Abstract
The potential influence of mechanical loading on transvascular transport in vascularized soft tissues has not been explored extensively. This experimental investigation introduced and explored the hypothesis that dynamic mechanical loading can pump solutes out of blood vessels and into the surrounding tissue, leading to faster uptake and higher solute concentrations than could otherwise be achieved under unloaded conditions. Immature epiphyseal cartilage was used as a model tissue system, with fluorescein (332 Da), dextran (3, 10 and 70 kDa) and transferrin (80 kDa) as model solutes. Cartilage disks were either dynamically loaded (±10% compression over a 10% static offset strain, at 0.2 Hz) or maintained unloaded in solution for up to 20 hours. Results demonstrated statistically significant solute uptake in dynamically loaded (DL) explants relative to passive diffusion (PD) controls for all solutes except unbound fluorescein, as evidenced by the DL:PD concentration ratios after 20 hours (1.0 ± 0.2, 2.4 ± 1.1, 6.1 ± 3.3, 9.0 ± 4.0, and 5.5±1.6 for fluorescein, 3, 10, and 70 kDa dextran, and transferrin). Significant uptake enhancements were also observed within the first 30 seconds of loading. Termination of dynamic loading produced dissipation of enhanced solute uptake back to PD control values. Confocal images confirmed that solute uptake occurred from cartilage canals into their surrounding extracellular matrix. The incidence of this loading-induced transvascular solute pumping mechanism may significantly alter our understanding of the interaction of mechanical loading and tissue metabolism.
Keywords: vascular transport, solute pumping, cartilage canals, active transport
1. Introduction
Solute transport in biological tissues is a fundamental process in support of cell metabolism, providing access to nutrients and a removal of waste products. In the native environment, solute exchange occurs primarily via the circulatory system, where it is understood that solutes transport down a concentration gradient from the blood stream into tissues. Over the years, considerable efforts have been made to explore the contributions of physiologic mechanisms for this exchange, such as passive diffusion, fluid convection, and transcytosis (Jennings and Florey, 1967; Michel and Curry, 1999; Pappenheimer, 1953; Pappenheimer and Soto-Rivera, 1948; Rippe and Haraldsson, 1994; Simionescu et al., 1975). However, the potential influence of mechanical loading on transvascular transport has not been explored significantly. This experimental investigation introduces and explores the hypothesis that cyclic mechanical loading can pump solutes out of blood vessels and into the surrounding tissue, leading to faster uptake and higher solute concentrations than could otherwise be achieved under unloaded conditions.
The concept of cyclic or dynamic loading enhancing solute transport in the extracellular matrix (ECM) of hydrated soft tissues has often been surmised to promote solute convection induced by oscillatory interstitial fluid flow. Studies have generally shown that dynamic loading can increase the rate of solute transport in avascular tissue explants or hydrogels (Bonassar et al., 2001; Evans and Quinn, 2006; Lee et al., 2000; O'Hara et al., 1990). However, more recently, it has been demonstrated that dynamic loading can pump solutes into hydrogels from an external bath, promoting substantially higher concentrations than observed under passive diffusion alone (Albro et al., 2008). These concentration enhancements were observed to be as high as 15-fold over passive equilibrium values, demonstrating that solutes are being transported against their concentration gradient. This phenomenon was shown, on a theoretical basis, to result from the momentum exchange between solutes and the deforming solid matrix induced by dynamic loading (Albro et al., 2010; Mauck et al., 2003). As the matrix expands and recoils under loading, it is able to pull solutes from an external bathing solution into the tissue, in a manner akin to pumping.
This solute-solid pumping mechanism may play an important nutritional role in various biological tissues. Various tissues possess a network of cells embedded in a dense ECM that can greatly hinder the diffusion of solutes in the tissue (Gu et al., 2004; Maroudas, 1970; Maroudas, 1976). As theoretically stated (Mauck et al., 2003), this hindrance is indicative of a high degree of momentum exchange between solutes and the ECM, suggesting the potential for effective solute-solid pumping under dynamic loading. Furthermore, studies have traditionally shown that dynamic loading is an important requisite for maintaining cellular metabolic activity in a variety of tissues, such as cartilage (Jurvelin et al., 1986; Kiviranta et al., 1988; Parkkinen et al., 1992; Sah et al., 1989; Salter and Field, 1960; Sood, 1971), muscle (Mackey et al., 2008), tendon (Woo et al., 1987), and bone (Woo et al., 1981). Generally, tissue immobilization leads to a loss of ECM products while dynamic loading has a stimulatory effect. While these results are primarily attributed to a mechanotransduction pathway, it is possible that an enhanced nutrient supply due to solute pumping may play a role as well.
Therefore, the goal of this current experimental study is to begin to explore the potential physiologic role of this transport phenomenon by testing whether dynamic loading of a vascularized tissue in vitro can pump solutes out of blood vessels and into the surrounding tissue. To this end, we implement immature epiphyseal cartilage as a model tissue system. It is well established that immature cartilage is vascularized with canals that provide nutrients to the developing tissue (Haines, 1937; Haines, 1974; Stockwell, 1971; Trueta, 1957; Wilsman and Van Sickle, 1972). Furthermore, cartilage possesses a dense ECM that has been shown to substantially hinder the transport of solutes in the tissue (Maroudas, 1970; Maroudas, 1976). This hindrance has been attributed to interactions with proteoglycans (Torzilli et al., 1997) and collagen fibers (Fetter et al., 2006; Filidoro et al., 2005; Meder et al., 2006), suggesting that the ECM may be effective at exchanging momentum with, and thus pumping, solutes.
