Abstract
Dopaminergic (DA) neurons exhibit a short-latency, phasic response to unexpected, biologically salient stimuli. The superior colliculus (SC) is also sensitive to such stimuli and sends a projection directly to DA-containing regions of the ventral midbrain. Recent evidence suggests that the SC is a critical relay for transmitting short-latency visual information to DA neurons. An important question is whether the ventral midbrain is an exclusive target of tectonigral neurons, or whether the tectonigral projection is a collateral branch of other tectofugal pathways. Double-label retrograde anatomical tracing techniques were used to address this issue. Injections of either Diamidino Yellow or Fluorogold into substantia nigra pars compacta (SNc) were combined with larger injections of True Blue into one of the following efferent projections of the SC: 1) target regions of the ipsilateral ascending projection to the thalamus; 2) the crossed descending tecto-reticulo-spinal pathway; 3) target structures of the ipsilateral descending projection; and 4) the contralateral superior colliculus. Moderate numbers of double-labeled neurons were observed following combined injections into substantia nigra and individual nuclei in the thalamus (ventromedial nucleus, 21.3%; central lateral, 18.4%; parafasicular nucleus 6.0%). Much less double-labeling was associated with injections into either of the descending projections (crossed, 1.0–3.2%; un-crossed, 0.2–2.7%) or the contralateral SC (0.7–1.9%). These results suggest: i) the SC may provide a coordinated input concerning the occurrence of unpredicted sensory events to both the substantia nigra and striatum (via the thalamus); and ii) few gaze-related motor signals are simultaneously relayed to DA-containing regions of the ventral midbrain.
Keywords: basal ganglia, dopamine, substantia nigra, thalamus, tectum, brainstem
Dopaminergic (DA) neurons located in the substantia nigra pars compacta (SNc) and the ventral tegmental area respond to unexpected biologically salient stimuli, including those associated with reward, with a stereotypic short-latency (<100 ms), short-duration (100 ms) increase in firing rate. Although this reaction has been extensively investigated in the context of stimuli associated with reward, the functional status of the phasic DA response has been difficult to discern (Wickelgren, 1997; Redgrave et al., 1999b). For example, simulation of dopamine responses provides effective “teaching signals” in models of reinforcement learning when they are assumed to convey information about errors in reward prediction (Schultz et al., 1997). However, the fact that these neurons can be activated by classes of stimuli other than those unambiguously associated with reward suggest that the phasic DA signal may have a broader role than reward alone (Schultz, 1998; Guarraci and Kapp, 1999; Redgrave et al., 1999b; Horvitz, 2000).
Given that DA neurons appear particularly sensitive to unpredicted sensory events, we have argued that a good strategy to achieve a better understanding of the role of DA neurons is to investigate the information-processing capacities of the afferent sensory structures that control their activity (Coizet et al., 2003, 2006; Comoli et al., 2003; Dommett et al., 2005). Consequently, we have shown that the midbrain superior colliculus (SC), well known for its direct multisensory inputs (Stein and Meredith, 1993), has a significant influence on the activity of neurons located in the ventral midbrain, including SNc and the ventral tegmental area (Coizet et al., 2003; Comoli et al., 2003; Dommett et al., 2005). These results may, in part, be understood in terms of the discovery of a previously unreported direct projection from the midbrain SC to DA-containing regions of the ventral midbrain. This “tectonigral” projection has now been reported in rat (Comoli et al., 2003), cat (McHaffie et al., 2005), and now has also be found in monkey (May, Stanford, McHaffie, Jaing, Coizet, Redgrave, and Haber, unpubl. obs.). Taken together, the results of these investigations suggest that the SC is the primary, if not unique, source of short latency visual afferents to DA neurons.
Identification of the SC as the most likely source of short latency visual input to the ventral midbrain carries with it the implication that the visual properties of DA neurons are likely to be heavily constrained by the visual processing capacities of the SC (Wurtz and Albano, 1980; Sparks, 1986; Stein and Meredith, 1993). Thus, in mammals, most visually responsive cells in SC are transiently activated 40–60 ms after the appearance, disappearance, or movement of a stimulus within a specific region of the visual field. Collicular neurons respond poorly, if at all, to contrast, velocity, wavelength, or geometric configuration of visual stimuli (Wurtz and Albano, 1980). These electrophysiological properties of SC neurons suggest that the visual information content of phasic short-latency dopamine responses may be largely restricted to stimuli that appear, disappear, or move.
With this sensitivity to biologically significant visual transients, emphasis has been placed on the role of the SC in orienting the head and eyes toward unexpected objects in the visual field (Grantyn, 1988; Munoz and Guitton, 1989; Wurtz, 1996; Sparks, 1999; Grantyn and Moschovakis, 2004). This concept was extended to include its involvement in directing the eyes, head, and body away from potentially threatening stimuli (Dean et al., 1989; Brandao et al., 1994, 1999). Shifts of gaze toward or away from sensory stimuli are produced by the SC through direct efferent descending projections to the motor systems in the brainstem and spinal cord responsible for generating eye, head, and body movements (May, 2006). These pathways can be subdivided into: 1) a crossed descending projection that contacts targets in the contralateral brainstem including precerebellar nuclei such as reticularis tegmentis pontis, the inferior olive, and premotor regions of the pontine and medullary reticular formation and spinal cord (Huerta and Harting, 1984; Dean et al., 1986; Redgrave et al., 1986, 1987; Yeomans and Tehovnik, 1988; Grantyn, 1988; Masino and Knudsen, 1992; Grantyn and Moschovakis, 2004); and 2) an ipsilateral descending projection with terminations in the periaqueductal gray, cuneiform area, lateral pons, and ventral pontine/medullary reticular formation (Huerta and Harting, 1984; Dean et al., 1988; Mitchell et al., 1988; Redgrave et al., 1986, 1987; Shehab et al., 1995). In the rat, the SC cells of origin of these two projections are separately localized subregions of the intermediate and deep layers of the SC (Redgrave et al., 1986, 1987, 1990; Bickford and Hall, 1989; Yasui et al., 1994). Experimental data have shown that approach responses are mediated primarily through the contralateral descending pathway (Yeomans and Tehovnik, 1988; Dean et al., 1989; Masino and Knudsen, 1992; Redgrave and Wang, 1996), whereas avoidance behaviors rely more heavily on the ipsilateral descending pathway (Dean et al., 1988; Mitchell et al., 1988; Shehab et al., 1995).
In addition to its descending pathways to the brainstem, the SC also has ascending efferent projections to the pretectum (Lugo-Garcia and Kicliter, 1987; Kubota, 1989; Lagares et al., 1994) and thalamus (Yamasaki et al., 1986; Abramson and Chalupa, 1988; Berson and Graybiel, 1991; Kolmac and Mitrofanis, 1998; Linke, 1999; Linke et al., 1999; Harting et al., 2001), some of which appear to be collateral branches of axons projecting to the brainstem (Chevalier and Deniau, 1984; Redgrave et al., 1986; Bickford and Hall, 1989). This third major efferent pathway directly links the intermediate and deep layers of SC with the central-lateral, paracentral, central-medial, and parafascicularis nuclei of the thalamus (Chevalier and Deniau, 1984; Yamasaki et al., 1986; Krout et al., 2001). It has been suggested that SC transmits an integrated, multimodal representation of the external environment to the thalamus (Grunwerg and Krauthamer, 1990; Krauthamer et al., 1992). However, it is significant that ascending projections from SC specifically target regions of the thalamus that provide the main thalamic input to the major input structure of the basal ganglia, the striatum (Mengual et al., 1999; Harting et al., 2001; Krout et al., 2001; Van der Werf et al., 2002; Kimura et al., 2004). This arrangement suggests that SC is an important afferent source of sensory information to the basal ganglia via relays in the thalamus (McHaffie et al., 2005).
Finally, another substantial collicular output connects the two SC on both sides of the brain (Edwards, 1977; Behan, 1985; Sahibzada et al., 1987; Appell and Behan, 1990; Olivier et al., 1998, 2000). This tecto-tectal pathway originates mainly from the deep and intermediate layers of the SC to project in the same areas on the contralateral side. The traditional view is that these commissural fibers may, in part, mediate mutually suppressive effects on the output of the contralateral colliculi to prevent competing responses in the opposite direction (Sprague, 1966; Munoz and Istvan, 1998).
This brief review of the output anatomy of the SC suggests that its major efferent connections are with premotor and precerebellar nuclei in the brainstem, and with prebasal ganglia relay regions in the thalamus (May, 2006). An immediate and important question, therefore, concerns the relationship between the recently discovered tectonigral projection and the well-established output projections of the SC (May, 2006). Specifically, do tectonigral fibers represent a collateral projection system that branches from one or more of the previously characterized efferent projections, or are they a functionally independent output system? The main purpose of the present study was to address this issue through the use of double-label retrograde anatomical tracing techniques. The injection of one fluorescent retrograde tracer into the SNc was combined with the injection of a different colored tracer into one of the SC’s established output projections. The resulting proportions of double-labeled neurons were used as a measure of collateralization between the tectonigral projection and the SC’s other principal output projections. Some of the present results have appeared in abstract form (Coizet et al., 2005).
MATERIALS AND METHODS
Hooded Lister rats (n = 32; male; 424–685 g) were injected with one of the retrograde fluorescent tracers Diamidino Yellow (Keizer et al., 1983; Conde, 1987) or Fluorogold (Richmond et al., 1994; Novikova et al., 1997; Lanciego et al., 1998) into SNc and a second injection of the fluorescent tracer True Blue (Illert et al., 1982; Payne and Peace, 1989) into either the contralateral descending predorsal bundle, target regions of the ipsilateral descending pathway, the contralateral SC, or target regions in the thalamus of the ipsilateral ascending projection. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act, 1986, and every effort was made to minimize suffering and reduce the number of animals used
Surgical procedures
The animals were anesthetized with an intraperitoneal injection of a mixture of Ketaset (0.765 mL/kg) and Rompum (1.1 mL/kg) and placed in a stereotaxic frame with the skull level in the plane employed by the stereotaxic atlas of Paxinos and Watson (1987). Throughout the surgery the temperature of the rat was maintained at 37°C by a thermostatically controlled heating blanket.
