Abstract
This study was designed to evaluate the clinical features of abdominal actinomycosis and to assess its therapeutic outcome. We reviewed patients with abdominal actinomycosis in Seoul St. Mary hospital, between January 1994 and January 2010. Twenty-three patients (5 male and 18 female, mean age, 47.8 yr; range, 6-75 yr), with abdominal actinomycosis were included. Emergency surgery was performed in 50% due to symptoms of peritonitis. The common presentation on preoperative computerized tomography was a mass with abscess, mimicking malignancy. The mean tumor size was 7.0 cm (range, 2.5-10.5). In all patients, actinomycotic masses were surgically removed. Mean duration of hospital stay was 17.8 days (range, 5-49). Long term oral antibiotic treatment (mean 4.2 months; range, 0.5-7.0 months) were administered to all patients. All patients were free of recurrence after a median follow up of 30.0 months (mean 35.5 ± 14.8 months, range, 10.0-70.0 months); recurrence was not seen in any patient. In conclusion, abdominal actinomycosis should be included as a differential diagnosis when an unusual abdominal mass or abscess presents on abdominal CT. Assertive removal of necrotic tissue with surgical drainage and long term antibiotic treatment provide a good prognosis in patients with actinomycosis.
Keywords: Abdominal Actinomycosis, Unusual Abdominal Mass
INTRODUCTION
Actinomycosis is a rare and insidious disease. The most common etiologic organism is the anaerobic, Gram-positive bacterium, Actinomyces israelii(1). It reveals invasive and chronic supportive in nature. And it spreads by direct extension across the tissue planes with the formation of multiple abscesses, abundant granulation tissue and sinuses (2). Its tendency to involve surrounding structures may mimic a tumor on imaging studies. Occasionally, hematogenous spread of the infection to the liver and lungs has been documented and can produce nodules, which on radiographic images can be mistaken for malignant neoplasm (3, 4). The three main clinical forms of this disease are cervicofacial, thoracic, and abdominopelvic (5-8). This study has been designed to evaluate clinical features of abdominal actinomycosis and to assess its therapeutic outcome.
MATERIALS AND METHODS
Patients
We reviewed patients with abdominal actinomycosis who had undergone surgical treatment in Seoul St. Mary's hospital between January 1994 and January 2010. The diagnostic standard of actinomycosis was made by pathological confirmation.
Study design and data acquisition
This work was designed to retrospectively review of medical records of patients with abdominal actinomycosis. Data collected from the internal database of Seoul St. Mary Hospital, Korea, included age, gender, disease histories, duration of symptoms, size of actinomycotic mass on abdominal computerized tomography (CT), first impression by the clinicians that performed the initial diagnosis, prior medication history, mode of surgery, presence of an intrauterine device (IUD) and total length of hospital duration. Prognosis data collected included clinical management, antibiotic treatment regimens after diagnosis and length of follow-up. The initial outcome was considered favorable if complete resolution was achieved after antibiotic treatment of least 6 months. Recurrence rate was surveyed through review of medical records.
Statistical analysis
We accessed whether initial demographic variables were associated with actinomycosis. Continuous variables were expressed by mean and range, and were compared using an unpaired t-test. Statistical analysis of categorical values was conducted by using a chi-squared test. The data were analyzed using SPSS version 12 for Windows (SPSS Inc, Chicago, IL, USA). For all tests, the significance level was established at a value of P < 0.05.
Ethics statement
This study was approved by the institutional review board of the Seoul St. Mary's Hospital, the Catholic University of Korea (IRB No. KC10OISI0103). Informed consent was exempted by the board. The study was carried out in accordance with the recommendations of the Declaration of Helsinki.
