Abstract
Prolactinomas are rare tumors in prepubertal children. A prolactinoma in a young child may be due to sequence variants in genes that are known to cause these tumors (MEN1, PRKAR1A, AIP). An 11-year-old boy with a macroprolactinoma was treated with cabergoline and the tumor receded. We studied the patient and his family for genetic causes of this tumor. No mutations were present in the coding sequence of PRKAR1A and AIP. A novel heterozygous substitution (IVS3–7 c>a) was identified in intron 3 of MEN1. We also found an additional PCR amplicon that incorporated the entire intron 3 of the gene (210 bp) in the patient's cDNA. The same amplicon was present with lower intensity in some of the control individuals who were not mutation carriers. Intron 3 harbors an in-frame stop codon and its incorporation is predicted to result in a prematurely terminated protein. We conclude that a novel MEN1 variation was identified in a young boy with prolactinoma and six of his relatives who did not present with prolactinoma or other MEN1 related symptoms. This novel MEN1 variation may be associated with low penetrance of the disease. The IVS3–7 c>a defect is suggested to be pathogenic because it is associated with lower menin levels in the cells of these patients, but its consequences may be mitigated by a variety of factors including changes in transcription and translation of the MEN1 gene.
Introduction
Pediatric pituitary adenomas are relatively rare1,2. Pituitary adenomas account for only 2.7% of supratentorial tumors in children; most are diagnosed after puberty, and tend to be more frequently functional3,4,5. Prolactinoma is the most common type of pituitary adenoma, but it presents predominantly in females of ages 20 to 50 years3. Prolactinomas are unusual in the pediatric age group; thus, the finding of a large prolactinoma in a prepubertal child should prompt consideration of a genetic syndrome such as multiple endocrine neoplasia type 1 (MEN1), Carney complex (CNC), the condition known as “familial isolated pituitary adenomas” (FIPA), or McCune-Albright syndrome6,7,8.
MEN1 is associated with parathyroid, enteropancreatic, pituitary, and other tumors. MEN1-associated endocrine tumors usually become clinically evident in late adolescence or young adulthood. However, in 2000 we reported a 2.3 cm pituitary macroadenoma in a 5-year-old boy with known family history of MEN18. That patient represented, at the time, the earliest manifestation of any endocrine tumor in MEN1 reported so far. Since this report additional cases of “early” MEN1 have been described, presenting mostly with pituitary tumors9,10.
Here we report on an 11-year-old boy with a macroprolactinoma. We tested his peripheral DNA for mutations in the genes known to cause MEN1 (menin, the MEN1 gene), CNC (protein kinase A regulatory subunit type 1A, the PRKAR1A gene), and FIPA (aryl hydrocarbon receptor interacting protein, the AIP gene). A sequence abnormality in the MEN1 gene was identified. In this report, we describe the case and the molecular analyses that point to the functional consequences of this genetic aberration.
