Abstract
Recurrent Clostridium difficile infection (CDI) is one of the most difficult problems in healthcare infection control. We evaluated the risk factors associated with recurrence in patients with CDI. A retrospective cohort study of 84 patients with CDI from December 2008 through October 2010 was performed at Pusan National University Yangsan Hospital. Recurrence occurred in 13.1% (11/84) of the cases and in-hospital mortality rate was 7.1% (6/84). Stool colonization with vancomycin-resistant enterococci (VRE) (P = 0.006), exposure to more than 3 antibiotics (P = 0.009), low hemoglobin levels (P = 0.025) and continued use of previous antibiotics (P = 0.05) were found to be more frequent in the recurrent group. Multivariate analysis indicated that, stool VRE colonization was independently associated with CDI recurrence (odds ratio, 14.519; 95% confidence interval, 1.157-182.229; P = 0.038). This result suggests that stool VRE colonization is a significant risk factor for CDI recurrence.
Keywords: Clostridium difficile, Recurrence, Risk factors, VRE
INTRODUCTION
Clostridium difficile infection (CDI) is one of the leading causes of nosocomial illness, and the incidence and severity of CDI have increased since 2000 (1). Most patients with CDI respond well to medical therapy including withdrawal of antibiotics and treatment with metronidazole or vancomycin. However, up to 30% of patients experience CDI recurrence (2). We retrospectively studied a cohort of patients with CDI at our institution and identified risk factors associated with recurrence. The purpose of this study was to identify patients at risk for recurrent CDI who may benefit from early preventive measures and therapeutic interventions.
MATERIALS AND METHODS
Identification of subjects and data collection
This retrospective study was performed at Pusan National University Yangsan Hospital, a 700-bed teaching hospital, between December 2008 and October 2010. All medical records were reviewed for patients who had been tested by analysis of stool cultures or toxin assays. In addition, all patients diagnosed with CDI, pseudomembranous colitis, or diarrhea were reviewed.
The exclusion criteria applied were: age of < 15 yr, failure to follow-up before completion of CDI treatment, presence of any other cause of diarrhea (such as laxative use), presence of any other diarrhea-causing pathogens, and inflammatory bowel disease.
Clinical data, including demographic information, comorbidities, prior therapeutic interventions (history of abdominal surgery within a month before CDI diagnosis, mechanical ventilation, or tube feeding before or during the treatment of CDI), recent medications within 30 days of diagnosis of CDI, the number and type of antibiotics prescribed before diagnosis of CDI, laboratory parameters, acid suppressive therapy, concurrent use of probiotics, therapy prescribed for CDI (discontinuation of antibiotics within 3 days of CDI diagnosis, metronidazole or oral vancomycin), and clinical outcomes were obtained from medical records. After excluding mortality cases, patients were classified into a recurrent group and non-recurrent group, based on recurrence within 60 days of cure.
Definitions
The diagnosis of CDI should include the following findings: 1) the presence of diarrhea, defined as passage of 3 or more unformed stools within 24 or fewer consecutive hours; and 2) a positive stool test result for the presence of toxigenic C. difficile or its toxins or colonoscopic or histopathological confirmation of pseudomembranous colitis (1). CDI was categorized according to the SHEA/IDSA guidelines (1): 1) healthcare facility (HCF)-onset HCF-associated CDI; 2) community-onset HCF-associated CDI; and 3) community-associated CDI. A score developed by Charlson et al. (3), was used to evaluate the prognosis based on age and comorbidities. CDI was considered severe if one of the following factors was found to be present: 1) leukocytosis with a white blood cell count of ≥ 15,000 cells/µL; or 2) a serum creatinine level of ≥ 1.5 times the premorbid level (1). Patients were regarded as cured when stool frequencies and consistencies were normal for at least 3 consecutive days. Recurrence was defined as the reappearance of either a symptom or a positive toxin assay within 60 days of the treatment. Treatment with proton pump inhibitors (PPIs) or histamine H2-blockers was defined as at least 3 days of treatment before the development of CDI, and continuous use thereafter. CDI-related mortality was defined as death that occurred during the treatment period with concurrent signs of CDI.
Statistical analysis
All data are presented as median and range. Comparisons between groups were performed using the Fisher exact test for categorical variables and the Mann-Whitney U-test for continuous variables. The relative risk of recurrence was calculated using a multivariate logistic regression. We simultaneously entered potential confounding variables with a P value of less than 0.1 in the univariate analysis in the final regression model. For all analyses, a P value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the institutional review board of Pusan National University Yangsan Hospital (IRB approval number: 2010-068). Informed consent was waived by the board.