RESULTS
Baseline characteristic of patients
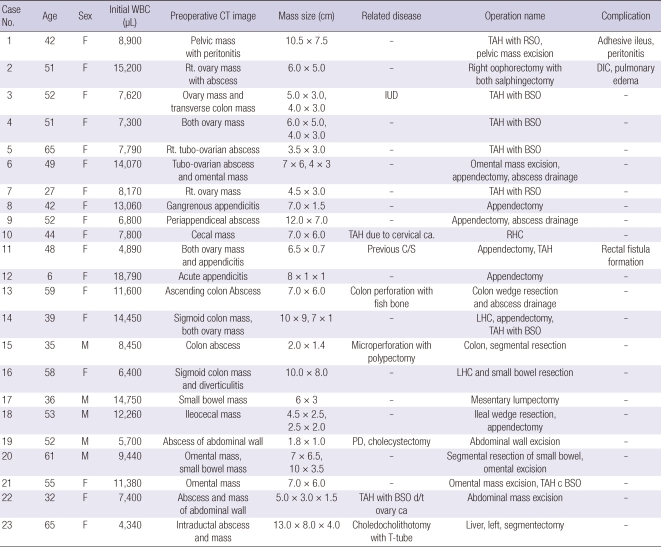
Between January 1995 and December 2008, 43 patients were received surgical resection and pathologically confirmed with actinomycosis at Seoul St. Mary hospital. Abdominopelvic involvement was most common (n = 23, 53.5%), followed by cervicofacial lesion (n = 12, 27.9%) and thoracic involvement was least common (n = 8, 18.6%). We excluded 20 extraabdominal actinomycosis. We reviewed about 23 patients with abdominopelvic actinomycosis, of which five were male and 18 were female (mean age, 47.8 yr; range, 6-75 yr) (Table 1).
Table 1.
Clinical characteristics in 23 patients of abdominopelvic actinomycosis
RLQ, right lower quadrant; LLQ, left lower quadrant; IUD, intrauterine device; TAH, total abdominal hysterectomy; C/S, caesarean section.
Clinical signs and symptoms of actinomycosis
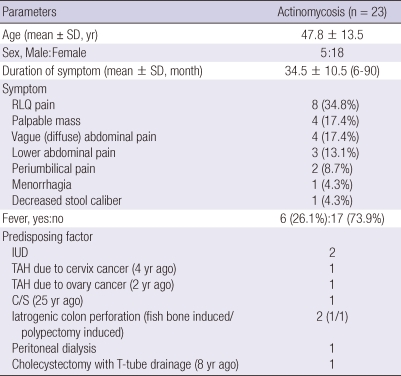
The chief complaints initiating a visit to the hospital were pain in the right upper abdomen (n = 8, 34.8%), a palpable abdominal mass (n = 4, 17.4%), pain in the left lower abdomen (n = 2, 8.7%), vague abdominal pain (n = 4, 17.4%), lower abdominal pain (n = 3, 13.1%), periumbilical pain with fever (n = 2, 8.7%), menorrhagia (n = 1, 4.3%), and decreased stool caliber (n = 1, 4.3%). Only six patients (26.1%) presented with fever (Table 1). The rate of emergency surgery due to peritonitis symptoms was 50%.
The duration from development of symptoms to a hospital visit ranged from 5 days to 3 months, with an average of 34.5 ± 10.5 days (Table 1). Medical history revealed a history of invasive abdominal surgery or procedure (n = 8, 34.8%), an intrauterine device (IUD) (n = 2, 8.7%), total abdominal hysterectomy (TAH) (n = 1, 4.3%), TAH due to ovarian cancer (2 yr ago) (n = 1, 4.3%), Caesarean section (C/S) (25 yr ago) (n = 1, 4.3%), a fish bone-induced rectal perforation (n = 1, 4.3%), polypectomy induced microperforation (n = 1, 4.3%), peritoneal dialysis (n = 1, 4.3%), and cholecystectomy with T-tube drainage (8 yr ago) (n = 1, 4.3%). The two patients with IUD had them for an average of 7 yr.