Substantia nigra pars compacta injection
All the animals (n = 29) received an injection of either Diamidino Yellow (Sigma, St. Louis, MO) or Fluorogold (Fluorochrome LCC, Molecular Probes, Eugene, OR) into SNc using the following stereotaxic coordinates: (AP: −5.2 to −6.04 mm from bregma; ML: 1.4-2.6 mm from midline; and DV: −7.3 to −8.2, from the surface of the brain). Because the density of tectonigral fibers is greatest in the lateral half of substantia nigra pars compacta (Comoli et al., 2003), most of our injections of retrograde tracer were aimed at this region. A contralateral approach using an angle of 38° was used to avoid damage or contamination of the ipsilateral SC. Diamidino Yellow was injected as a 2% suspension in distilled water (100–250 nL; 100 nL/min; n = 11) via a sharpened 30G injection cannula connected with polyethylene tubing to a 10-μl Hamilton syringe driven by a Harvard infusion pump. Fluorogold was injected as a 4.0% solution in distilled water (45–100 nL; n = 18) via a glass micropipette (tip diameter: 10–20 μm) using a compressed air pressure-injection system. The SNc is a thin (0.5–0.8 mm), tilted sheet of neurons positioned between fibers of the medial lemniscus and substantia nigra pars reticulata. Because of the right-angled approach to the long axes of pars compacta, we used electrophysiological recording procedures to improve the successful depth placement of these injections. Therefore, the pipette/cannula was joined to a Parylene-C-insulated tungsten microelectrode and the assembly lowered with a microdrive (David Kopf Instruments, Tujunga, CA) until the electrophysiological record showed an absence of activity (usually ~−8.00 mm below the surface of the brain) normally corresponding to the lemniscal fiber tract. Shortly after (0.5–1.0 mm deeper), the record typically revealed the presence of fast-firing low-amplitude activity characteristic of neurons in substantia nigra pars reticulata (Chevalier and Deniau, 1990). As soon as this activity was detected the tracer injection was made at this level.
True Blue injections
Once the substantia nigra pars compacta injection had been made, each rat was given one, or sometimes a series, of comparatively large True Blue injections (Sigma) (5% in water; 500–2000 μL; 250 nL/min) via a 28G injector needle connected to a 10-μL Hamilton syringe placed in a Harvard infusion pump. Targets for the True Blue injections included the following (all numbers are expressed in mm with AP measurements relative to bregma, ML measurements relative to midline, and DV measurements relative to the surface of the brain):
Ipsilateral ascending projection: Rostral intralaminar thalamus (n = 3) (AP: −3.3 to −3.8; ML: 1.0 to 1.8; DV: −4.4 to −6.4). Caudal intralaminar and ventromedial thalamus (n = 4) (AP: −3.8 to −4.2 and −4.5; ML: 1.00; DV: −4.1 to −6.2).
Ipsilateral descending projection: Cuneiform nucleus (n = 2) (AP: −8.00 to −8.3; ML: 1.8 to 2.0; DV: −5.8 to −6.3). Parabigeminal nucleus (n = 1) (AP: −8.00; ML: 3.00; DV: −6.2). Pontine reticular formation (n = 2) (AP: −8.3; ML: 2.0; DV: −10.0). Pedunculopontine nucleus (n = 2) (AP: −8.0 to −8.3; ML: 1.8 to 2.0; DV: −7.2 to −7.4).
Crossed descending projection: Contralateral descending fibers at the level of the medial pontine reticular formation (n = 7) (AP: −9.2 to −10.3; ML: 0.4 to 1.2; DV: −5.4 to −10.0).
Tectotectal projection: The contralateral SC (n = 3) (AP: −6.3 to −6.8; ML: 0.4 to 0.8; DV: −3.6 to −3.8).
Histology
After 7 days to allow time for the retrograde transport of the fluorescent labels, animals were deeply anesthetized and perfused transcardially with 0.9% isotonic saline followed by 10% formalin in 0.1 M phosphate buffer at pH 7.5 (0.1 M, PB). The brains were placed immediately in the formalin for 4 hours before being cryoprotected by immersion in a sucrose solution (20% in 0.1 M PB) overnight. The next day, 30-μm coronal sections were cut on a freezing microtome and collected directly onto slides, allowed to dry in a light-protected box, then coverslipped with DPX mountant. The injection sites and retrogradely labeled cells in the SC were examined with a fluorescence microscope equipped with episcopic illumination (Nikon Elipse E800M) and a UV excitation filter (330–380 nm). Photomicrographs were captured with digital photography (Spot-2 digital camera, Diagnostic Instruments, Sterling Heights, MI) and all image files were processed using Photoshop (Adobe, San Jose, CA), and FreeHand (Macromedia, San Francisco, CA) software. The images were corrected for contrast, brightness, and color balance.
Injection sites
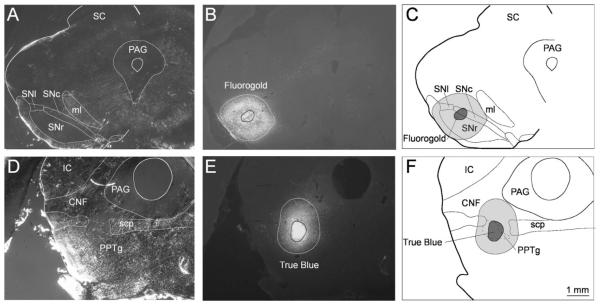
All injection sites were determined by taking two photographs containing the largest manifestation of the tracer injection (Fig. 1). The first picture (Fig. 1A,D) was taken with polarizing filters in the light path, which highlighted the brain’s internal structures and fiber tracts. The second picture (Fig. 1B,E) was taken with the appropriate fluorescence filters in place. From this picture the various zones of the injection site were plotted. Previous experience with the fluorescent retrograde tracers (Redgrave et al., 1986, 1987, 1988, 1990) indicated that reliable retrograde transport occurs mainly from the densely labeled central zone of the injection site, while transport from the surrounding fluorescent halo is minimal (Fig. 1). In the present study, this observation was confirmed in several control cases (not illustrated) where the central zone of the Fluorogold injection was centered ventrally in SN pars reticulata and the surrounding halo extended dorsally covering large parts of SNc. In these examples SC cells retrogradely labeled with Fluorogold were largely absent.
Fig. 1.
Examples of injection site reconstruction. A-C: The reconstruction of a Fluorogold injection into SNc. D-F: An injection of True Blue into the pedunculopontine nucleus. For sections containing the largest dimensions of the injection site, structure boundaries were identified from a photomicrograph taken with polarizing filters in the light path (A,D). Zones of the injection sites were then estimated from a second picture taken with the appropriate fluorescence filters in place (B,E). Illustrations of the injection sites represent a combination of the two plots (C,F). CNF, cuneiform nucleus; IC, inferior colliculus; ml, medial lemniscus; PAG, periaqueductal gray; PPTg, pedunculopontine nucleus; SNc, SNl, and SNr: substantia nigra pars compacta, lateralis and reticulata, respectively; SC, superior colliculus; scp, superior cerebellar peduncle. Scale bar = 1 mm.
Retrograde tracing
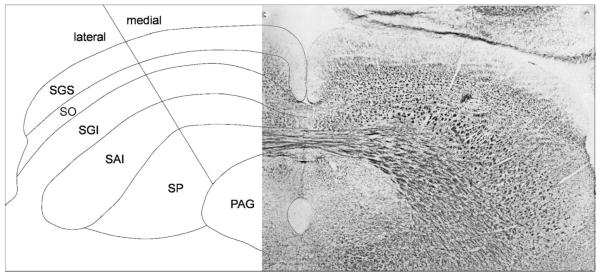
Three coronal sections through the SC separated by ~0.5 mm (equivalent to −6.3, −6.8, and −7.3 mm caudal to bregma in the atlas of Paxinos and Watson, 1986) were selected for detailed analysis. A series of pictures were taken at high magnification (100×) with the Spot-2 digital camera. The pictures were imported into a graphics program (Macromedia FreeHand) and montaged to provide an entire photographic representation of the SC on one side of the brain. Using the graphics facilities of Free-Hand, the outlining borders and layers of the SC were drawn over the montage. The scheme used for layering within the rodent SC is the one used previously in this laboratory and is based on the morphometric study of Albers et al. (1988) (i.e., SGS, stratum griseum superficiale; SO, stratum opticum; SGI, stratum griseum intermediate; SAI, stratum album intermediate; SP, stratum profunda). Each layer was then bisected into a medial and a lateral zone as described previously by Telford et al. (1996). The resulting subdivisions of the SC are illustrated in Figure 2. For each section, single- and double-labeled tectonigral cells that appeared in each SC subdivision were plotted using a combination of the underlying photomontage and visual inspection of the tissue with the fluorescence microscope. Also, for each of the SC subdivisions in each section, the number of single-labeled cells containing blue dye transported from one of the other SC output projections was, in most cases, counted but not plotted. However, the regional distribution of True Blue-labeled cells was recorded for the central section (AP −6.8) of the SC of illustrated cases and included in Figure 5. Because the sectors of the SC differ in area, a density measure of single and double retrograde labeling was calculated (cells/mm2) for comparative purposes. The area of each sector was measured using the area-tool in the Spot 2 image processing and analysis software (Diagnostic Instrument).
Fig. 2.
