Abstract
When associations between certain visual stimuli and particular actions are learnt, those stimuli become capable of automatically and unconsciously activating their associated action plans. Such sensorimotor priming is assumed to be fundamental for efficient responses, and can be reliably measured in masked prime studies even when the primes are not consciously perceived. However, when the delay between prime and target is increased, reversed priming effects are often found instead (the negative compatibility effect, NCE). The main accounts of the NCE assume that it too is a sensorimotor phenomenon, predicting that it should occur only when the initial positive priming phase also occurs. Alternatively, reversed priming may reflect a perceptual process entirely independent from positive motor priming (which is simply evident at a different temporal delay), in which case no dependency is expected between the NCE and positive priming. We tested these predictions while new sensorimotor associations were learnt, and when learnt associations were suddenly reversed. We found a remarkable symmetry between positive and reversed priming during all such learning phases, supporting the idea that reversed priming represents a sensorimotor process that is contingent on, and automatically follows, the positive priming phase. We discuss also whether the NCE mechanism is subject to a trigger threshold.
Introduction
Although we regard most human behaviour as voluntary, many actions may be underpinned by unconscious and automatic associations between certain stimuli and particular responses. It is known that visual stimuli can automatically activate motor plans associated with them, and that such motor priming can occur even when the stimuli are masked so that they are not consciously perceived (e.g. Leuthold & Kopp, 1998; Neumann & Klotz, 1994; Tucker & Ellis, 2004). Generally, primes speed responses to subsequent “target” stimuli if they are associated with the same response (compatible) and slow responses if prime and target are associated with different responses (incompatible). This positive compatibility effect (PCE) has been taken to demonstrate that a prime can partially activate the response associated with it, even though the participant had no intention of responding to the prime, and may not even have perceived it.
In some masked priming paradigms, a counterintuitive negative compatibility (NCE) effect has been measured, such that responses are faster and more accurate for incompatible primes than for compatible primes (for reviews see Eimer & Schlaghecken, 2003; Sumner, 2007). For example, in the classic task, participants respond with left or right button presses to leftward or rightward pointing arrows, which are preceded by irrelevant and masked prime arrows. With brief intervals between prime and target (up to about 100 ms), a PCE tends to be measured, but when the prime-target interval is longer (normally 150-200 ms) a NCE is often measured instead. Thus an early positive priming phase is followed by a negative phase.
This reversed priming effect has been the subject of much interest, and has been explained both by sensorimotor mechanisms and by purely perceptual processes. In the sensorimotor accounts, the NCE occurs because in a compatible trial, by the time the target arrives the motor representations needed to make the appropriate response are in an unfavourable state, relative to an incompatible trial. In the perceptual accounts, the NCE occurs because perceptual processing of the target stimulus is slower following a compatible prime. Various different mechanisms have been proposed for how motor or perceptual disadvantage may occur on compatible trials, which we outline below, but our purpose here is to distinguish between sensorimotor and perceptual classes of account, not between different sensorimotor accounts or different perceptual accounts.
Sensorimotor accounts
In two of the main sensorimotor theories, the NCE represents an inhibitory mechanism in the motor system, which acts to suppress the initial sub-threshold motor activation evoked by the prime, providing a mechanism to keep automatic motor activation in check without the intervention of top-down control processes. After the inhibition has been triggered it becomes more difficult to make the primed response than the alternative response. It was originally suggested that such “self inhibition” automatically follows any sub-threshold motor activation that is not further supported by continuing sensory input (e.g. Bowman, Schlaghecken, & Eimer, 2006; Eimer & Schlaghecken, 1998; Klapp & Hinkley, 2002; Schlaghecken, Bowman, & Eimer, 2006; Schlaghecken & Eimer, 2002). Alternatively, Jaskowski and colleagues (Jaskowski, 2007, 2008; Jaskowski & Przekoracka-Krawczyk, 2005) have proposed that inhibition is triggered when the prime is immediately followed by another potentially relevant stimulus (i.e. the mask stimulus). A similar idea was also suggested by Lleras and Enns (Lleras & Enns, 2006).
A different sensorimotor theory, often referred to as “object updating” (Lleras & Enns, 2004, 2005, 2006), argued that in some circumstances the stimulus sequence of prime and mask could cause an NCE without the need for motor inhibition (Lleras & Enns, 2004, 2005; Verleger, Jaskowski, Aydemir, van der Lubbe, & Groen, 2004). For example, although a rightward prime arrow would initially cause activation of the right hand response, the mask might then prime the leftward response, so that by the time the target appears there is motor priming in the opposite direction from the prime. How such mask-induced priming might occur when the mask contains features of both possible targets relies on perceptual interactions between prime and mask, and we return to this in Discussion. However, despite this critical perceptual aspect of the theory, for our purposes it belongs in the sensorimotor class because ultimately the NCE depends on the state of the motor system encountered during response selection for the target.
Perceptual accounts
In contrast to the sensorimotor accounts, it has been suggested that the NCE may have a perceptual or attentional source in which perceptual processing of targets is delayed when they share features with the primes (Bavelier, Deruelle, & Proksch, 2000; Huber, 2008; Sohrabi & West, 2008; see also van Leeuwen & Lachmann, 2004). Lleras and Enns (2005) also discussed this idea alongside their object updating theory. Such perceptual delay could occur through adaptation-like processes such as “repetition blindness”, (Hochhaus & Johnston, 1996; Johnston, Hochhaus, & Ruthruff, 2002), whereby the visual system becomes less sensitive to stimuli it has just previously been exposed to. It has been argued that such adaptation, or habituation, is not just a passive side effect of sensory cells, but is ubiquitous at all levels of perceptual and cognitive processing, and serves the essential purpose of resolving source confusion (Huber, 2008). For example, Huber et al. (Huber, Shiffrin, Lyle, & Ruys, 2001; Huber, Shiffrin, Quach, & Lyle, 2002) explained a pattern of reversed priming measured during a word recognition task with a computational theory for ‘responding optimally with unknown sources of evidence’ (ROUSE). Essentially they argued that feature representations activated noisily by a series of stimuli (e.g. a prime and a target) are subject to source confusion – it is difficult for the system to know whether an activated unit is responding due to a feature in the first or second stimulus. When participants are required to recognise only one of the stimuli (the target), they must employ a ‘discounting’ mechanism that estimates the feature activity associated with the prime and removes it from the decision about the target. The discounting mechanism may overestimate the prime-related activity, and thus overcompensate, resulting in a bias against targets that share these features (i.e. a form of NCE).
Huber (2008) has recently developed this idea into a neural habituation model that can potentially explain a variety of priming, masking, attentional and interference phenomena between perceptual stimuli of many kinds, and which was explicitly applied to explain the NCE. Note that although it has previously been argued that perceptual source for the NCE should require that primes and masks occupy the same location (Sumner, 2007), this does not have to be the case. Many cells in visual cortex and temporal cortex respond to certain objects or features regardless of their location over a relatively large receptive field area. Indeed, this is one of the reasons why source confusion may occur, and thus one of the motivators for Huber’s model. Thus evidence that the NCE occurs when prime and target occur in different locations cannot be taken as evidence against all perceptual sources for the NCE.
An alternative perceptual account of the NCE has recently been developed as a computational model by Sohrabi & West (2008). In this model the NCE emerges due to an attentional refractory period, and it claims to account for human data better than does the computational model of Bowman et al. (2006) based on the self-inhibition sensorimotor hypothesis. For our purposes, an attentional refractory period is essentially the same as perceptual habituation – both act to slow the perceptual processing of the target in compatible trials. Thus, in sum, the NCE may have an entirely perceptual or attentional source, occurring through modulation of the processing of target features, rather than a sensorimotor source relying on priming of responses associated with those features. One reason for doubting a perceptual source for the NCE is that in a cross modal study in which visual primes were followed by either visual or auditory targets, an NCE occurred for both types of target (Klapp & Hinkley, 2002). It is hard to explain why perceptual processing of auditory targets would be delayed following visual primes. However, the mask stimuli in this experiment were likely to have elicited mask-induced priming (object updating) as the main source of the NCE, but more recent studies have measured the NCE in circumstances that do not favour mask-induced priming (e.g. Jaskowski, 2009; Klapp, 2005; Schlaghecken & Eimer, 2006; Sumner, 2008). Thus in cases where mask-induced priming is not the explanation, the perceptual hypotheses may still stand.