Subject and Methods
Patient
An 11-year-old Caucasian boy from Chile presented to his local physician with right eye ptosis and decreased peripheral vision. Magnetic resonance imaging (MRI) revealed a pituitary macroadenoma. The lesion occupied an enlarged sella turcica with extension into the right cavernous sinus; the pituitary stalk was deviated to the left (Figure 1A). Laboratory evaluation revealed serum prolactin level of 14 642 mIU/l (normal range 47–473). Other studies of anterior pituitary function (IGF-I 254 ng/ml, BP3 2.9 mg/L, DHEAS 382 mcg/dl) were significant for secondary hypothyroidism with a TSH of 1.13 uIU/ml, free T4 0.9 ng/dl, and T3 110 ng/dl (normal range 0.3–4, 1.1–1.7, and 75–190, respectively). Morning cortisol was 11 ng/ml. Replacement with levothyroxine (50 mcg daily) and treatment with cabergoline 0.5 mg twice a week were initiated. After 2 months of treatment, the tumor decreased in size and PRL levels declined (275 mIU/l); the patient was continued on treatment and referred to our center for further investigation. At that time, he was 11.5 years of age at which point the tumor had almost completely regressed (see below). There was no family history of diabetes, hormonal diseases, or MEN1-related tumors; a paternal cousin had died from Ewing's osteosarcoma and a paternal grandmother had been diagnosed with skin cancer. At the age of 11, he was prepubertal with a height in the 25th and a weight in the 50th percentile. Multiple junction nevi and freckles were detected on the face, chest, back, and ears, but no lesions were observed on the lips, fingers, oral or ocular mucosa. The patient had two small café-au-lait spots and a congenital 2 mm fibroma but no angiofibromas, collagenomas, fibrous displasia, or lipomas. The rest of the physical examination was unremarkable, including normal peripheral vision and no evidence for gynecomastia or galactorrhea. No clinical signs consistent with McCune-Albright syndrome were found except for two small café-au-lait spots. PTH, total ionized calcium and fasting gastrin and insulin levels were within the normal range. Other endocrine and imaging studies including serum cortisol levels, growth hormone studies, glucose tolerance tests, thyroid and testicular ultrasounds and cardiac echocardiogram are also normal to this date. Today at the age of 15 the tumor is not visible on MRI (Figure 1B) and his PRL levels are in the normal range. Cabergoline was recently discontinued and the patient is being followed by biannual measurements of his PRL levels; he remains normoprolactinemic to date.
Figure 1.
A) Post contrast T1-weighted MRI scan of the pituitary. There is a large adenoma demonstrating homogeneous enhancement throughout its parenchyma. The adenoma has invaded the right cavernous sinus encasing the internal carotid artery; it has also extended into the suprasellar cistern compressing the optic chiasm. B) Post contrast T1-weighted MRI scan of the pituitary after treatment: the adenoma is not longer present; a remnant of pituitary tissue is identified in close proximity to the floor of the sella turcica which is filled with cerebral spinal fluid. The pituitary stalk is deviated towards the left side and the optic chiasm has migrated inferiorly and assumed a V shape.
For the purposes of this study, the patient and his family members were enrolled in protocol 97-CH-0076 for the genetic investigation of pituitary tumors; the patient and his family signed the appropriate consent and assent forms. The study and all forms have been approved by the institutional review board of the National Institute of Child Health and Development.
DNA and RNA analyses
Genomic DNA was obtained from peripheral blood from the patient and available family members. Direct bi-directional sequencing was employed to analyze all coding regions and the flanking exon/intron junctions of the MEN1, PRKAR1A, and AIP genes by standard methods11,12,13,14. RNA was extracted from peripheral blood cells using Trizol LS according to the manufacturer's protocol (Invitrogen) and subsequently treated with DNAse. For the analysis of the MEN1 gene defect effects on cDNA, RT-PCR was performed on 1 μg total RNA using two different specific couples primers of primers (5′-3′): F1: GAGCAGACAGCTGAGGTCAC, R1: CCGATAGTAGGTCTTGGCTGA, F2: AGACAGTCAATGCCGGTGTG, and R2: GTGGTAGCCAGCCAGGTACA. RT-PCR products were analyzed on 1.2% agarose gel electrophoresis, visualized with ethidium bromide, gel-purified according to the manufacturer recommendations (QIA QUICK, Qiagen Inc), and subjected to automated sequencing (Sequence Analyzer 3130xl, Applied Biosistems, Inc).
Western blotting: whole cell lysate and nuclear extract
Western blot analysis was performed as previously described15. The polyclonal antibody against menin was generated by Guru et al.16. Actin antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Whole cell lysates were prepared from lymphocytes homogenized in 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% NP40, 0.5% sodium deoxycholate, protease inhibitor cocktail I (EMD Biosciences, La Jolla, CA, USA) with subsequent centrifugation at 10 000 rpm, 10 min, 4°C. Nuclear lysates were prepared by the method of Dignam et al.17. For Western blotting analysis equal amounts of protein lysate were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies. Complexes were visualized with the appropriate horseradish peroxidase-conjugated secondary antibody and developed by enhanced chemiluminescence procedure (Santa Cruz Biotechnology, Santa Cruz, CA).
