Abstract
Purpose
To assess the relationship between donor factors and 5-year corneal graft survival in the Cornea Donor Study (CDS).
Methods
Donor corneas met criteria established by the Eye Bank Association of America, had an endothelial cell density of 2300–3300/mm2, and were determined to be of good to excellent quality by the eye banks. Donor corneas were assigned using a random approach and surgeons were masked to information about the donor cornea including donor age. Surgery and post-operative care were performed according to the surgeons’ usual routines and subjects were followed for five years. Donor and donor cornea factors were evaluated for their association with graft failure, which was defined as a regraft or a cloudy cornea that was sufficiently opaque to compromise vision for a minimum of three consecutive months.
Results
Graft failure was not significantly associated with the type of tissue retrieval (enucleation versus in situ), processing factors, timing of use of the cornea, or to characteristics of the donor or the donor cornea. Adjusting for donor age did not affect the results.
Conclusion
Donor and donor cornea characteristics do not impact graft survival rates for corneas comparable in quality to those used in this study.
Keywords: cornea transplant, graft failure, cornea donor
INTRODUCTION
The Cornea Donor Study (CDS) recently published results demonstrating no difference in corneal graft survival at 5 years related to donor age.1 Although eye banks routinely gather information on both the donor and the quality of the corneal tissue prior to distributing tissue for transplantation, relatively little information is present in the literature concerning the impact of these factors on graft survival.2 In the CDS, information on cornea donors, tissue handling, and tissue findings was obtained and assessed in order to examine any potential impact on graft survival at 5 years. The results of these analyses are presented here.
MATERIALS AND METHODS
Study Protocol
Details of the CDS protocol have been reported previously1, 3, 4 and the key aspects are briefly summarized. The study protocol was approved by institutional review boards for each eye bank and at each investigational site.
Eligible subjects were between 40 and 80 years old and had corneal disease associated with endothelial dysfunction and moderate risk of failure (principally Fuchs’ dystrophy and pseudophakic corneal edema). Written informed consent was obtained from each subject. Eligible donor corneas met Eye Bank Association of America (EBAA) standards for human corneal transplantation.5, 6 Eligibility criteria for the donor corneas assigned in the study are listed in Table 1. Eye banks obtained information about the cornea donor, including age, gender, race, history of diabetes and cause of death from medical records, health care provider interviews, and family members. Type of tissue recovery, either as whole eye (enucleation) or corneo-scleral rim removal (in situ), time from death to placement in preservative medium, body refrigeration time, and death to surgery time were recorded. Specific characteristics of the donor tissue were recorded, including epithelial slit lamp findings, stromal edema, arcus, folds in Descemet’s membrane, the presence of “snail tracks” (linear ruptures of endothelial cells), and endothelial morphology by specular microscopy.
Table 1.
Cornea Donor Study Donor Tissue Eligibility Criteria
| Age of donor at time of death: 10–75 years |
| Death to preservation time: ≤12 hrs if body refrigerated or eyes iced and ≤8 hrs if not |
| Death to surgery time: ≤5 days |
| Donor medical exclusions including cause of death: meets EBAA standards |
| Donor ocular exclusions: meets EBAA standards for excluding tissue plus no prior intraocular surgery (must be phakic) |
Specular microscopy:
|
|
Slit lamp examination criteria: Epithelium
|
Stroma
|
Descemet’s membrane
|
Endothelium
|
Clinical investigators and subjects were masked to all characteristics of the donor cornea, including age and endothelial cell density. Preoperative management, surgical technique, and postoperative care (including prescription of medications), were provided according to each investigator’s customary routine. The visit schedule during the first 6 post-operative months was at the discretion of the investigator. Thereafter, the minimum follow-up visit schedule included a visit between six and 12 months and then annual visits through 5 years. Because of the trial’s simple design, data collection at each visit was limited and included an assessment of graft clarity, signs of graft rejection, and intraocular pressure. The definition of graft failure, based on the definition used in the Collaborative Corneal Transplantation Studies (CCTS)7, 8, was a regraft or, in the absence of regraft, a cloudy cornea in which there was loss of central graft clarity sufficient to compromise vision for a minimum of three consecutive months. Further details of the classification scheme for graft failures has been published.1
Statistical Methods
The analysis included the 1,090 eligible subjects in the CDS. Baseline endothelial cell density was evaluated by the Reading Center for 658 cases. Donor race/ethnicity was excluded from the analysis due to the small number of subjects per group (41 African Americans, 11 Hispanics, 3 Asians, and 11 other).