Dependency of NCE on positive priming
A key distinction between the sensorimotor and perceptual classes of explanation for the NCE concerns how the NCE is related to the positive priming measured at very short prime-target intervals. All the sensorimotor theories make the fundamental assumption that the NCE relies on strong sensorimotor associations between primes and responses. This in turn predicts that the NCE occurs only when the prime is capable of eliciting the early phase of positive priming. In the self-inhibition theory, motor inhibition is a direct consequence of the early priming phase and occurs precisely because this early activation is detected within the motor system. In the stimulus-triggered inhibition theory, although inhibition may be triggered by the mask rather than by detecting the presence of motor activation, directional inhibition leading to an NCE would only occur when the initial activation is there to suppress. In the mask-induced priming (object updating) account, priming from features of the mask would only be expected when priming can also occur from the prime itself. Thus according to these theories, the NCE should occur only when an initial positive priming phase also occurs. The aim of this study is to test such dependency, arguing that if the NCE occurs independently of positive priming, then none of the sensorimotor theories outlined above can be the correct account.
We investigate the dependency of the NCE on the initial motor priming phase during the learning of new sensorimotor associations. Positive priming takes time to emerge when a new and arbitrary relationship between a stimulus and responses is required, because subliminal motor priming relies on the stimulus-response (S–R) association becoming strong enough that it can be detectibly activated by very weak subliminal primes. If the NCE is sensorimotor in origin, then its appearance during learning should depend on the appearance of positive priming (Fig 1A). This dependency has never been tested, mainly because the usual stimuli have been arrows, which elicit priming very quickly, presumably due to their intuitive response associations (Jaskowski & Slosarek, 2007). With more arbitrary S-R mappings, and even sometimes with arrows, there is some indication from previous research that practice can be required to obtain a robust NCE (Klapp & Hinkley, 2002; Sumner, 2008). It has also been found that an NCE can develop gradually for incidental learning of S-R contingencies (Schlaghecken, Blagrove, & Maylor, 2007). In this study participants were instructed to respond to colour, but there was also a consistent relationship between the colour and the shape of the targets. Masked primes corresponded to the shapes but not the colour, but nevertheless they produced priming effects (an NCE) by the third block (even for participants who had no awareness of the shape-colour association). Although this data indicate that some association between shape and response had been implicitly learnt, they are actually consistent with both sensorimotor and perceptual accounts of the NCE. Under the perceptual account, the primes would affect perceptual processing of target shape, but while target shape remains unassociated with any response, only colour processing will affect response speed, so no priming effects would be expected. Only when target shape acquires some response association (whether implicitly or explicitly), will delaying shape processing cause an NCE.
Figure 1.
Illustrated predictions of the hypotheses under examination. (A) If the negative compatibility effect (NCE) reflects any perceptual process it should be present in all blocks (dotted line) and not depend on learning stimulus-response associations. However, if the NCE reflects a sensorimotor mechanism that depends on associations between primes and responses it should appear only when positive priming appears (solid black line).
(B) If the PCE reflects automatic activation of previously learnt S-R associations, when the S-R requirements are suddenly reversed, an inverse PCE should initially occur because priming still reflects the previous S-R association. Such inverse PCE should then reduce and a normal PCE should emerge as the new association is gradually acquired. Likewise, if the NCE has a sensorimotor origin, it two should reverse and recover in mirror image to the PCE. However, a perceptual locus of the NCE would not predict any reversal.
Crucially, however, any perceptual source for the NCE would not be dependent on learning the S-R association when the primes carry the features required to perform the task. In this case, as soon as the participant attempts to process the relevant perceptual features of the targets, any influence of the prime on such perceptual processing should be evident. Thus the NCE would be expected to occur before the S-R-dependent positive priming has appeared, rather than appearing at the same time as (or after) the PCE is evident, as predicted by the sensorimotor theories (see Fig 1A). We test these predictions in four experiments in which S-R learning is manipulated.
Overview of experiments
In all experiments participants learnt arbitrary associations between stimuli (vertical and horizontal lines) and responses (left/right button presses), while masked prime stimuli were presented to elicit positive or reversed priming (Fig 2). We used two different intervals (stimulus onset asynchrony, SOA) between masked primes and targets, short (40 ms, see Fig 2B) and long (150 ms, see Fig 2A), which are expected to elicit positive and negative compatibility effects (PCE and NCE), respectively (by convention the SOAs are specified as mask-target SOAs). Rather than using the more standard 0 ms SOA, the choice of 40 ms came from previous piloting, and seemed to produce the most robust PCE across participants with our particular prime-mask stimuli. In Experiment 1, trials with short and long SOA were presented in a random order so that we could measure the NCE in long SOA trials while positive priming (the PCE) emerged gradually in short SOA trials as the S–R association was learnt. In this way we could test whether the NCE is present before the PCE emerges, whether the NCE emerges with the PCE, or emerges only after the PCE. In Experiment 2 we presented short and long SOA trials in short blocks of 20 trials, which produces more rapid emergence of the PCE relative to random ordering (because error rates are lower, so associative learning proceeds more rapidly (Guthrie, 1952). In this way we could test whether the NCE is still locked to the PCE when its rate of emergence is changed. In Experiments 3 and 4, having established asymptotic priming with one S-R association, the required S-R relationship was reversed. Under the assumption that the PCE reflects automatic activation of previously learnt S-R associations, when the S-R requirements are suddenly reversed, an inverse PCE should initially occur because priming still reflects the previous S-R association. This inverse PCE should reduce and then a normal PCE should emerge as the new association is gradually acquired. Measuring the NCE during this period provides a stringent test of whether it too is a consequence of previously established S-R associations, and remains locked in mirror image to the PCE during such inversion and recovery (Fig. 1B). If the NCE is perceptual in origin, there is no reason for it to reverse when the S-R relationship reverses, because perceptual requirements have not changed – it should still be more difficult to process a vertical target after a vertical prime, regardless of whether the target is mapped to left or right response (Fig 1B).
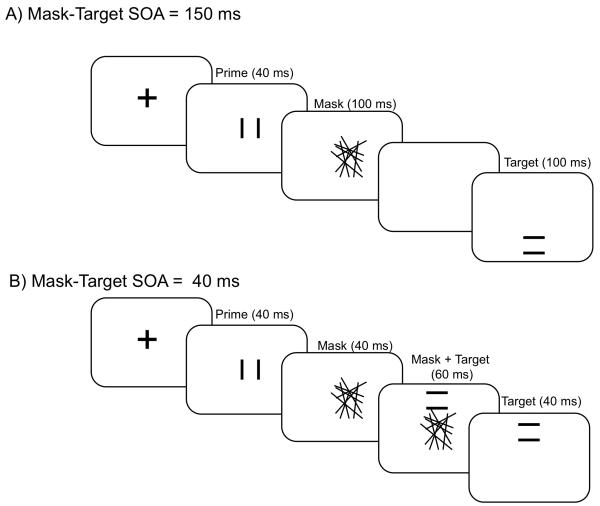
Figure 2.
(A, B) Illustration of the stimulus sequence (and relevant timings) for the two types of trials used. (B) As the mask-target SOA is shorter than the mask duration, both mask and target need to be presented at the same time. For this reason targets did not appear in the same location as the prime of the mask in any trial. The mask was always centred on fixation, the prime appeared somewhere within the area covered by the mask, and the target appeared at a random location in an annulus just outside the area of the mask. Note that the SOA refers to the time elapsed between mask and target appearances.
Methods
Participants (all experiments)
48 participants (38 women; age 17–41) from Cardiff University participated in the four experiments (12 subjects in each). Eight other participants underwent a control experiment assessing whether they could discriminate between the two types of mask-target SOA. All self-reported having normal or corrected-to-normal vision and were right-handed.
Apparatus (all experiments)
Stimulus presentation was performed by a PC-controlled Cambridge Research Systems (CRS) Visage® connected to a 21″ Sony GDM-F520 Trinitron monitor. Stimulus presentation was synchronized with the screen refresh rate of 100 Hz, and timings were controlled and measured by the CRS clock and thus not subject to the errors produced by normal PC operating systems. Manual responses were collected using a CRS-CB6 button box.
Experiment 1
Participants had to make speeded responses with a left- or right-hand key press (counterbalanced) to the orientation of horizontal and vertical targets (parallel lines 1° × 1°), which occurred in random order and located at 2.5° from fixation, in a random direction from fixation (see Fig. 2). A fixation cross was visible at the center of the screen at the beginning of each trial. The primes were identical to either one or the other targets, but presented for only 40 ms, and appeared within 0.5° of fixation (i.e., in the same vicinity as the target, but not in an identical location on any trial). In all conditions the prime was followed by a mask of 2.2° × 2.2° and constructed of 35 randomly orientated lines, excluding any orientation closer than ± 5° to the vertical or the horizontal. A new mask was constructed on each trial but appeared always in the same place, centred on fixation. Half of the trials had a short mask-target SOA of 40 ms and the other half a long SOA of 150 ms. 480 trials of both types were presented in a random order, with brief breaks every 40 trials. Participants were not informed about the different mask-target SOAs. Stimulus sequence and timing for the two trial types is described in Fig. 2.
