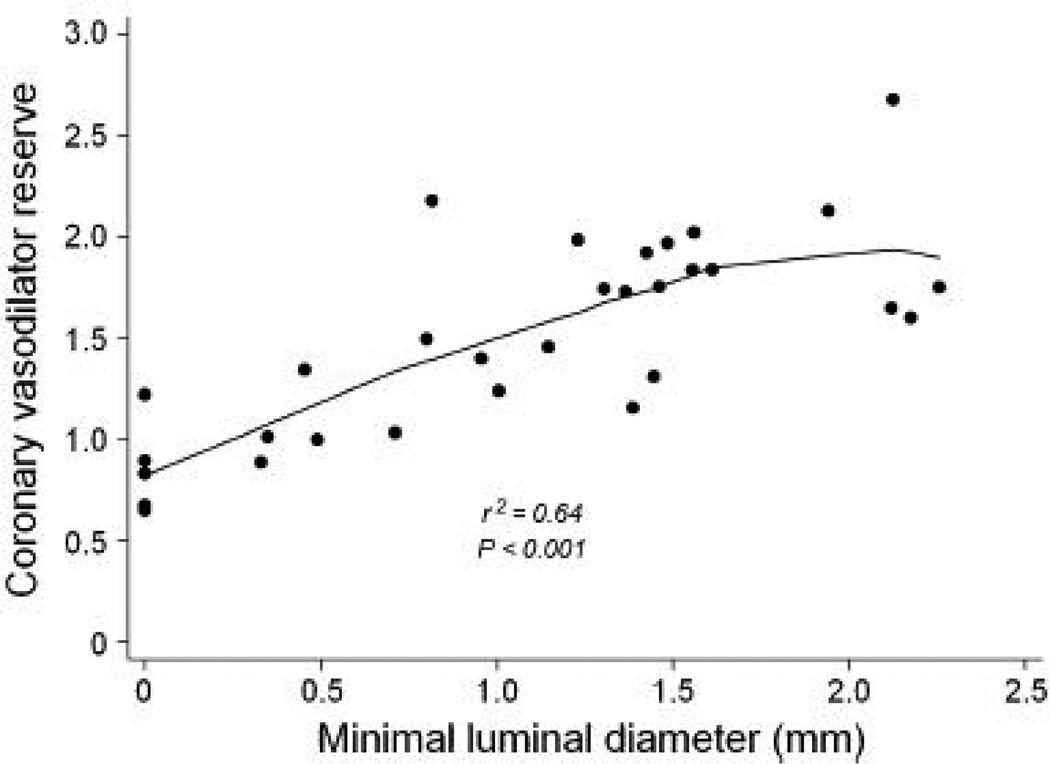
Consequently, CVR also showed a similar progressive decline with increasing stenosis severity (Figures 3a and 3b). As with MBF, the reduction was more pronounced when an additional stenosis of at least 50% was also present in a different vessel (Table 4). CVR in regions supplied by vessels with <50% diameter stenosis (1.9, IQ range 1.7 to 2.7) was comparable to that in the healthy volunteers (2.2, IQ range 2.0 to 3.4). However, CVR progressively declined in regions supplied by vessels with 50–69% diameter stenosis (1.7, IQ range 1.4 to 2.0), 70–89% (1.0, IQ range 1.0 to 1.3), and those with >90% diameter stenosis (0.8, IQ range 0.7 to 0.9). A similar relationship was observed between CVR and minimal luminal diameter (Figure 4).
FIGURE 3.
(A) Median values of coronary vasodilator reserve with IQ range (25–75percentiles) and with upper and lower adjacent values; *p value from Kruskal Wallis analysis for four groups (<50%, 50%–69%, 70%–89% and >90% diameter stenosis). (B) Scatter plot of relation of coronary vasodilator reserve and quantitative coronary angiography measurements of percent diameter stenosis.
FIGURE 4.
Scatter plot of relation of coronary vasodilator reserve and quantitative coronary angiography measurements of minimal luminal diameter.