Subdivisions of the superior colliculus. The collicular layers (left) are identified as a reflected schematic of a photomicrograph of the superior colliculus (right) in which myelinated fibers appear dark. The medial/lateral subdivision approximates the horizontal meridian in the collicular map of visual space. SGS, stratum griseum superficiale; SO, stratum opticum; SGI, stratum griseum intermediate; SAI, stratum album intermediate; SP, stratum profunda.
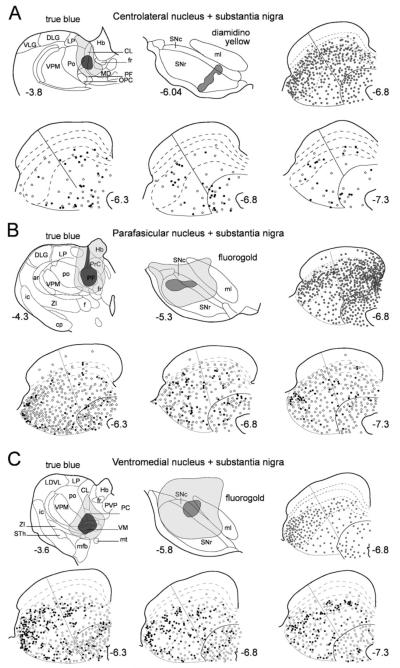
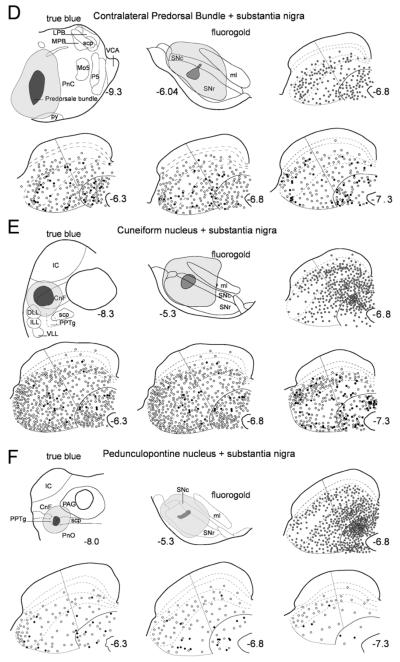
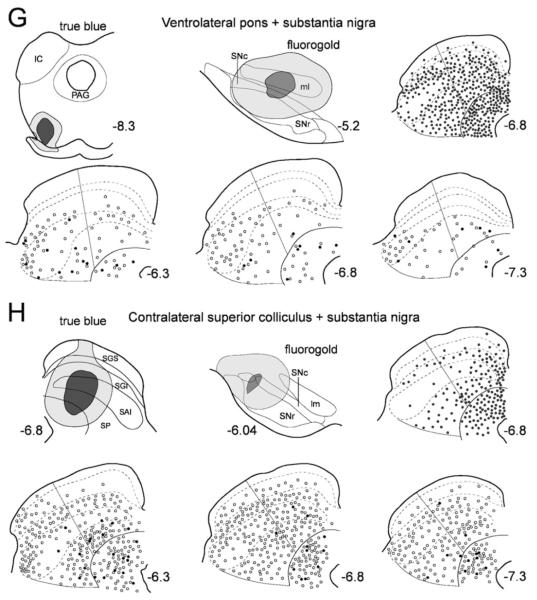
Fig. 5.
Representative examples of retrograde labeling in the superior colliculus following combinations of injections into substantia nigra and targets of the other major collicular efferent projections. Each case is represented in a subsection of the figure (A–H) comprising six panels that are organized as follows. Top left: a schematic illustration of the True Blue injection site in one of the target regions of the major ascending or descending projections of the superior colliculus. Top central: a schematic representation of the nigral injection site. Top right: a single section from central superior colliculus illustrating the distribution of cells retrogradely labeled from the True Blue injection site (one gray dot represents five labeled cells). Bottom three panels contain rostral (AP6.3), central (AP6.8), and caudal (AP7.3) sections of the superior colliculus on which single- (one open symbol represents five labeled cells) and double-labeled (one filled symbol represents one double-labeled neuron) tectonigral cells are plotted. A–C: ar, acoustic radiation; CL, centrolateral thalamic nucleus; cp, cerebral peduncle, basal part; DLG, dorsal lateral geniculate nucleus; f, fornix; fr, fasciculus retroflexus; Hb, habenula nucleus; ic, internal capsule; LDVL, laterodorsal thalamic nucleus ventrolateral; LP, Lateral posterior nucleus; MD, mediodorsal thalamic nucleus; mfb, medial forebrain bundle; mt, mammillothalamic tract; OPC, oval paracentral thalamic nucleus; PC, paracentral thalamic nucleus; PF, parafascicular thalamic nucleus; Po, posterior thalamic nucleus group; PVP, paraventricular thalamic nucleus posterior; PrC, precommissural nucleus; STh, subthalamic nucleus; VLG, ventral lateral geniculate nucleus; VM, ventromedial thalamic nucleus; VPM, ventral posteromedial thalamic nucleus; ZI, zona incerta. D–F: CnF, cuneiform nucleus; DLL, dorsal nucleus of lateral lemniscus; ILL, intermediate nucleus of lateral lemniscus; IC, inferior colliculus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; Mo5, motor 5 nucleus; P5, peritrigeminal zone; PnC, pontine reticular nucleus; PPTg, pedunculopontine tegmental nucleus; py, pyramidal tract; scp, superior cerebellar peduncle; VCA, ventral cochlear nucleus; VLL, ventral nucleus of lateral lemniscus. G–H: IC, inferior colliculus; PAG, periaqueductal gray. In all cases, abbreviations for the collicular layers see legend to Figure 2.
Data analysis
Statistical analyses were conducted, first to characterize the distribution of tectonigral cells of origin within the SC, then to provide a comparative evaluation and analysis of the distribution of double-labeled cells arising from the various combinations of Fluorogold and True Blue injections.
Regional distribution of tectonigral neurons
Cases were selected for this analysis when the Diamidino Yellow/Fluorogold injection site was localized in SNc, although encroachment of the injection site into underlying SN pars reticulata was accepted. This was because anterograde tracing data show that projections from SC to SN pars reticulata are minor (Comoli et al., 2003) and may provide input to the population of ventrally displaced DA neurons (Gonzalez-Hernandez and Rodriguez, 2000). Quantitative differences in the regional distribution of tectonigral cell densities (single label yellow + double-labeled neurons) within the SC subdivisions were analyzed using an analysis of variance (ANOVA) with the following three factors:
“Rostro-caudal” factor with 3 levels (−6.3, −6.8, −7.3);
“Medio-lateral” factor with 2 levels (medial or lateral);
“Layers” factor 5 levels (SGS, SO, SGI, SAI, and SP).
The Fisher post-hoc test was used to analyze significance between specified levels of the factors that underlie significant main effects in the ANOVA.
Regional distribution of double-labeling
Cases were selected for this analysis according to the following criteria: 1) There was robust retrograde labeling with True Blue in the SC confirming that the “other” projection was well-labeled. This maximized the likelihood of observing double-labeling in tectonigral neurons, if axon branching was present. 2) In cases where sparse double-labeling was observed, encroachment of the nigral injection site from SNc into overlying tissue was accepted. In such cases the largely negative result could only reflect an overestimation of the degree of collateralization of the tectonigral projection. 3) In cases where significant numbers of double-labeled cells were found, the injection of yellow dye into SNc had to be confined to pars compacta, with or without encroachment into underlying pars reticulata. These criteria were adopted to maximize the number of useful cases. The relative proportions (%) of single- and double-labeled tectonigral cells were determined for each of the SC subdivisions. A quantitative analysis of the percentage of double-labeled cells in cases with injections into the SC’s ascending and descending projections was conducted using the Mann–Whitney U-test. A subsequent multiple factor ANOVA was used to compare the distributions of double-labeled cells associated with ascending and descending projections, according to their location in the SC (in terms of layer, mediolateral, and rostrocaudal position).
RESULTS
Injection sites
Our use of electrophysiological recording procedures to guide the final location of injections into SNc ensured that, in most cases, at least part of the central zone of the nigral injection was located in pars compacta (Figs. 1, 5). Although the nigral injection sites involving Diamidino Yellow (e.g., Fig. 5A) were more restricted than those of Fluorogold (e.g., Fig. 5B–H), the Diamidino dye was associated with significantly fewer retrogradely labeled in the SC (Diamidino Yellow, mean density = 26.5 cells/mm2; Fluorogold, mean density = 69.7 cells/mm2;F = 44.7; P < 0.0001). However, the relative distribution of retrogradely labeled cells among the different regional sectors of the SC was similar for both tracers. Although considerably larger, the True Blue injections were also well directed to their intended targets (Fig. 5). In most cases, very large numbers of neurons retrogradely labeled with True Blue were found in the SC, indicating that the relevant efferent projection had been well-labeled (Fig. 5).
Identification of retrogradely labeled neurons
As Diamidino Yellow is a nuclear stain (Keizer et al., 1983), cases of double-labeling with this tracer were revealed in the form of a white nucleus surrounded by blue neuronal cytoplasm (Fig. 3A). When double-labeling was produced by combined injections of Fluorogold and True Blue, which are both cytoplasmic stains, judgments had to be made between single- (yellow or blue) and double-labeled (white) neurons (Fig. 3B,C). Given that the purpose of the study was to determine the extent of axonal branching in the tectonigral projection system, together with the consequent size differences in the yellow and blue injections, we saw no advantage of plotting the distributions of cells single-labeled with True Blue on the same diagram. Finally, so that the distribution of double-labeled neurons was not overwhelmed, single-labeled tectonigral neurons were plotted using a ratio of one dot for five labeled neurons, while each double-labeled neuron is presented by a single dot (Fig. 5).
