Abstract
The lateral part of intermediate layer of superior colliculus (SCl) is a critical substrate for successful predation by rats. Hunting-evoked expression of the activity marker Fos is concentrated in SCl while prey capture in rats with NMDA lesions in SCl is impaired. Particularly affected are rapid orienting and stereotyped sequences of actions associated with predation of fast moving prey. Such deficits are consistent with the view that the deep layers of SC are important for sensory guidance of movement. Although much of the relevant evidence involves visual control of movement, less is known about movement guidance by somatosensory input from vibrissae. Indeed, our impression is that prey contact with whiskers is a likely stimulus to trigger predation. Moreover, SCl receives whisker and orofacial somatosensory information directly from trigeminal complex, and indirectly from zona incerta, parvicelular reticular formation and somatosensory barrel cortex. To better understand sensory guidance of predation by vibrissal information we investigated prey capture by rats after whisker removal and the role of superior colliculus (SC) by comparing Fos expression after hunting with and without whiskers. Rats were allowed to hunt cockroaches, after which their whiskers were removed. Two days later they were allowed to hunt cockroaches again. Without whiskers the rats were less able to retain the cockroaches after capture and less able to pursue them in the event of the cockroach escaping. The predatory behaviour of rats with re-grown whiskers returned to normal. In parallel, Fos expression in SCl induced by predation was significantly reduced in whiskerless animals. We conclude that whiskers contribute to the efficiency of rat prey capture and that the loss of vibrissal input to SCl, as reflected by reduced Fos expression, could play a critical role in predatory deficits of whiskerless rats.
Keywords: predatory behaviour, vibrissae, somatosensory information, sensorimotor integration
Insect predation is a fundamental motivated behaviour of many rodents, including rats. As such it is an interesting paradigm which has been used to study patterns of innate predatory hunting. Cockroaches have been considered a suitable prey because they are relatively innocuous, easily overcome, and appear very palatable with both hunting and subsequent consumption contributing to a high hedonic value associated with the activity (Comoli et al., 2005).
Previous studies have shown that insect predation in rats is associated with the expression of Fos protein at the lateral part of intermediate layer of superior colliculus (SCl) (Comoli and Canteras, 1998). Moreover, rats with local bilateral NMDA lesions in SCl typically fail to orient towards and chase the cockroaches with the series of stereotyped movements commonly seen in the predatory hunting of intact controls (Comoli and Canteras, 2000; Furigo et al., 2010).
The superior colliculus (SC) is known to be responsible for detecting and orienting the head and eyes toward visual stimuli (Grantyn, 1988; Munoz and Guitton, 1989; Wurtz, 1996; Sparks, 1999; Boehnke and Munoz, 2008). In the case of rodents it is also important for positioning the head in space during a variety of oral tasks, such as predation, foraging for food, and nocifensive responses directed to localized pain (Redgrave et al., 1990; Welzl et al., 1984; Steiner and Huston, 1992; Wang and Redgrave, 1997; Ewert et al., 1999). It is also involved in gaze-shifts directed towards auditory, somatosensory and even olfactory cues (Grobstein, 1988; Dean et al., 1989; King, 2004; Boehnke and Munoz, 2008; Felsen and Mainen, 2008). Moreover SC is critical for the detection and guidance of initial responses to unexpected objects in the visual field (Grantyn, 1988; Munoz and Guitton, 1989; Wurtz, 1996; Sparks, 1999; Coizet et al., 2003; Comoli et al., 2003), and orienting the head away from potentially threatening stimuli (Dean et al., 1989; Brandão et al., 1994, 1999).
In the light of our previous behavioural observations, the movement of prey, in particular their contacts with the rats’ whiskers seem to be the critical stimulus responsible for evoking hunting and to the up-regulation of Fos in the SCl. Anatomical and electrophysiological data show that SCl receives whisker and orofacial-related somatosensory information from a range of sources including the spinal (SpV) and principal trigeminal nuclei (PrV) (Killackey and Erzurumlu, 1981; Huerta et al., 1983; Comoli and Canteras, 2000; Hemelt and Keller, 2007), zona incerta (ZI) (Kolmac et al., 1998; Comoli and Canteras, 2000, parvicelular reticular nucleus (PARN) and somatosensory cortex (SSp) (Comoli and Canteras, 2000; Cohen et al., 2008). In line with this view, the fast attacks associated with prey capture by wild shrews depend on information from the whiskers rather than visual and olfactory cues (Anjum et al., 2006).
Although SCl has an important role in predatory hunting in rats (Comoli and Canteras, 2000; Furigo et al., 2010), the link to somatosensory input to the SCl from the vibrissae is still missing. The purpose of the present study was, therefore, to determine the effects of removing the whiskers both on the behavioural performance of predatory hunting and on immediate early gene (c-Fos) activation in the SCl which has shown to be elicited by hunting (Comoli and Canteras, 1998).
EXPERIMENTAL PROCEDURES
The basic design of the present study involved behavioural observations of predatory hunting together with measurements of associated Fos expression in the SCl in rats with intact whiskers, rats that had their whiskers subsequently removed, and rats whose whiskers had been allowed to re-grow to their original size (35 days after removal).
Animal housing
All experimental procedures were performed with adults male Wistar rats (n=22) each weighting aproximately 230 g obtained from local Ribeirão Preto breeding facilities. The rats were kept under controlled temperature (23±2 °C) and 12-h light/dark cycle (lights on at 7 am), and had free access to water and standard laboratory diet. Experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996), and were approved by the Committee on Care and Use of Laboratory Animals of the School of Medicine of Ribeirão Preto—University of São Paulo, Brazil (Protocol number 133/2007). Effort was made to minimize the number of animals used and their suffering.