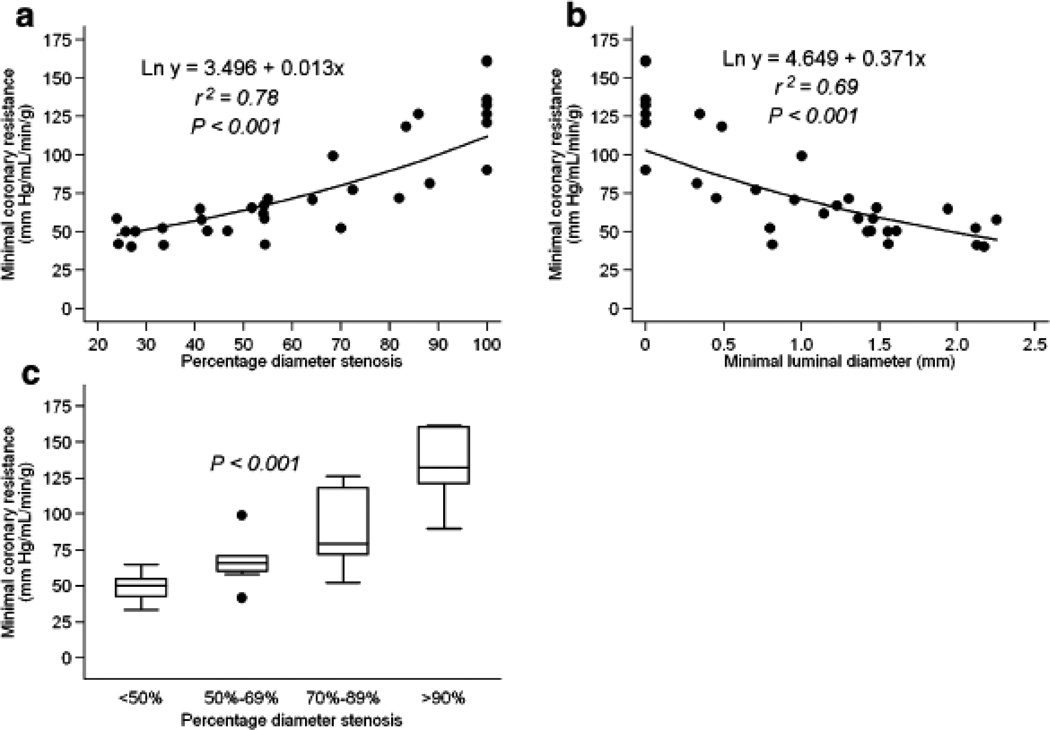
To relate the hyperaemic blood flow to one of its major determinants, the coronary driving pressure, the mean aortic blood pressure was divided by the hyperaemic blood flow and an index of the "minimal coronary resistance" (MCR) obtained. Significant correlations were observed between the estimated MCR and percent diameter stenosis (r2=.78, p<0.001, Figure 5), as well as with minimal luminal diameter (r2=.69, p<0.001, Figure 5).
FIGURE 5.
(A) Scatter plot of relation of “minimal coronary resistance” and quantitative coronary angiography measurements of percent diameter stenosis. (B) Scatter plot of relation of “minimal coronary resistance” and quantitative coronary angiography measurements of minimal luminal diameter. (C) Median values of “minimal coronary resistance” with IQ range (25–75percentiles) and with upper and lower adjacent values; *p value from Kruskal Wallis analysis for four groups (<50%, 50%–69%, 70%–89% and >90% diameter stenosis).
DISCUSSION
Rubidium-82 PET imaging is being increasingly used as an alternative to single photon emission computed tomography (SPECT) to evaluate selected patients with known or suspected CAD. As with SPECT, however, relative estimates of myocardial perfusion with PET also underestimate the underlying extent of CAD. Because clinically CAD presents as a continuous spectrum of severity rather than a binary (present or absent) condition, it has been suggested that absolute measures of MBF and CVR can offer a unique advantage to PET compared to SPECT for assessing the anatomic extent of CAD. The underlying assumption is that absolute measures of MBF and CVR represent a superior tool to uncover the entire spectrum of coronary artery disease severity. Our results demonstrate that absolute measurements of MBF during vasodilator stress and CVR with Rubidium-82 PET imaging are closely associated with arteriographic measurements of luminal stenosis severity as measured by quantitative coronary angiography. More importantly, coronary artery stenoses of intermediate severity display differences in haemodynamics that can be detected by absolute measurements of MBF and CVR
Relationship beween angiographic stenosis and MBF
According to our results, resting blood flow remains unchanged despite increasingly severe stenosis, in agreement with previous studies (3,4). Regarding the hyperaemic response to dipyridamole, there is an inverse non-linear relationship between maximal blood flow and percent diameter stenosis that results from a progressive reduction of hyperaemic response as stenosis severity increases. A similar but not identical, non-linear relationship between hyperaemic response to dipyridamole and coronary stenosis was observed by Di Carli et al (4) using 13N-ammonia in 18 patients with CAD. After minimising confounding factors such as collateral circulation or multiple stenoses-in-series, the investigators reproduced the classic curvilinear perfusion-stenosis relationship first observed in the experimental setting by Gould, Lipscomb and Hamilton (15). Uren et al (3) also studied the same relationship in 35 patients using 15O-H2O and demonstrated that maximum MBF decreases in a non-linear fashion as stenosis severity increases.