To test this hypothesis, cartilage explants were subjected to either unloaded passive diffusion (PD) conditions or sustained dynamic compressive loading (DL), and solute uptake was assessed through two methods: Fluorescent assays that quantitatively measure solute concentrations in the entire aggregate tissue, and confocal imaging that qualitatively assesses the spatial distribution of solutes in various regions of the tissue. Firstly, the uptake of fluorescein-conjugated dextran polysaccharides of various molecular weights (70 kDa, 10 kDa and 3 kDa and unbounded fluorescein, 332 Da, serving as a control) was tested. These solutes were implemented in our earlier hydrogel investigations (Albro et al., 2008; Chahine et al., 2009) and are commonly used in cartilage transport studies (Evans and Quinn, 2005; Fetter et al., 2006; Leddy et al., 2004; Maroudas, 1970; Nimer et al., 2003; Quinn et al., 2000; Torzilli et al., 1987; Torzilli et al., 1998) due to their availability in a wide range of molecular weights. Additionally, to explicitly study the biological relevance of this transport phenomenon, the uptake of the plasma carrier protein, transferrin (80 kDa), was also measured. Transferrin is responsible for the cellular uptake of iron ions (Laurell, 1951) and is essential for the metabolic activity of most biological cells (Chua et al., 2007).
2. Methods
Materials
Articular cartilage explants (Ø6 × 3.5 mm) were harvested from the femoral condyles and patellar grooves of 4–6 week old bovine calves with a biopsy punch (N=21 joints from 14 animals, n=572 explants). All explants were stored frozen in phosphate buffered saline (PBS) until testing.
Solutions of dextran polysaccharides (3kDa, 10kDa, and 70 kDa, Invitrogen, CA) conjugated to fluorescein, as well as reference fluorescein solution (332 Da Invitrogen) were prepared in phosphate buffered saline (PBS) at concentrations of 0.25 mg/mL. Bovine transferrin (80 kDa, APO form, Sigma, MO) was prepared in PBS at 6 μM and conjugated with carboxyfluorescein succinimidyl ester using the Invitrogen amine-reactive probes conjugation protocol. Following the reaction, the conjugate was separated from the unreacted labeling reagent with a centrifuge filter (Amicon Ultra, 10 kDa cutoff, Millipore, MA). All the solutes tested in this study possess a net negative charge. Before each test, solutions were supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail Tablets, Roche, Germany, 1 tablet per 50mL solution).
While immersed in testing solution at 22° C, disks were subjected to unconfined compression with a prescribed dynamic strain of ± 10% amplitude superposed over a 10% static strain offset at a frequency of 0.2 Hz. This loading configuration intentionally produces platen lift-off on the upstroke, mimicking the physiological loading regimen cartilage experiences under reciprocating sliding motion.
Solute Uptake
Cartilage disks were subjected to dynamic loading (DL) while immersed in a solution bath of 3 kDa, 10 kDa, 70 kDa dextran, fluorescein, or transferrin. Concurrently, control disks (PD) were immersed in a solution-filled Petri dish under unloaded conditions and gently agitated on an orbital shaker (Bellco, NJ). Tests were conducted for time periods ranging from 30 seconds to 20 hours of loading. At the completion of each test, disks were removed and processed for their interstitial solute content (see Appendix A: Supplementary data).
Recovery Test
An additional study was conducted where the transient solute concentration in cartilage disks was measured upon termination of dynamic loading. Disks were subjected to dynamic loading (DL) for 20 hours in transferrin solution, alongside a group of unloaded control disks (PD). At the end of this period, disks were transferred to a solution-filled Petri dish and maintained unloaded. At three time points during this recovery period (0, 6 and 40 hours), disks were removed and processed for their interstitial solute content. After the completion of each 24- hour testing period, solutions were replenished with a new dose of protease inhibitors.
Confocal Imaging
Cartilage explants were subjected to the aforementioned DL or PD routines while immersed in 3 kDa, 70 kDa dextran, fluorescein, or transferrin. After 30 seconds, 1 hour, or 20 hours of testing, samples were removed from solution, gently blotted, and axially sectioned (approx 0.5 mm thick) with a custom made cutting device. Sections were then placed on a glass coverslip and imaged in air on a confocal microscope (Leica TCS SP5 Multiphoton) at excitation and emission wavelengths of 488 and 535 nm and at a scanning depth of 35 μm into the tissue. Preliminary studies have demonstrated this to be an optimal acquisition depth, which prevents excessive laser signal attenuation while avoiding potential artifacts from imaging exuded solution on the surface of the sample. Images were acquired under a 20 × objective through automated tile scanning to recreate the entire cross-section of each sample (30 seconds per tile scan). Image acquisition settings were consistently maintained within each solute type only, and therefore, no attempt was made to make comparisons of sections between different solutes.