Result of laboratory test and Pre-operative diagnosis
According to blood chemistry tests, the mean numbers of WBC averaged 10,850.4 109/L, which were slightly higher than normal (normal value; 4,000-10,000 109/L). Mean segmented neutrophil count was 70.6% (normal value; 55.0%-70.0%). Liver function test results were within the normal range in most patients. ESR averaged 42.9 mm/hr, an elevation of four times the normal value (normal value, 0-10 mm/hr). C-reactive protein (CRP) averaged 6.8 mg/L (normal value, less than 5 mg/L).
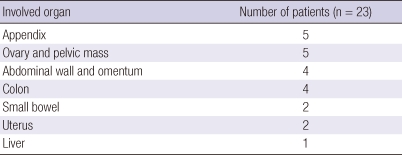
A preoperative abdominal CT scan was performed on all patients, which detected either an intra-abdominal mass or abscess, mimicking malignancy. Locations of the mass were appendix (n = 5), ovarian mass (n = 5), abdominal wall mass (n = 4), colonic mass (n = 4), small bowel mass (n = 2), uterus mass (n = 2) and liver (n = 1) (Table 2).
Table 2.
Involved organs of abdominal actinomycosis
The first impression of the clinicians who acute appendicitis (n = 10), pelvic inflammatory disease (n = 8) and acute tubo-ovarian abscess (n = 4), ovarian cancer or colon cancer. None of the patients underwent percutaneous biopsy.
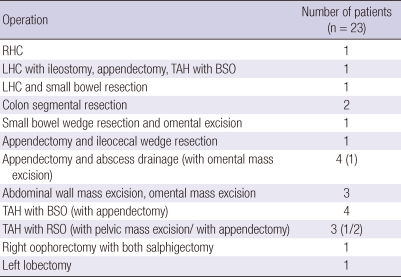
Mode of surgical treatment and pathologic characteristics
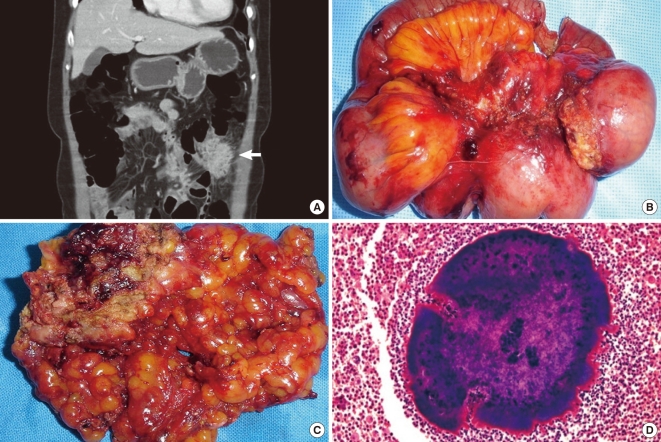
All patients underwent surgical treatment. The mean tumor size was 7.0 cm (range, 2.5-10.5). The diagnosis of actinomycosis was confirmed histologically in all cases. Microscopically, each of the specimens exhibited chronic inflammatory reactions to sulfur granules (Fig. 1). Modes of surgical treatment are summarized in Table 3.
Fig. 1.
Fifty eight years old female presented with lower left quadrant pain for 15 days. (A) Ovoid mass of sigmoid colon mimics a malignant tumor on computerized tomography. (B) 10.0 × 8.0 cm ovoid mass on the sigmoid colon and diverticulitis with severe inflammatory adhesion to descending colon and mesentery. (C) The cut surface demonstrates typical abscess with yellowish-brown color. (D) A magnified view of the characteristic sulfur granule in the middle of purulent exudates (H&E, × 200).
Table 3.
Mode of surgical treatment
RHC, right hemicolectomy; LHC, left hemicolectomy; TAH, total abdominal hysterectomy; BSO, bilateral saphingo-oophorectomy; RSO, right salphingo-oophorectomy.