Results
DNA and RNA analyses
Complete bi-directional sequencing of exon 2–10 and the flanking exon-intron junctions of MEN1 identified a heterozygote substitution located six nucleotides before the beginning of exon 4 (IVS3–7c>a). No other sequence changes were seen in the coding regions of this gene or PRKAR1A and AIP. The IVS3–7c>a substitution has not been seen in several hundreds of normal samples that have been tested by our institution for MEN1 mutations; it has not been previously reported and it is not listed in the on-line databases. We also looked at more than 300 controls and did not find this sequence alteration.
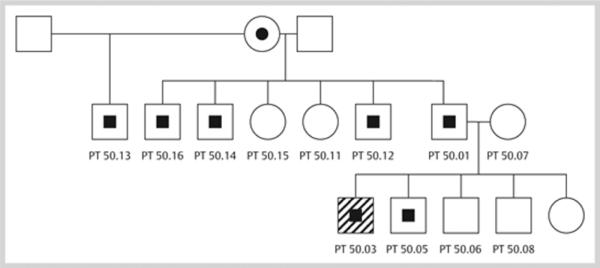
Available family members were tested: the mutation was present in the father, one of the patient's brothers, and four male members of the father's family (Figure 2).
Figure 2.
Family pedigree. The index case is indicated with an arrow; half-filled black symbols indicate members who are heterozygote for the MEN1 IVS3–7 c>a mutation. Open symbols are family members without this mutation.
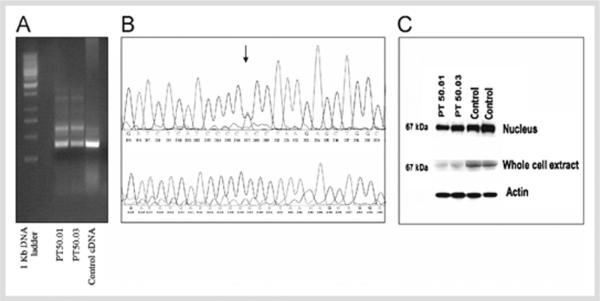
RT-PCR amplification of exons 3–7 of the cDNA of the proband and the affected family members revealed the presence of two bands with similar intensity: the expected wild-type product (372 bp) and an additional fragment of 582 bp (Figure 3A). The same two bands were also seen in most (but not all) control cDNA samples; however, the intensity of the alternative product was significantly lower (about an 8-fold decrease compared to the band in the carriers of the mutant MEN1 gene). The alternate band was not detected below 30 cycles of amplification upon repeated experiments (data not shown). Similar results were obtained with alternative set of forward and reverse primers. Gel purification and subsequent sequencing of the shorter amplicon revealed the expected wild type part of MEN1 cDNA. Sequencing of the longer fragment showed retention of the entire intron 3 (210 bp) between exons 3 and 4 (Figure 3B). The junctions between exons 4 and 5, 5 and 6, and 6 and 7 were present, as expected, thus excluding contamination with genomic DNA. The substitution was not seen in the control cDNA amplicons which had also retained intron 3. Retention of intron 3 (the entire sequence between exon 3 and 4), containing an in-frame stop codon, is predicted to lead to a prematurely terminated menin protein; thus, the effect of the mutation at the cDNA and protein level is as follows: c.669ins210/p.R223+61X.
Figure 3.