3. Results
Solute Uptake
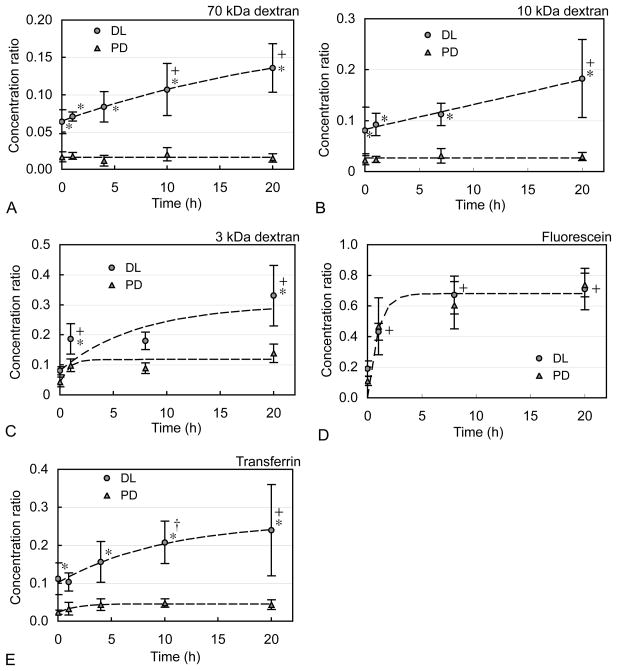
For all uptake measurements, the solute concentration in each cartilage disk was normalized to the concentration in the external bathing solution and expressed as the normalized ratio ĉ (mean ± standard deviation). Under passive diffusion conditions, all solutes were partially excluded from the tissue, as evidenced by steady-state normalized concentration ratios less than unity. These values generally decreased with increasing solute molecular weight, ranging from ĉPD = 0.737 ± 0.078 for fluorescein to 0.015 ± 0.006 for 70 kDa dextran (Figure 1). In fact, the absence of a passive temporal rise in concentration for the larger solutes (70 kDa, 10 kDa dextran and transferrin, Figure 1A, B, & E) suggests that they may be almost entirely excluded from the cartilage tissue. Partitioning of solutes in cartilage has been generally attributed to steric volume exclusion and short-range electrostatic interactions, and in the case of charged solutes, long-range electrostatic interactions between solutes and the highly negatively charged proteoglycans in the cartilage ECM. Similar near-zero partition coefficients in cartilage, as observed for the larger solutes in this study, have previously been reported for negatively charged high molecular weight dextrans (Fetter et al., 2006; Maroudas, 1970) and large proteins (Maroudas, 1976). Previous studies have also demonstrated an inverse relationship between partitioning in cartilage and solute molecular weight as seen here (Maroudas, 1970; Maroudas, 1976; Quinn et al., 2000).
Figure 1.
Ratio cö of internal to external solute concentration over time in cartilage disks for (A) 70 kDa dextran, (B) 10 kDa dextran, (C) 3 kDa dextran, (D) fluorescein (332 Da), and (E) transferrin (80 kDa) under dynamic loading (DL) and unloaded passive diffusion (PD) conditions (mean ± standard deviation). Results demonstrate that DL progressively pumps solutes into cartilage at concentrations that significantly exceed those achieved under PD; this pumping mechanism is most effective for larger molecular weight solutes, but insignificant for unconjugated fluorescein. *p<0.02: significant increase above respective PD time point. +p<0.001, †p<0.05: significant increase above initial DL time point.
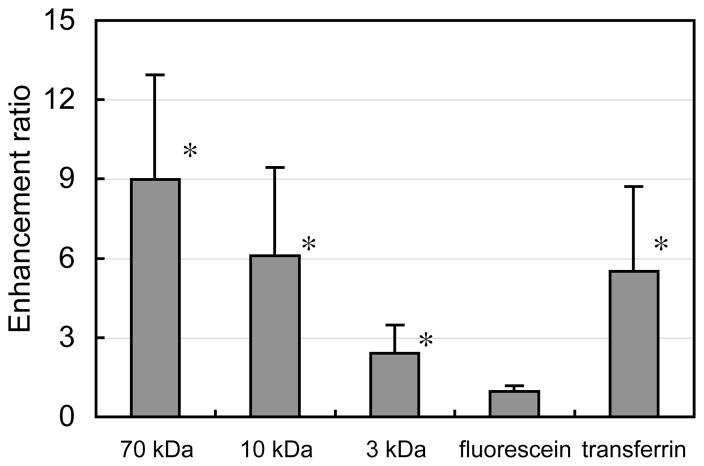
Under dynamic loading, solute uptake in cartilage disks exhibited a two phase response: a jump in concentration above passive values within 30 seconds of loading initiation (p<0.02 for 70 kDa, 10 kDa dextran, transferrin, Figure 1A, B, &E), followed by a continued rise under additional loading, reaching concentrations significantly higher than the initial dynamic loading time point after 20 hours (p<0.001, Figure 1A, B, &E). After 20 hours of loading, all solutes except for fluorescein exhibited significantly higher concentrations relative to passive control values (p<0.001, Figure 1). Furthermore, the observed enhancement factors (ĉDL / ĉPD in this study after 20 hours were found to increase with solute molecular weight, reaching values of 1.0 ± 0.2, 2.4 ± 1.1, 6.1 ± 3.3, and 9.0 ± 4.0 for fluorescein, 3 kDa, 10 kDa, and 70 kDa dextran, respectively (Figure 2).