RESULTS
Demographic characteristics, clinical characteristics, and clinical course in patients with CDI
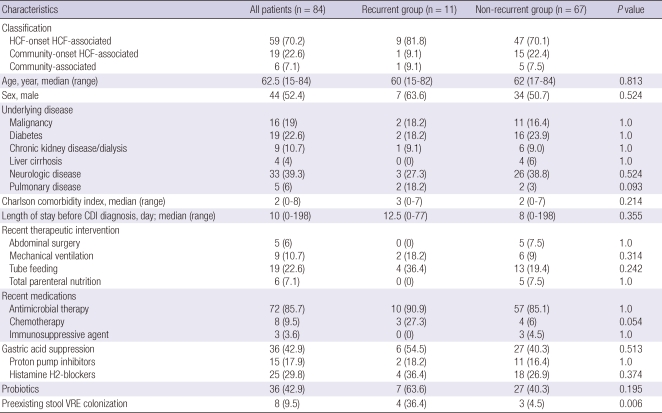
A total of 84 patients with CDI were identified during the study period: 59 (70.2%) were HCF-onset HCF-associated infections; 19 (22.6%), community-onset HCF-associated infections; and 6 (7.1%), community-associated infections (Table 1). The median age of the patients was 62.5 yr (range, 15-84). Forty-four patients were male and 40 were female. The median duration of hospitalization before the diagnosis of CDI was 10 days (range, 0-198) and 14 days (range, 2-198) in HCF-onset cases.
Table 1.
Demographic and clinical characteristics of the patients with and without recurrence of Clostridium difficile infection
Data are presented as number (%) of patients unless otherwise specified. HCF, healthcare facility; CDI, Clostridium difficile infection; VRE, vancomycin-resistant enterococci.
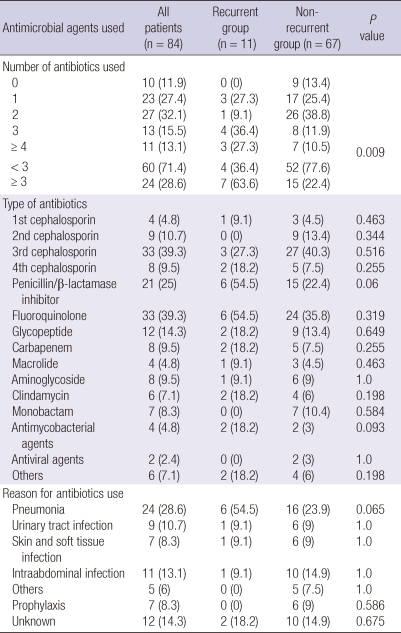
Seventy-two patients (85.7%) were treated with antibiotics. The most common antibiotics administered before diagnosis of CDI were third-generation cephalosporins (39.3%) and fluoroquinolones (39.3%) (Table 2). The main causes of previous antibiotic prescription were pneumonia (28.6%), intra-abdominal infection (13.1%), and urinary tract infection (10.7%).
Table 2.
Number and type of antimicrobial agents used before diagnosis of Clostridium difficile infection and the conditions for which the treatment was indicated
Data are presented as number (%) of patients.
Stool vancomycin-resistant enterococci (VRE) colonization was identified in 8 patients (9.5%) at the time of treatment initiation. Among the recurrent group, 1 patient's baseline culture was negative for VRE, and new detection of VRE stool colonization was identified after completion of antimicrobial therapy for CDI. We considered this new detection case as a preexisting VRE negative. No VRE infections occurred in any of the patients during the treatment and follow-up.
The in-hospital mortality rate was 7.1% (6/84), and none of these cases was related to CDI. Eleven patients (13.1%) experienced recurrence (recurrent group) within 60 days of cure, and 67 (79.8%) did not experience recurrence (non-recurrent group).
Treatment, complications, and outcomes of CDI
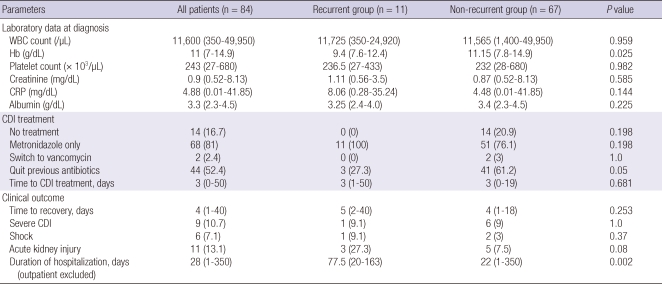
Fourteen patients (16.7%) were not treated due to a self-limiting course (Table 3). Metronidazole was the initial therapeutic regimen for the remaining 70 patients (83.3%). Oral vancomycin was substituted thereafter for 2 patients because of unsatisfactory responses to metronidazole therapy. Only 3 patients (27.3%) were able to stop using previous non-C. difficile antibiotics in the recurrent group, while 41 patients (61.2%) stopped antibiotics after CDI diagnosis in the non-recurrent group (P = 0.05).