Experimental procedure
Seven days before the experiments began, rats were handled repeatedly by the same investigator who conducted the behavioural tests. Rats were individually placed into an acrylic cage (40×30×16 cm3) for 2 h each day in which free access to rat chow and water was available.
Subsequently, hunting sessions were carried out between 09:00 and 12:00 h, during the light phase of the cycle. Rats were induced to hunt by simultaneously introducing five mature intact cockroaches (Leurolestes circunvagans), raised for this purpose in our laboratory, into the experimental cage. The rapid movement of the prey was sufficient to elicit hunting and no additional inducement was needed. A hunting trial for each rat lasted until it had caught all five cockroaches or 20 min had elapsed at which point the trial was ended by the experimenter. Hunting was videotaped using an overhead camera and the resulting record retained for additional behavioural analysis.
For the analysis of predatory behaviour seven animals were allowed to hunt cockroaches during three successive sessions: (i) An initial baseline session (Vib) in which rats were allowed to hunt with their whiskers intact. (ii) Rats then had their whiskers removed while under anaesthesia (ketamine, xylazine and acepromazine; 45:5:0.5 mg/kg, i.p.) (União Farmacêutica Nacional S/A, Embu-Guaçu, SP, Brazil; Laboratórios Calier S/A Barcelona, Espanha; Rhobifarma Indústria Farmacêutica Ltda, São Paulo, SP, Brazil). Forty-eight hours later they were allowed to hunt for a second time (VibX). (iii) A period of 35 days was then allowed during which the rats’ whiskers re-grew to their original size, at which point they were allowed to hunt for a third and final time (VibR).
For the histological analysis of c-Fos induced by hunting with and without whiskers we used 20 animals. These included: a group of five animals who experienced the same Vib experimental condition of hunting with whiskers; a second group of five who experienced the VibX experimental condition of hunting without whiskers; five animals from the final behavioural VibR session in which hunting was conducted with re-grown whiskers; and a control group (n=5) who were similarly housed and handled, but were never allowed to hunt nor had their whiskers removed (they were placed undisturbed in the experimental cage during the test period). After behavioural testing the brain tissue of all animals was taken for immunohistochemical analysis (see below).
Behavioural analysis
A trained observer scored behavioural characteristics using the ethological analysis software “The Observer XT®”, (version XT 7; Noldus Information Technology, Wagenigen, The Netherlands). The following events were measured: (i) Latency for the first attack. (ii) Total time spent hunting—forward head and body movements directed towards a cockroach. (iii) The duration of each occasion the rat caught and held one of the prey—time from when the prey disappeared below the rat from the extension of its shoulder to its snout. This measure included the time spent handling and eating prey. (iv) Frequency of occasions when the rat immediately followed cockroach after it had escaped (been released)—the nose of the rat moving in the same direction as the cockroach in successive video frames. (v) Frequency of occasions when the rat failed to follow the cockroach after an escape—the rat’s nose either remained stationary or moved in a different direction to that of the cockroach in successive video frames. (vi) The total number of cockroaches killed and eaten. (vii) Other states—including grooming, drinking and resting.
Immunohistochemical procedures
After all behavioural procedures had been completed, rats whose brain tissue was to be analysed for c-Fos were deeply anesthetized (by ketamine, xylazine and acepromazine; 100:20:3 mg/kg, i.p.) (União Farmacêutica Nacional S/A, Embu-Guaçu, SP, Brazil; Laboratórios Calier S/A Barcelona, Espanha; Rhobifarma Indústria Farmacêutica Ltda, São Paulo, SP, Brazil) 90 min after their final hunting session. They were perfused transcardially with a brief saline rinse followed by an ice-cold solution of 4% paraformaldehyde in a 0.1 M phosphate buffer at pH 7.4. Two hours later the brains were removed from the skull and cryoprotected with 20% sucrose in 0.1 M phosphate buffer at 4 °C. The brains were then frozen and sectioned coronally on a sliding microtome (Leica Microsystems, Germany) (30 μm) and sorted into four series. One series was processed (rabbit anti-Fos, dilution 1:20,000; Calbiochem, San Diego, CA, USA, lot # D00007099) with the avidin–biotin-peroxidase immunohistochemical technique (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA, USA) according to the method of Gerfen and Sawchenko (1984) to reveal Fos-like immunoreactivity. Briefly, sections were washed in potassium phosphate-buffered saline (KPBS; pH 7.4) and incubated for 90 min at room temperature in a solution of biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA). They were then exposed to a mixed avidin–biotin horseradish peroxidase (HRP) complex solution (ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA) for the same period. The peroxidase reaction product was visualized using the glucose oxidase procedure (Itoh et al., 1979) and the metal free 3,3′ diaminobenzidine tetrahydrochloride (DAB, Sigma, St Louis, MO, USA) as a chromogen. Sections were exposed for 10 min to a 0.02% chromogen solution containing 0.3% nickel ammonium sulfate in 0.05 M phosphate buffer (pH 7.4) and glucose oxidase, followed by the addition of β-d-glucose to produce a blue-black product. The reaction was stopped by extensive washing in KPBS, pH 7.4. The sections were mounted on gelatinized slides, and then dehydrated and coverslipped with DPX (Sigma, St. Louis, MO, USA). An adjacent series was stained with Thionin to serve as a cytoarchitectonic reference for the distribution of Fos labeling.
Fos-immunoreactive neurons were evaluated by an observer ignorant of the rat’s experimental status. Sections were viewed under a 10×objective of a Leika DM 2500 microscope equipped with a camera lucida, and a graticule (0.3×0.3 mm2 area=0.09 mm2) was used to manually quantify the number of Fos-positive neurons in the lateral and medial sectors of the SC at mid rostrocaudal levels, AP ≅ 6.0 mm. For a cell to be considered as expressing Fos-like immunoreactivity, the nucleus of the neurons had to be of appropriate size (ranging approximately, from 8 to 15 μm) and shape (oval or round), show the characteristic blue-black staining of oxidized DAB-Ni, and be distinct from the background at magnification of ×10. Both regions of interest were counted in three sections from each rat.