Figure 2.
Enhancement ratios (ĉDL / ĉPD) of 70 kDa dextran, 10 kDa dextran, 3 kDa dextran, fluorescein, and transferrin in cartilage disks after 20 hours of dynamic loading (mean ± standard deviation). These ratios demonstrate that the pumping mechanism is more effective with larger solutes, for a given molecular species. *p<0.01: significant increase above unity.
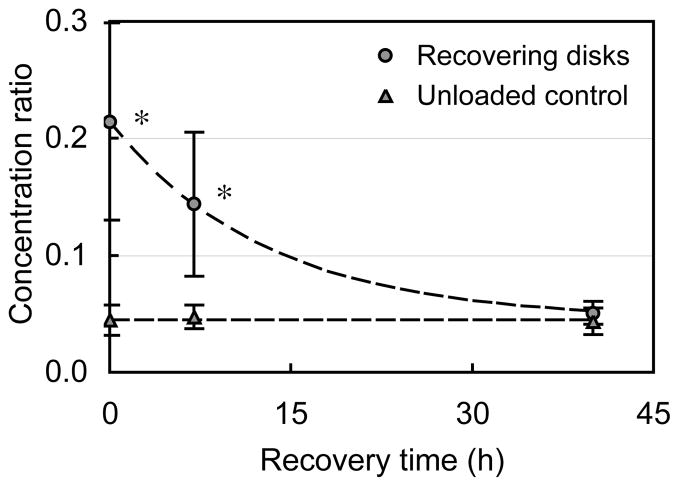
Recovery Test
After 20 hours of loading the transferrin concentration in cartilage disks relative to the external bath reached a value of cöDL = 0.21 + 0.08. During the unloaded recovery period, the transferrin concentration exponentially decayed back down to cöPD = 0.05+ 0.01, a value not statistically different from the corresponding concentration under maintained passive diffusion control conditions (p> 0.5, Figure 3).
Figure 3.
Recovery response of cartilage disks in transferrin following 20 hours of dynamic loading (DL) and corresponding response in never-loaded control samples (mean ± standard deviation). These results demonstrate that DL is the primary cause for increased solute uptake, since termination of loading returns solute concentration back to PD levels. *p<0.02: significant increase above respective PD time point.
Confocal Imaging
Confocal images illustrated the localized presence of 70 kDa dextran in cartilage canals after only 30 seconds of loading (Figure 4A). In contrast, 70 kDa dextran was only faintly present in cartilage canals after any duration of passive uptake (Figure 4B, D & F). Images also demonstrated that under prolonged periods of dynamic loading, solutes spread from the cartilage canals deeper into the tissue, as became evident after 2 hours (Figure 4C) and after 20 hours, when it occupied most of the surrounding tissue (Figure 4E). The concentration of dextran in the surrounding tissue was visibly higher than that under passive uptake at any time point. Similar results were also observed for 3 kDa and transferrin (Figure 4G–J). These images also depicted enhanced solute concentrations in dynamically loaded tissue (Figure 4G & I) relative to passive diffusion (Figure 4H & J). On the other hand, there was no observable difference in fluorescein concentration between dynamic loading and passive diffusion (Figure 4K & L).
Figure 4.
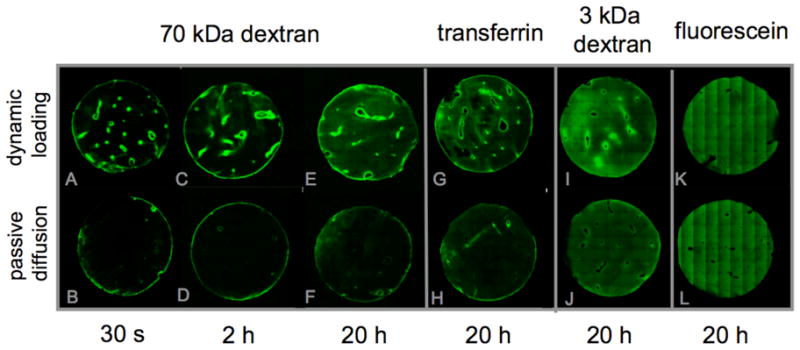
Confocal images of various solutes in cartilage sections after testing under dynamic loading or passive diffusion conditions. These images demonstrate that solute pumping under DL occurs at cartilage canals as well as the outer boundaries of the explant, as evidenced from the narrow boundary layers of high solute concentrations at 30 s and 2 h (70 kDa dextran). Over time, solute concentration spreads out from the canals into the surrounding ECM (20 h). In contrast, PD results in much lower solute concentrations for dextrans and transferrin. Only fluorescein shows no visible difference between DL and PD.
4. Discussion
Results demonstrated that dynamic loading pumps solutes out of cartilage canals and into the ECM, since solute concentrations in the ECM were found to be considerably higher under loading than under steady-state passive diffusion (Figures 1, 2 & 4). These concentration enhancements dissipated upon termination of dynamic loading (Figure 3), proving that solute transport against a concentration gradient was indeed occurring.