In agreement with these studies, we also observed a progressive attenuation of CVR with a stepwise statistically significant reduction in segments supplied by vessels with stenoses of increasing severity. Our results also agree with an earlier report by Goldstein et al, who studied 41 patients with CAD using either Rubidium-82 or 13N-ammonia and demonstrated a strong curvilinear relationship between relative perfusion reserve (defined as the ratio of hyperaemic to resting perfusion in the stenosis-related region divided by the ratio in a remote region) and percentage of vessel diameter or area stenosis (16). Extending further these observations in the clinical setting, we found that despite the fact that most of our patients were on treatment for diabetes, hypertension or hyperlipidaemia, factors known to affect the CVR (17–19), the previously defined non-linear relationship between angiographic stenosis and CVR was maintained and we were able to distinguish between mild, moderate and severe angiographic stenoses as effectively as previous investigators who studied patients with a reduced risk burden compared to ours. In addition in our study, we observed a significant decline of stress MBF compared to rest in regions subtended by arteries with stenoses above 90% of the luminal diameter. This is probably due to coronary steal consequential to development of collateral circulation in four out of seven arteries showing greater than 90% stenosis. The latter is also likely to explain maintenance of resting flow in the same regions.
As in previous studies (3–5), we have also shown that both peak MBF and CVR vary significantly among vessels with coronary stenoses of comparable severity. Multiple factors influence the shape and associated scatter of this relationship, including left ventricular haemodynamics, myocardial contractility, age-related changes in myocardial blood flow, collateral flow not visible on conventional angiography, and possibly others (20–25). There are also inherent discrepancies between anatomical descriptors and functional behaviour of the human circulation. In contrast to the morphologically ideal coronary stenosis in experimental models of coronary stenosis, human lesions exhibit a great variation in morphology and geometry. Consequently, factors such as shape, length and eccentricity of a lesion, the presence of stenoses in series and even vasospasm are likely to contribute to the variability in the myocardial blood flow response to the same pharmacological stimulus (26).
In further agreement with the studies by Uren et al (3) and Di Carli et al (4), we found that the magnitude of scatter around the curve expressing the relationship between minimal coronary resistance and angiographic stenosis was reduced compared to that depicting the relationship between hyperaemic response or CVR and coronary stenosis, at least for lesions with 20–80% diameter stenosis (figure 5). As with the previous two studies, MCR was used to normalize MBF during dipyridamole vasodilation to the coronary perfusion pressure, and the reduction in data scatter suggests that the latter was an important determinant of these relations.
Quantitative assessment of MBF by Rubidium-82
In this study, CVR was higher in healthy volunteers (median: 2.2) and in vessels with a stenosis less than 50% of the lumen diameter (median: 1.9), than in vessels with more severe coronary stenoses (median 1.2). The former are somewhat lower than previously reported values with 15O-H2O and 13N-ammonia (3–5). This difference could be related to the age of our population, since it is known that CVR declines with age (22), and/or to a small underestimation of hyperaemic flow and CVR with Rubidium-82. The latter is only partially extracted by the myocardium. After a single capillary pass and active transport across the sarcolemmal membrane, it competes with back diffusion of tracer from the interstitial to the vascular space. Because the rate of back diffusion is related to MBF, first -pass extraction fraction declines in a non-linear fashion with increasing MBF. This flow dependency of the first-pass extraction fraction makes it difficult to obtain precise measurements but it can be modelled with our methodology after estimating the LV and RV input functions with GFADS. To validate this approach in a robust way, an experimental study using an acute ischemic canine model is presently underway comparing Rubidium-82 MBF measurements with absolute MBF values obtained from radioactive microspheres (27)
Limitations of the study