A) RT-PCR of MEN1 (exons 3–7): RT-PCR showed two bands with similar intensity with the expected wild type product (372 bp) and an additional fragment of 582 bp. The same two bands were also seen in the control; however, the intensity of the alternative product was significantly lower. The same results were obtained with alternative set of forward and reverse primers (data not shown). B) Upper panel shows the sequencing chromatogram of the alternative cDNA product of PT50.03. The substitution (IVS3–7, c>a) is indicated with an arrow. The sequencing revealed similar intensity for the wild type and the alternative peaks. The same result is observed in PT 50.01 (data not shown). Lower panel shows the sequencing of the alternative band in the control individual revealed the same cDNA sequence that incorporated intron 3, without presence of the substitution. C) Western blot of MEN1 in nucleus and whole cell extracts of lymphocytes from patients with mutations (PT 50.01 and PT 50.03) and from wild-type control individuals; the experiment is controlled by β-actin.
Two independent in silico splice analyzing algorithms (spliceport.cs.umd.edu/; http://www.cbs.dtu.dk/services/NetGene2/) predicted approximately 10% decreased probability of the newly identified sequence variant to act as an acceptor site, compared to the wild type. However, since we have seen intron 3 incorporated also in the wild type sequence (although less frequently) the correlation between the variation and splice site features remains unclear18,19.
Protein analyses
We prepared nuclear and whole cell extracts from the patients lymphocytes in both carriers of the mutation as well as in normal control donors. In both whole cell and nuclear extracts, we detected only one protein product, corresponding to the expected size of the menin (67 kDa). A decrease in the menin levels was seen in the carriers of the mutation; this decrease was more significant in the whole cell extracts.
Discussion
To date, more than one thousand inactivating germline mutations of the MEN1 gene have been identified20. Most of them are unique, seen in single families only; a small number of mutations are reported in more than 2% of the affected individuals17,18,19,20. In patients with MEN1 mutations, the lifetime chance of developing a pituitary adenoma is approximately 40%21. Interestingly, there are no age differences between MEN1-associated and sporadic prolactinomas: both appear most frequently during the forth decade of life, although some children with MEN1 present in early childhood8,9,10,21,22. MEN1 patients and sporadic pediatric patients with prolactinomas generally do not respond to dopamine agonist as well as adult patients with sporadic PRL-producing tumors9,21,23. However, several reports show remarkable effect of cabergoline treatment in children and young adults with large prolactinomas24,25.
The mutation in our patient is unusual and its pathogenic effect could be questioned. First, it was detected in six apparently unaffected family members. We had the opportunity to screen the father biochemically (including for fasting glucose and insulin levels) and by MRI (of head, neck and abdomen): although he had a large pituitary for his age, he had no tumor and he was biochemically normal. Clinical screening including the examination by experienced dermatologist for the presence of collagenomas and/or angiofibromas was negative for any skin findings. The other “positive” members of the family have been tested only biochemically and have shown no abnormality. So we could not find any other manifestations of MEN1 in this family, but the patient is still young and being followed and it is not unlikely that he will show signs of the disease as he grows older. Second, at the cDNA level, the patient and his father who both carried the IVS-7 c>a substitution, were found to have an additional MEN1 fragment of 582 bp, a fragment occasionally seen in control samples. However, when present in controls, the 582 bp band had decreased intensity when compared to the patient and his father. Sequencing of this band revealed retention of intron 3 in both mutation carriers and controls but, as expected, the substitution was present (in the heterozygote state) in the retained intron 3 sequence only in the carriers of the genetic defect and not in any control cDNA samples.
Incorporation of intron 3 in the MEN1 mRNA predicts premature termination of the protein (c.669ins210/p.R223+61X). We looked at our kindred's samples: in heterozygotes, the menin protein levels were significantly decreased in whole cell lysates (as one would expect from the lack of expression of the shorter, mutant form); nuclear wild-type menin levels were also decreased in our patients. In the Western blot we did not find any difference between mutated and control samples in terms of finding a new smaller product. One explanation is the specificity of our antibodies. Another explanation can be connected to the position of the mutation and possibly to the express mutant protein and size of the protein.