These concentration enhancements were similar to those observed earlier in dynamically loaded (avascular) hydrogel disks (Albro et al., 2008; Albro et al., 2010), suggesting that they also resulted from the same mechanism of momentum exchange with the tissue solid matrix. Solute enhancement factors (ĉDL / ĉPD) increased with solute molecular weight (Figure 2). This dependency was similarly observed in loaded hydrogels (Albro et al., 2008) and was expected since larger solutes exchange momentum more effectively with a deforming solid matrix due to increased hindrance (Mauck et al., 2003). Transferrin (80 kDa) concentration was enhanced less than the 70 kDa dextran, possibly due to the smaller hydrodynamic radius and reduced hindrance of this compact globular protein (4.3 nm for transferrin (Sakajiri et al., 2009) versus 7.4 nm for 70 kDa dextran, Lebrun and Junter, 1993). Since the enhancement of transferrin was similar to that of the smaller 10 kDa dextran (2.7 nm, Lebrun and Junter, 1993), other factors may play a role as well.
Solute pumping against a concentration gradient was further verified through the recovery test (Figure 3). Transferrin was selected for this test because, unlike dextran and fluorescein, it is a biologically relevant molecule. This result was also expected from theory (Mauck et al., 2003), since cessation of loading would stop pumping, and enhanced concentrations would dissipate as solutes diffuse back out of the tissue down their concentration gradient. Therefore, the observed enhanced concentrations were due to solute pumping and not some hypothetical permanent alteration to the system, such as binding or degradation.
Images of the tissue using confocal microscopy confirmed that pumping from the cartilage canals into the ECM did occur. These images showed localized presence of 70 kDa dextran in a narrow boundary layer around canals after only 30 seconds of loading (Figure 4A). As noted in the Methods, no solution remained in the canal lumen at the depth of the confocal imaging plane. These results suggested that rapid fluid convection into the canal upon initiation of loading provided a plentiful supply of solutes that were subsequently pumped into the ECM due to momentum exchange with the solid matrix. This rapid uptake over the multitude of canals in these tissue explants was responsible for the initial concentration jumps observed in assay measurements at 30 seconds of loading (Figure 1).
In contrast, 70 kDa dextran was only faintly present at the canal-ECM interface after any duration of passive uptake (Figures 4B, D & F), confirming the availability of solutes in the canals but demonstrating that concentration enhancement did not occur at these interfaces in the absence of loading. Images also demonstrated that under prolonged periods of dynamic loading, solutes spread from the boundary layers surrounding canals deeper into the tissue, as became evident after 2 hours (Figure 4C) and after 20 hours, when it occupied most of the ECM surrounding the canals (Figure 4E). The concentration of dextran in the surrounding tissue was evidently higher than that under passive uptake at any time point. Similar results were also observed for 3 kDa dextran and transferrin (Figures 4G–J). In contrast, there was no observable difference in fluorescein concentration between dynamic loading and passive diffusion (Figure 4K & L), consistent with assay measurements (Figure 2).
This demonstration that dynamic loading can pump solutes out from channels localized in the interior of biological tissues may have broad physiological implications. It has traditionally been understood that solutes can transport into or out of blood vessels through a multitude of pathways such as transcytosis (Jennings and Florey, 1967; Simionescu et al., 1975; Simionescu et al., 1973), passive diffusion, or through transvascular fluid convection induced by hydrostatic or oncotic pressure gradients (Michel and Curry, 1999; Pappenheimer, 1953; Pappenheimer et al., 1951; Pappenheimer and Soto-Rivera, 1948; Renkin, 1977; Renkin et al., 1977a; Renkin et al., 1977b; Rippe and Haraldsson, 1994; Rippe et al., 1979). All of these pathways are believed to occur down the natural solute transvascular concentration gradient. The results of this study suggest that solutes may get pumped out of the microvasculature through dynamic loading as well, and that this mechanism can give rise to enhanced concentrations in the surrounding tissue. Concentration enhancements in this study were observed for a wide range of molecular weights (3 kDa to 80 kDa), suggesting that solute pumping may occur in physiologic settings for a variety of proteins, hormones, and growth factors. This mechanism may potentially occur in any vascularized tissue that is subjected to cyclic mechanical forces.
Solute pumping may potentially be influenced by the type of vasculature involved, such as the type of fenestrations and continuity of the basal lamina. In terms of cartilage transport, investigations through electron microscopy have demonstrated that cartilage canals possess enlarged fenestrated capillaries surrounded by a highly discontinuous basement membrane (Hunt et al., 1979). These structural observations suggest that testing live versus dead tissue may not significantly influence solute transport and that a fully intact endothelium may not qualitatively influence active pumping into the cartilage matrix, though this assumption may need to be confirmed in future studies. Similarly, blood pressure gradients within cartilage canals are negligible compared to pressure gradients induced by dynamic loading of the tissue. Therefore, it is unlikely that results will be different in vivo due to this factor.