Fig. 3.
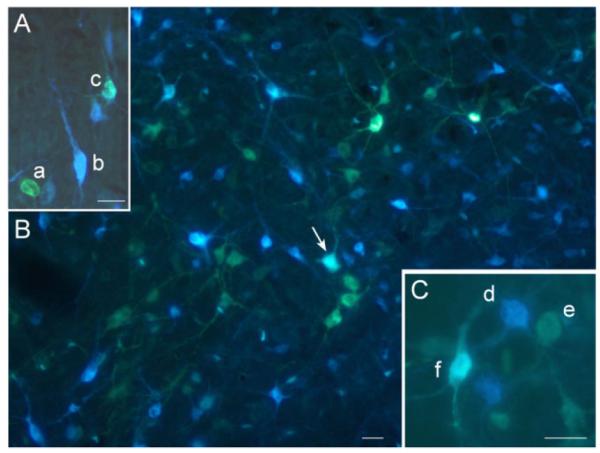
Examples of collicular neurons, single- and double-labeled with the retrograde tracers used in the present study. A: A photomicrograph showing a single Diamidino Yellow (a), a single True Blue (b), and double retrogradely labeled neuron (c). B: A lower-power photomicrograph showing a larger population of single Fluorogold, single True Blue, and a double-labeled neuron (arrow). C: A photomicrograph showing a single True Blue (d), a single Fluorogold (e), and double-retrogradely labeled neuron (f). Scale bars = 20 μm.
Regional distribution of tectonigral neurons
Of the animals used in this study (n = 29), 21 were included for the analysis of the regional distribution of tectonigral neurons in the SC. As intended, the injection sites of these animals were mainly localized in the central and lateral part of SNc (n = 17), with fewer located more medially (n = 4). The main results of the distribution analysis were as follows: 1) The numbers of retrogradely labeled tectonigral cells varied significantly across the layers of the SC (Fig. 4) [Layers factor: F = 91.661; P < 0.0001]. They were mainly concentrated in the intermediate and deep layers, with very few retrogradely labeled neurons found in the superficial layers. 2) Significantly larger numbers of tectonigral neurons were found in lateral sectors of the SC [Medio-Lateral factor: F = 37.129; P < 0.0001]. 3) No reliable differences were found between the numbers of tectonigral cells in different rostrocaudal sections of the SC (F = 1.959; P > 0.10). 4) A significant interaction between the Layers and Medio-Lateral factors [Interaction: F = 4.287; P < 0.01] indicates that more labeled neurons were found in lateral sectors only for the intermediate layers (Fig. 4).
Fig. 4.
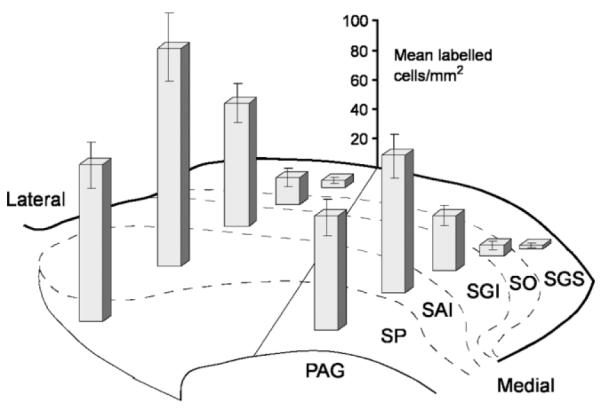
Regional distribution of retrogradely labeled tectonigral cells in different sectors of the superior colliculus. The bars illustrate the average density in each sector (mean labeled cells/mm2 ± SEM, n = 21 cases) of singleplus double-labeled cells after injections of either Diamidino Yellow or Fluorogold into substantia nigra. For collicular layers abbreviations, see legend to Figure 2. PAG, periaqueductal gray.
Double-labeled cells
Although for each of the major efferent projections we have several confirming cases, the double-labeling results are presented on a typical case basis with the example best representing the group selected for presentation. Thus, the selected examples all had large numbers of retrogradely labeled blue cells in the SC combined with well-restricted injections of yellow dyes into SNc. It should be noted that the total numbers of retrogradely labeled cells in the SC refer to the number of single-labeled, plus double-labeled cells counted on the three selected sections of the SC (−6.3, −6.8, and −7.3) for any specific injection of True Blue or Diamidino Yellow/Fluorogold.
1. Ascending projections
Central-lateral nucleus (Fig. 5A)
This case contained a large injection of True Blue that was centered on the rostral central-lateral thalamic nucleus, with a small encroachment into the adjacent mediodorsal thalamus. This injection labeled 3,478 cells in the selected sections of the SC. These cells were spread over the intermediate and deep layers of the SC, with a tendency to concentrate in SGI. Because the central lateral nucleus is principally targeted by the medial SC (Krout et al., 2001), together with the with medial–medial topography in the tectonigral projections (Comoli et al., 2003), this injection was combined with an injection of Diamidino Yellow into medial SN, which involved medial parts of pars reticulata extending dorsally into pars compacta. This small injection labeled a relatively small population of 389 SC neurons. The distribution of the tectonigral cells in this case was similar to that of the general tectonigral population (Fig. 4). However, even within this small population of retrogradely labeled cells, a relatively high proportion were double-labeled (18.4% overall), rising to 26.5% in the region containing the highest proportion of double-labeled cells (lateral SAI).
Rostral thalamic injections (additional cases, not illustrated)
An injection, similar in many respects to the central-lateral injection described above but displaced ~0.5 mm medially into the medial dorsal nucleus, gave rise to substantially fewer (2,061) retrogradely labeled cells in the SC. This injection was combined with an injection of Fluorogold into lateral SNc. While this injection retrogradely labeled significantly more tectonigral cells (2,390), a much lower proportion were double-labeled (4.7%, cf. 18.4% for the case illustrated in Fig. 5A). A second control case contained a True Blue injection that was displaced medially and dorsally into the lateral posterior thalamic region, and an injection of Diamidino Yellow centered on the medial SN. Again, in this case the thalamic injection produced numerous retrogradely labeled cells in the SC (5,401), although this time with a heavy concentration in the superficial layers. However, the cells in the intermediate and deep layers contained a far lower proportion of double-labeled tectonigral neurons (2.0%).
Parafasicular nucleus (Fig. 5B)
This animal received a large injection of True Blue into the caudal parafasicular thalamic nucleus, with some encroachment of the injected dye into the overlying anterior pretectum. This injection produced extensive retrograde labeling throughout the SC (5,400), including the medial superficial layers, presumably as a result of the injection involving the pretectum (Lieberman et al., 1985; Kubota, 1989). The thalamic injection was combined with one of the larger injections of Fluorogold, the central zone of which was confined to the lateral substantia nigra including both pars compacta and pars reticulata. The retrograde transport from the nigral injection extended throughout the SC, with some concentration in the rostrolateral intermediate layers. Together, the two injections produced a lower level of double-labeled tectonigral cells (6.0%), but again with a clear concentration in the lateral SAI sector (14.9%).
Caudal thalamic injections (other cases not illustrated)
Two other animals had injections of True Blue into the parafasicular thalamus combined with injections of Fluorogold into SNc. In both cases, similar proportions of double-labeled cells to those described for the case in Figure 5B were observed (4.3% and 8.6%).
Ventromedial nucleus (Fig. 5C)
A large injection of True Blue was centered on the ventromedial nucleus of thalamus, with some spread dorsally into the ventral posterior and paracentral nuclei. Although this injection was at least as large as those in the previous illustrated cases (Fig. 5A,B), considerably fewer SC cells were retrogradely labeled (2,338), of which about half were concentrated in the lateral SAI sector. This injection was combined with an injection of Fluorogold whose central core was confined to the lateral substantia nigra pars compacta. Again the overall distribution of retrogradely labeled cells in the SC conformed to that of the general tectonigral population (Fig. 4). Moreover, this case had both the highest overall proportion (21.3%) and the highest regional proportion (29.1%) of double-labeled cells (lateral SAI) observed in the present study.
2. Crossed descending projection
Contralateral predorsal bundle (Fig. 5D)
A large injection of True Blue was placed into descending fibers of the contralateral predorsal bundle at the level of the pontine reticular formation. This injection labeled 2,008 cells in the SC, with a clear concentration in lateral SAI. The injection of Fluorogold in this case was in dorsal SNr extending into lateral SNc. Significant numbers (1,940) of retrogradely labeled tectonigral cells, again concentrated in lateral intermediate layers, were also observed in this case. However, compared with the ascending projection examples (see above), a far smaller proportion of double-labeled neurons was observed (3.2%), with the maximum area for double-labeling (lateral SAI) only reaching 7.2% of tectonigral cells double-labeled. Of particular note was that none of the large and numerous tecto-reticulo-spinal neurons identified in this and previous studies by the injection of True Blue into the brainstem (Redgrave et al., 1986; Bickford and Hall, 1989) were double-labeled with Fluorogold.
Crossed descending projection (other cases not illustrated)
Nigral injections of either Diamidino Yellow or Fluorogold were combined with True Blue injections into the contralateral ventromedial pontine reticular formation, medial medulla (n = 3), and periabducens area. Despite the presence of numerous cells singly labeled with either of the two dyes, the overall proportion of double-labeled tectonigral neurons in each case never exceeded 1%.
3. Ipsilateral descending projection
Cuneiform nucleus (Fig. 5E)
A large injection of True Blue centered on the cuneiform nucleus produced a high density of retrogradely labeled cells concentrated in the SGI and SP layers of the SC. This injection was combined with an injection of Fluorogold centered on the lateral part of SNc. Despite large numbers of singly labeled blue (3961) and yellow cells (3630) in the SC, only 2.6% of the tectonigral cells were double-labeled. This result was confirmed by a second case involving an injection into the cuneiform nucleus in which even fewer (0.2%) tectonigral neurons were double-labeled (not illustrated).