The figures were prepared using the Adobe Photoshop CS3 (version 10.0.1; Adobe Systems, Mountain View, CA, USA) for photomicrographs and Adobe Illustrator CS3 (Version 13.0.1; Adobe System, Mountain View, CA, USA) for line drawings. Only contrast and brightness were adjusted.
Statistical analysis
For behavioural measurements we used a one-way or two-way analysis of variance (ANOVA) with repeated measures. However, because the hold-duration data were not normally distributed comparisons were made with the non-parametric Kolmogorov–Smirnov test. Data reflecting the density of Fos labeled cells were analysed using the one-way ANOVA followed by the Fisher’s PLSD multiple comparisons test for independent samples for lateral or medial SC. In each case the significance level was set at α=5%. All the values are expressed as means±SEM.
RESULTS
Behavioural analysis
The purpose of the behavioural analysis was to determine the effects of removing rats’ whiskers on their ability to hunt cockroaches. We will first describe the normal hunting of rats with intact whiskers, before outlining the effects whisker removal had on this behaviour.
Rats were placed individually in the experimental cage after which five intact mature cockroaches were introduced. Initially, a rat sniffed around the cage until a prey was located, often by the whiskers, at which point the rat rushed towards the cockroach and tried to capture it, either with the forepaws or immediately with the mouth. While hunting the rats were able to orient themselves optimally with respect to the prey and immediately after capture, rapidly manipulated the cockroach into a position where a killing bite could be directed towards the prey’s head. More often than not the cockroach was then eaten. On some occasions, rats with intact whiskers were so motivated they would leave the cockroach they had just caught to catch another that happened to distract it. Alternatively, the rats with intact whiskers would sometimes appear to play with the prey in much the same way as a cat would play with a mouse—letting it escape only to re-capture it again. Results of the quantitative analysis of hunting by rats with intact whiskers are presented in first data column of Table 1 and Figs. 1 and 3.
Table 1.
Behavioural measurements during experimental session
| Behavioural measurements |
Experimental conditions (n=7) |
||
|---|---|---|---|
| Vib | VibX | VibR | |
| Latency to attack (s) | 18.9±4.6 | 25.7±6.9 | 11.3±6.0 |
| Hunt-duration (s) | 235.3±71.2 | 262.0±67.1 | 234.3±66.5 |
| Total contact time (s) | 467.6±33.9 | 429.0±64.7 | 338.9±41.0 |
| Total # of cockroaches- killed/consumed |
1.2±0.1 | 1.3±0.1 | 1.1±0.1 |
| Mean-attacks/kill | 4.5±0.8 | 4.7±0.4 | 3.8±0.7 |
Values are mean±SEM. Differences between conditions were assessed by one-way repeated measures ANOVA.
Fig. 1.
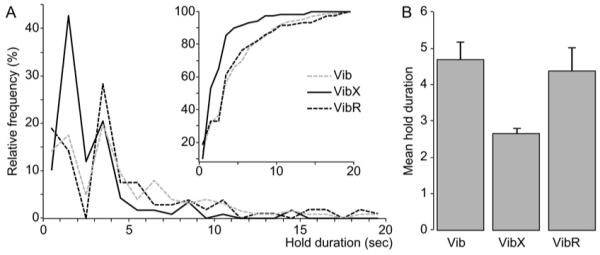
Increased frequency of short-duration holds when rats hunt without whiskers. (A) The frequency distribution of hold durations <20 s for the three experimental conditions. A cumulative representation of the relative frequency of hold durations <20 s (inset). (B) Mean hold duration of values from 1 to 20 s for the three experimental conditions. Data (n=7) are expressed as mean±SEM; one-way ANOVA test for differences between experimental conditions: F=8.78; df=2.12; P=0.0045. Vib, intact whiskers; VibX, whiskers removed; VibR, whiskers re-grown.
Fig. 3.
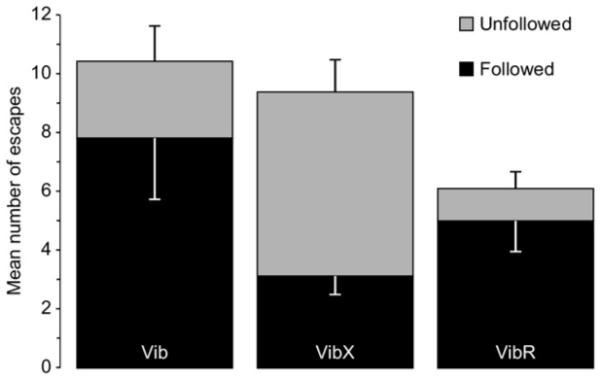
Increased proportion of un-followed escapes by rats without whiskers. Data (n=7) are expressed as mean±SEM. Interaction between experimental condition (Vib, VibX, VibR) and behaviour after escape (followed, un-followed) highly significant (F=28.3, df=2.12; P<0.0001).