The results of this study may have more specific implications for cartilage metabolic activity. Over the years, there has been considerable interest as to the physiologic role and mode of formation of vascular canals in the immature cartilage epiphyses. These canals extend into the tissue from the vasculature of the perichondrium and each canal consists of a single arteriole and venule, interconnected by a series of large capillaries. Most evidence attributes their function to promoting the osteogenesis of secondary ossification centers, the growth of the epiphysis through the supplementation of mesenchymal stem cells, and the metabolic exchange of nutrients and waste products (Blumer et al., 2008; Blumer et al., 2005; Carlson et al., 1986; Carlson et al., 1989; Ganey et al., 1995; Haines, 1933; Haines, 1974; Lutfi, 1970; Ytrehus et al., 2004a; Ytrehus et al., 2004b). The results of this study provide further evidence towards the nutritional role of cartilage canals based on their potential to significantly enhance the concentrations of nutrients in cartilage tissue under a physiologic loading regimen.
Upon skeletal maturation vascular canals undergo a physiologic regression process, whereby the tissue becomes entirely avascular and nutrients are subsequently limited to accessing the tissue from synovial fluid via the articular surface (Honner and Thompson, 1971; Maroudas et al., 1968). Considerable efforts have been made to understand how chondrocytes are provided sufficient access to large macromolecules, despite this avascularity and the transport hindrance caused by the dense ECM (Maroudas, 1970; Maroudas, 1976; Nimer et al., 2003; Quinn et al., 2000; Torzilli et al., 1987). The earliest suggestion that cyclic compression could increase the concentration of large solutes in cartilage appeared in a brief report by Maroudas et al., who also noted a similar response under static loading conditions, and thus attributed it to temporary pore alterations (Maroudas, 1980). More recently, in vitro studies have demonstrated that dynamic loading can increase the desorption or uptake rate of solutes in articular cartilage explants (Bonassar et al., 2001; Evans and Quinn, 2006; O'Hara et al., 1990). In this context, the results of this study suggest that dynamic loading of cartilage may also actively pump solutes across the articular surface from the surrounding synovial fluid.
In summary, the incidence of this loading-induced transvascular solute pumping mechanism may significantly alter our understanding of the interaction of mechanical loading and tissue metabolism. In cartilage, as well as other biological tissues, it has been well recognized that mechanical stimulation can greatly enhance cellular metabolic activity (Jurvelin et al., 1986; Parkkinen et al., 1992; Sah et al., 1989; Salter and Field, 1960; Sood, 1971). The results of this study suggest that these observations may be an important manifestation of enhanced nutrient concentrations induced by dynamic loading.
Supplementary Material
Acknowledgments
This study was supported with funds from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the U.S. National Institutes of Health (AR46532, AR46568).
Appendix (Supplementary Data)
Methods
Materials
Articular cartilage explants were harvested from the femoral condyles and patellar grooves of 4–6 week old bovine calves with a biopsy punch (N=21 joints from 14 animals, n=572 explants). The bottom 1–5 mm of these explants was excised with a scalpel blade and approximately 750 μm of superficial cartilage was removed with a freezing stage microtome, producing cartilage disks with uniform dimensions (Ø6 × 3.5 mm), whose thickness spanned the vascularized middle and deep zones of this immature tissue (Klein et al., 2003). In the solute uptake study, explants were randomized into 44 testing groups: 5 solutes × 2 loading conditions (PD and DL) × 4 (fluorescein, 3 and 10 kDa dextran) or 5 (70 kDa dextran and transferrin) time points. Each group included 10 samples, for a total of 440 explants. In the recovery test, described below, samples were randomized into 6 testing groups: 1 solute (transferrin) × 2 loading conditions (PD and DL) × 3 time points. Each group included 6 samples, for a total of 36 explants. For the confocal imaging study, explants were randomized into 24 testing groups: 4 solutes × 2 loading conditions (PD and DL) × 3 time points. Each group included 4 samples, for a total of 96 explants. All explants were stored frozen in phosphate buffered saline (PBS) until testing.
One face of each cartilage disk was fixed to a thin acrylic square plate (6 × 6 × 1.5 mm) using a small amount of cyanoacrylate glue immediately before testing. This method of mechanical fixation was found to prevent the occurrence of abrasion during loading. Additionally, this configuration better mimics the articular cartilage physiological environment where cartilage is anchored to the underlying bone.
Dynamic Loading protocol
Dynamic compression was applied with a custom-made device that consisted of a stainless steel loading platen actuated through a motorized linear translation stage (Tolomatic, Hamel, MN). During testing, platen displacement was controlled continuously under a feedback loop using a displacement transducer to ensure accurate application of the desired deformation profile. Cartilage explants affixed to acrylic plates were placed in a stainless steel loading chamber.
Fluorescent Assay
At the completion of each test, samples of bathing solution were collected and stored for subsequent analysis while cartilage samples were removed and immediately processed. To measure the solute concentration in the disks, samples were excised from their acrylic plate, blotted with a Kimwipe, radially sectioned into quadrants, and placed in PBS-filled desorption tubes. Following a 48-hour desorption period, the interstitial solute content of samples was determined through a fluorescent assay as described previously (Albro et al., 2008).
Statistical Analysis
A two-way analysis of variance (α=0.05) was performed for the factors of time and loading condition (PD and DL) to determine differences in concentration for each tested solute. Statistical significance was set at p≤0.05. A one-tailed test using a T-statistic was performed to determine whether the enhancement ratio for each solute was significantly greater than unity.