In summary, our results suggest that the IVS-7 c>a mutation is associated with low penetrance and probably lower expression of the MEN1 phenotype, but it is a pathogenic MEN1 defect and hence most probably responsible for our patient's prolactinoma. Its molecular effects appear to relate to 1) a more efficient transcription of a normally found alternate MEN1 cDNA isoform (which incorporates intron 3 of the sequence into the mRNA), and 2) a consequent decrease in normal menin protein levels in the patients. Menin is a mostly nuclear protein, known for its tumor suppression properties22,26. Any decrease in its nuclear presence would lead to relative loss of its function which is, most likely, the reason for the described tumor in our proband.
Acknowledgement
This work was supported by the intramural program of NICHD, NIH.
References
- 1.Webb C, Prayson RA. Pediatric pituitary adenoma. Arch Pathol Lab Med. 2008;132:77–80. doi: 10.5858/2008-132-77-PPA. [DOI] [PubMed] [Google Scholar]
- 2.Jagannathan J, Kanter AS, Sheehan JP, Jane JR, Jr, Laws ER., Jr Benign brain tumors: sellar/paraselar tumors. Neurol. 2007;25:1231–1249. doi: 10.1016/j.ncl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ciccarelli A, Daly AF, Beckers A. The epidemiology of prolactinomas. Pituitary. 2005;8:3–6. doi: 10.1007/s11102-005-5079-0. [DOI] [PubMed] [Google Scholar]
- 4.Diamond FB., Jr Pituitary adenomas in children: development and diagnosis. Fetal Pediatric Pathol. 2006;25:339–356. doi: 10.1080/15513810701209603. [DOI] [PubMed] [Google Scholar]
- 5.De Menis E, Visentin A, Billeci D, Tramontin P, Agostini S, Marton E, Conte N. Pituitary adenomas in childhood and adolescence. Clinical analysis of 10 cases. J Endocrinol Invest. 2001;24:92–97. doi: 10.1007/BF03343820. [DOI] [PubMed] [Google Scholar]
- 6.Beckers A, Daly AF. The clinical, pathological, and genetic features of familial isolated pituitary adenomas. Eur J Endocrinol. 2007;157:371–382. doi: 10.1530/EJE-07-0348. [DOI] [PubMed] [Google Scholar]
- 7.Daly AF, Jaffrain-Rea ML, Beckers A. Clinical and genetic features of familial pituitary adenomas. Horm Metab Res. 2005;37:347–354. doi: 10.1055/s-2005-870135. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, Skarulis MC, Weil RJ, Lubensky IA, Zhuang Z, Oldfield EH, Marx SJ. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2000;85:4776–4780. doi: 10.1210/jcem.85.12.7064. [DOI] [PubMed] [Google Scholar]
- 9.Oiwa A, Sakurai A, Sato Y, Sakuma T, Yamashita K, Katai M, Aizawa T, Hashizume K. Pituitary adenomas in adolescent patients with multiple endocrine neoplasia type 1. Endocr J. 2002;49:635–640. doi: 10.1507/endocrj.49.635. [DOI] [PubMed] [Google Scholar]
- 10.Rix M, Hertel NT, Nielsen FC, Jacobsen BB, Hoejberg AS, Brixen K, Hangaard J, Kroustrup JP. Cushing's disease in childhood as the first manifestation of multiple endocrine neoplasia syndrome type 1. Eur J Endocrinol. 2004;151:709–715. doi: 10.1530/eje.0.1510709. [DOI] [PubMed] [Google Scholar]