Despite already having had the experience of hunting, several aspects of the predatory behaviour of rats were quite different when their whiskers were removed. However, we will describe first the similarities. Rats without whiskers appeared equally well motivated to catch cockroaches. Thus, in terms of latency to first attack, time during the session spent hunting, total time in contact with the prey, together with the number of cockroaches caught, killed and consumed, rats without whiskers did not differ significantly from the previous session when their whiskers were intact (data column 2 of Table 1). However, despite being well motivated, rats without whiskers were clearly less proficient in the sensorimotor aspects of hunting and prey capture. First, they appeared to have difficulty orienting themselves correctly towards the prey to facilitate an efficient strike, either with the mouth or the paws. Second, having caught or made contact with a cockroach they had difficulty manipulating it into a position where it could be easily killed. Thus, on many occasions the cockroach was able to escape at which point the whiskerless rats often failed to follow the escaping prey. To provide quantitative measures of these behavioural problems we measured first, the duration prey were as held after each attack, and secondly, in the event of the cockroach escaping, whether or not it was immediately followed. Hold durations >20 s were excluded from the analysis, because by this time a caught cockroach was most often dead or injured, and therefore unlikely to escape. The distribution of hold durations across the three experimental sessions are illustrated in Fig. 1A. Very short-hold durations (1–2 s) were more frequent when rats were hunting without whiskers, thus compared with the initial control condition, the distribution of hold-durations <20 s was significantly different for rats without whiskers (Chi-square=28.035, P<0.0001), but not after the whiskers were allowed to re-grow (Chi-square=1.626, P=0.8869). This was confirmed with an analysis of the mean duration of holds <20 s which was significantly shorter for rats hunting without whiskers (Fig. 1B; F=8.78; df=2.12; P=0.0045). These data are consistent with the failure of rats without whiskers being able to orient themselves appropriately during an attack and subsequently manipulating the prey to prevent its escape. However, the short-hold duration data confounded whiskerless incompetence with “cat-and-mouse” play where the cockroach was “deliberately” released by rats with whiskers, then only to be recaptured. To disambiguate this we analysed the frequency of “following” during the 1st second after an escape. Rats were deemed to be “following” if the nose moved in the same direction as the escaping cockroach in successive video frames (Fig. 2A). If the rat’s nose either remained stationary or moved in a different direction the cockroach was considered “un-followed” (Fig. 2B). The proportion of un-followed cockroaches was significantly higher when the rats were hunting without whiskers (Fig. 3: Interaction between experimental condition×followed:un-followed escapes–F=28.3; df=2.12; P<0.0001).
Fig. 2.
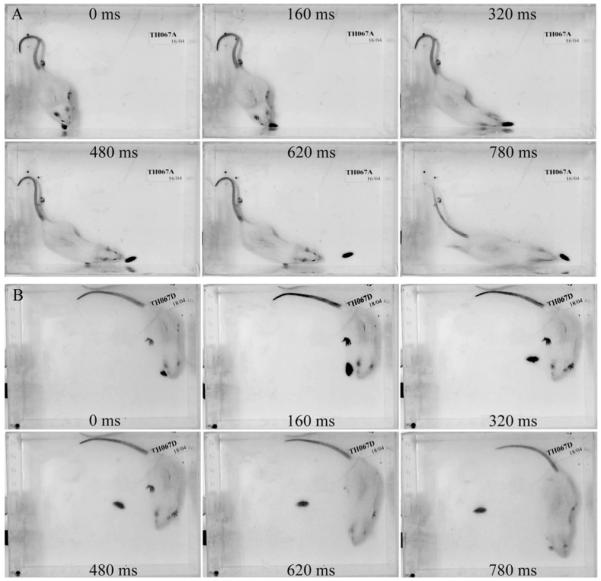
Typical examples of a rat with whiskers immediately following an escaping cockroach (A), and a rat without whiskers failing to follow a similarly escaping cockroach (B). Time (ms) following the cockroach’s escape is shown in each image and runs in horizontal lines from the top left to bottom right.
After the whiskers had been allowed to re-grow, all aspects of hunting behaviour returned to the pre-whisker removal baseline (Table 1 and Figs. 1 and 3).
Immunohistochemical analysis
During each phase of the experiment, five subjects were taken, following the hunting experience, for an analysis of the expression of Fos-like-immunoreactivity (FLI) in the SC. In addition to the groups of animals with intact whiskers, whiskers removed and re-grown whiskers, an additional group of non-hunting control animals was used to establish baseline expression of FLI in the SC.
To characterise the regional pattern of FLI labeling in the SC we followed the convention used in previous publications (Sahibzada et al., 1986; Telford et al., 1996) in which the superficial layers comprise: stratum zonale (SZ); stratum griseum superficiale (SGS); stratum opticum (SO); the intermediate layers included: stratum griseum intermediale (SGI); stratum album intermediale (SAI); and the deep layers were: stratum griseum profundum (SGP); and stratum album profundum (SAP).
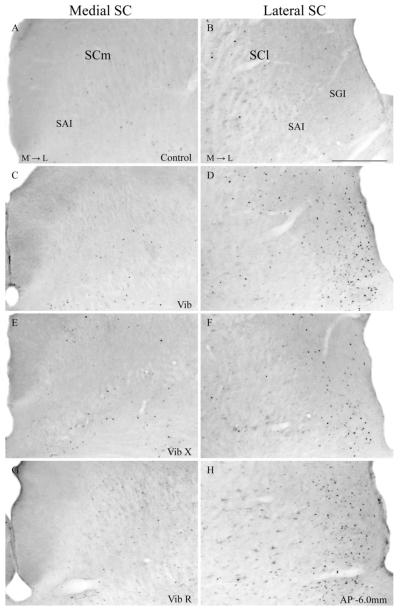
Compared with non-hunting controls, rats with intact whiskers that had performed predatory hunting revealed a general increase of FLI expression (F=131.87; df=3.16; P<0.0001). The hunting-induced increase in FLI expression was, however, particularly prominent in the lateral SC where labeled cells were concentrated in the lateral sectors of SGI and to a lesser extent SAI (see Figs. 4 and 5). These observations were true for both the initial group with intact whiskers (Vib) and the group whose whiskers had been allowed to re-grow (VibR). The main finding, however, was that the expression of FLI in the lateral SC in animals who had been allowed to hunt without whiskers (VibX) had significantly less FLI expressed in lateral SC compared with the animals with intact whiskers (Figs. 4F, 5C and 6) (Interaction between experimental condition (Vib, VibX and VibR)×region within the SC (lateral, medial): F=21.2; df=2.12; P=0.0001). In medial sectors of the SC there was a small but significant increase in FLI expression in all the groups that were allowed to hunt compared with the non-hunting controls (Figs. 4-6) (F=32.5; df=3.16; P<0.0001). However, there was no reliable difference in FLI expression in the medial SC between animals hunting with and without whiskers (Fig. 6) (Interaction between experimental condition (Vib, VibX and VibR)×region within the SC (lateral, medial): F=21.2; df=2.12; P=0.0001).