Beyond the factors related to the methodology used to assess MBF, other parameters also need to be considered as potential limitations of our study. First, assessment of MBF was not performed in a “blinded” manner but our methodology was similar to that used in previous studies with 15O-H2O and 13N-ammonia (3, 4). Second, quantitative coronary angiography was chosen to validate PET-based assessment of CVR instead of other functional methods such as, for instance, intracoronary Doppler-velocity wire flow reserve or coronary pressure wire measurements. Although a comparison with such parameters could have been ideal for a comparative assessment of functional behavior of coronary lesions, this was not the aim of our study. Moreover, such measurements are not yet performed routinely in busy clinical centers. Third, we found that myocardial regions subtended by coronary arteries with less than 50% diameter stenosis had a median CVR of 1.9. Although, this was not statistically different (p=0.09) from the CVR in our age-matched control population (median 2.2), this lack of difference is likely to be related to the relatively small size of our observation groups. A higher number of analysed patients might have also allowed a better assessment of the functional behaviour of coronary stenoses in patents with single-vessel compared to those with multi-vessel disease. However, our total number of analysed vascular territories (n=38) is similar to that (41 vessels) of Di Carli et al (4), who also studied patients both with single- and multi-vessel disease and also similar to that of Goldstein et al (16) and Uren et al (3) who studied patients only with single-vessel disease (41 and 35, respectively). Our results are in agreement with the findings of these studies and in addition, we have observed that for the same level of stenosis both the hyperaemic response and the CVR were reduced further when additional significant stenoses were present in remote arteries, probably reflecting a greater disease burden in these patients. Fourth, the inclusion of patients with prior myocardial infarction may have affected the underestimated MBF and CVR in the non-infarcted territories. However, because the relationship between MBF and stenosis severity without these subjects is expected to improve, our results would still hold and thus would not compromise the validity of our conclusions. Finally, to assess the role of Rubidium-82 in clinical practice we recruited individuals who were referred for assessment of myocardial perfusion on clinical grounds. Inevitably, all patients had at least one risk factor for CAD and seven of our patients at least two. Reduced hyperaemic response and CVR have been reported in asymptomatic individuals with diabetes, hypertension or hyperlipidaemia (17–19), hence their presence in our patients might have affected our measurements. However, our finding that the previously defined relationship between angiographic stenosis and CVR was maintained and the fact that we were able to distinguish between mild, moderate and severe angiographic stenoses strengthen rather than diminish the significance of our results. Indeed, such distinction between moderate and severe stenoses based on CVR measurements was possible in the great majority of myocardial territories. A limited overlap of CVR values is inevitable and it has also been observed previously (4). This is probably the result of non idealized conditions in the clinical setting where factors such as severe disease in remote vessels, collateral circulation, serial lesions, diffuse coronary disease and even morphological differences of coronary stenoses can all affect CVR measurements of individual lesions (26).
CONCLUSION
Measurements of MBF and CVR by Rubidium-82 PET are inversely and non-linearly related to stenosis severity as assessed by quantitative coronary angiography. This is in agreement with prior studies performed with 15O-H2O and 13N-ammonia and indicates that Rubidium-82 PET can be a clinically useful tool for accurate functional assessment of CAD.
ACKNOWLEDGMENTS
We thank Martha Coughlan and Lisa Cantagallo, research coordinators, and also Jon Hainer, IT consultant in the Nuclear Medicine/PET division, Department of Radiology, Brigham and Women’s Hospital, for their assistance during this project.
REFERENCES
- 1.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331(4):222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- 2.Yoshinaga K, Katoh C, Noriyasu K, et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: a study using oxygen 15-labeled water PET. J Nucl Cardiol. 2003;3:275–283. doi: 10.1016/s1071-3581(02)43243-6. [DOI] [PubMed] [Google Scholar]
- 3.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330(25):1782–1788. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 4.Di Carli M, Czernin J, Hoh CK, et al. Relation Among Stenosis Severity, Myocardial Blood Flow, and Flow Reserve in Patients With Coronary Artery Disease. Circulation. 1995;91:1944–1951. doi: 10.1161/01.cir.91.7.1944. [DOI] [PubMed] [Google Scholar]