Control Experiment: Discrimination of mask-target SOA
To test whether participants were able to consciously identify the type of mask-target SOA that was used in a given trial, we placed eight naïve subjects in the same conditions as in experiment 1. After a demonstration of the two different SOAs (3 trials for each type of SOA) they were asked to try to identify which SOA occurred on each trial. Thus, instead of responding to the identity of the target, they were asked to press a button to indicate which type of trial (“short” or “long”) was employed.
Experiment 2
Experiment 2 was identical to Experiment 1 except in the way that trials were ordered. Here, the different masked-target SOAs were blocked in sets of 20 trials. Thus there were 24 blocks, 12 with short SOA, and 12 with long SOA, presented alternately (order counterbalanced). Participants were not informed about the different mask-target SOAs. The transition between blocks was not marked, except that there were opportunities for the participants to take breaks every 40 trials.
Experiment 3
For the first 240 trials, Experiment 3 was identical to Experiment 2, comprising six 20-trial blocks each for short and long mask-target SOA. Then a message presented on the screen instructed participants to reverse the correspondence between a given target and the associated button press (they were not warned that this would happen beforehand). These instructions could be presented for a maximum of 25 s (allowing rephrasing the instructions if needed) but the majority of participants decided to continue after only few seconds. A further 240 trials occurred with the new S-R mapping, following the same procedure of alternating blocks for short and long SOA. As before, participants were not informed about the different SOAs.
Experiment 4
Experiment 4 replicated Experiment 3, but with shorter blocks of 5 trials, in order to capture with a finer grain the performance after the motor reversal.
Prime identification
Whether the primes were consciously perceived does not alter the logic of the current study. However, for completeness we assessed prime visibility at the end of each experiment. Prime duration was 40 ms (as in Experiments 1, 2, 3 and 4) and targets were omitted. The participants’ task was to guess the identity of the prime (forced choice). If the main experiment had involved a reversal of the S-R mapping (as in Experiment 3 and 4), participants completed the prime identification task using the motor mapping last employed. In order to minimize the potential influence of non-conscious motor priming on the prime identification response, participants were instructed to make their responses after a short delay rather than in a speeded manner. It is arguable that target stimuli ought to be included rather than omitted from the prime identification test, in order to maintain exactly the same stimulus sequence as in the main task. However, in practice this can confuse participants, who often report pressing the button according to the target stimulus by mistake (see also Schlaghecken & Eimer, 1997). Note that in addition to this, the likely influence of the target stimulus would be to render the prime less visible, by distracting attention from it (see e.g., Sumner, Tsai, Yu, & Nachev, 2006; Naccache, Blandin & Dehaene, 2002). Thus our prime identification procedure is likely to overestimate, if anything, the visibility of the prime in the main experiments.
Results
Discrimination of mask-target SOA
In the initial control experiment subjects showed no evidence that they could detect which mask-target SOA occurred in a given trial. Mean accuracy was 58.3% ± 13% (95% confidence interval, CI); comparison to chance, t(7) = 1.1, p > .05. This difficulty in distinguishing trial types is partly due to the small difference of only 90 ms between trial types. It may also be related to the unpredictability of target location, which may partly obscure the differences in timing. Whatever the reason, the result means that participants in the main experiment probably did not notice our manipulations of SOA, and while the logic of the study does not depend on this, it is useful so that normal task performance was not disrupted by conscious task-switching strategies, for example.
Prime identification
In all four experiments forced-choice responses in the prime identification blocks did not differ from 50% (Expt. A: 53.1% [± 15%; CI], t(11) = 1.16, p > .05; Expt. B: 54.2% [± 18%; CI], t(11) = 1.8, p > .05; Expt. C: 54% [± 19%; CI], t(11) = 1.56, p > .05) and Expt D. 56.9% [± 21%; CI], t(11) = 1.2, p > .05). There was no difference in identification level across the four experiments (F(3, 44) < 1).
Experiment 1 (shuffled presentation)
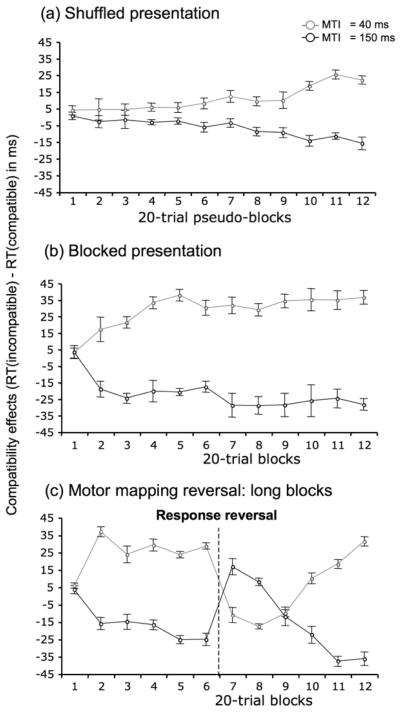
For analysis of Experiment 1 data for each trial type was pooled into “pseudo blocks” of 20 trials. Doing so allowed us to process the data similarly for Experiments 1, 2 and 3. As shown in Fig. 3a we observed a main effect of mask-target SOA on the compatibility effect as expected, F(1,11)= 420.4, p < 0.01, MSE = 51.8, η2p = .97. Compatibility effects were positive (PCE), for trials with the short SOA (mean=10.9 ms, SD = 11.7) and negative (NCE) for long SOA trials (mean=−6.6 ms, SD = 8.9). There was no main effect of the trial position (i.e. pseudo-block) on the compatibility effect, F(11,121)= 1.2, p > 0.05, since learning effects were approximately equal and opposite, leading to a significant interaction between trial position and mask-target SOA; F(11,121)= 10.7, p < 0.01, MSE = 83, η2p = .49. The key aspect of the results is that the NCE is not present from the start, but grows during sensorimotor learning (the NCE does not become significantly different from zero until the 8th pseudo block – i.e. after on average 320 trials (thereafter all t(11) > 3.5, all ps < 0.01; similarly, the PCE becomes different from zero from the 6th block onward, thereafter all t(11) > 2.3, all ps < 0.05). Secondly, the growth of the NCE appears to mirror the growth of positive priming (the absolute values of NCE and PCE differ only in the 11th blocks, t(11) = 3.7; p < 0.01). Such mirroring is consistent with a sensorimotor mechanism that is contingent on the strength of positive priming. Mean reaction times and error rates are inserted in Tables 1 and 2.
Figure 3.
(A, B & C) Evolution of Compatibility Effects (in ms) for short and long SOAs as function of training (expressed in “pseudo-blocks” or blocks) in Expt. 1, 2 and 3. Error bars plot the standard error of the mean for each block. Note that in experiment 3, the response mapping was reversed after the sixth block.
Table 1.
Mean reaction times (Standard Deviations in italic) for Expts 1, 2 & 3.