Fig. 4.
Photomicrographs of transverse sections of the rat brain at the level of SC showing Fos-stained from a control rat (A, B) and from rats that performed predatory behaviour: a rat with intact whiskers (Vib) (C, D), a rat with whiskers-removed (VibX) (E, F) and a rat with re-grown whiskers (VibR) (G, H). Abbreviations: see list. Scale bars=300 μm. M → L, direction from medial to lateral axis.
Fig. 5.
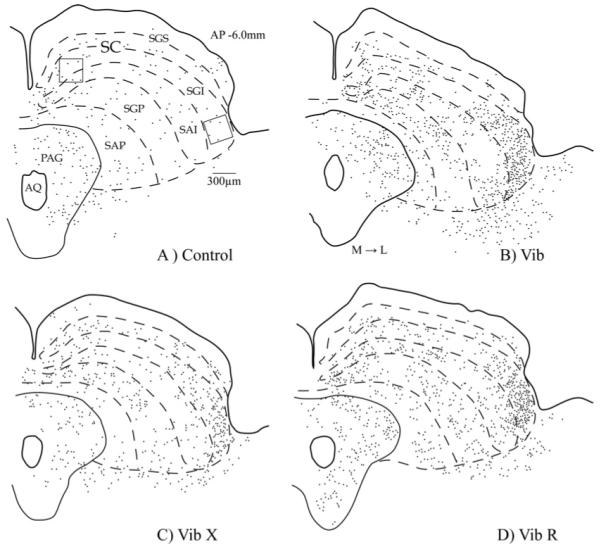
Camara lucida drawings from transverse sections of representative cases illustrating the distribution of Fos staining in the SC. Control rat (A) and rats that performed predatory behaviour: a rat with intact whiskers (Vib) (B), a rat with whiskers-removed (VibX) (C), a rat with re-grown whiskers (VibR) (D). Open squares indicate the position of the 0.09 mm2 grid (exactly to scale) within which the Fos counts were made. Abbreviations: see list. Scale bars=300 μm. M → L, direction from medial to lateral axis.
Fig. 6.
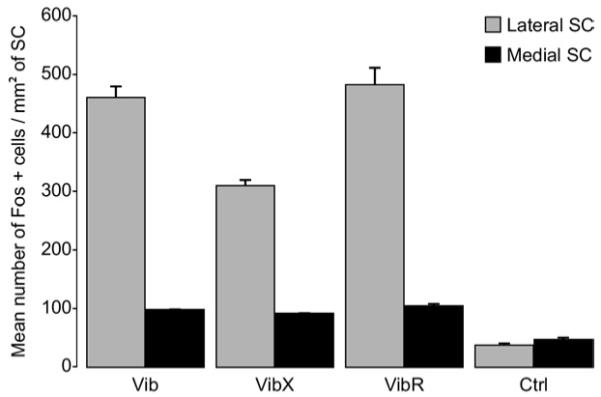
Histograms showing the mean density of Fos-immunoreactive cells in the lateral and medial parts of the SC in control animals (n=5) and animals that had performed predatory behaviour: rats with intact whiskers (Vib) (n=5), rats with whiskers-removed (VibX), and rats with re-grown whiskers rat (VibR) (n=5). Values represent the number of Fos-labeled cells/mm2. Data are expressed as mean±SEM. (Separate analysis of interaction between experimental condition (Vib, VibX and VibR)×region within the SC (lateral, medial): F=21.2; df=2.12; P=0.0001).
DISCUSSION
The aim of the present study was to determine how far the predatory behaviour of rats relies on whisker-related somatosensory information, and the role of the midbrain SC as an important target structure involved in the somatosensory guidance of predatory responses. The main behavioural findings were that removal of the whiskers significantly impaired the ability to hold on to the cockroaches and to follow rapidly an escaping cockroach. However, competent hunting was re-established after the whiskers had been allowed to re-grow. In parallel with the behavioural observations the experience of hunting with whiskers induced a significant elevation of FLI which was concentrated in the lateral intermediate layers of the SC. In rats allowed to hunt without whiskers the activation of FLI in the lateral SC was significantly reduced. Before discussing these findings in detail we will consider technical issues that could influence their interpretation.
Visual inspection of rats hunting with and without whiskers gave the clear impression, those with whiskers were supremely competent while those without were not. Because the whiskerless rats were clearly motivated to hunt, the latency to first contact, the time they spent hunting, the numbers of cockroaches caught and killed and the time they spent doing so did not differ significantly from sessions when their whiskers were intact. However, without whiskers, they failed to orient towards and strike at the cockroaches in the most effective manner and once caught, the cockroach stood a fair chance of escaping (Fig. 1) and not being immediately re-captured (Figs. 2 and 3). Fos protein expression has been used as a sensitive cellular marker for neuronal activation induced by a variety of stimuli (Morgan and Curran, 1991), however the absence of Fos expression cannot be evaluated as lack of influence on neuronal activity (Sheng and Greenberg, 1990). For the present study, this is not a problem as we report a significant increase in FLI expression associated with hunting and its subsequent suppression following removal of the whiskers. Because the rats with and without whiskers spent approximately the same amount of time hunting, the conclusion that whisker-related somatosensory input is responsible for the activation of neurons in the lateral SC as reflected by their expression of FLI is sound. The important observation was that the behavioural competence of animals whose whiskers were allowed to re-grow was re-established and with it, the re-activation of neurons in the lateral SC while hunting.