Table 3.
Laboratory parameters, treatment, and clinical outcome for Clostridium difficile infection
Data are presented as number (%) of patients or median (range). WBC, white blood cell count; Hb, hemoglobin; CRP, C-reactive protein; CDI, Clostridium difficile infection.
Three patients were treated at an outpatient clinic. Among the hospitalized patients, the median hospital stay was 28 days (range, 1-350 days). The recurrent group required prolonged hospitalization during first CDI episode (median 77.5 days vs 22 days; P = 0.002). The 2 study groups were similar in terms of the time lag for recovery, CDI severity and complications such as acute kidney injury or shock.
Risk factors for recurrence of CDI
There was no significant difference in age, gender, comorbidity, recent therapeutic interventions and medications between the 2 groups (Table 1). Even though statistically significant differences were not observed, more patients of the recurrent group had underlying pulmonary disease (18.2% vs 3%, P = 0.093) and chemotherapy history (27.3% vs 6%, P = 0.054) as compared to the non-recurrent group. The length of stay before CDI diagnosis, tube feeding, gastric acid suppression, and concurrent use of probiotics were not found to be significantly associated with recurrence. The patients with CDI recurrence had greater prevalence of preexisting stool VRE colonization (36.4% vs 4.5%, P = 0.006).
With regard to antimicrobial therapy, patients who received more than 3 antibiotics were more common in the recurrent group as compared to the non-recurrent group (63.6% vs 22.4%, P = 0.009) (Table 2). In contrast to a previous study (4), fluoroquinolone exposure was found to be not significantly different between the 2 groups. Patients treated for pneumonia were more commonly found in the recurrent group as compared to the non-recurrent group (54.5% vs 23.9%, P = 0.065), although this observation was not statistically significant.
The white blood cell count, the levels of serum albumin, and the levels of C-reactive protein at diagnosis were not significantly different between the 2 groups. However, the hemoglobin level was significantly low in the recurrent group (P = 0.025) (Table 3).
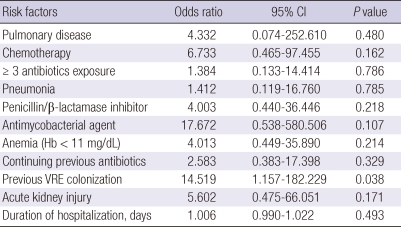
Multivariate analysis showed that stool VRE colonization (odds ratio [OR], 14.519; 95% confidence interval [CI], 1.157-182.229; P = 0.038) was the only independent and significant risk factor for CDI recurrence (Table 4).
Table 4.
Multivariate analysis of risk factors for recurrence of Clostridium difficile infection
Hb hemoglobin; VRE, vancomycin resistant enterococci.
DISCUSSION
Despite an initial successful response, CDI recurs in 15%-30% of the cases (2). Recurrence typically occurs within 1 to 3 weeks after completion of treatment, but late recurrences of up to 2 months are not infrequent (2, 5, 6). In this study, 13.1% of patients experienced recurrence within 60 days. This rate is lower than those in other Western studies, but is higher than those in previous Korean reports (1.2%-12%) (7-9).
Risk factors for recurrent CDI described in previous studies include old age (6, 10, 11), low serum albumin level (11), poor quality-of-health index (6), fecal incontinence (12), lower levels of immunoglobulin against toxin B or toxin A (13, 14), infection with the B1/NAP1/027 strain (14), hospital-acquired disease (15), history of surgery (16), concomitant treatment with antacid medication (10-12), continued treatment with non-C. difficile antibiotics after CDI (5, 10), and fluoroquinolone use (4). These studies did not identify an association between stool VRE colonization and recurrent CDI. To the best of our knowledge, the present study is the first investigation of the effect of stool VRE colonization on the recurrence of CDI. Interestingly, stool VRE colonization was found to be the only reliable risk factor for recurrence of CDI. Our results suggest a link between colonization of stool with VRE and recurrent CDI.