Pedunculopontine tegmental nucleus (Fig. 5F)
An injection of True Blue centered on the pedunculopontine nucleus retrogradely labeled many cells in the SC (4,285). These neurons were mainly distributed in SGI and SP, with a clear concentration in the medial SC. This injection was combined with a smaller injection of Fluorogold into lateral substantia nigra, producing retrogradely labeled cells (890) throughout the SC, with the typically higher concentration in lateral SAI. Again, only a comparatively small proportion of double-labeled cells were recorded in this case (2.7%). This result was confirmed in a further two cases (not illustrated) in which 3.6% and 2.6% of tectonigral neurons were double-labeled.
Ventrolateral pons (Fig. 5G)
A large injection of True Blue was placed in the SC ipsilateral descending efferent fibers and terminal zones in the ventrolateral pons. Again, a large amount of retrograde labeling was observed (4,502) in SGI and SP of the ipsilateral SC. This injection was combined with an injection of Fluorogold, the central zone of which was located slightly more dorsally in SNc and extended into overlying fibers of the medial lemniscus. Note that this injection was associated with a smaller than normal number of retrogradely labeled tectonigral cells (890), although again their distribution was similar to that of the general population (Fig. 4). As with the previous cases involving targets of the SC’s ipsilateral descending projection, only a small proportion of the tectonigral cells were double-labeled (2.7%).
Parabigeminal nucleus (not illustrated)
A small injection of True Blue into the ipsilateral parabigeminal nucleus produced an enormous number of retrogradely labeled cells across all layers of the SC (6,155). This injection was combined with an injection of Diamidino Yellow into the lateral SNc. Again, despite significant levels of single-labeling, only very few (0.5%) tectonigral cells were double-labeled.
4. Tecto-tectal projection
Contralateral SC (Fig. 5H)
A large injection of True Blue was placed medially in the contralateral SC with the intention of involving a large proportion of the crossing tecto-tectal fibers. This injection produced a substantial number (1,639) of retrogradely labeled cells that were concentrated in medial SGI and SP. The injection of Fluorogold into the lateral substantia nigra produced a total of 2,690 tectonigral cells whose distribution conformed to that of the general population (Fig. 4), and of which only 0.7% were double-labeled. This result was replicated in a further two cases (not illustrated) in which the double-labeling of tectonigral neurons was 1.9% and 0.9%, respectively.
Quantitative analysis of double-labeled cells
To assess the difference in levels of double-labeling associated with the ascending and descending SC projections, we compared the overall percentages of double-labeled cells in all the above cases (ascending cases n = 7; descending cases n = 16). A nonparametric statistical analysis confirmed that a significantly higher proportion of double-labeling was associated with combinations of nigral and ascending projection injections (ascending projection mean = 9.3%; descending projection mean = 1.5%: Mann–Whitney U = 6.0; P = 0.0008). Note that the comparatively low overall mean values, especially for the ascending projections, reflects the inclusion of all regions of the SC (Fig. 2), some of which were sparsely populated with double-labeled neurons, and cases where the True Blue injection was not ideally centered on SC target zones.
To test whether the distribution of double-labeled cells within the SC subregions varied systematically, we confined our analysis to the two groups of illustrated cases that contained the best targeted injection sites: 1) those with True Blue injections into targets of the ipsilateral ascending projections (cases in Fig. 5A–C); and 2) those with injections into the descending projection fibers and targets of the SC (cases in Fig. 5D–H). Because the superficial layers contained no double-labeled cells, they were excluded from the analysis. Figure 6 presents the mean numbers of double-labeled tectonigral cells in the different sectors of the SC for ascending and descending projection groups. The multifactorial ANOVA confirmed that the ascending projection group was associated with significantly higher levels of double-labeling [Group factor: F = 38.94; P < 0.0001]. The overall distribution of the double-labeled cells in SC for both groups varied as follows: i) significant differences in the numbers of double-labeled cells were observed across the SC layers [Layer factor: F = 8.725; P < 0.01], with the highest density found in SAI; ii) significantly larger numbers of double-labeled cells were encountered in lateral sectors of SC [Medio-Lateral factor: F = 11.422; P < 0.001]; iii) no reliable differences were found within the different rostrocaudal sections of SC. Two interactions between Group and Layers factors [F = 5.038; P < 0.01] as well as between Group and MedioLaterality factors [F = 5.03; P < 0.05] revealed subtle differences in the distributions of double-labeled cells between the ascending and the descending projection groups. The distribution of double-labeled cells for the ascending projection group was similar to that of the overall distribution of tectonigral cells with the high density in the lateral SAI sector. In contrast, the double-labeled cells of the descending projection group were more evenly distributed among the regional sectors of the SC (Fig. 6).
Fig. 6.
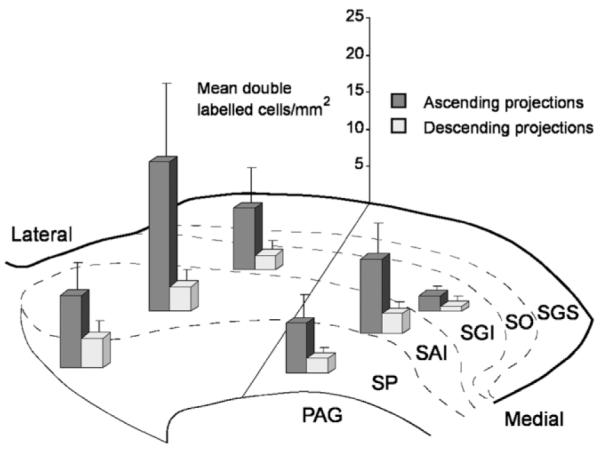
Regional distribution of double-labeled tectonigral cells in different sectors of the intermediate and deep layers of the superior colliculus. The bars illustrate the average density of cells in each sector (mean labeled cells/mm2 ± SEM) for cases in which the nigral injection was combined with an injection into thalamic targets of the ipsilateral ascending projections (n = 3) and into the descending projections (n = 5). Abbreviations: see legend to Figure 4.
DISCUSSION
In this study we used retrograde double-label anatomical tracing to investigate whether the recently discovered tectonigral pathway (Comoli et al., 2003) is an independent output pathway of the SC or a collateral branch of one or more of its other major output projections (May, 2006). Our principal findings were: 1) substantial numbers of tectonigral neurons were double-labeled when nigral injections were combined with second tracer injections into the thalamus; and 2) comparatively few double-labeled neurons were found with combined injections into SN and any of the fiber pathways or targets of the SC’s descending projections to the brainstem or the contralateral SC. We will consider these findings first in terms of the methodological issues that could influence their interpretation. We will then discuss our results in terms of the previous anatomical literature and conclude with an evaluation of their functional implications.
Methodological considerations
The fluorescent double-labeling retrograde tracing technique has proven useful to demonstrate branching axonal architectures in different areas of brain (i.e., Kuypers et al., 1980; Cavada et al., 1984; Gonzalo-Ruiz and Leichnetz, 1987; Conde et al., 1990; Feger et al., 1994; Nogueira et al., 2000; Erro et al., 2002) including those associated with the SC (Redgrave et al., 1986, 1987, 1988, 1990; Sahibzada et al., 1987; Bickford and Hall, 1989). Combinations of fluorescent dyes like Diamidino Yellow or Fluorogold with True Blue, all of which have been shown to be efficient retrograde markers (Illert et al., 1982; Keizer et al., 1983; Conde, 1987; Payne and Peace, 1989; Richmond et al., 1994; Novikova et al., 1997; Lanciego et al., 1998) produce single-labeled (yellow or blue) or double-labeled cells (white) that can be viewed simultaneously using the same UV excitation filter (Kuypers et al., 1980). However, the use of fluorescent dyes for retrograde double-labeling is not without difficulty, and some of the most prevalent issues can be itemized as follows.
The efficiency of uptake and retrograde transport of injected dyes can be variable both for an individual tracer and also between tracers (Illert et al., 1982; Haase and Payne, 1990; Gunturkun et al., 1993; Richmond et al., 1994). In the present study, potential variability in uptake and transport of dyes was assessed through the use of confirming cases. Thus, the multiple injections of Diamidino Yellow and Fluorogold into the SN that differed slightly in size, location, and efficiency of uptake were associated with a reliable pattern of retrograde labeling in the SC (Fig. 5A–H).
A problem common to most anatomical tracing is the variable uptake and transport of tracers by individual neuronal elements. Thus, in the present study the intensity of emitted blue and yellow fluorescence by retrogradely labeled cells in the SC varied from intense to barely detectable (Fig. 3). The variable combinations of variable intensities of the two tracers in double-labeled cells means that the process of identifying double-labeled cells can be quite difficult and is necessarily subjective.
Again, a problem that affects some tracers more than others is the potential uptake and transport of the dye from the injection site by nonterminating fibers of passage (Skirboll et al., 1989). In the present study we used this feature to label simultaneously many components of the SC’s crossed descending projection. Thus, effective transport to the SC was recorded when True Blue was injected directly into fibers of the PDB at the level of the medial pontine reticular formation (Fig. 5D) (see also Redgrave et al., 1986). On the other hand, it has been reported that some of the forward projecting fibers from the SC to the rostral intralaminar nuclei traverse regions of the caudal intralaminar thalamus (parafasicular nucleus) (Chevalier and Deniau, 1984). Although it is uncertain whether such fibers also terminate at the level of the parafasicular nucleus, we cannot rule out the possibility that some of the retrograde labeling seen after injections into the caudal thalamus derives from uptake into rostrally projecting, nonterminating fibers of passage.