| Expt 1 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Pseudo-Block | Mean | SD | Mean | SD |
| 1 | 542.7 | 73.3 | 546.7 | 74.3 |
| 2 | 534.8 | 73.1 | 539.3 | 74.8 |
| 3 | 532.8 | 65.8 | 537.2 | 72.4 |
| 4 | 519.5 | 62.2 | 525.5 | 64.9 |
| 5 | 519.7 | 57.7 | 525.3 | 61.0 |
| 6 | 515.2 | 54.8 | 523.4 | 58.0 |
| 7 | 507.6 | 54.7 | 519.8 | 56.4 |
| 8 | 510.0 | 53.9 | 519.4 | 57.8 |
| 9 | 505.2 | 53.9 | 515.2 | 55.9 |
| 10 | 497.6 | 51.2 | 516.2 | 51.4 |
| 11 | 491.7 | 50.1 | 517.1 | 49.3 |
| 12 | 491.5 | 41.8 | 513.5 | 45.5 |
| Expt 1 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Pseudo-Block | Mean | SD | Mean | SD |
| 1 | 567.1 | 51.8 | 567.8 | 48.6 |
| 2 | 554.0 | 63.1 | 551.3 | 60.2 |
| 3 | 543.7 | 79.5 | 542.2 | 78.3 |
| 4 | 530.2 | 63.3 | 527.0 | 61.8 |
| 5 | 525.5 | 61.2 | 523.1 | 59.9 |
| 6 | 532.2 | 53.3 | 526.0 | 52.6 |
| 7 | 531.6 | 39.4 | 528.1 | 39.3 |
| 8 | 528.9 | 52.2 | 520.1 | 52.0 |
| 9 | 529.4 | 56.5 | 520.1 | 56.4 |
| 10 | 528.8 | 52.4 | 514.5 | 52.3 |
| 11 | 530.7 | 44.1 | 519.9 | 43.7 |
| 12 | 531.9 | 42.6 | 516.1 | 42.6 |
| Expt 2 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 519.5 | 62.2 | 522.3 | 63.6 |
| 2 | 500.4 | 51.9 | 517.6 | 54.2 |
| 3 | 493.6 | 46.8 | 514.9 | 51.6 |
| 4 | 474.9 | 35.0 | 508.1 | 34.2 |
| 5 | 462.4 | 36.1 | 500.1 | 42.8 |
| 6 | 449.4 | 41.3 | 479.5 | 42.5 |
| 7 | 437.8 | 36.4 | 469.4 | 45.8 |
| 8 | 431.5 | 38.7 | 460.4 | 39.7 |
| 9 | 426.8 | 25.2 | 461.9 | 31.7 |
| 10 | 429.6 | 46.8 | 464.7 | 46.8 |
| 11 | 423.5 | 40.6 | 458.3 | 52.9 |
| 12 | 421.6 | 36.3 | 459.0 | 45.1 |
| Expt 2 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 515.9 | 68.5 | 519.4 | 71.2 |
| 2 | 519.4 | 61.3 | 500.3 | 62.2 |
| 3 | 513.6 | 60.7 | 494.9 | 61.2 |
| 4 | 496.9 | 58.5 | 476.6 | 55.5 |
| 5 | 486.3 | 71.2 | 465.4 | 64.2 |
| 6 | 482.0 | 52.8 | 464.3 | 48.0 |
| 7 | 476.3 | 61.5 | 455.8 | 61.2 |
| 8 | 469.9 | 62.8 | 441.0 | 63.2 |
| 9 | 472.5 | 55.9 | 443.9 | 58.8 |
| 10 | 466.9 | 61.5 | 440.9 | 54.7 |
| 11 | 472.3 | 43.6 | 447.7 | 41.7 |
| 12 | 468.5 | 45.7 | 448.3 | 50.8 |
| Expt 3 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 528.1 | 67.1 | 534.0 | 73.0 |
| 2 | 505.0 | 51.6 | 542.0 | 52.1 |
| 3 | 499.3 | 45.6 | 523.1 | 45.5 |
| 4 | 478.8 | 34.1 | 508.1 | 30.7 |
| 5 | 467.4 | 36.2 | 491.1 | 35.2 |
| 6 | 454.6 | 40.7 | 482.4 | 32.0 |
| 7 | 451.7 | 28.5 | 440.7 | 29.8 |
| 8 | 451.1 | 40.2 | 433.8 | 45.1 |
| 9 | 431.6 | 24.6 | 441.4 | 41.3 |
| 10 | 435.4 | 47.9 | 445.5 | 50.8 |
| 11 | 427.8 | 40.1 | 446.2 | 46.2 |
| 12 | 426.9 | 36.9 | 456.0 | 51.3 |
| Expt 3 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 519.5 | 53.1 | 523.1 | 49.3 |
| 2 | 524.7 | 67.8 | 508.8 | 70.8 |
| 3 | 518.9 | 60.3 | 506.0 | 58.0 |
| 4 | 502.7 | 57.8 | 486.0 | 51.4 |
| 5 | 491.8 | 70.6 | 466.6 | 68.2 |
| 6 | 487.0 | 51.8 | 462.0 | 46.8 |
| 7 | 464.3 | 57.5 | 480.5 | 41.7 |
| 8 | 475.0 | 62.2 | 483.1 | 63.7 |
| 9 | 476.8 | 55.7 | 465.0 | 75.2 |
| 10 | 469.7 | 52.2 | 447.4 | 52.8 |
| 11 | 477.7 | 43.0 | 440.2 | 51.1 |
| 12 | 473.0 | 45.4 | 436.9 | 41.6 |
Table 2.
Mean error rate (Standard Deviations in italic) for Expts 1, 2 & 3.
| Expt 1 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Pseudo-Block | Mean | SD | Mean | SD |
| 1 | 17.3 | 4.8 | 16.9 | 4.4 |
| 2 | 14.6 | 4.4 | 14.2 | 6.7 |
| 3 | 14.5 | 4.8 | 14.2 | 6.5 |
| 4 | 14.4 | 4.8 | 13.5 | 2.9 |
| 5 | 13.3 | 7.4 | 11.3 | 5.1 |
| 6 | 12.8 | 6.2 | 12.8 | 3.9 |
| 7 | 10.2 | 3.8 | 11.3 | 5.3 |
| 8 | 10.1 | 5.2 | 10.5 | 6.6 |
| 9 | 10.1 | 4.8 | 10.0 | 4.8 |
| 10 | 11.0 | 6.3 | 10.3 | 6.2 |
| 11 | 10.5 | 4.3 | 10.9 | 6.2 |
| 12 | 11.0 | 7.0 | 10.5 | 5.0 |
| Expt 1 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Pseudo-Block | Mean | SD | Mean | SD |
| 1 | 15.52 | 4.30 | 18.34 | 5.36 |
| 2 | 13.11 | 5.75 | 12.21 | 4.60 |
| 3 | 11.48 | 6.70 | 13.13 | 4.48 |
| 4 | 11.40 | 4.38 | 13.43 | 4.65 |
| 5 | 9.56 | 3.77 | 11.38 | 5.86 |
| 6 | 10.03 | 3.68 | 10.33 | 4.62 |
| 7 | 10.53 | 4.52 | 8.71 | 3.60 |
| 8 | 8.93 | 6.13 | 10.12 | 3.65 |
| 9 | 8.26 | 5.20 | 9.06 | 5.27 |
| 10 | 9.08 | 5.79 | 9.17 | 7.92 |
| 11 | 8.48 | 6.05 | 9.78 | 4.83 |
| 12 | 9.65 | 4.49 | 9.60 | 4.41 |
| Expt 2 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 11.7 | 4.5 | 10.8 | 5.2 |
| 2 | 9.7 | 5.9 | 10.4 | 4.4 |
| 3 | 11.0 | 4.5 | 8.7 | 4.3 |
| 4 | 9.6 | 3.3 | 7.7 | 3.6 |
| 5 | 8.3 | 4.1 | 5.4 | 1.9 |
| 6 | 7.7 | 5.3 | 6.0 | 3.0 |
| 7 | 9.3 | 5.1 | 5.1 | 3.8 |
| 8 | 8.8 | 3.4 | 5.6 | 1.7 |
| 9 | 7.5 | 4.4 | 4.7 | 3.5 |
| 10 | 9.5 | 5.3 | 4.7 | 2.4 |
| 11 | 8.3 | 3.4 | 5.0 | 3.5 |
| 12 | 8.1 | 2.7 | 5.6 | 2.3 |
| Expt 2 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 10.2 | 4.9 | 11.4 | 3.8 |
| 2 | 8.5 | 3.9 | 11.0 | 5.8 |
| 3 | 7.8 | 3.1 | 8.7 | 4.5 |
| 4 | 5.9 | 3.3 | 7.5 | 3.5 |
| 5 | 4.9 | 3.1 | 7.2 | 1.5 |
| 6 | 5.3 | 2.2 | 6.5 | 4.3 |
| 7 | 4.6 | 4.0 | 8.6 | 1.4 |
| 8 | 3.7 | 2.7 | 5.6 | 3.8 |
| 9 | 2.9 | 1.9 | 5.7 | 3.0 |
| 10 | 5.4 | 3.5 | 6.3 | 4.0 |
| 11 | 3.1 | 3.3 | 5.8 | 2.0 |
| 12 | 4.2 | 2.1 | 6.2 | 3.2 |
| Expt 3 SOA = 40 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 12.1 | 7.3 | 11.4 | 5.9 |
| 2 | 11.7 | 3.1 | 12.9 | 6.5 |
| 3 | 10.9 | 6.4 | 8.3 | 6.1 |
| 4 | 10.0 | 5.2 | 10.9 | 5.9 |
| 5 | 8.3 | 2.8 | 8.8 | 4.3 |
| 6 | 7.7 | 5.7 | 6.6 | 2.0 |
| 7 | 18.0 | 8.7 | 15.7 | 3.3 |
| 8 | 10.7 | 6.8 | 11.4 | 3.0 |
| 9 | 9.9 | 5.2 | 10.3 | 5.9 |
| 10 | 8.3 | 5.2 | 6.1 | 3.2 |
| 11 | 8.3 | 4.9 | 7.8 | 4.6 |
| 12 | 7.2 | 3.2 | 5.6 | 3.0 |
| Expt 3 SOA = 150 ms | ||||
|---|---|---|---|---|
| Compatible | Incompatible | |||
|
| ||||
| Block | Mean | SD | Mean | SD |
| 1 | 13.9 | 6.4 | 12.0 | 6.6 |
| 2 | 10.8 | 6.5 | 11.0 | 2.2 |
| 3 | 7.9 | 5.4 | 9.7 | 6.4 |
| 4 | 7.9 | 5.8 | 9.1 | 4.3 |
| 5 | 8.3 | 4.7 | 8.7 | 2.3 |
| 6 | 5.9 | 7.0 | 6.3 | 5.1 |
| 7 | 21.9 | 3.2 | 18.1 | 4.0 |
| 8 | 11.3 | 4.7 | 10.6 | 6.0 |
| 9 | 8.8 | 5.1 | 10.0 | 6.2 |
| 10 | 5.6 | 2.8 | 7.9 | 4.3 |
| 11 | 7.7 | 4.3 | 10.1 | 4.3 |
| 12 | 5.1 | 2.9 | 6.4 | 3.1 |
Experiment 2 (Blocked presentation)
Again, as expected, when pooling all blocks together we observe a mean PCE (28.6 ms) for short SOA trials and a mean NCE (−22 ms) for long SOA trials – see Fig. 3b; main effect, F(1,11)=758.8, p < 0.01, MSE = 243, η2p = .98. As in Experiment 1, the key aspect of the results is that the NCE is not present from start, but grows during sensorimotor learning – the NCE does not become significantly different from zero until the 2nd block, t(11) > 2.71; p < 0.02. The PCE starts becoming different from zero at the same block (t(11) > 2.5; p < 0.05). This growth again appears to mirror the growth of positive priming, even though the rate of growth has been markedly changed by the change in blocking protocol. Thus there was no main effect of the block position, F(11,121) < 1, and a significant interaction between block and SOA; F(11,121) = 6, p < 0.001, MSE = 330, η2p = .35. Here again, the absolute values of the PCE and NCE in each block differed significantly only in two sporadic blocks – 4th and 11th blocks; all t(11) > 3.9; all ps < 0.01, although note that while the sensorimotor accounts of the NCE require that its existence should be systematically related to the PCE, no theory requires that the NCE should have identical absolute values as the PCE (and in any case we may not have employed the exact mask-target SOAs yielding maximum PCE and NCE). Mean reaction times and error rates can be found in Tables 1 and 2.