The vibrissae system is a vital sensorimotor system for the rat and has been recognized as an important source of sensory information to rats and other rodents for a long time (Vincent, 1912). The development of a sophisticated somatosensory system based on whiskers in rats may have occurred in part to compensate for their comparatively poor vision and demands imposed by a nocturnal environment. In such conditions, the ability to build texture-maps by which to navigate and to receive signals indicating the immediate presence of potential prey or con-specifics, afforded by whisker-related somatosensation, would likely confer significant evolutionary advantage. Support for these ideas can be found in literature suggesting the active use of whiskers allows rats to distinguish texture differences of surfaces, and shapes at a level comparable to and perhaps exceeding the use of fingertips by human and non-human primates (Guic-Robles et al., 1989, 1992; Carvell and Simons, 1990; Prigg et al., 2002; Arabzadeh et al., 2005; Kleinfeld et al., 2006; Wolfe et al., 2008). Moreover the sensitivity of the whisker system to direction and speed of whisker deflections would be essential for guiding the fore-paws or the mouth to a moving target (Brecht et al., 1997).
Rat facial vibrissae are characterized by long and laterally oriented macrovibrissae and shorter, more numerous frontal microvibrissae. Information from the macrovibrissae is relayed successively through a spatially preserved architecture at all levels within the neuraxis (Kleinfeld et al., 2006). For rats operating in the dark these are its object detecting sense organs which can determine the presence of an object and provide critical spatial information that can be used to guide exploratory movements that bring the microvibrissae into contact with the object so it can be recognized (Brecht et al., 1997).
Tactile orientation was studied in cricket capture by wild Etruscan shrews (Anjum et al., 2006). This is a very small mammal which is forced to capture and kill large numbers of prey, in the dark. This study established that prey capture by Etruscan shrew is also based on active use of the whiskers, information from which supports fast movements of the head directed towards its prey. A drop of 52% of attacks on prey by shrews was observed after their whiskers were removed.
According to our observations, during predatory hunting rats use a combination of body, head and whisker movements to locate the prey and capture them. Because of the comparatively low resolution of our overhead video recordings we were not able to determine whether prey location is based on passive deflection of whiskers caused by the prey moving into them, or whether active whisking was used to detect the presence of the cockroaches. Studies based on high speed video recordings showed that object localization by rats can certainly be achieved by active touch (Knutsen et al., 2005; Towal and Hartmann, 2006; Mitchinson et al., 2007; Ahissar and Knutsen, 2008; Grant et al., 2009) as well as for prey capture by the Etruscan shrew (Anjum et al., 2006). However, in our experimental situation it is likely that both active and passive deflection of whiskers was used in prey capture; active when the animals were moving forward hunting and passive when a cockroach had the misfortune to enter the whisker field while the rat was either pausing or consuming a cockroach it had captured.
In our study we observed that VibX group often failed to initiate pursuit after a cockroach had escaped. Perhaps this was because, in the absence of whiskers information the rat was unable to initiate the fast translational head and body movements required for immediate re-capture (Fig. 2B). In contrast, the groups with intact whiskers would often immediately re-orient themselves in order to facilitate the cockroache’s re-capture (Fig. 2A). Although our data demonstrate that whiskers provide important information necessary for effective prey detection and capture, further studies with high resolution video capability are necessary to clarify the extent to which active and passive deflection of the whiskers may elicit different components of the predatory response.
The SC is an ancient midbrain structure considered the mammalian homologue of the optic tectum. It is widely acknowledged that the SC is responsible for the sensorimotor transformations required to direct gaze towards or away from unexpected, biologically salient events (Wurtz and Albano, 1980; Sparks, 1986; Dean et al., 1989; Stein and Meredith, 1993). The SC contains a variable number of alternating cell and fiber rich layers (Kanaseki and Sprague, 1974; Reiner, 1994; May, 2006), although a functional distinction is often made (Casagrande et al., 1972) between the superficial layers, which receive spatially organized retinotopic input giving rise to a retinotopically coded map, and deep layers, which receive numerous multisensory (including somatosensory input from the whiskers) and non-sensory modulatory inputs (Stein and Meredith, 1993; Sefton and Dreher, 1995; Boehnke and Munoz, 2008). In rodents a critical distinction between the medial and lateral sectors of SC has been made on functional grounds (Dean et al., 1989). Thus, the ecology of the rat is such that most of its predators (e.g. larger mammals, hawks, etc.), are detected initially as a movement in the upper visual field. Since in the retinotopic representations it is the medial SC that such movement will be detected, appropriate stimulation of this region can elicit a fully integrated defensive reactions (Dean et al., 1988, 1991). On the other hand, appetitive stimuli for the rodent (prey, offspring, etc.) generally appear or can be found in the lower visual field which is represented in the lateral SC. Consequently, appropriate stimulation of this region typically elicits approach and appetitive behaviours. Of particular relevance to the present study, are reports demonstrating that integrity of the lateral SC is essential for effective predatory hunting (Comoli and Canteras, 2000; Furigo et al., 2010). This work is extended by the present investigation by showing that signals from the whiskers relayed to this region are likely to be a critical component of the circuitry responsible for efficient predation. In particular, our behavioural and immunohistochemical results highlight the importance of whisker input to the lateral SC as a source of information necessary to target a prey and generate the movements required for a successful attack.