Complications and long-term prognosis
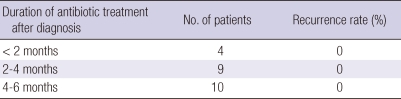
Mean hospital duration was 17.4 days (range, 4-50 days). The antibiotic of choice was intravenous (IV) penicillin G or IV ampicillin. The duration of treatment was less than 2 months in 4 patients, 2 to 4 months in 9, and 4 to 6 months in 10 (Table 4). Postoperative complications occurred in three patients: an adhesive ileus in one, disseminated intravascular coagulation in one, and a rectal perforation with fistula formation in one. Of the 23 patients, 20 patients (91%) improved following surgery and combinatorial antibiotic therapy. All patients were free of recurrence after 1 yr, and after a median follow up of 30.0 months (mean 35.5 ± 14.8 months, range, 10.0-70.0 months), recurrence was not seen in any patient. Clinical details are summarized in Table 5.
Table 4.
Duration of antibiotic treatment and recurrence rate
Table 5.
Demography and clinical characteristics of 23 patients with abdominopelvic actinomycosis
IUD, intrauterine device; PD, peritoneal dialysis; TAH, Total abdominal hysterectomy; BSO, both salphingo oophorectomy; RSO, right salphingo oophorectomy; RHC, right hemicolectomy; LHC, left hemicolectomy; C/S, caesarean section.
DISCUSSION
Our study clearly demonstrated that abdominal actinomycosis usually exhibited an indolent course with non-specific symptoms and signs, resulting in delayed diagnosis. In this study, IUD use was less frequently associated with abdominopelvic actinomycosis, as only two patients had an IUD. Our case reviews suggest that sustained inflammatory conditiona of the appendix, diverticula and salphingo-ovary can be common causes of abdominal actinomycosis.
Actinomyces israelii is the most common etiologic organism of actinomycosis. And it exists in the normal flora of the oral cavity, throughout the gastrointestinal tract and the female genital tract (1). It is also commonly cultured from carious teeth and tonsila crypts (9, 10). This infection usually spreads locally in an indolent manner, and it may take months to years before any symptoms manifest (11). The organism is unable to cross normal mucosal barriers and, therefore, opportunistic infections can occur only in the context of underlying local disease. The destruction of the mucosal barrier by trauma, operations, immunosuppression, a foreign body that penetrates the barrier, or a history of perforated viscera are recognized as predisposing factors. Acute perforated appendicitis was one of the most common predisposing events (12). Other predisposing factors may include diverticulitis and gastrointestinal perforations (13). Pelvic actinomycosis usually occurs following a bowel perforation, especially the perforation of the appendix due to appendicitis, or following ascending infections through use of an IUD (14). Pelvic actinomycosis has recently become more prevalent and is associated almost exclusively with women who use an IUD, which may increase the risk of infection through injury to the normal uterine mucosa (10, 15). In our review of 23 cases, appendicitis was most commonly associated with actinomycosis; eight patients revealed gangrenous appendicitis with periappendiceal abscess formation and three patients revealed appendicitis with right tubo-ovarian abscess formation. Sustained diverticulitis was a frequent cause of actinomycosis with pericolic abscess formation; two patients revealed colon diverticulitis with pericolic abscess formation and two patients revealed small bowel diverticulitis with pericolic abscess formation. Iatrogenic bowel perforation was associated with actinomycosis: one patient with rectal perforation by a fish bone and one with a polypectomy site perforation.
Clinically, the disease follows an indolent course and the initial presentation usually includes lower abdominal pain and fever, with or without a palpable mass; as was seen in our study. Since the disease exhibits vague clinical features, it is difficult to distinguish from other intrabdominal inflammatory diseases. Moreover, it can simulate malignancies in many cases because of the presence of a solid invasive mass and lack of marked leukocytosis (16). Diagnosis is made preoperatively in less than 10% of patients because of the low index of suspicion, unusual presentations, and difficulty in confirming Actinomyces (17-19).