Experiment 3 (rule reversal)
Before response reversal (first 12 blocks)
As in Experiment 2, we observe a rapid growth of PCE and NCE to asymptotic levels in short and long SOA trials, respectively – main effect of SOA, F(1,11)= 316.5, p < 0.01, MSE = 185, η2p = .96; interaction with block, F(5,55)= 22.2, p < 0.01, MSE = 102, η2p = .66. The absolute values of PCE and NCE differ only in the 3rd block, t(11) = 3.9; p < 0.01.
Response mapping reversal
At the start of block 7, the S-R mapping was inverted, which caused both PCE and NCE to invert (Fig 3c) – for blocks 7 and 8 the compatibility effects were significantly negative for short SOA trials – block 7: t(11) = 4.8; p < 0.01; block 8: t(11) = 2.9; p < 0.02, and significantly positive for long SOA trials – block 7: t(11) = 4.7; p < 0.01; block 8: t(11) = 4.0; p < 0.01. In subsequent blocks, the normal pattern of PCE and NCE reasserted itself (for blocks 10-12, PCEs and NCEs are all significantly different from zero; all t(11) > 2.19; all ps < 0.05). The most notable feature of the results is the symmetry between PCE and NCE during both inversion and recovery (the absolute values of PCE and NCE did not differ in any block). Mean reaction times and error rates are inserted in Tables 1 and 2.
The symmetrical evolution of PCE and NCE can be measured by computing the correlation between the individual learning gradients. In other words, whether subjects whose PCEs emerge quickly also show a quickly emerging NCEs. We calculated the gradients for the “recovery” period (blocks 8 to 12) rather than on the initial learning period in order to minimize possible variabilities / inconsistancies related to the discovery of a novel task, and also because the initial learning was so rapid. Doing so, we found that these slopes for (re-) emerging PCE and NCE were negatively correlated (R = −.54, t(10) = 2.01, p < .036), as predicted if the emergence of the NCE depends on the emergence of the PCE.
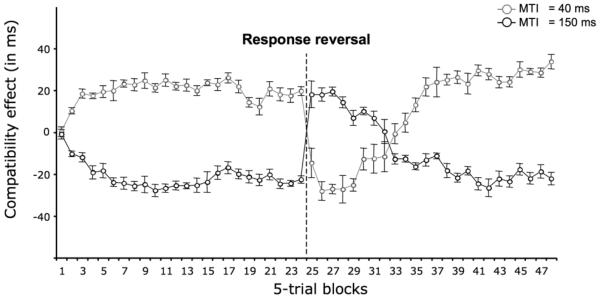
Experiment 4 (rule reversal with smaller blocks)
Experiment 4 (see Fig. 4) replicated Experiment 3, but was designed to capture with a finer resolution the performance after the motor reversal. Importantly following motor reversal, both PCE and NCE remain inverted for several blocks symmetrically. Similarly the recovery to “normal” PCE and NCE is remarkably symmetric.
Figure 4.
Evolution of Compatibility Effects (in ms) for short and long SOAs as function of training (in blocks) in Expt. 4. Error bars show the standard error of the mean. Note that the response mapping was reversed after the 24th block.
Before response reversal (first 24 blocks)
As in Experiment 2 and 3, we observe a rapid growth of PCE and NCE to asymptotic levels in short and long SOA trials, respectively; main effect of SOA, F(1,11)= 523.2, p < 0.01, MSE = 448, η2p = .98; interaction with block, F(23,253)= 11, p < 0.01, MSE = 64, η2p = .5. The absolute values of PCE and NCE differ only in the 3rd, 10th, 17th, 20th and 23rd blocks, all t(11) > 2.3; p < 0.04, and no evidence of a difference in the magnitude of PCE and NCE across blocks was found, t(23) = 1.4; p > 0.2.
Response mapping reversal
The S-R mapping was inverted from the start of block 25, which caused both PCE and NCE to invert – for blocks 25 to 30 the compatibility effects were significantly negative for short SOA trials, all t(11) > 2.3; all ps < 0.05, and for blocks 25, 26, 27, 28, 30 and 31 the compatibility effects were significantly positive for long SOA trials, all t(11) > 2.3; all ps < 0.05. In subsequent blocks, the normal pattern of PCE and NCE reasserted itself (from blocks 35 onward, PCEs and NCEs are all significantly different from zero; all t(11) > 2.19; all ps < 0.05). Again, the most notable feature of the results is the symmetry between PCE and NCE during both inversion and recovery (the absolute values of PCE and NCE did not differ in any other block). Mean reaction times and error rates are inserted in Tables 1 and 2.
As in Expt 3, we calculated the correlation across participants of the slopes of the emergence of positive and negative priming in the recovery phase (blocks 27 to 35). We found that these gradients were negatively correlated (R = −.53, t(10) = 1.96, p < .039), as predicted if the emergence of the NCE depends on the emergence of the PCE.
Discussion
Perceptual vs. sensorimotor source for the NCE
Masked-prime studies have been widely employed to investigate the consequences of sub-threshold activation in both sensorimotor and perceptual (recognition) domains. In one class of masked prime paradigm, succeeding phases of positive and negative compatibility effects (PCE and NCE) occur following presentation of prime stimuli that are strongly associated with responses (for reviews see Eimer & Schlaghecken, 2003; Sumner, 2007). However, the crucial question of whether the negative phase is dependent on the earlier positive phase, or whether it is an independent process, has not previously been examined. Sensorimotor accounts of the NCE (e.g. Eimer & Schlaghecken, 1998; Jaskowski & Przekoracka-Krawczyk, 2005; Lleras & Enns, 2004; Verleger et al., 2004) predict that the occurrence of the NCE should be systematically related to the occurrence of the earlier PCE, which is also assumed to have a sensorimotor source. However it has also been suggested that the NCE may have a perceptual source (e.g. Bavelier et al., 2000; Huber, 2008; Lleras & Enns, 2005) or an attentional source (Sohrabi & West, 2008), in which case it would not be expected to show dependence on the PCE.
Here, we have found a remarkable symmetry between the growth of the PCE and NCE during sensorimotor learning. This was replicated over four experiments, and occurred whether priming emerged slowly or more quickly, and even as priming was inverted and recovered when the S–R relationships were suddenly reversed. Together, this provides evidence that the NCE is tightly coupled to the positive priming.