Perhaps the most serious problem when estimating the proportion of collateral branching within a projection architecture occurs when the pathways to relevant target regions are topographically organized. To establish a true value for both target regions, tracer uptake should be limited to precisely equivalent target zones (which may or may not be the same size/volume in different target structures) of a given population of projection neurons. Clearly, this ideal is rarely (never) achieved. Consequently, the levels of collateralization reported in many studies invariably underestimate the true levels of axon branching. In the present investigation, two strategies were used to minimize this problem: i) The precise location of injection sites was selected according to known projection topographies. For example, the central lateral nucleus has been identified as one of the main thalamic targets of neurons in the medial SC (Krout et al., 2001). Also, we have previously described a medial–medial, lateral–lateral topography in the tectonigral pathway (Comoli et al., 2003). For present purposes, we therefore combined injections of True Blue into the central lateral nucleus with injections of yellow dye into medial SN (Fig. 5A). ii) Wherever possible, the injections of True Blue into target regions or fibers of the main output pathways of the SC were sufficiently large to “fill” the intended structure. Thus, when combined with much smaller subtotal injections into SNc, a better estimate of the extent of the axonal branching of identified tectonigral neurons would be ensured.
Despite these methodological caveats, the present study has established reliable differences in the levels of collateralization between the tectonigral projection and the major ascending and descending projections of the SC. Potentially high levels of axonal branching occurs between SC projections to SNc and various targets in the thalamus, while very little is associated with the SC’s descending projections to the brainstem. However, to be confident of this conclusion it is necessary that all our tracer injections were correctly positioned in major target structures of the SC. We shall therefore consider the present retrograde tracing data in the context of previous reports of efferent projections from the SC.
Comparison with previous tracing studies
Tectonigral projection
Using the fluorescent tracers Fluorogold and Diamidino Yellow, we have shown that tectonigral neurons have a regional distribution that largely excludes retrograde labeling of the SC superficial layers (Fig. 4). Their distribution in the intermediate and deep layers accords well with the original description of the tectonigral projection in rat in which Cholera Toxin subunit b was the retrograde tracer (Comoli et al., 2003). The observed rostrolateral bias of rat tectonigral neurons (Fig. 4) corresponds to regions of the SC’s somatosensory topography that represent the vibrissae (Bruce et al., 1987; Grunwerg and Krauthamer, 1990; Kleinfeld et al., 1999). In more visually based species such as the cat (McHaffie et al., 2005) and monkey (May, Stanford, McHaffie, Jaing, Coizet, Redgrave, and Haber, unpubl. obs.), a lateral bias in the distribution of tectonigral neurons is largely absent.
Ascending projections
Ascending projections from the SC to target regions in the thalamus have been established with tract-tracing techniques in a wide range of vertebrate species (Harting et al., 1980, 2001; Lysakowski et al., 1986; Abramson and Chalupa, 1988; Berson and Graybiel, 1991; Butler, 1994; Veenman, 1997) including rat (Chevalier and Deniau, 1984; Yamasaki et al., 1986; Kobayashi and Nakamura, 2003). Of particular relevance to the current investigation was the detailed retrograde tracing study of Krout et al. (2001) in which small injections of Cholera Toxin subunit b were into individual midline and intralaminar nuclei. In general terms, relevant results of the present study accord well with those reported by Krout et al. (2001), with the widespread and dense labeling of the SC’s intermediate and deep layers showing that much of the SC’s input to the thalamus will have been included in the uptake zone of the relevant injection sites.
Descending projections
The SC has two major descending projection systems (the ipsilateral and contralateral pathways), both of which contact precerebellar and premotor nuclei in the brainstem (Huerta and Harting, 1984; Dean et al., 1986, 1988; Redgrave et al., 1986, 1987; Grantyn, 1988; Yeomans and Tehovnik, 1988; Mitchell et al., 1988; Masino and Knudsen, 1992; Shehab et al., 1995; Grantyn and Moschovakis, 2004). The cells of origin giving rise to these projections in rat are largely segregated and differentially distributed within the SC (Redgrave et al., 1986; Bickford and Hall, 1989). Neurons giving rise to the crossed descending projection are located predominantly in SAI, and are sandwiched between the cells of origin of the ipsilateral descending projection in SGI and SP layers. These differential distribution patterns are also clearly evident in the present data (compare Fig. 5D with 5E–G), and in all cases the dense retrograde labeling of the SC suggests that the intended projections were comprehensively filled.
Tecto-tectal projection
Previous tracing data (Edwards, 1977; Behan, 1985; Sahibzada et al., 1987; Appell and Behan, 1990; Olivier et al., 1998, 2000) indicate that the sites of termination of the tecto-tectal projection in the contralateral SC mirrors the locations of the cells of origin. In the present study, our intention was to place injections in the medial contralateral SC to facilitate tracer uptake into local terminals and fibers passing laterally through the injection site. Unfortunately, uptake of True-Blue into nonterminating fibers projecting to the opposite lateral SC was less effective than that achieved when the tracer was injected into the contralateral predorsal bundle. Consequently, only sparse retrograde labeling of the SC’s lateral sectors was observed (Fig. 5H). Following combined injections into the contralateral SC and SNc, low levels of double-labeling in medial regions of the SC were recorded; however, at the present time we cannot be certain this is the case in the SC’s lateral sectors.
Organization of ascending projections from the superior colliclus
The main finding of the present study was that significantly more axonal branching was found to occur between tectonigral fibers and ipsilateral ascending projections of the SC than with its descending projections to the brainstem. Within certain subregions of the SC (principally the lateral SAI sector) more than 25% of tectonigral neurons were double-labeled (18.4% overall) following a combination of injections into the central lateral nucleus of the thalamus and SNc. For the injection into the parafasicular nucleus the proportion of double-labeled tectonigral neurons in SAI was close to 15%, while in the case of the ventromedial nucleus it was nearly 30%. These figures raise the question of whether the double-labeling associated with different projection targets in the thalamus should be considered as separate or overlapping. If they are separate, and ascending projections from the SC to the central lateral, parafasicular, and ventromedial nuclei arise from different populations of SAI neurons, the proportions of double-labeled cells could add to give a total of ~70%. However, the extent to which tectonigral collateral projections to the different thalamic targets are themselves branched would reduce this figure. At present a detailed knowledge of tecto-thalamic architecture is unavailable (McHaffie et al., 2005) and we have work currently in progress to resolve this issue. However, once resolved, the value of conducting a more technically demanding triple-label study involving injections of retrograde tracers into the SNc and two of the identified thalamic targets of the SC would become clear.
Functional implications
Midbrain DA neurons are known to exhibit short-latency, short-duration phasic responses specifically to the onset of unpredicted, multisensory, biological salient events (visual, auditory, and tactile) (Schultz, 1998). These reliable sensory responses occur very generally across species and experimental situations. In contrast, the activity of DA neurons appears largely unrelated to motor-related events (Schultz, 1998). Because of this specific sensitivity to sensory stimuli, we have investigated the source of the short-latency sensory input to DA neurons (Coizet et al., 2003, 2006; Comoli et al., 2003; Dommett et al., 2005; McHaffie et al., 2005). In the case of vision, it appears that the SC is the primary if not exclusive source of short-latency afferent signals. It is within this context that the present results have several important functional implications: 1) Our finding that comparatively few identified tectonigral neurons were double-labeled following True Blue injections into any of the descending fiber systems or brainstem targets of the SC is entirely consistent with the electrophysiological findings that DA neuronal activity appears unrelated to motor output (Schultz, 1998). This is because the SC’s projections to the brainstem carry the motor signals responsible for gaze-shifts toward, or away from, unexpected biologically salient events (Grantyn, 1988; Dean et al., 1989; Grantyn and Moschovakis, 2004; May, 2006). 2) Many of the descending fibers that carry motor information from the SC to the brainstem have an ascending collateral that terminates in the thalamus (Chevalier and Deniau, 1984; Moschovakis and Karabelas, 1985; Redgrave et al., 1986; Moschovakis et al., 1988; Bickford and Hall, 1989). It is widely assumed (Yamasaki, 1986; McHaffie et al., 2005) that these collaterals carry “efference copies” of gaze-related motor commands issued to the brainstem. The present finding that comparatively few of the sensory dominated tectonigral neurons are double-labeled by injections into the brainstem suggests, by inference, that the tectothalamic pathway contains sensory-components that are unrelated to motor output. 3) The finding that in some subregions of the SC possibly up to 70% of the tectonigral cells could be part of a projection system with branched axons that contact both DA cells in SNc (Comoli et al., 2003) and those regions of the thalamus that contain thalamostriatal projection neurons (Takada et al., 1985; Mengual et al., 1999; Gimenez-Amaya et al., 2000; Van der Werf et al., 2002; Kimura et al., 2004; McHaffie et al., 2005) is particularly significant. It suggests that shortlatency sensory signals originating from the SC could be directed simultaneously to two of the principal input stations of the basal ganglia; indirectly via the thalamus to the striatum and directly to SNc (Fig. 7) (Matsumoto et al., 2001; Minamimoto and Kimura, 2002; Morris et al., 2004; Cragg, 2006). Interestingly, the latencies of visual activity recorded in the striatum (100–250 ms) (Hikosaka et al., 1989; Matsumoto et al., 2001; Morris et al., 2004) suggest that short-latency glutamatergic input from the thalamus (Matsumoto et al., 2001; Minamimoto and Kimura, 2002; Paquet and Smith, 2003; Smith et al., 2004) is likely to be temporally coincident with the phasic DA input from substantia nigra (Wightman and Robinson, 2002; Morris et al., 2004; Roitman et al., 2004; Dommett et al., 2005; Cragg, 2006). Thus, a likely temporal convergence of glutamatergic and DA input to the striatum, both initiated subcortically by the same sensory event, could have important implications for experience-dependent plasticity in the basal ganglia (Calabresi et al., 1997; Arbuthnott et al., 2000; Silkis, 2000; Centonze et al., 2001; Reynolds and Wickens, 2002). Consequently, the processes most likely to be involved are those associated with selecting responses to (Hikosaka, 1994; Mink, 1996; Redgrave et al., 1999a) and learning about the behavioral significance of biologically salient sensory events (Redgrave et al., 1999b; Matsumoto et al., 2001; Horvitz, 2002; Minamimoto and Kimura, 2002; Wickens et al., 2003; Graybiel, 2005).