Confirming electrophysiological experiments in rodents have shown that neurons in the lateral SC are responsive to somatosensory stimuli applied to face and whiskers (Tiao and Blackemore, 1976; Dräger and Hubel, 1976). Further studies have demonstrated that the lateral SC is differently responsive to perioral somatosensory stimuli that can be used to guide objects towards the mouth in the form of a “bitting reflex” (Redgrave et al., 1990; Keay et al., 1990). There are several potential sources of whisker-related somatosensory input to the lateral SC. The most obvious are the direct afferent projections from the brainstem trigeminal system (Hemelt and Keller, 2007). Thus the lateral SC receives whisker and orofacial-related somatosensory information from both the SpV and PrV (Killackey and Erzurumlu, 1981; Huerta et al., 1983; Rhoades et al., 1989; Comoli and Canteras, 2000; Hemelt and Keller, 2007). Other indirect inputs originate from the zona incerta (Kolmac et al., 1998; Comoli and Canteras, 2000), parvicelular reticular nucleus and somatosensory barrel cortex (Comoli and Canteras, 2000; Cohen et al., 2008). An important recent paper by Felsen and Mainen (2008) reported that the lateral SC is also critical for orienting to olfactory stimuli. Olfactory cues are likely to play a critical motivational role in predatory behavioural responses (Comoli et al., 2005). Consequently it was not surprising to observe that VibX group was highly motivated to hunt, showing no delays to start chasing the cockroaches and spending similar amounts of time hunting and engaging with the prey. Their problem was one of competence. It is therefore likely that whiskers removal resulted in a loss of effective spatial localization of fast moving prey and consequent lack of specific sensory information required to direct orienting movements of head, body and mouth towards a moving target. Thus, our data are consistent with the large body of work showing that phasic whisker-related input to the lateral SC is specialised for the initial detection of biologically salient stimuli that require immediate appetitive action (Cohen et al., 2007, 2008; Hemelt and Keller, 2007), including the detection and capture of cockroaches.
Acknowledgments
This research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, n°. 07/03655-4) and Fundação Apoio ao Ensino e Pesquisa e Assistência Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto awarded to E.C.. P.N.F., T.S.G., and S.R.O. were supported by FAPESP fellowships. P.R. was in receipt of support from the Wellcome Trust.
Abbreviations
- AQ
cerebral aqueduct
- FLI
Fos-like-immunoreactivity
- PAG
periaqueductal gray
- SAI
stratum album intermediale
- SAP
stratum album profundum
- SC
superior colliculus
- SCl
superior colliculus, lateral part
- SCm
superior colliculus, medial part
- SGI
stratum griseum intermediale
- SGP
stratum griseum profundum
- SGS
stratum griseum superficiale
- SZ
stratum zonale
REFERENCES
- Ahissar E, Knutsen PM. Object localization with whiskers. Biol Cybern. 2008;98:449–458. doi: 10.1007/s00422-008-0214-4. [DOI] [PubMed] [Google Scholar]
- Anjum F, Turni H, Mulder PG, van der Burg J, Brecht M. Tactile guidance of prey capture in Etruscan shrews. Proc Natl Acad Sci U S A. 2006;103:16544–16549. doi: 10.1073/pnas.0605573103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Zorzin E, Diamond ME. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol. 2005;3:e17. doi: 10.1371/journal.pbio.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol. 2008;18:1–8. doi: 10.1016/j.conb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Cardoso SH, Melo LL, Motta V, Coimbra NC. Neural substrate of defensive behavior in the midbrain tectum. Neurosci Biobehav Rev. 1994;18:339–346. doi: 10.1016/0149-7634(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Anseloni VZ, Pandóssio JE, De Araújo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science. 1972;177:444–447. doi: 10.1126/science.177.4047.444. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci. 2007;27:7762–7776. doi: 10.1523/JNEUROSCI.1124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci. 2008;28:11205–11220. doi: 10.1523/JNEUROSCI.2999-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Comoli E, Westby GW, Redgrave P. Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 2003;17:28–40. doi: 10.1046/j.1460-9568.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- Comoli E, Canteras NS. Characterization of the neural systems mobilized during predatory behavior in rats; Proceedings of the 28th Annual Meeting of the Society for Neuroscience, vol 24; Los Angeles, CA. November 1998; 1998. p. 678. Abstract 265.17. [Google Scholar]
- Comoli E, Canteras NS. Lateral region of intermediate layer of superior colliculus: a key site involved in the motor control of predatory hunting; Proceedings of the 30th Annual Meeting of the Society for Neuroscience, vol 26; New Orleans, LA. November 2000; 2000. p. 2257. Abstract 847.5. [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Negrão N, Goto M, Canteras NS. Functional mapping of the prosencephalic systems involved in organizing predatory behavior in rats. Neuroscience. 2005;130:1055–1067. doi: 10.1016/j.neuroscience.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Dean P, Mitchell IJ, Redgrave P. Contralateral head movements produced by microinjection of glutamate into superior colliculus of rats: evidence for mediation by multiple output pathways. Neuroscience. 1988;24:491–500. doi: 10.1016/0306-4522(88)90344-2. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GWM. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Dean P, Simkins M, Hetherington L, Mitchell IJ, Redgrave P. Tectal induction of cortical arousal: evidence implicating multiple output pathways. Brain Res Bull. 1991;26:1–10. doi: 10.1016/0361-9230(91)90184-l. [DOI] [PubMed] [Google Scholar]