In our series, only four patients were presumed to actinomycosis before surgery. Intra-abdominal actinomycosis can appear as an abdominal mass of ambiguous benignity and can mimic a malignant tumor. For example, actinomycosis of the colon or the greater omentum is a rare differential diagnosis of colonic carcinoma or peritoneal tumor (20, 21). Radiological techniques are inadequate for the diagnosis of abdominal actinomycosis, except for CT, which shows the site and content of the lesions and their relation to adjacent tissues (20, 22). CT can be used as a visualizing method, but never as a conclusive diagnostic tool for abdominal actinomycosis. CT appearance of actinomycetoma more frequently resembles the solid mass with focal low-attenuation areas rather than the cystic form (6, 23). On CT images, tubo-ovarian actinomycosis usually appears as a predominantly solid, or a solid and cystic mass in the adnexal region. It may be well or poorly defined, according to the acute or chronic stage of the infection. Contrast enhancement is very prominent in the solid portion of the mass (23). Small, rim-enhancing lesions are sometimes found in the solid portion of the mass, which are thought to be small abscesses. Thick, linear, and well-enhancing lesions that extend directly from the mass into the adjacent tissue planes, which reflect the invasive nature of actinomycosis, are frequently found and may be characteristic findings. Sometimes, these inflammatory extensions form peri-rectal masses or masses in the cul-de-sac, mimicking seeding masses from ovarian malignancies (24). When actinomycosis is suspected, CT-guided aspiration, with or without core biopsy of suspicious lesions, can be a useful investigative tool (25).
The definitive diagnosis of actinomycosis requires microscopic proof. The presence of sulfur granules, as well as multiple Gram-positive branching hyphae, during pathological examination is important in diagnosing this infection (20, 22). The 'gold standard' is the microbiological culture, which, unfortunately, is rarely positive in the clinical setting. There are some limits in the culturing of Actinomyces species include: requiring freshly obtained material, such as pus or tissue, which should be transported in specific anaerobic containers, or ideally, be processed immediately; and even then testing is capricious and has a negative outcome in 76% of cases (25). The acquired colonies resemble a molar tooth and it takes a week or so for their growth in anaerobic incubation (25). Sulfur granules are seen in pus in only 50% of cases (2). Actinomycotic granules regularly exhibit a positive reaction to periodic acids, Schiff and Grocott's dye, but the Kossa reaction is negative (26).
Surgery is valuable as a definitive diagnosis and also as a therapeutic adjunct because it enables the removal of necrotic tissue and persistent sinuses (20). If recognized preoperatively or confirmed by intra-operative frozen section, extensive resection can be avoided. A limited surgical procedure with surgical drainage and antibiotic treatment may spare the patient from an extensive operation. Whereas there are avascular spaces present due to severe tissue reaction, medical therapy may be less effective, resulting in a longer duration of antibiotic treatment (6). Assertive removal of necrotic tissue with surgical drainage is enough to potentiate the antibiotic effect, thereby shortening the duration of antibiotic use. Penicillin has been shown to be effective in treating abdominal actinomycosis but the duration of treatment varies from several weeks to months to achieve permanent recovery (27, 28). The therapeutic policy of our hospital is to give IV ampicillin during admission. Then, a switch is made to oral ampicillin for more than 4 months. The time of finishing antibiotic therapy is decided by whether resolution is achieved on follow up CT. Recent studies have shown that a combination of complete surgical resection followed by short-term antibiotic treatment is an effective therapy (2). In our analysis, 8 of 23 patients received short term antibiotic therapy for less than 2 months after complete surgical resection, and the disease did not recur during the 34 months follow-up. CT imaging can be a useful tool to monitor resolution or recurrence of the disease. We think that duration of antibiotic therapy should be individualized and optimized according to the individual response.
In conclusion, abdominal actinomycosis should be included as a differential diagnosis when an unusual mass or abscess presents on abdominal CT. Preoperative needle aspiration should be considered in these cases to allow optimization of therapy. That being said, surgical eradication is an important component of disease control. Once the diagnosis is established, the infection can be treated, with good results, by combining surgical and medical methods. Assertive removal of necrotic tissue and surgical drainage may spare the patient from an extensive operation. Lastly, antibiotic therapy is essential to minimize the risk of recurrence after surgical excision.