Fig. 7.

A schematic illustration of the proposed convergence of short-latency phasic inputs to the striatum elicited by an unpredicted visual event. Direct retinal input to the superior colliculus could be redirected, via the branched projections described in the present study, to the intralaminar thalamic nuclei and to the substantia nigra pars compacta. At present, the identity of the neurotransmitter(s) used in these branched connections to substantia nigra and the thalamus are unknown. However, consequent phasic inputs to the striatum from intralaminar nuclei (glutamate) and substantia nigra (dopamine) may converge on specific populations of striatal neurons. The timing of visual responses in the striatum and the latencies of phasic dopaminergic input are appropriate for this proposed convergence (see text for details).
Finally, on a practical note, the possibility of having the same sensory event initiate coincident afferent convergence of glutamatergic and DA input to the striatum could have important implications for how functional magnetic resonance imaging (MRI) studies investigating basal ganglia responses to biologically salient sensory events, including reward, are interpreted (Knutson et al., 2001, 2005; O’Doherty et al., 2001; McClure et al., 2003; Zink et al., 2003; Zink et al., 2004). The possibility that indirect afferent collaterals from the SC to the striatum via the thalamus could act in concert with phasic input from ascending dopaminergic neurons to influence striatal hemodynamic responses to unpredicted visual events should now be considered.
ACKNOWLEDGMENT
The authors thank Natalie Walton for excellent histological assistance.
Grant sponsor: Wellcome Trust; Grant numbers: 068021 (to P.R.), 062742 (to P.O.).
LITERATURE CITED
- Abramson BP, Chalupa LM. Multiple pathways from the superior colliculus to the extrageniculate visual thalamus of the cat. J Comp Neurol. 1988;271:397–418. doi: 10.1002/cne.902710308. [DOI] [PubMed] [Google Scholar]
- Albers FJ, Meek J, Nieuwenhuys R. Morphometric parameters of the superior colliculus of albino and pigmented rats. J Comp Neurol. 1988;274:357–370. doi: 10.1002/cne.902740306. [DOI] [PubMed] [Google Scholar]
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–158. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. J Anat. 2000;196:587–596. doi: 10.1046/j.1469-7580.2000.19640587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M. An EM-autoradiographic and EM-HRP study of the commissural projection of the superior colliculus in the cat. J Comp Neurol. 1985;234:105–116. doi: 10.1002/cne.902340108. [DOI] [PubMed] [Google Scholar]
- Berson DM, Graybiel AM. Tectorecipient zone of cat lateral posterior nucleus—evidence that collicular afferents contain acetylcholinesterase. Exp Brain Res. 1991;3:478–486. doi: 10.1007/BF00230959. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. Collateral projections of predorsal bundle cells of the superior colliculus in the rat. J Comp Neurol. 1989;283:86–106. doi: 10.1002/cne.902830108. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Cardoso SH, Melo LL, Motta V, Coimbra NC. Neural substrate of defensive behavior in the midbrain tectum. Neurosci Biobehav Rev. 1994;18:339–346. doi: 10.1016/0149-7634(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Anseloni VZ, Pandossio JE, DeAraujo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Bruce LL, McHaffie JG, Stein BE. The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol. 1987;262:315–330. doi: 10.1002/cne.902620302. [DOI] [PubMed] [Google Scholar]
- Butler AB. The evolution of the dorsal thalaums of jawed vertebrates, including mammals: cladistic analysis and a new hypothesis. Brain Res Rev. 1994;19:29–65. doi: 10.1016/0165-0173(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Cavada C, Huisman AM, Kuypers HGJM. Retrograde double labeling of neurons: the combined use of horseradish peroxidase and diamidino yellow dihydrochloride (DY.2HCl) compared with true blue and DY.2HCl in rat descending brainstem pathways. Brain Res. 1984;308:123–136. doi: 10.1016/0006-8993(84)90923-5. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Spatio-temporal organization of a branched tecto-spinal/tecto-diencephalic neuronal system. Neuroscience. 1984;12:427–439. doi: 10.1016/0306-4522(84)90063-0. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–281. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Coizet V, Comoli E, Westby GWM, Redgrave P. Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 2003;17:28–40. doi: 10.1046/j.1460-9568.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurons are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Conde F. Further studies on the use of the fluorescent tracers Fast blue and Diamidino yellow: effective uptake area and cellular storage sites. J Neurosci Methods. 1987;21:31–43. doi: 10.1016/0165-0270(87)90100-2. [DOI] [PubMed] [Google Scholar]
- Conde F, Audinat E, Mairelepoivre E, Crepel F. Afferent connections of the medial frontal-cortex of the rat: a study using retrograde transport of fluorescent dyes. 1. Thalamic afferents. Brain Res Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Sahibzada N, Tsuji K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience. 1986;19:367–380. doi: 10.1016/0306-4522(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Dean P, Mitchell IJ, Redgrave P. Responses resembling defensive behaviour produced by microinjection of glutamate into superior colliculus of rats. Neuroscience. 1988;24:501–510. doi: 10.1016/0306-4522(88)90345-4. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GWM. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Edwards SB. The commissural projection of the superior colliculus in the cat. J Comp Neurol. 1977;173:23–40. doi: 10.1002/cne.901730103. [DOI] [PubMed] [Google Scholar]
- Erro ME, Lanciego JL, Gimenez-Amaya JM. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci Res. 2002;42:45–55. doi: 10.1016/s0168-0102(01)00302-9. [DOI] [PubMed] [Google Scholar]
- Feger J, Bevan M, Crossman AR. The projections from the parafascicular thalamic nucleus to the subthalamic nucleus and the striatum arise from separate neuronal populations—a comparison with the corticostriatal and corticosubthalamic efferents in a retrograde fluorescent double-labeling study. Neuroscience. 1994;60:125–132. doi: 10.1016/0306-4522(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Gimenez-Amaya JM, de las Heras S, Erro E, Mengual E, Lanciego JL. Considerations on the thalamostriatal system with some functional implications. Histol Histopathol. 2000;15:1285–1292. doi: 10.14670/HH-15.1285. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Rodriguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol. 2000;421:107–135. doi: 10.1002/(sici)1096-9861(20000522)421:1<107::aid-cne7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Leichnetz GR. Collateralization of cerebellar efferent projections to the paraoculomotor region, superior colliculus, and medial pontine reticular formation in the rat—a fluorescent double-labeling study. Exp Brain Res. 1987;68:365–378. doi: 10.1007/BF00248802. [DOI] [PubMed] [Google Scholar]
- Grantyn R. Gaze control through superior colliculus: structure and function. In: Buttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Elsevier; Amsterdam: 1988. pp. 273–333. [PubMed] [Google Scholar]
- Grantyn AA, Moschovakis AK. Structure-function relationships in the superior colliculus of higher mammals. In: Hall WC, Moschovakis A, editors. Superior colliculus: new approaches. CRC Press; Boca Raton, FL: 2004. pp. 107–145. [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grunwerg BS, Krauthamer GM. Vibrissa-responsive neurons of the superior colliculus that project to the intralaminar thalamus of the rat. Neurosci Lett. 1990;111:23–27. doi: 10.1016/0304-3940(90)90338-a. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Gunturkun O, Melsbach G, Horster W, Daniel S. Different sets of afferents are demonstrated by the fluorescent tracers fast blue and rhodamine. J Neurosci Methods. 1993;49:103–111. doi: 10.1016/0165-0270(93)90114-7. [DOI] [PubMed] [Google Scholar]
- Haase P, Payne JN. Comparison of the efficiencies of true blue and diamidino yellow as retrograde tracers in the peripheral motor system. J Neurosci Methods. 1990;35:175. doi: 10.1016/0165-0270(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Frankfurter AJ, Strominger NL, Royce GJ. Ascending pathways from the monkey superior colliculus: an autoradiographic analysis. J Comp Neurol. 1980;192:853–882. doi: 10.1002/cne.901920414. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, VanLieshout DP. The visual-oculomotor striatum of the cat: functional relationship to the superior colliculus. Exp Brain Res. 2001;136:138–142. doi: 10.1007/s002210000606. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Role of basal ganglia in control of innate movements, learned behavior and cognition—a hypothesis. In: Percheron G, McKenzie JS, Feger J, editors. The basal ganglia IV: new ideas and data on structure and function. Plenum Press; New York: 1994. pp. 589–596. [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J Neurophysiol. 1989;61:799–813. doi: 10.1152/jn.1989.61.4.799. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Connectional organization of the superior colliculus. Trends Neurosci. 1984;7:286–289. [Google Scholar]
- Illert M, Fritz N, Aschoff A, Hollander H. Fluorescent compounds as retrograde tracers compared with horseradish peroxide (HRP). 1. A parametric study in the peripheral motor system of the cat. J Neurosci Methods. 1982;6:199–218. doi: 10.1016/0165-0270(82)90084-x. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG, Huisman AM, Dann O. Diamidino yellow dihydrochloride (DY. 2HCl); a new fluorescent retrograde neuronal tracer, which migrates only very slowly out of the cell. Exp Brain Res. 1983;51:179–191. doi: 10.1007/BF00237193. [DOI] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamostriatal system. Neurosci Res. 2004;48:355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Berg RW, O’Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Motor Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nakamura Y. Synaptic organization of the rat parafascicular nucleus, with special reference to its afferents from the superior colliculus and the pedunculopontine tegmental nucleus. Brain Res. 2003;980:80–91. doi: 10.1016/s0006-8993(03)02921-4. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Mitrofanis J. Patterns of brainstem projection to the thalamic reticular nucleus. J Comp Neurol. 1998;396:531–543. [PubMed] [Google Scholar]