Footnotes
Conflict of interest statement
We do not have any conflicts of interest with regard to this study and the materials contained herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152–3157. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Li R, Banerjee RE, Hung CT, Ateshian GA. Validation of theoretical framework explaining active solute uptake in dynamically loaded porous media. J Biomech. 2010;43:2267–2273. doi: 10.1016/j.jbiomech.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190:305–315. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Blumer MJ, Longato S, Richter E, Perez MT, Konakci KZ, Fritsch H. The role of cartilage canals in endochondral and perichondral bone formation: are there similarities between these two processes? J Anat. 2005;206:359–372. doi: 10.1111/j.1469-7580.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Hilley HD, Henrikson CK, Meuten DJ. The ultrastructure of osteochondrosis of the articular-epiphyseal cartilage complex in growing swine. Calcif Tissue Int. 1986;38:44–51. doi: 10.1007/BF02556594. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Hilley HD, Meuten DJ. Degeneration of cartilage canal vessels associated with lesions of osteochondrosis in swine. Vet Pathol. 1989;26:47–54. doi: 10.1177/030098588902600108. [DOI] [PubMed] [Google Scholar]
- Chahine NO, Albro MB, Lima EG, Wei VI, Dubois CR, Hung CT, Ateshian GA. Effect of dynamic loading on the transport of solutes into agarose hydrogels. Biophys J. 2009;97:968–975. doi: 10.1016/j.bpj.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44:413–459. doi: 10.1080/10408360701428257. [DOI] [PubMed] [Google Scholar]
- Evans RC, Quinn TM. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch Biochem Biophys. 2005;442:1–10. doi: 10.1016/j.abb.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Evans RC, Quinn TM. Solute convection in dynamically compressed cartilage. J Biomech. 2006;39:1048–1055. doi: 10.1016/j.jbiomech.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Fetter NL, Leddy HA, Guilak F, Nunley JA. Composition and transport properties of human ankle and knee cartilage. J Orthop Res. 2006;24:211–219. doi: 10.1002/jor.20029. [DOI] [PubMed] [Google Scholar]
- Filidoro L, Dietrich O, Weber J, Rauch E, Oerther T, Wick M, Reiser MF, Glaser C. High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med. 2005;53:993–998. doi: 10.1002/mrm.20469. [DOI] [PubMed] [Google Scholar]
- Ganey TM, Ogden JA, Sasse J, Neame PJ, Hilbelink DR. Basement membrane composition of cartilage canals during development and ossification of the epiphysis. Anat Rec. 1995;241:425–437. doi: 10.1002/ar.1092410318. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: effect of porosity. Ann Biomed Eng. 2004;32:1710–1717. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- Haines RW. Cartilage Canals. J Anat. 1933;68:45–64. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. Growth of Cartilage Canals in the Patella. J Anat. 1937;71:471–479. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. The pseudoepiphysis of the first metacarpal of man. J Anat. 1974;117:145–158. [PMC free article] [PubMed] [Google Scholar]
- Honner R, Thompson RC. The nutritional pathways of articular cartilage. An autoradiographic study in rabbits using 35S injected intravenously. J Bone Joint Surg Am. 1971;53:742–748. [PubMed] [Google Scholar]
- Hunt CD, Ollerich DA, Nielsen FH. Morphology of the perforating cartilage canals in the proximal tibial growth plate of the chick. Anat Rec. 1979;194:143–157. doi: 10.1002/ar.1091940110. [DOI] [PubMed] [Google Scholar]
- Jennings MA, Florey L. An investigation of some properties of endothelium related to capillary permeability. Proc R Soc Lond B Biol Sci. 1967;167:39–63. doi: 10.1098/rspb.1967.0012. [DOI] [PubMed] [Google Scholar]
- Jurvelin J, Kiviranta I, Tammi M, Helminen HJ. Effect of physical exercise on indentation stiffness of articular cartilage in the canine knee. Int J Sports Med. 1986;7:106–110. doi: 10.1055/s-2008-1025743. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Tammi M, Jurvelin J, Saamanen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6:188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, Thonar EJ, Sah RL. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- Laurell CB. What is the function of transferrin in plasma? Blood. 1951;6:183–187. [PubMed] [Google Scholar]
- Lebrun L, Junter GA. Diffusion of sucrose and dextran through agar gel membranes. Enzyme Microb Technol. 1993;15:1057–1062. doi: 10.1016/0141-0229(93)90054-6. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- Lutfi AM. Mode of growth, fate and functions of cartilage canals. J Anat. 1970;106:135–145. [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Heinemeier KM, Koskinen SO, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 2008;49:165–168. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10:365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Transport of solutes through cartilage: permeability to large molecules. J Anat. 1976;122:335–347. [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. In: The Joints and Synovial Fluid II. Sokoloff L, editor. Academic Press; New York: 1980. [Google Scholar]