- Dräger UC, Hubel DH. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol. 1976;39(1):91–101. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- Ewert JP, Buxbaum-Conradi H, Glagow M, Röttgen A, Schürg-Pfeiffer E, Schwippert WW. Forebrain and midbrain structures involved in prey-catching behaviour of toads: stimulus-response mediating circuits and their modulating loops. Eur J Morphol. 1999;37(2–3):172–176. doi: 10.1076/ejom.37.2.172.4743. [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60:137–148. doi: 10.1016/j.neuron.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo IC, de Oliveira WF, de Oliveira AR, Comoli E, Baldo MVC, Mota-Ortiz SR, Canteras NS. The role of supeior colliculus in predatory behavior. Neuroscience. 2010;165:1–15. doi: 10.1016/j.neuroscience.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290(2):219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Mitchinson B, Fox CW, Prescott TJ. Active touch sensing in the rat: anticipatory and regulatory control of whiskers movements during surface exploration. J Neurophysiol. 2009;101:862–874. doi: 10.1152/jn.90783.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R. Gaze control through superior colliculus: structure and function. In: Buttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Elsevier; Amsterdam: 1988. pp. 273–333. [PubMed] [Google Scholar]
- Grobstein P. Between the retinotectal projection and directed movement: topography of a sensorimotor interface. Brain Behav Evol. 1988;31:34–48. doi: 10.1159/000116574. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Valdivieso C, Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav Brain Res. 1989;31(3):285–289. doi: 10.1016/0166-4328(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Jenkins WM, Bravo H. Vibrissal roughness discrimination is barrelcortex-dependent. Behav Brain Res. 1992;48(2):145–152. doi: 10.1016/s0166-4328(05)80150-0. [DOI] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci. 2007;8:12. doi: 10.1186/1471-2202-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol. 1983;220(2):147–167. doi: 10.1002/cne.902200204. [DOI] [PubMed] [Google Scholar]
- Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979;175(2):341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Kanaseki T, Sprague JM. Anatomical organization of pretectal and tectal laminae in the cat. J Comp Neurol. 1974;158:319–337. doi: 10.1002/cne.901580307. [DOI] [PubMed] [Google Scholar]
- Keay K, Westby GW, Frankland P, Dean P, Redgrave P. Organization of the crossed tecto-reticulo-spinal projection in rat–II. Electrophysiological evidence for separate output channels to the periabducens area and caudal medulla. Neuroscience. 1990;37(3):585–601. doi: 10.1016/0306-4522(90)90093-j. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Erzurumlu RS. Trigeminal projections to the superior colliculus of the rat. J Comp Neurol. 1981;201(2):221–242. doi: 10.1002/cne.902010207. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol. 2004;14(9):R335–R338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Power BD, Mitrofanis J. Patterns of connections between zona incerta and brainstem in rats. J Comp Neurol. 1998;396(4):544–555. doi: 10.1002/(sici)1096-9861(19980713)396:4<544::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Knutsen PM, Derdikman D, Ahissar E. Tracking whisker and head movements in unrestrained behaving rodents. J Neurophysiol. 2005;93(4):2294–2301. doi: 10.1152/jn.00718.2004. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc R Soc B. 2007;274:1035–1041. doi: 10.1098/rspb.2006.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Fixation and orientation control by the tecto-reticulo-spinal system in the cat whose head is unrestrained. Rev Neurol (Paris) 1989;145:567–579. [PubMed] [Google Scholar]
- Prigg T, Goldreich D, Carvell GE, Simons DJ. Texture discrimination and unit recordings in the rat whiskers/barrel system. Physiol Behav. 2002;77:671–675. doi: 10.1016/s0031-9384(02)00917-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Westby GW. Organization of the crossed tecto-reticulo-spinal projection in rat–I. Anatomical evidence for separate output channels to the periabducens area and caudal medulla. Neuroscience. 1990;37(3):571–584. doi: 10.1016/0306-4522(90)90092-i. [DOI] [PubMed] [Google Scholar]
- Reiner A. Laminar distribution of the cells of origin of ascending and descending tectofugal pathways in turtles: implications for the evolution of tectal lamination. Brain Behav Evol. 1994;43(4–5):254–292. doi: 10.1159/000113639. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Fish SE, Chiaia NL, Bennett-Clarke C, Mooney RD. Organization of the projections from the trigeminal brainstem complex to the superior colliculus in the rat and hamster: anterograde tracing with Phaseolus vulgaris leucoagglutinin and intra-axonal injection. J Comp Neurol. 1989;289(4):641–656. doi: 10.1002/cne.902890409. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of the primate superior colliculus. Physiol Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, Dreher B. Visual system. In: Paxinos G, editor. The rat nervous system. Academic Press Inc.; 1995. pp. 833–898. [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. The MIT Press; 1993. [Google Scholar]
- Steiner H, Huston JP. Control of behavior under apomorphine by sensory input from the face. Psychopharmacol (Berl) 1992;109(4):390–394. doi: 10.1007/BF02247713. [DOI] [PubMed] [Google Scholar]
- Telford S, Wang S, Redgrave P. Analysis of nociceptive neurons in the rat superior colliculus using c-fos immunohistochemistry. J Comp Neurol. 1996;375:601–617. doi: 10.1002/(SICI)1096-9861(19961125)375:4<601::AID-CNE4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tiao YC, Blackemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol. 1976;168(4):483–503. doi: 10.1002/cne.901680404. [DOI] [PubMed] [Google Scholar]
- Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci. 2006;26(34):8838–8846. doi: 10.1523/JNEUROSCI.0581-06.2006. Erratum in: J Neurosci 26(40):10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SB. The function of vibrissae in the behavior of the white rat. Behav Monogr. 1912;1:1–85. [Google Scholar]
- Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81(4):967–988. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Welzl H, Schwarting R, Kulajta J, Huston JP. Perioral bitting and turning after intranigral injection of a GABA- or metenkephalin-agonist: role of the thalamus and superior colliculus. Exp Brain Res. 1984;55:438–444. doi: 10.1007/BF00235274. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol. 2008;6(8):e215. doi: 10.1371/journal.pbio.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci. 1980;3:189–226. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Vision for the control of movement. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1996;11:2130–2145. [PubMed] [Google Scholar]