Cumulative probabilities of graft failure (subsequently referred to as “graft failure rates”) were calculated using the Kaplan–Meier method. Univariate Cox proportional hazards regression models were used to individually assess the association of each donor factor with graft failure. No significant deviations from the proportional hazards assumptions were detected. All reported p-values are two-sided. Because of multiple comparisons, P values ≥ 0.01 were not considered statistically significant. Statistical analyses were conducted using SAS version 9.1 software (SAS Institute Inc., Cary, NC).
RESULTS
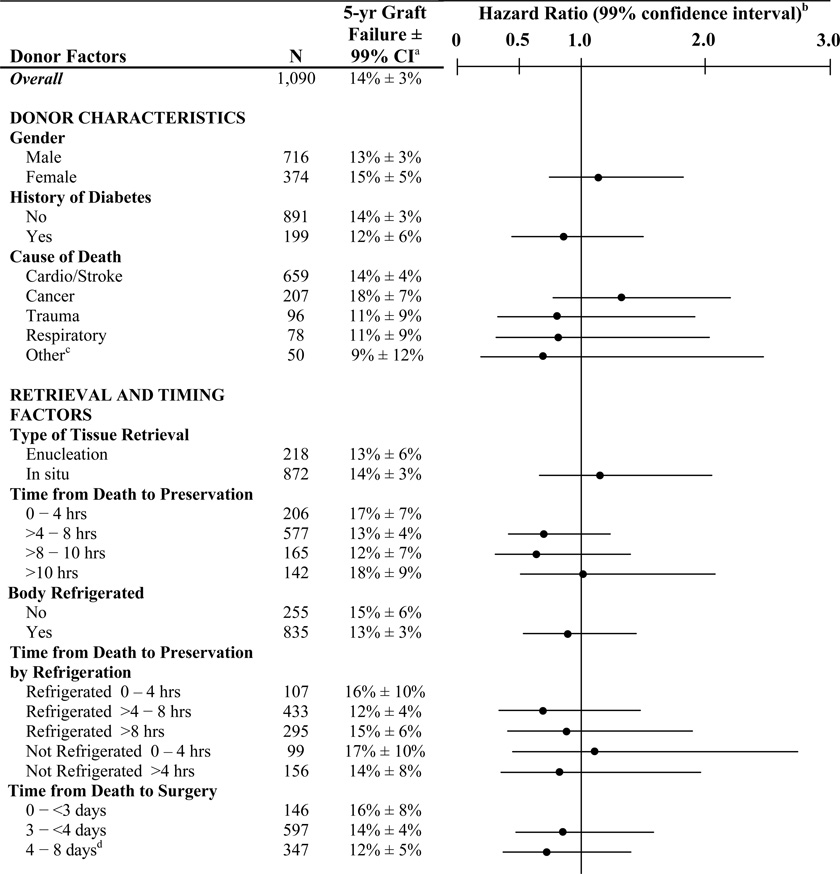
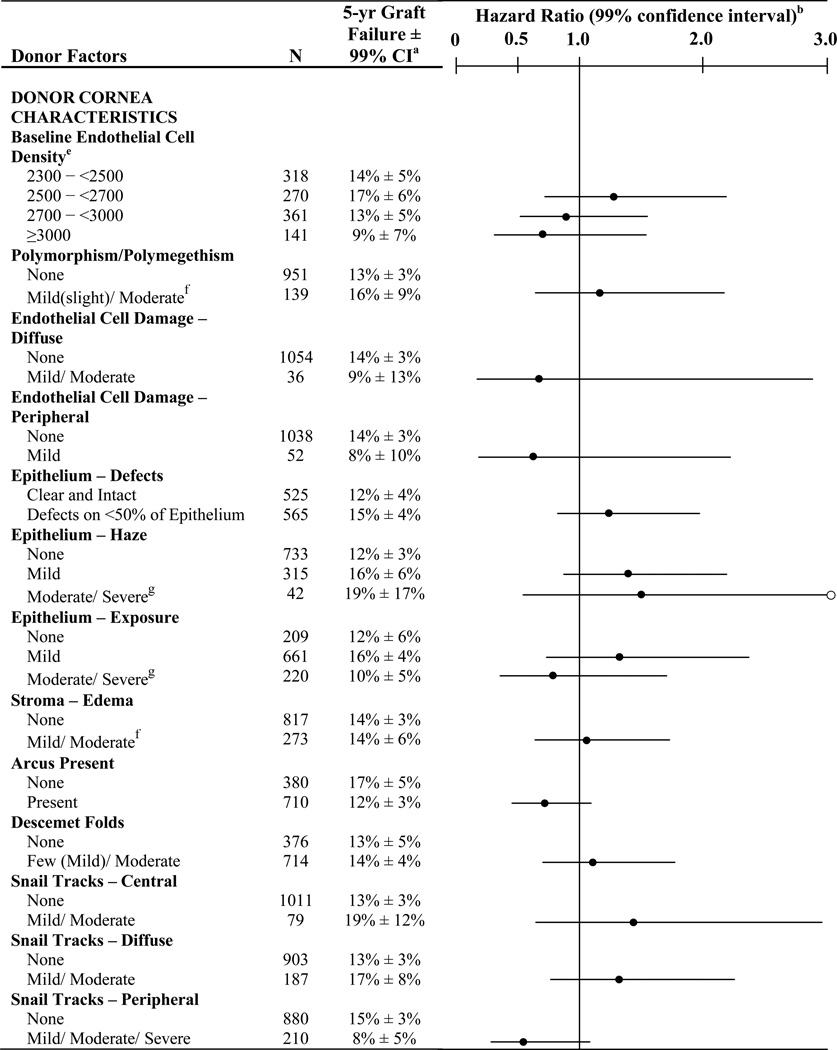
The donor characteristics and slit-lamp characteristics of the corneas have been reported in detail previously.3 The distribution of each characteristic is indicated in Table 2.