- Maroudas A, Bullough P, Swanson SA, Freeman MA. The permeability of articular cartilage. J Bone Joint Surg Br. 1968;50:166–177. [PubMed] [Google Scholar]
- Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder R, de Visser SK, Bowden JC, Bostrom T, Pope JM. Diffusion tensor imaging of articular cartilage as a measure of tissue microstructure. Osteoarthritis Cartilage. 2006;14:875–881. doi: 10.1016/j.joca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- Nimer E, Schneiderman R, Maroudas A. Diffusion and partition of solutes in cartilage under static load. Biophys Chem. 2003;106:125–146. doi: 10.1016/s0301-4622(03)00157-1. [DOI] [PubMed] [Google Scholar]
- O'Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR. Passage of molecules through capillary wals. Physiol Rev. 1953;33:387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Soto-Rivera A. Effective osmotic pressure of the plasma proteins and other quantities associated with the capillary circulation in the hindlimbs of cats and dogs. Am J Physiol. 1948;152:471–491. doi: 10.1152/ajplegacy.1948.152.3.471. [DOI] [PubMed] [Google Scholar]
- Parkkinen JJ, Lammi MJ, Helminen HJ, Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992;10:610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- Quinn TM, Kocian P, Meister JJ. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys. 2000;384:327–334. doi: 10.1006/abbi.2000.2077. [DOI] [PubMed] [Google Scholar]
- Renkin EM. Multiple pathways of capillary permeability. Circ Res. 1977;41:735–743. doi: 10.1161/01.res.41.6.735. [DOI] [PubMed] [Google Scholar]
- Renkin EM, Joyner WL, Sloop CH, Watson PD. Influence of venous pressure on plasma-lymph transport in the dog's paw: convective and dissipative mechanisms. Microvasc Res. 1977a;14:191–204. doi: 10.1016/0026-2862(77)90018-8. [DOI] [PubMed] [Google Scholar]
- Renkin EM, Watson PD, Sloop CH, Joyner WM, Curry FE. Transport pathways for fluid and large molecules in microvascular endothelium of the dog's paw. Microvasc Res. 1977b;14:205–214. doi: 10.1016/0026-2862(77)90019-x. [DOI] [PubMed] [Google Scholar]
- Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994;74:163–219. doi: 10.1152/physrev.1994.74.1.163. [DOI] [PubMed] [Google Scholar]
- Rippe B, Kamiya A, Folkow B. Transcapillary passage of albumin, effects of tissue cooling and of increases in filtration and plasma colloid osmotic pressure. Acta Physiol Scand. 1979;105:171–187. doi: 10.1111/j.1748-1716.1979.tb06329.x. [DOI] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Sakajiri T, Yamamura T, Kikuchi T, Ichimura K, Sawada T, Yajima H. Absence of Binding Between the Human Transferrin Receptor and the Transferrin Complex of Biological Toxic Trace Element, Aluminum, Because of an Incomplete Open/Closed Form of the Complex. Biol Trace Elem Res. 2009 doi: 10.1007/s12011-009-8547-y. [DOI] [PubMed] [Google Scholar]
- Salter R, Field P. The Effects of Continuous Compression on Living Articular Cartilage: An Experimental Investigation. J Bone Joint Surg [Am] 1960;42:31–90. [Google Scholar]
- Simionescu N, Siminoescu M, Palade GE. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N, Simionescu M, Palade GE. Permeability of muscle capillaries to exogenous myoglobin. J Cell Biol. 1973;57:424–452. doi: 10.1083/jcb.57.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood SC. A study of the effects of experimental immobilisation on rabbit articular cartilage. J Anat. 1971;108:497–507. [PMC free article] [PubMed] [Google Scholar]
- Stockwell RA. The ultrastructure of cartilage canals and the surrounding cartilage in the sheep fetus. J Anat. 1971;109:397–410. [PMC free article] [PubMed] [Google Scholar]
- Torzilli PA, Adams TC, Mis RJ. Transient solute diffusion in articular cartilage. J Biomech. 1987;20:203–214. doi: 10.1016/0021-9290(87)90311-3. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. J Biomech. 1997;30:895–902. doi: 10.1016/s0021-9290(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Grande DA, Arduino JM. Diffusive properties of immature articular cartilage. J Biomed Mater Res. 1998;40:132–138. doi: 10.1002/(sici)1097-4636(199804)40:1<132::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br. 1957;39-B:358–394. doi: 10.1302/0301-620X.39B2.358. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Van Sickle DC. Cartilage canals, their morphology and distribution. Anat Rec. 1972;173:79–93. doi: 10.1002/ar.1091730107. [DOI] [PubMed] [Google Scholar]
- Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200–1211. [PubMed] [Google Scholar]
- Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, Akeson WH. The effect of prolonged physical training on the properties of long bone: a study of Wolff's Law. J Bone Joint Surg Am. 1981;63:780–787. [PubMed] [Google Scholar]
- Ytrehus B, Andreas Haga H, Mellum CN, Mathisen L, Carlson CS, Ekman S, Teige J, Reinholt FP. Experimental ischemia of porcine growth cartilage produces lesions of osteochondrosis. J Orthop Res. 2004a;22:1201–1209. doi: 10.1016/j.orthres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ytrehus B, Ekman S, Carlson CS, Teige J, Reinholt FP. Focal changes in blood supply during normal epiphyseal growth are central in the pathogenesis of osteochondrosis in pigs. Bone. 2004b;35:1294–1306. doi: 10.1016/j.bone.2004.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.