ACKNOWLEDGMENTS
All pathologic slides were reviewed and confirmed by pathologist Dr. Su Young Kim.
Footnotes
This study was supported by the Catholic Medical Center Research Foundation made in the program year of 2008.
AUTHOR SUMMARY
Clinical Features of Abdominal Actinomycosis: A 15-year Experience of A Single Institute
Hye Young Sung, In Seok Lee, Sang Il Kim, Seung Eun Jung, Sang Woo Kim, Su Young Kim, Mun Kyung Chung, Won Chul Kim, Seong Tack Oh and Won Kyung Kang
Abdominal actinomycosis should be considered when an unusual mass or abscess is observed on abdominal CT. Preoperative needle aspiration is requested for optimization of therapy. After surgical eradication, once the diagnosis is established, the infection could be treated by appropriate medications. Assertive removal of necrotic tissue and surgical drainage could spare the patients from an extensive operation. Lastly, antibiotic therapy is essential to minimize the risk of recurrence after surgical excision.
References
- 1.Stringer MD, Cameron AE. Abdominal actinomycosis: a forgotten disease? Br J Hosp Med. 1987;38:125–127. [PubMed] [Google Scholar]
- 2.Koren R, Dekel Y, Ramadan E, Veltman V, Dreznik Z. Periappendiceal actinomycosis mimicking malignancy report of a case. Pathol Res Pract. 2002;198:441–443. doi: 10.1078/0344-0338-00279. [DOI] [PubMed] [Google Scholar]
- 3.Apothéloz C, Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis. 1996;22:621–625. doi: 10.1093/clinids/22.4.621. [DOI] [PubMed] [Google Scholar]
- 4.Mesgarzadeh M, Bonakdarpour A, Redeck PD. Case report 365: Hematogenous actinomyces oeteomyelitis (calcaneus) Skeletal Radiol. 1986;15:584–588. doi: 10.1007/BF00361061. [DOI] [PubMed] [Google Scholar]
- 5.Belmont MJ, Behar PM, Wax MK. Atypical presentations of actinomycosis. Head Neck. 1999;21:264–268. doi: 10.1002/(sici)1097-0347(199905)21:3<264::aid-hed12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Berchtenbreiter C, Brüning R, Auernhammer A, Reiser M. Misleading diagnosis of retroperitoneal actinomycosis. Eur Radiol. 1999;9:1869–1872. doi: 10.1007/s003300050937. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari TC, Couto CA, Murta-Oliveira C, Conceição SA, Silva RG. Actinomycosis of the colon: a rare form of presentation. Scand J Gastroenterol. 2000;35:108–109. doi: 10.1080/003655200750024623. [DOI] [PubMed] [Google Scholar]
- 8.Russo TA. Agents of actinomycosis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. New York: Churchill Livingstone; 2005. pp. 2924–2934. [Google Scholar]
- 9.Uchiyama N, Ishikawa T, Miyakawa K, Iinuma G, Nakajima H, Ushio K, Yokota T, Akasu T, Shimoda T. Abdominal actinomycosis: barium enema and computed tomography findings. J Gastroenterol. 1997;32:89–94. doi: 10.1007/BF01213302. [DOI] [PubMed] [Google Scholar]
- 10.Cintron JR, Del Pino A, Duarte B, Wood D. Abdominal actinomycosis. Dis Colon Rectum. 1996;39:105–108. doi: 10.1007/BF02048278. [DOI] [PubMed] [Google Scholar]
- 11.Meyer P, Nwariaku O, McClelland RN, Gibbons D, Leach F, Sagalowsky AI, Simmang C, Jeyarajah DR. Rare presentation of actinomycosis as an abdominal mass: report of a case. Dis Colon Rectum. 2000;43:872–875. doi: 10.1007/BF02238030. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh N, Heaney SJ. Actinomycosis at multiple colonic sites. Am J Gastroenterol. 1986;81:1212–1214. [PubMed] [Google Scholar]