VRE-colonized patients with CDI have an increased risk of skin contamination and environmental shedding of VRE (17-19). In previous reports (18, 19), VRE colonized patients with diarrhea have an increased prevalence of environmental VRE contamination. Moreover, Sethi et al. (17) showed that treatment of CDI with metronidazole or vancomycin may promote transmission of VRE, by promoting persistent high-density colonization of VRE. It is interesting to note that shedding of VRE remained common even after diarrhea was resolved (17). Therefore, patients with stool colonization of VRE require careful medical supervision if they exhibit CDI symptoms such as diarrhea. There is a concern that oral vancomycin may be more likely to promote acquisition and overgrowth of VRE (20, 21). Because multiple genes are necessary to generate vancomycin resistance in enterococci, acquisition of VRE colonization does not occur via mutations in the susceptible enterococci in the intestinal tract. Rather, selective pressure exerted by oral vancomycin may facilitate the exogenous acquisition of VRE or the transfer of vancomycin resistance genes from other organisms to the enterococci in the intestinal tract (22, 23). However, several studies have failed to identify an increased risk of VRE emergence in patients treated with oral vancomycin (21, 24, 25). Similarly, newly detected VRE colonization was found to be uncommon in this study (with only a single case observed). It should be noted that we did not routinely monitor stool VRE colonization after recovery from CDI.
In this study, it appears that patients colonized with VRE may be at an increased risk for CDI recurrence. We cannot conclusively state that stool VRE colonization is responsible for the recurrence of CDI because many patients of the recurrent group had multiple severe coexisting conditions. C. difficile and VRE have emerged as major nosocomial pathogens that require rigorous monitoring and control. VRE and C. difficile share risk factors and putative causes, such as antimicrobial therapy and prolonged hospitalization (26). Stool VRE colonization may represent the final consequence of several CDI risk factors. However, VRE colonization has remained a significant risk factor after adjusting for confounding variables such as the number of antibiotics and duration of hospitalization in this study. Our results suggest that gastrointestinal bacterial colonization plays an important role in the development of recurrent CDI. Although the pathogenesis of CDI recurrence is poorly understood, it has been proposed to involve alterations of constituents of normal bowel flora (27, 28). We cautiously propose that patients with stool VRE colonization are more prone to experience alterations of the bowel flora after CDI.
This study has several limitations. First, this is a single center study with a relatively small number of patients. Second, we could not evaluate the effect of the initial regimen on CDI recurrence. Studies have shown that the rates of treatment failure and recurrence are greater for patients initially treated with metronidazole than for patients initially treated with vancomycin (29). Except for the untreated patients, all patients in this study were initially treated with metronidazole. Two patients whose treatment was switched to vancomycin did not experience recurrence. Therefore, we cannot evaluate the effect of the initial treatment regimen in this study. Third, we were unable to perform ribotyping of stool C. difficile isolates. Therefore, we could not differentiate between reinfection and recurrence. In addition, we cannot rule out the possibility that the B1/NAP/027 strain existed in our cohort. The first case of isolation of C. difficile PCR ribotype 027 in Korea was recently reported in a patient with refractory CDI (30). Finally, this study may be affected by all of the limitations of a retrospective design. Further prospective studies may be needed to provide further confirmation of our results. Although the results do not allow us to conclude that stool VRE colonization increases CDI recurrence, further prospective studies with a larger number of patients should be performed to validate this relationship and the pathophysiology between VRE colonization and recurrent CDI.
In conclusion, stool VRE colonization appears to be an independent risk factor for CDI recurrence. Preventing initial acquisition of VRE and C. difficile, both in terms of VRE and CDI control, should be emphasized.
Footnotes
This study was supported by Medical Research Institute Grant (2010), Pusan National University Yangsan Hospital.
AUTHOR SUMMARY
Risk Factors for Recurrence of Clostridium difficile Infection: Effect of Vancomycin-resistant Enterococci Colonization
Hee Kyoung Choi, Kye Hyung Kim, Sun Hee Lee and Su Jin Lee
We evaluated the risk factors associated with recurrence in patients with Clostridium difficile infection (CDI). A retrospective cohort study of 84 patients with CDI was performed. Recurrence occurred in 13.1% (11/84) of the cases and in-hospital mortality rate was 7.1% (6/84). On multivariate analysis, stool vancomycin-resistant enterococci colonization was independently associated with CDI recurrence.