Table 2.
Baseline Donor Factors Predictive of Graft Failure (N=1,090)
 |
 |
CI = confidence interval
○ signifies an upper 99% confidence limit > 3.00 (for moderate/severe epithelium – haze group the upper 99% confidence limit = 4.03)
12 anoxia/asphyxiation, 10 renal, 6 hepatic, 4 neurological, 4 drug overdose, 3 gastrointestinal, 3 shock, 2 pancreatitis, 2 seizure, 2 undetermined, 1adrenal insufficiency, 1electrocution
Includes 17 subjects who received corneas where time from death to transplant longer than five days (15 were six days, 1 was seven days and 1 was eight days)
658 values from the reading center and 432 from the eye bank. Results were similar when excluding the cases without a Reading Center value (data not shown).
Only one subject classified as moderate
Only one subject classified as severe
Among the 135 eyes with graft failures, 102 (76%) had a regraft and 33 (24%) met the cloudy cornea failure criteria defined for the study without a regraft (30 had a cloudy cornea for at least three months and 3 had a cloudy cornea for less than three months without additional available follow up). Three graft failures were due to primary donor failure, 8 to uncorrectable refractive error, 48 to graft rejection, 46 to endothelial decompensation, and 30 to other causes.
As shown in Table 2, graft failure rates were not significantly impacted by any donor characteristics (gender, history of diabetes, or cause of death), by any factors related to the type of tissue retrieval, processing, timing of use of the cornea (time from death to preservation or time from death to surgery), or by any characteristics of the donor cornea (presence of endothelial polymorphism, endothelial cell damage, Descemet folds, snail tracks, baseline ECD, epithelial defects, epithelial haze, epithelial exposure, stromal edema, or arcus). Adjusting for donor age did not affect the results (data not shown). When analyses were conducted separately for rejection and non-rejection graft failures, no baseline donor factors were found to be associated with the rate of graft failure, based upon our pre-specified level of significance (data not shown).
DISCUSSION
While a number of factors evaluated here have been assessed in other studies, this prospective study is one of the few to address their impact on graft survival. The limitation of this study is that the tissue selection criteria excluded extremes such as prolonged death to preservation time and death to surgery time. Only mild to moderate epithelial, stromal, Descemet and endothelial variations were accepted. Nonetheless, the information obtained is useful in demonstrating the lack of any adverse impact of the abnormalities found in the ranges studied.
Data on donor cause of death and presence or absence of diabetes mellitus show no impact on five-year graft outcomes. Earlier studies have shown a similar lack of impact of donor cause of death on five-year graft survival.2 Recently, deaths due to cancer have been implicated in post-operative endophthalmitis9 but there were no cases of endophthalmitis attributable to the donor cornea in the CDS. Additional studies have shown no reason to exclude donors with cancer10 but these did not look at graft survival.
Graft outcome did not differ by method of tissue procurement (enucleation vs. in situ retrieval). Rootman and co-investigators reported no difference in initial donor tissue quality rating by procurement methodology but they did not look at graft outcomes beyond primary graft failure.11 A recent study showed no difference in graft clarity at 3 months with either procurement method.12
Timing of tissue procurement, refrigeration, and use has been studied in the past and has also been shown, within limited ranges, to have no effect on graft outcome2 although prolonged storage times, not studied here, may well have a deleterious effect13. Endothelial characteristics likewise had no impact on graft outcomes using the donor criteria of the CDS.14
Epithelial and stromal changes were generally mild with only one case of severe epithelial exposure and one with moderate stromal edema. While over half of the donor corneas had epithelial defects, these all involved less than 50% of the epithelium and had no statistically significant effect. Because of the relatively low prevalence of endothelial trauma related to tissue preparation, as manifested by snail tracks in the central cornea and present in only 7% of donor corneas, the impact of this type of trauma cannot be fully assessed.