- Krauthamer GM, Krol JG, Grunwerg BS. Effect of superior colliculus lesions on sensory unit responses in the intralaminar thalamus of the rat. Brain Res. 1992;576:277–286. doi: 10.1016/0006-8993(92)90691-2. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD, Westby GWM, Redgrave P. Superior colliculus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2001;431:198–216. doi: 10.1002/1096-9861(20010305)431:2<198::aid-cne1065>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kubota T. Projection from the superficial layers of the tectum to the pretectal complex in the cat. Brain Res Bull. 1989;22:373. doi: 10.1016/0361-9230(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Bentivoglio M, Catsman-Berrevoets CE, Bharos AT. Double retrograde neuronal labeling through divergent axon collaterals, using two fluorescent tracer with the same excitation wavelength which label different features of the cell. Exp Brain Res. 1980;40:383–392. doi: 10.1007/BF00236147. [DOI] [PubMed] [Google Scholar]
- Lagares C, Caballerobleda M, Fernandez B, Puelles L. Reciprocal connections between the rabbit suprageniculate pretectal nucleus and the superior colliculus—tracer study with horseradish peroxidase and fluorogold. Vis Neurosci. 1994;11:347–353. doi: 10.1017/s0952523800001681. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Luquin M, Guillen J, Gimenez-Amaya JM. Multiple neuroanatomical tracing in primates. Brain Res Protoc. 1998;2:323–332. doi: 10.1016/s1385-299x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Lieberman AR, Taylor AM, Campbell G. Axon terminal of the projection from the superior colliculus to the olivary pretectal nucleus in the rat. Neurosci Lett. 1985;56:235–239. doi: 10.1016/0304-3940(85)90135-1. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci. 1999;11:187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R, DeLima AD, Schwegler H, Pape HC. Direct synaptic connections of axons from superior colliculus with identified thalamoamygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 1999;403:158–170. [PubMed] [Google Scholar]
- Lugo-Garcia N, Kicliter E. Superior colliculus efferents to five subcortical visual system structures in the ground squirrel. Brain Res. 1987;426:131–141. doi: 10.1016/0006-8993(87)90432-x. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Standage GP, Benevento LA. Histochemical and architectonic differentiation of zones of pretectal and collicular inputs to the pulvinar and dorsal lateral geniculate nuclei in the macaque. J Comp Neurol. 1986;250:431–448. doi: 10.1002/cne.902500403. [DOI] [PubMed] [Google Scholar]
- Masino T, Knudsen EI. Anatomical pathways from the optic tectum to the spinal cord subserving orienting movements in the barn owl. Exp Brain Res. 1992;92:194–208. doi: 10.1007/BF00227965. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- May PJ. Neuroanatomy of the oculomotor system. Elsevier; Amsterdam: 2006. The mammalian superior colliculus: laminar structure and connections; pp. 321–378. [DOI] [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26:423–428. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet W, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mengual E, de las Heras S, Erro E, Lanciego JL, Gimenez-Amaya JM. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat. 1999;16:187–200. doi: 10.1016/s0891-0618(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Dean P, Redgrave P. The projection from superior colliculus to the cuneiform area in the rat. II. Defense-like responses to stimulation with glutamate in cuneiform nucleus and surrounding structures. Exp Brain Res. 1988;72:626–639. doi: 10.1007/BF00250607. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol. 1985;239:276–308. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. J Neurophysiol. 1988;60:232–262. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Fixation and orientation control by the tectoreticulo-spinal system in the cat whose head is unrestrained. Rev Neurol. 1989;145:567–579. [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Nogueira MI, deRezende BD, doVale LER, Bittencourt JC. Afferent connections of the caudal raphe pallidus nucleus in rats: a study using the fluorescent retrograde tracers fluorogold and true-blue. Ann Anat. 2000;182:35–45. doi: 10.1016/s0940-9602(00)80118-1. [DOI] [PubMed] [Google Scholar]
- Novikova L, Novikov L, Kellerth JO. Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods. 1997;74:9–15. doi: 10.1016/s0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–202. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Olivier E, Porter JD, May PJ. Comparison of the distribution and somatodendritic morphology of tectotectal neurons in the cat and monkey. Vis Neurosci. 1998;15:903–922. doi: 10.1017/s095252389815513x. [DOI] [PubMed] [Google Scholar]
- Olivier E, Corvisier J, Pauluis Q, Hardy O. Evidence for glutamatergic tectotectal neurons in the cat superior colliculus: a comparison with GABAergic tectotectal neurons. Eur J Neurosci. 2000;12:2354–2366. doi: 10.1046/j.1460-9568.2000.00132.x. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JN, Peace JM. Fluorometric comparisons of the retrograde axonal transport of true blue and diamidino yellow from the rat caudate putamen. Exp Brain Res. 1989;75:169–182. doi: 10.1007/BF00248540. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Wang SM. Microinjections of muscimol into lateral superior colliculus disrupt oral orientation to the site of injection in the formalin model of pain. Soc Neurosci. 1996;22 doi: 10.1016/s0306-4522(97)00191-7. Abstr. 51.12. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Odekunle A, Dean P. Tectal cells of origin of predorsal bundle in rat: location and segregation from ipsilateral descending pathway. Exp Brain Res. 1986;63:279–293. doi: 10.1007/BF00236845. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Mitchell IJ, Dean P. Further evidence for segregated output channels from superior colliculus in rat: ipsilateral tectopontine and tecto-cuneiform projections have different cells of origin. Brain Res. 1987;413:170–174. doi: 10.1016/0006-8993(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Mitchell IJ, Odekunle A, Clark A. The projection from superior colliculus to cuneiform area in the rat. I. Anatomical studies. Exp Brain Res. 1988;72:611–625. doi: 10.1007/BF00250606. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Westby GWM. Organization of the crossed tecto-reticulo-spinal projection in rat. I. Anatomical evidence for separate output channels to the periabducens area and caudal medulla. Neuroscience. 1990;37:571–584. doi: 10.1016/0306-4522(90)90092-i. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott T, Gurney KN. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999a;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short latency dopamine response too short to signal reward error? Trends Neurosci. 1999b;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Richmond FJR, Gladdy R, Creasy JL, Kitamura S, Smits E, Thomson DB. Efficacy of seven retrograde tracers, compared in multiple-labeling studies of feline motoneurons. J Neurosci Methods. 1994;53:35–46. doi: 10.1016/0165-0270(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahibzada N, Yamasaki D, Rhoades RW. The spinal and commissural projections from the superior colliculus in rat and hamster arise from distinct neuronal populations. Brain Res. 1987;415:242–256. doi: 10.1016/0006-8993(87)90206-x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shehab S, Dean P, Redgrave P. The dorsal midbrain anticonvulsant zone. II. Efferent connections revealed by the anterograde transport of wheatgerm agglutinin-horseradish peroxidase from injections centered on the intercollicular area in the rat. Neuroscience. 1995;65:681–695. doi: 10.1016/0306-4522(94)00516-8. [DOI] [PubMed] [Google Scholar]
- Silkis I. The cortico-basal ganglia-thalamocortical circuit with synaptic plasticity. I. Modification rules for excitatory and inhibitory synapses in the striatum. Biosystems. 2000;57:187–196. doi: 10.1016/s0303-2647(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Skirboll LR, Thor K, Helke C, Hökfelt T, Robertson B, Long R. Use of retrograde fluorescent tracers in combination with immunochemical methods. In: Heimer L, Záborszky L, editors. Neuroanatomical tracttracing methods 2 recent progress. Plenum Press; New York: 1989. [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of the primate superior colliculus. Physiol Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in the mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Takada M, Itoh K, Yasui Y, Sugimoto T, Mizuno N. Topographical projections from the posterior thalamic regions to the striatum in the cat, with reference to possible tecto-thalamo-striatal connections. Exp Brain Res. 1985;60:385–396. doi: 10.1007/BF00235934. [DOI] [PubMed] [Google Scholar]
- Telford S, Wang S, Redgrave P. Analysis of nociceptive neurons in the rat superior colliculus using c-fos immunohistochemistry. J Comp Neurol. 1996;375:601–617. doi: 10.1002/(SICI)1096-9861(19961125)375:4<601::AID-CNE4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Veenman CL. Pigeon basal ganglia: insights into the neuroanatomy underlying telencephalic sensorimotor processes in birds. Eur J Morphol. 1997;35:220–233. doi: 10.1076/ejom.35.4.220.13079. [DOI] [PubMed] [Google Scholar]
- Wickelgren I. Getting the brain’s attention. Science. 1997;278:35–37. doi: 10.1126/science.278.5335.35. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JNJ, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward.’. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Vision for the control of movement—the Friedenwald lecture. Invest Ophthalmol Vis Sci. 1996;37:2131–2145. [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci. 1980;3:189–226. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]
- Yamasaki DS, Krauthamer GM, Rhoades RW. Superior collicular projection to intralaminar thalamus in rat. Brain Res. 1986;378:223–233. doi: 10.1016/0006-8993(86)90925-x. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Tsumori T, Ando A, Domoto T, Kayahara T, Nakano K. Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res. 1994;656:420–426. doi: 10.1016/0006-8993(94)91489-3. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Tehovnik EJ. Turning responses evoked by stimulation of visuomotor pathways. Brain Res Rev. 1988;13:235–259. doi: 10.1016/0165-0173(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, MartinSkurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]