Hypotheses based on perceptual inhibition, perceptual habituation, or an attentional refractory period share the core feature that the source of the NCE stems from modulations of perceptual processing, rather than modulations of motor activation. We believe this could not explain the present results. If perceptual processing of the target is slowed when it is similar to the prime (compatible trials), this would be independent from the establishment of a strong S–R link. Therefore, a perceptual NCE should occur immediately rather than build up like positive sensorimotor priming. Further, it should not have inverted in Experiments 3 and 4 because when the motor mapping reversed, the only modification concerned the target–response correspondence while the perceptual aspects of the prime-target relationships were kept unchanged. Thus the inversion of the NCE highlights its motoric origins and that it is the automatic consequence of previously established perceptuo–motor associations, rather than any mechanism that acts on or within perceptual processing.
Thus our main conclusion is rather simply stated: the NCE shows very tight coupling to the PCE, as predicted by a sensorimotor source. This conclusion is consistent with previous converging data that are better explained by a sensorimotor account than a perceptual account. Electrophysiological measurements of the lateralized readiness potential (LRP) have shown oscillations that appear to track the balance of activation between response alternatives during the prime-mask-target interval (e.g. Eimer & Schlaghecken, 1998, 2003; Praamstra & Seiss, 2005). These traces show an initial deflection associated with the prime, followed by a reversal consistent with inhibition, and lastly a strong deflection associated with the response. Behaviourally, a further oscillation in priming has been measured at longer SOAs, such that following the NCE, there was a small rebound back to a PCE (Sumner & Brandwood, 2008). This is difficult for perceptual habituation to account for, but can be explained by sensorimotor inhibition if not only the initial priming is inhibited, but also the subsequent reversal is inhibited, creating a further reversal. Lastly, an NCE has been measured in a cross-modal situation in which visual primes were followed by auditory targets (Klapp & Hinkley, 2002). Since these primes and targets were in different perceptual modalities, but associated with the same responses, their interaction is most likely to have occurred in a motor (or responses selection) locus.
The three sensorimotor accounts
While the present study was not designed to distinguish between accounts within the sensorimotor class, it is worth briefly discussing the three main sensorimotor theories. In the original “self inhibition” theory, the NCE represents an inhibitory mechanism acting to suppress the initial sub-threshold motor activation evoked by the prime (e.g. Bowman et al., 2006; Eimer & Schlaghecken, 1998; Klapp & Hinkley, 2002; Schlaghecken et al., 2006; Schlaghecken & Eimer, 2002). It was suggested that such self inhibition automatically follows any sub-threshold motor activation that is not further supported by continuing sensory input, providing a mechanism to keep automatic motor activation in check without the intervention of top-down control processes. It is clear that in this theory the NCE only occurs when the initial PCE phase occurs, because it is the (subconscious) detection of the initial priming phase that triggers the inhibition.
The second theory argued against the need for inhibition, and postulated that the stimulus sequence used in some masked priming experiments could actually reverse the priming from positive to negative relative to the direction of the prime (Lleras & Enns, 2004, 2005; Verleger et al., 2004). For example, although a prime might be expected to activate the right hand response, the mask could potentially prime the leftward response, even though the mask contained features of both possible targets. Such directional mask-induced priming could occur because, as discussed for the perceptual accounts of the NCE, the perceptual system tends to enhance new features – i.e. those not already displayed in the prime. This emphasis on new features has been referred to as “object updating” (Lleras & Enns, 2004, 2005, 2006). Thus the object updating theory uses essentially the same logic as the perceptual theories, but applies it to the processing of the mask, not the target. So while masks may contain features of both primes, on any particular trial the features shared with the prime may be less perceptually salient, and therefore the features of the alternative prime more salient. But while the first stage in the theory is a perceptual interaction akin to neural habituation (see Huber 2008), the consequences of this perceptual modulation of mask features is envisaged to be relatively greater motor priming for the response opposite to the prime. It is this motor priming that causes the NCE, and thus places the theory in the sensorimotor category. In turn this predicts that, consistent with our results, the NCE occurs only when a sensorimotor association between certain features and certain responses has become established.
Thirdly, Jaskowski and colleagues (Jaskowski, 2007, 2008; Jaskowski & Przekoracka-Krawczyk, 2005) have proposed that the NCE does reflect motor inhibition, but that it is not self-triggered as initially proposed. Rather, it is triggered by the mask stimulus. Sub-threshold activation caused by the prime is suppressed when immediately followed by another potentially relevant stimulus (e.g. a mask). A similar idea was also suggested by Lleras and Enns (Lleras & Enns, 2006).
Although our data do not distinguish these three sensorimotor accounts, other studies have endeavored to do so. Currently, the evidence appears to indicate that mask-induced priming (object updating) is an important factor when the masks are similar to a combination of the two primes (Bennett, Lleras, Oriet, & Enns, 2007; Klapp, 2005; Lleras & Enns, 2004, 2005; Verleger et al., 2004), as occurred in most early experiments. But in many recent experiments where this has not been the case, object updating is probably not the main source of NCE (Jaskowski, 2009; Schlaghecken & Eimer, 2006; Sumner, 2008). Between the self-inhibition and the stimulus-triggered inhibition accounts, the balance of evidence appears to favour the latter (Boy, Clarke, & Sumner, 2008; Jaskowski, 2007, 2008), but Sumner and Brandwood’s (2008) finding of a further oscillation from NCE back to PCE is more consistent with self-inhibition, since there was no further stimulus to trigger that reversal. It may be that all these sensorimotor accounts make contributions, which differ in importance depending of the stimuli and procedures employed.
Trigger threshold for inhibition?
Eimer and Schlaghecken’s model of automatic inhibition (Bowman et al., 2006; Eimer & Schlaghecken, 2003) contains a trigger threshold, so that inhibition does not occur unless the initial priming phase reaches a certain strength (Schlaghecken & Eimer, 2000, 2002). Although our study was not designed to investigate this issue, it is possible to interrogate our data with respect to what a trigger threshold would predict. In this case we would not expect to measure an NCE during learning until the PCE has built up to the threshold level. Further, in Experiments 3 and 4, a threshold mechanism would set inhibition to zero for a period while the PCE crossed over zero in its recovery following inversion. However our data show a consistent symmetry between the emergence of PCE and NCE during learning and reversal of S–R responses (Figs 3 and 4), with no sign that inhibition did not occur when the initial motor activation was below a certain threshold. Of course, it remains possible that the threshold is too small to detect in our experiments, although there was a significant 1-tailed NCE when a significant PCE was as small as 6 ms (as in Expt 1, “pseudo–block” no. 4: t(11) = 2.12; p < 0.03, Cohen’s d = 1.31), and significant (2-tailed) NCEs occurred when the PCE was as small as 9 ms (as in Expt 1, “pseudo–block” no. 8: t(11) = 3.5; p < 0.01).
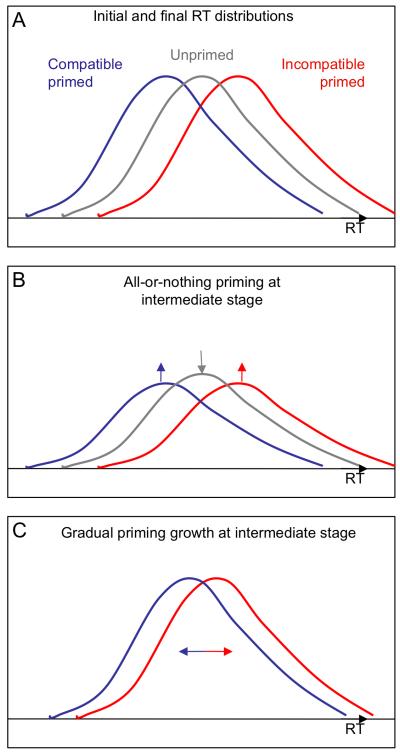
There is also the possibility that priming may in fact be ‘all or nothing’ on any individual trial or in individual participants. The graded mean values, as reported in Figures 3 and 4, could arise through changes in either the proportion of trials or proportion of participants showing priming in that block. In this case, blocks with small mean PCE values would not mean that priming was small, but that priming was rare (across trials or participants). But every time positive priming did occur it would be above threshold, and thus the NCE would be expected in the same proportion of trials/participants as the PCE, and the mean NCE would track the mean PCE, as we have found. However, we can rule out the possibility that priming appears in an all or nothing manner for each participant, by plotting the data for each individual for every experiment (Figure 5).
Figure 5.