While study of more extreme alterations of tissue would be of benefit, this study demonstrates effectively, as have the other reports from the CDS, that all tissue meeting the donor criteria used performs equally well. Continued follow-up of this cohort through 10 years is ongoing in order to assess any potential differences in longer-term survival.
Acknowledgments
Funding/Support: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services EY12728 and EY12358. Additional support provided by: Eye Bank Association of America, Bausch & Lomb, Inc., Tissue Banks International, Vision Share, Inc., San Diego Eye Bank, The Cornea Society, Katena Products, Inc., ViroMed Laboratories, Inc., Midwest Eye-Banks (Michigan Eye-Bank, Illinois Eye-Bank), Konan Medical Corp., Eye Bank for Sight Restoration, SightLife, Sight Society of Northeastern New York (Lions Eye Bank of Albany), Lions Eye Bank of Oregon
APPENDIX
A listing of the Cornea Donor Study Investigator Group, including clinical site investigators, eye bank staff, coordinating center staff, specular microscopy reading center staff, and committees, has been previously published online.1
The following CDS Publications Committee members independently reviewed and approved this manuscript for submission: John Affeldt, MD, Michael W. Belin, MD, Terry E. Burris, MD, Richard Eifermann, MD, Jonathan Macy, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Cornea Donor Study Investigator Group. The effect of donor age on corneal transplantation outcome: results of the cornea donor study. Ophthalmology. 2008;115:620–626. doi: 10.1016/j.ophtha.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KA, Lowe MT, Bartlett CM, et al. The Australian Corneal Graft Registry, 2007 report. Flinders University Press; 2007. [Google Scholar]
- 3.Cornea Donor Study Group. Baseline donor characteristics in the Cornea Donor Study. Cornea. 2005;24:389–396. doi: 10.1097/01.ico.0000151503.26695.f0. [DOI] [PubMed] [Google Scholar]
- 4.Cornea Donor Study Group. Clinical profile and early surgical complications in the Cornea Donor Study. Cornea. 2006;25:164–170. doi: 10.1097/01.ico.0000164832.69668.4b. [DOI] [PubMed] [Google Scholar]
- 5.EBAA. Washington, DC: 2000. Eye Bank Association of America. Medical Standards. [Google Scholar]
- 6.Eye Bank Association of America. Medical Standards. 2006 doi: 10.1001/jamaophthalmol.2015.3127. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Corneal Transplantation Studies Research Group. The Collaborative Corneal Transplantation Studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 8.Collaborative Corneal Transplantation Studies Research Group. Design and methods of the Collaborative Corneal Transplantation Studies. Cornea. 1993;12:93–103. doi: 10.1097/00003226-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hassan SS, Wilhelmus KR, Dahl P, et al. Infectious disease risk factors of corneal graft donors. Arch Ophthalmol. 2008;126:235–239. doi: 10.1001/archophthalmol.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagoner MD, Dohlman CH, Albert DM, et al. Corneal donor material selection. Ophthalmology. 1981;88:139–145. doi: 10.1016/s0161-6420(81)35070-2. [DOI] [PubMed] [Google Scholar]
- 11.Rootman DB, Wankiewicz E, Sharpen L, Baxter SA. In situ versus whole-globe harvesting of corneal tissue from remote donor sites: effects on initial tissue quality. Cornea. 2007;26:270–273. doi: 10.1097/ICO.0b013e31802c9e05. [DOI] [PubMed] [Google Scholar]
- 12.Jhanji V, Tandon R, Sharma N, et al. Whole globe enucleation versus in situ excision for donor corneal retrieval - a prospective comparative study. Cornea 2008;27(10):1103-8Sharma N, et al. Whole globe enucleation versus in situ excision for donor corneal retrieval - a prospective comparative study. Cornea. 2008;27(10):1103–1108. doi: 10.1097/ICO.0b013e31817f812e. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelmus KR, Stulting RD, Sugar J, Khan MM. Primary cornea graft failure: a national reporting system. Medical Advisory Board of the Eye Bank Association of America. Arch Ophthalmol. 1995;113:1497–1502. doi: 10.1001/archopht.1995.01100120027002. [DOI] [PubMed] [Google Scholar]
- 14.Cornea Donor Study Investigator Group. Donor age and corneal endothelial cell loss five years after successful cornea transplantation: specular microscopy ancillary study results. Ophthalmology. 2008;115:627–632. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