References
- 1.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Maroo S, Lamont JT. Recurrent clostridium difficile. Gastroenterology. 2006;130:1311–1316. doi: 10.1053/j.gastro.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 4.Cadena J, Thompson GR, 3rd, Patterson JE, Nakashima B, Owens A, Echevarria K, Mortensen EM. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci. 2010;339:350–355. doi: 10.1097/MAJ.0b013e3181d3cdaa. [DOI] [PubMed] [Google Scholar]
- 5.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Lee SY, Kim YS, Park SW, Jo SY, Ryu SH, Moon JS, Whang DH, Shin BM. The incidence and clinical features of Clostridium difficile infection; single center study. Korean J Gastroenterol. 2010;55:175–182. doi: 10.4166/kjg.2010.55.3.175. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Choi MG, Lim CH, Jung WR, Cho HS, Sung HY, Nam KW, Chang JH, Cho YK, Park JM, Kim SW, Chung IS. Change of Clostridium difficile colitis during recent 10 years in Korea. Korean J Gastroenterol. 2010;55:169–174. doi: 10.4166/kjg.2010.55.3.169. [DOI] [PubMed] [Google Scholar]
- 9.Byun TJ, Han DS, Ahn SB, Cho HS, Kim TY, Eun CS, Jeon YC, Sohn JH, Kang JO. Clinical characteristics and changing epidemiology of Clostridium difficile-associated disease (CDAD) Korean J Gastroenterol. 2009;54:13–19. doi: 10.4166/kjg.2009.54.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Lee KL, Jeong JB, Kim BG, Shin S, Kim JS, Jung HC, Song IS. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16:3573–3577. doi: 10.3748/wjg.v16.i28.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tal S, Gurevich A, Guller V, Gurevich I, Berger D, Levi S. Risk factors for recurrence of Clostridium difficile-associated diarrhea in the elderly. Scand J Infect Dis. 2002;34:594–597. doi: 10.1080/00365540210147525. [DOI] [PubMed] [Google Scholar]
- 13.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 14.Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–969. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 15.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 16.Jung KS, Park JJ, Chon YE, Jung ES, Lee HJ, Jang HW, Lee KJ, Lee SH, Moon CM, Lee JH, Shin JK, Jeon SM, Hong SP, Kim TI, Kim WH, Cheon JH. Risk factors for treatment failure and recurrence after metronidazole treatment for Clostridium difficile-associated diarrhea. Gut Liver. 2010;4:332–337. doi: 10.5009/gnl.2010.4.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sethi AK, Al-Nassir WN, Nerandzic MM, Donskey CJ. Skin and environmental contamination with vancomycin-resistant Enterococci in patients receiving oral metronidazole or oral vancomycin treatment for Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2009;30:13–17. doi: 10.1086/592710. [DOI] [PubMed] [Google Scholar]
- 18.Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter-Bynoe G, Sherman CB, Romulo RL, Fortna S, Medeiros AA. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–1153. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drees M, Snydman DR, Schmid CH, Barefoot L, Hansjosten K, Vue PM, Cronin M, Nasraway SA, Golan Y. Antibiotic exposure and room contamination among patients colonized with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2008;29:709–715. doi: 10.1086/589582. [DOI] [PubMed] [Google Scholar]
- 20.Gerding DN. Metronidazole for Clostridium difficile-associated disease: is it okay for Mom? Clin Infect Dis. 2005;40:1598–1600. doi: 10.1086/430317. [DOI] [PubMed] [Google Scholar]
- 21.Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008;52:2403–2406. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harbarth S, Cosgrove S, Carmeli Y. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 2002;46:1619–1628. doi: 10.1128/AAC.46.6.1619-1628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester CH, Frimodt-Møller N, Sørensen TL, Monnet DL, Hammerum AM. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob Agents Chemother. 2006;50:596–599. doi: 10.1128/AAC.50.2.596-599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado CD, Giannetta ET, Farr BM. Failure to develop vancomycin-resistant Enterococcus with oral vancomycin treatment of Clostridium difficile. Infect Control Hosp Epidemiol. 2004;25:413–417. doi: 10.1086/502415. [DOI] [PubMed] [Google Scholar]
- 25.Miller M, Bernard L, Thompson M, Grima D, Pepin J. Lack of increased colonization with vancomycin-resistant enterococci during preferential use of vancomycin for treatment during an outbreak of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31:710–715. doi: 10.1086/653613. [DOI] [PubMed] [Google Scholar]
- 26.Gerding DN. Is there a relationship between vancomycin-resistant enterococcal infection and Clostridium difficile infection? Clin Infect Dis. 1997;25(Suppl 2):S206–S210. doi: 10.1086/516247. [DOI] [PubMed] [Google Scholar]
- 27.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 28.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 29.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549–557. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 30.Tae CH, Jung SA, Song HJ, Kim SE, Choi HJ, Lee M, Hwang Y, Kim H, Lee K. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009;24:520–524. doi: 10.3346/jkms.2009.24.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]