Evolution of Compatibility Effects (in ms) for each individual participant in short and long SOAs as function of training (in blocks) in Expts 1, 2, 3 & 4. Individual data is noisy (each point now represents RT in only 10 incompatible trials minus 10 compatible trials, except Expt. 4 where there were only 2 or 3 trials of each type in a block), but it appears that each participants’ data approximately follows the pattern of the mean (Figures 3 and 4), rather than making step transitions from absent to present priming. This is especially clear after the response reversal in Experiments 3 and 4, where the graded change in priming strength lasts several blocks.
The possibility that individual trials show all-or-nothing priming is much harder to separate from the idea of gradual growth in priming strength. Before learning, both all-or-nothing priming and gradual growth expect that RT on all trials is drawn from the same “unprimed” distribution. After priming asymptotes, both possibilities expect that RTs on compatible and incompatible trials come from different “fully primed” distributions, separated by approximately 20-30 ms (separation will differ between participants). During learning, all-or-nothing priming expects that the proportion of trials drawn from the unprimed distribution diminishes, while the proportion drawn from the “fully primed” distributions increases. This predicts that the variance for each type of trial (compatible and incompatible) will be higher during learning than when priming asymptotes and one distribution becomes dominant. Gradual growth, on the other hand, does not predict such a pattern because the trials would be drawn from two “slightly-primed” distributions that gradually move further apart (see Figure 6 A, B & C). We therefore calculated, for each participant, the standard deviation (SD) of response time in each trial type in blocks when the PCE was growing, and compared these (averaged over trial type) to the SD in blocks when the PCE was stable. Mean SD for ‘growing’ blocks was 11.0 for Expt 2 (blocks 2 & 3) and 9.7 for Expt 3 (blocks 9, 10 & 11), while mean SD for ‘stable’ blocks was 10.6 for Expt 2 (blocks 6 & 7) and 10.8 for Expt 3 (blocks 3, 4 & 5). Neither difference is significant (all ts(11) < 1.3, all ps > .05), see also Table 3. This does not support a random sampling from two distributions during the growth phase, but admittedly the conclusion rests on a null result – not detecting an increase in variance with intermediate mean priming values.
Figure 6.
(A & B) Illustrated comparison of the all-or-nothing theory of priming vs gradual emergence. During learning, the all-or-nothing theory expects that the proportion of trials drawn from the unprimed distribution diminishes, while the proportion drawn from the “fully primed” distributions increases. (C) The gradual theory expects that trials are drawn from two “slightly-primed” distributions that gradually move further apart.
Table 3.
Mean Standard Deviation of the reaction times averaged over participants and over trial types for Expts 2 & 3. Averages were carried out separately for blocks in which the Compatibility effect is either ‘Growing’ or ‘Stable’. Paired student T-tests compared pairs of blocks (in italic).
| Expt 2 SOA = 40ms | |||
|---|---|---|---|
| ‘Growing’ | ‘Stable’ | ||
| Block 2 | 5.3 | Block 6 | 13.4 |
| 29.4 | 3.2 | ||
| 11.4 | 6.2 | ||
| 10.8 | 4.6 | ||
| 7.3 | 15.5 | ||
| 5.1 | 9.6 | ||
| 14.8 | 16.5 | ||
| 7.9 | 5.3 | ||
| 29.0 | 7.3 | ||
| 15.1 | 9.4 | ||
| 10.3 | 7.4 | ||
| 17.6 | 12.6 | ||
| t(11) = 1.3 p > 0.05 | |||
|
| |||
| Block 3 | 4.7 | Block 7 | 18.5 |
| 17.9 | 4.4 | ||
| 6.1 | 19.6 | ||
| 4.8 | 17.7 | ||
| 3.5 | 6.8 | ||
| 7.7 | 13.4 | ||
| 20.5 | 7.8 | ||
| 1.3 | 9.1 | ||
| 10.8 | 9.3 | ||
| 9.3 | 15.3 | ||
| 4.5 | 6.7 | ||
| 7.8 | 14.8 | ||
| t(11) = 1.2 p > 0.05 | |||
| Expt 3 SOA = 40ms | |||
|---|---|---|---|
| ‘Growing’ | ‘Stable’ | ||
| Block 3 | 12.4 | Block 9 | 7.9 |
| 6.5 | 4.6 | ||
| 3.0 | 7.5 | ||
| 11.0 | 4.0 | ||
| 4.5 | 2.0 | ||
| 15.1 | 33.6 | ||
| 2.3 | 10.8 | ||
| 20.7 | 6.9 | ||
| 16.9 | 18.6 | ||
| 6.8 | 7.6 | ||
| 16.4 | 15.4 | ||
| 5.5 | 27.3 | ||
| t(11) = 0.715; p > 0.05 | |||
|
| |||
| Block 4 | 5.2 | Block 10 | 3.7 |
| 6.8 | 14.7 | ||
| 21.3 | 6.3 | ||
| 5.3 | 5.0 | ||
| 7.9 | 11.2 | ||
| 3.0 | 8.8 | ||
| 13.0 | 23.4 | ||
| 5.1 | 2.0 | ||
| 14.3 | 10.0 | ||
| 12.2 | 7.1 | ||
| 3.6 | 10.3 | ||
| 11.3 | 4.7 | ||
| t(11) = 0.94; p > 0.05 | |||
|
| |||
| Block 5 | 20.1 | Block 11 | 7.4 |
| 4.4 | 7.4 | ||
| 15.7 | 34.0 | ||
| 18.2 | 37.0 | ||
| 4.2 | 8.1 | ||
| 5.4 | 1.7 | ||
| 6.3 | 1.5 | ||
| 15.5 | 5.2 | ||
| 6.9 | 3.4 | ||
| 5.5 | 8.6 | ||
| 4.2 | 14.7 | ||
| 13.4 | 5.5 | ||
| t(11) = 0.4; p > 0.05 | |||
In sum our data do not offer any evidence for a trigger threshold for the NCE, which is consistent with the conclusions of Lingnau and Vorberg (2005), who advocated a continuum of priming effects. The possibility that a trigger threshold could be hidden by priming in an all-or-nothing fashion, either across participants or across trials, does not appear to be supported by the pattern of individual priming growth (Fig 5) or trial-by-trial variance during that growth (Table 3).
Contiguity in learning masked priming?
In order to obtain different rates of priming growth in Experiments 1 and 2, we used a simple manipulation of randomising or blocking the order of trial types. It is worth briefly considering why this manipulation should be so effective, when the blocked/randomised factor (mask-target SOA) was irrelevant to learning the S-R mapping, and most participants did not even notice the difference between these two SOAs (see control experiment). There are three (non-exclusive) reasons that may contribute to faster learning of automatic S–R associations when trial timing was blocked. Firstly, error rates were lower for blocked presentation. Every time an error is made, the wrong response is temporarily associated with the stimulus – a set-back to the associative pairing of the correct S-R mapping. Secondly, S-R learning may depend not only on the actual stimulus, but on its context. Guthrie (1952), for example, attributed a central role to the repetition of context when learning a novel task – in his view it is the repetition of the same type of stimulation within the same context that permits rapid and strong learning. In our case the context is provided by the timing of the mask presented before the target. Lastly, when long and short SOA trials are randomly intermixed, the uncertainty (even unconscious uncertainty) about the moment at which the response has to be given may activate some control processes to stop responses being triggered too soon. This may impede the development of strong priming effects, because the influence of automatic S-R activation may be down–regulated relative to a more cognitive route.
Conclusions
In four experiments we have found a remarkable correspondence between the PCE measured at short SOA and the NCE measured at long SOA during S-R learning in a masked prime task. Both effects abruptly reversed when the S-R mapping was reversed, and then recovered at the same rate, providing strong evidence for the sensorimotor accounts of the NCE. Our results do not support a perceptual locus for the NCE, such as repetition blindness, neural habituation or an attentional refractory period, in this kind of masked prime paradigm.
Acknowledgments
The authors wish to thank Friederike Schlaghecken, Martin Eimer and one anonymous reviewer for their helpful comments on previous versions of this manuscript. Supported by the Wellcome Trust and the Wales Institute of Cognitive Neuroscience (WICN).
This work was supported by the BBSRC, the Wales Institute of Cognitive Neuroscience (WICN) and the Wellcome Trust.