- 5.Beanlads RS, Muzik O, Melon P, et al. Noninvasive quantification of regional myocardial flow reserve in patients with coronary atherosclerosis using nitrogen-13 ammonia positron emission tomography. Determination of extent of altered vascular reactivity. J Am Coll Cardiol. 1995;26(6):1465–1475. doi: 10.1016/0735-1097(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 6.Gould KL. Coronary Artery Stenosis and Reversing Atherosclerosis. New York, NY: Arnold; 1999. Absolute myocardial perfusion and coronary flow reserve; pp. 247–273. [Google Scholar]
- 7.Coxson PG, Huesman RH, Borland L. Consequences of using a simplified kinetic model for dynamic PET data. J Nucl Med. 1997;38:660–667. [PubMed] [Google Scholar]
- 8.Herrero P, Markham J, Shelton ME, Bergmann SR. Implementation and evaluation of a two-compartment model for quantification of myocardial perfusion with rubidium-82 and positron emission tomography. Circ Res. 1992;70:496–507. doi: 10.1161/01.res.70.3.496. [DOI] [PubMed] [Google Scholar]
- 9.Lin JW, Sciacca RR, Chou RL, Laine FA, Bergmann SR. Quantification of myocardial perfusion in human subjects using 82Rb and wavelet-based noise reduction. J Nucl Med. 2001;42:201–208. [PubMed] [Google Scholar]
- 10.Lin JW, Laine FA, Akinboboye O, Bergmann SR. Use of wavelet transforms in analysis of time–activity data from cardiac PET. J Nucl Med. 2001;42:194–201. [PubMed] [Google Scholar]
- 11.El Fakhri G, Sitek A, Guérin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative Dynamic Cardiac 82Rb PET Using Generalized Factor and Compartment Analyses. J Nucl Med. 2005;46(8):1264–1271. [PubMed] [Google Scholar]
- 12.Marshall RC, Taylor SE, Powers-Risius P, et al. Kinetic analysis of rubidium and thallium as deposited myocardial blood flow tracers in isolated rabbit heart. Am J Physiol. 1997;272:H1480–H1490. doi: 10.1152/ajpheart.1997.272.3.H1480. [DOI] [PubMed] [Google Scholar]
- 13.El Fakhri G, Guérin B, Sitek A, et al. Absolute Myocardial Blood Flow Quantitation in Rb-82 Cardiac PET Using Generalized Factor and Compartment Analysis: A Reproducibility Study [abstract] Eur J Nucl Med Mol Imag. 2006;33(2):S217. [Google Scholar]
- 14.van der Zwet PM, Reiber JH. A new approach for the quantification of complex lesion morphology: the gradient field transform: basic principles and validation results. J Am Coll Cardiol. 1994;24:216–224. doi: 10.1016/0735-1097(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 15.Gould LK, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33(1):87–94. doi: 10.1016/0002-9149(74)90743-7. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein RA, Kirkeeide RL, Demer LL, et al. Relation between geometric dimensions of coronary artery stenoses and myocardial perfusion reserve in man. J Clin Invest. 1987;79:1473–1478. doi: 10.1172/JCI112976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitkänen OP, Nuutila P, Raitakari OT, et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47(2):248–254. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- 18.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32(1):147–153. doi: 10.1016/s0735-1097(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996;94:3232–3238. doi: 10.1161/01.cir.94.12.3232. [DOI] [PubMed] [Google Scholar]
- 20.Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res. 1977;41:648–653. doi: 10.1161/01.res.41.5.648. [DOI] [PubMed] [Google Scholar]
- 21.McGinn AL, White CW, Wilson RF. Interstudy variability of coronary flow reserve: influence of heart rate, arterial blood pressure, and ventricular preload. Circulation. 1990;81:1319–1330. doi: 10.1161/01.cir.81.4.1319. [DOI] [PubMed] [Google Scholar]
- 22.Rossen JD, Winniford MD. Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J Am Coll Cardiol. 1993;21:343–348. doi: 10.1016/0735-1097(93)90673-o. [DOI] [PubMed] [Google Scholar]
- 23.Czernin J, Müller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–69. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Mosher P, Ross J, Jr, McFate PA, Shaw RF. Control of coronary blood flow by an autoregulatory mechanism. Circ Res. 1964;14:250–256. doi: 10.1161/01.res.14.3.250. [DOI] [PubMed] [Google Scholar]
- 25.Marzilli M, Goldstein S, Sabbah HN, Lee T, Stein PD. Modulating effect of regional myocardial performance on local myocardial perfusion in the dog. Circ Res. 1979;45:634–641. doi: 10.1161/01.res.45.5.634. [DOI] [PubMed] [Google Scholar]
- 26.Shellbert HR, Prior JO. Positron Emission Tomography. In: Fuster V, et al., editors. Hurst’s, The Heart. New York, NY: McGraw-Hill Companies Inc; 2004. pp. 557–693. [Google Scholar]
- 27.Fakhri G, Mekkaoui C, Sitek A, et al. Experimental validation using radiolabeled microspheres of absolute quantitation of regional myocardial blood flow using dynamic Rubidium 82 Positron Emission Tomography [abstract] Circulation. 2007 (in press) [Google Scholar]