- 13.Fowler RC, Simpkins KC. Abdominal actinomycosis: a report of three cases. Clin Radiol. 1983;34:301–307. doi: 10.1016/s0009-9260(83)80341-9. [DOI] [PubMed] [Google Scholar]
- 14.Taga S. Diagnosis and therapy of pelvic actinomycosis. J Obstet Gynaecol Res. 2007;33:882–885. doi: 10.1111/j.1447-0756.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 15.Luff RD, Gupta PK, Spence MR, Frost JK. Pelvic actinomycosis and the intrauterine contraceptive device. A cyto-histomorphologic study. Am J Clin Pathol. 1978;69:581–586. doi: 10.1093/ajcp/69.6.581. [DOI] [PubMed] [Google Scholar]
- 16.Weese WC, Smith IM. A study of 57 cases of actinomycosis over a 36-year period. A diagnostic 'failure' with good prognosis after treatment. Arch Intern Med. 1975;135:1562–1568. [PubMed] [Google Scholar]
- 17.Harris LF, Kakani PR, Selah CE. Actinomycosis. Surgical aspects. Am Surg. 1985;51:262–264. [PubMed] [Google Scholar]
- 18.Kaya M, Sakarya MH. A rare cause of chronic abdominal pain, weight loss and anemia: abdominal actinomycosis. Turk J Gastroenterol. 2007;18:254–257. [PubMed] [Google Scholar]
- 19.Harris LA, DeCosse JJ, Dannenberg A. Abdominal actinomycosis: evaluation by computed tomography. Am J Gastroenterol. 1989;84:198–200. [PubMed] [Google Scholar]
- 20.Huang CJ, Huang TJ, Hsieh JS. Pseudo-colonic carcinoma caused by abdominal actinomycosis: report of two cases. Int J Colorectal Dis. 2004;19:283–286. doi: 10.1007/s00384-003-0568-y. [DOI] [PubMed] [Google Scholar]
- 21.Rose G, Franke FE, Weimar B, Buhr J, Padberg W. Actinomycosis of the colon as a rare differential diagnosis of colonic carcinoma. Chirurg. 2000;71:93–97. doi: 10.1007/s001040050016. [DOI] [PubMed] [Google Scholar]
- 22.Abela J, Sciberras J, Meilak M, Felice AG, Degaetano J. Omental actinomycosis presenting with right lower quadrant abdominal pain. J Clin Pathol. 2004;57:671. [PMC free article] [PubMed] [Google Scholar]
- 23.Ha HK, Lee HJ, Kim H, Ro HJ, Park YH, Cha SJ, Shinn KS. Abdominal actinomycosis: CT findings in 10 patients. AJR Am J Roentgenol. 1993;161:791–794. doi: 10.2214/ajr.161.4.8372760. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim SH, Yang DM, Kim KA. Unusual causes of tubo-ovarian abscess: CT and MR imaging findings. Radiographics. 2004;24:1575–1589. doi: 10.1148/rg.246045016. [DOI] [PubMed] [Google Scholar]
- 25.Spagnuolo PJ, Fransioli M. Intrauterine device-associated actinomycosis simulating pelvic malignancy. Am J Gastroenterol. 1981;75:144–147. [PubMed] [Google Scholar]
- 26.Wagenlehner FM, Mohren B, Naber KG, Männl HF. Abdominal actinomycosis. Clin Microbiol Infect. 2003;9:881–885. doi: 10.1046/j.1469-0691.2003.00653.x. [DOI] [PubMed] [Google Scholar]
- 27.Anteby E, Milvidsky A, Goshen R, Ben-Chetrit A, Ron M. IUD associated abdominopelvic actinomycosis. Harefuah. 1991;121:150–153. [PubMed] [Google Scholar]
- 28.Atad J, Hallak M, Sharon A, Kitzes R, Kelner Y, Abramovici H. Pelvic actinomycosis. Is long-term antibiotic therapy necessary? J Reprod Med. 1999;44:939–944. [PubMed] [Google Scholar]