References
- Bavelier D, Deruelle C, Proksch J. Positive and negative compatibility effects. Perception & Psychophysics. 2000;62(1):100–112. doi: 10.3758/bf03212064. [DOI] [PubMed] [Google Scholar]
- Bennett JD, Lleras A, Oriet C, Enns JT. A negative compatibility effect in priming of emotional faces. Psychonomic Bulletin & Review. 2007;14(5):908–912. doi: 10.3758/bf03194120. [DOI] [PubMed] [Google Scholar]
- Bowman H, Schlaghecken F, Eimer M. A neural network model of inhibitory processes in subliminal priming. Visual Cognition. 2006;13(4):401–480. [Google Scholar]
- Boy F, Clarke K, Sumner P. Mask stimulus triggers inhibition in subliminal visuomotor priming. Experimental Brain Research. 2008;190(1):111–116. doi: 10.1007/s00221-008-1515-5. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schlaghecken F. Effects of masked stimuli on motor activation: Behavioral and electrophysiological evidence. Journal of Experimental Psychology-Human Perception and Performance. 1998;24(6):1737–1747. doi: 10.1037//0096-1523.24.6.1737. [DOI] [PubMed] [Google Scholar]
- Eimer M, Schlaghecken F. Response facilitation and inhibition in subliminal priming. Biological Psychology. 2003;64(1-2):7–26. doi: 10.1016/s0301-0511(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Guthrie ER. The psychology of learning. Peter Smith Pub Inc.; Gloucester, MA: 1952. [Google Scholar]
- Hochhaus L, Johnston JC. Perceptual repetition blindness effects. Journal of Experimental Psychology-Human Perception and Performance. 1996;22(2):355–366. doi: 10.1037//0096-1523.22.2.355. [DOI] [PubMed] [Google Scholar]
- Huber DE. Immediate priming and cognitive aftereffects. Journal of Experimental Psychology-General. 2008;137(2):324–347. doi: 10.1037/0096-3445.137.2.324. [DOI] [PubMed] [Google Scholar]
- Huber DE, Shiffrin RM, Lyle KB, Ruys KI. Perception and preference in short-term word priming. Psychological Review. 2001;108(3):652–652. doi: 10.1037/0033-295x.108.1.149. [DOI] [PubMed] [Google Scholar]
- Huber DE, Shiffrin RM, Quach R, Lyle KB. Mechanisms of source confusion and discounting in short-term priming: 1. Effects of prime duration and prime recognition. Memory & Cognition. 2002;30(5):745–757. doi: 10.3758/bf03196430. [DOI] [PubMed] [Google Scholar]
- Jaskowski P. The effect of nonmasking distractors on the priming of motor responses. Journal of Experimental Psychology - Human Perception and Performance. 2007;33:456–468. doi: 10.1037/0096-1523.33.2.456. [DOI] [PubMed] [Google Scholar]
- Jaskowski P. The negative compatibility effect with nonmasking flankers: A case for mask-triggered inhibition hypothesis. Consciousness and Cognition. 2008;17(3):765–777. doi: 10.1016/j.concog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Jaskowski P. Negative compatibility effect: the object-updating hypothesis revisited. Exp Brain Res. 2009;193(1):157–160. doi: 10.1007/s00221-008-1700-6. [DOI] [PubMed] [Google Scholar]
- Jaskowski P, Przekoracka-Krawczyk A. On the role of mask structure in subliminal priming. Acta Neurobiologiae Experimentalis. 2005;65(4):409–417. doi: 10.55782/ane-2005-1569. [DOI] [PubMed] [Google Scholar]
- Jaskowski P, Slosarek M. How important is a prime’s gestalt for subliminal priming? Consciousness and Cognition. 2007;16:485–497. doi: 10.1016/j.concog.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Johnston JC, Hochhaus L, Ruthruff E. Repetition blindness has a perceptual locus: Evidence from Online processing of targets in RSVP streams. Journal of Experimental Psychology-Human Perception and Performance. 2002;28(2):477–489. [PubMed] [Google Scholar]
- Klapp ST. Two versions of the negative compatibility effect: Comment on Lleras and Enns (2004) Journal of Experimental Psychology-General. 2005;134(3):431–435. doi: 10.1037/0096-3445.134.3.431. [DOI] [PubMed] [Google Scholar]
- Klapp ST, Hinkley LB. The negative compatibility effect: Unconscious inhibition influences reaction time and response selection. Journal of Experimental Psychology-General. 2002;131(2):255–269. doi: 10.1037//0096-3445.131.2.255. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Kopp B. Mechanisms of priming by masked stimuli: Inferences from event-related brain potentials. Psychological Science. 1998;9(4):263–269. [Google Scholar]
- Lingnau A, Vorberg D. The time course of response inhibition in masked priming. Perception & Psychophysics. 2005;67(3):545–557. doi: 10.3758/bf03193330. [DOI] [PubMed] [Google Scholar]
- Lleras A, Enns JT. Negative compatibility or object updating? A cautionary tale of mask-dependent priming. Journal of Experimental Psychology-General. 2004;133(4):475–493. doi: 10.1037/0096-3445.133.4.475. [DOI] [PubMed] [Google Scholar]
- Lleras A, Enns JT. Updating a cautionary tale of masked priming: Reply to Klapp (2005) Journal of Experimental Psychology-General. 2005;134(3):436–440. doi: 10.1037/0096-3445.134.3.431. [DOI] [PubMed] [Google Scholar]
- Lleras A, Enns JT. How much like a target can a mask be? Geometric, spatial, and temporal similarity in priming: A reply to Schlaghecken and Eimer (2006) Journal of Experimental Psychology-General. 2006;135(3):495–500. doi: 10.1037/0096-3445.135.3.495. [DOI] [PubMed] [Google Scholar]
- Neumann O, Klotz W. Motor-Responses to Nonreportable, Masked Stimuli - Where Is the Limit of Direct Parameter Specification. In: Umiltà C, Moscovitch M, editors. Attention and Performance XV: Conscious and nonconscious information processing. MIT Press; Cambridge, MA: 1994. pp. 123–150. [Google Scholar]
- Praamstra P, Seiss E. The neurophysiology of response competition: Motor cortex activation and inhibition following subliminal response priming. Journal of Cognitive Neuroscience. 2005;17(3):483–493. doi: 10.1162/0898929053279513. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Blagrove E, Maylor EA. Incidental learning of S-R contingencies in the masked prime task. Journal of Experimental Psychology-Human Perception and Performance. 2007;33(5):1177–1188. doi: 10.1037/0096-1523.33.5.1177. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Bowman H, Eimer M. Dissociating local and global levels of perceptuo-motor control in masked priming. Journal of Experimental Psychology-Human Perception and Performance. 2006;32(3):618–632. doi: 10.1037/0096-1523.32.3.618. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Eimer M. The influence of subliminally presented primes on response preparation. Sprache & Kognition. 1997;16(3-4):166–175. [Google Scholar]
- Schlaghecken F, Eimer M. A central-peripheral asymmetry in masked priming. Perception & Psychophysics. 2000;62(7):1367–1382. doi: 10.3758/bf03212139. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Eimer M. Motor activation with and without inhibition: Evidence for a threshold mechanism in motor control. Perception & Psychophysics. 2002;64(1):148–162. doi: 10.3758/bf03194564. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F, Eimer M. Active masks and active inhibition: A comment on Lleras and Enns (2004) and on Verleger, Jaskowski, Aydemir, van der Lubbe, and Groen (2004) Journal of Experimental Psychology-General. 2006;135(3):484–494. doi: 10.1037/0096-3445.135.3.484. [DOI] [PubMed] [Google Scholar]
- Sohrabi A, West RL. A biologicallly-plausible cognitive model (BPCM) of positive and negative congruency effects in masked priming. Poster session presented at the annual meeting of the Cognitive Science Society; Washinton DC, USA. 2008. [Google Scholar]
- Sumner P. Negative and positive masked-priming – implications for motor inhibition. Advances in Cognitive Psychology. 2007;3:317–326. doi: 10.2478/v10053-008-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P. Mask-induced priming and the negative compatibility effect. Experimental Psychology. 2008;55(2):133–141. doi: 10.1027/1618-3169.55.2.133. [DOI] [PubMed] [Google Scholar]
- Sumner P, Brandwood T. Oscillations in motor priming: Positive rebound follows inhibitory phase in the masked prime paradigm. Journal of Motor Behavior. 2008 doi: 10.3200/JMBR.40.6.484-490. in press. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. Action priming by briefly presented objects. Acta Psychol (Amst) 2004;116(2):185–203. doi: 10.1016/j.actpsy.2004.01.004. [DOI] [PubMed] [Google Scholar]
- van Leeuwen C, Lachmann T. Negative and positive congruence effects in letters and shapes. Perception & Psychophysics. 2004;66(6):908–925. doi: 10.3758/bf03194984. [DOI] [PubMed] [Google Scholar]
- Verleger R, Jaskowski P, Aydemir A, van der Lubbe RH, Groen M. Qualitative differences between conscious and nonconscious processing? On inverse priming induced by masked arrows. J Exp Psychol Gen. 2004;133(4):494–515. doi: 10.1037/0096-3445.133.4.494. [DOI] [PubMed] [Google Scholar]