- 11.Tala HP, Carvajal CA, Gonzales AA, Garrido JL, Tobar J, Solar A, Campino C, Arteaga E, Fardella CE. New splicing mutation of MEN1 gene affecting the translocation of menin to the nucleus. J Endocrinol Invest. 2006;29:888–893. doi: 10.1007/BF03349192. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekharappa SC, Guru SC, Manickamp P, Olufemi SC, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Ccrabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloninig of the gene for multiple endocrine neoplasia type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 13.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 14.Daly AF, Vanbellinghen JF, Khoo SK, Jaffrain-Rea ML, Naves LA, Guitelman MA, Murat A, Emy P, Gimenez-Roqueplo AP, Tamburrano G, Raverot G, Barlier A, De Herder W, Penfornis A, Ciccarelli E, Estour B, Lecomte P, Gatta B, Chabre O, Sabaté MI, Bertagna X, Garcia Basavilbaso N, Stalldecker G, Colao A, Ferolla P, Wémeau JL, Caron P, Sadoul JL, Oneto A, Archambeaud F, Calender A, Sinilnikova O, Montañana CF, Cavagnini F, Hana V, Solano A, Delettieres D, Luccio-Camelo DC, Basso A, Rohmer V, Brue T, Bours V, Teh BT, Beckers A. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92:1891–1896. doi: 10.1210/jc.2006-2513. [DOI] [PubMed] [Google Scholar]
- 15.Neary CL, Nesterova M, Cho YS, Cheadle C, Becker KG, Cho-Chung YS. Protein kinase A isozyme switching: eliciting differential cAMP signaling and tumor reversion. Oncogene. 2004;23:8847–8856. doi: 10.1038/sj.onc.1208165. [DOI] [PubMed] [Google Scholar]
- 16.Guru SC, Goldsmith PK, Burns AL, Marx SJ, Spiegel AM, Collins FS, Chandrasekharappa SC. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci USA. 1998;95:1630–1634. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription inhibition by RNA polymerase 11 in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunak S, Engelbrecht J, Knudsen S. Prediction of human mRNA donor and acceptor sites from the DNA sequence. J Mol Biol. 1991;5220:49–65. doi: 10.1016/0022-2836(91)90380-o. [DOI] [PubMed] [Google Scholar]
- 19.Dogan RI, Getoor L, Wilbur WJ, Mount SM. SplicePort-an interactive splice-site analysis tool. Nucleic Acids Res. 2007;35(Web Server issue):W285–W291. doi: 10.1093/nar/gkm407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 21.Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvenay C, Calender A. Pituitary disease in MEN1 type 1 (MEN1):data from the Franch Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457–465. doi: 10.1210/jcem.87.2.8145. [DOI] [PubMed] [Google Scholar]
- 22.Horvath A, Stratakis CA. Clinical and molecular genetics of acromegaly: MEN1, Carney complex, McCune-Albright syndrome, Familial acromegaly and genetic defects in sporadic tumors. Rev Endocr Metab Disord. 2007;9:1–11. doi: 10.1007/s11154-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 23.Bowden SA, Sotos JF, Stratakis CA, Weil RJ. Successful treatment of an invasive growth hormone-secreting pituitary macroadenoma in an 8-year-old boy. Pediatr Endocrinol Metab. 2007;20:643–647. doi: 10.1515/jpem.2007.20.5.643. [DOI] [PubMed] [Google Scholar]
- 24.Howell DL, Wasilewski K, Mazewski CM, Hudgins RJ, Meacham LR. The use of high-dose daily cabergoline in an adolescent patient with macroprolactinemia. J Pediatr Hematol Oncol. 2005;27:326–329. doi: 10.1097/01.mph.0000168727.41896.d2. [DOI] [PubMed] [Google Scholar]
- 25.Eyal O, Naffaa LN, Elder DA. A case of macroprolactinoma and elevated insulin-like growth factor-I in a young boy. Acta Paediatr. 2005;94:1852–1854. doi: 10.1111/j.1651-2227.2005.tb01869.x. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Kennedy PA, Scacheri PC, Novotny EA, Hickman AB, Cerrato A, Rice TS, Moore JB, Rao S, Ji Y, Mateo C, Libutti SK, Oliver B, Chandrasekharappa SC, Burns AL, Collins FS, Spiegel AM, Marx SJ. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res. 2005;37:369–370. doi: 10.1055/s-2005-870139. [DOI] [PubMed] [Google Scholar]