Abstract
MicroRNAs comprise a novel group of gene regulators implicated in the development of different types of cancer; however, their role in primary pigmented nodular adrenocortical disease (PPNAD) has not been elucidated. PPNAD is a bilateral adrenal hyperplasia often associated with Carney Complex (CNC), a multiple neoplasia syndrome; both disorders are caused by protein kinase A (PKA) regulatory subunit type 1A (PRKARIA)-inactivating mutations. We identified a 44 microRNA gene signature of PPNAD after comparing PPNAD and normal adrenal samples. Specifically, 33 microRNAs were up-regulated and 11 down-regulated in PPNAD relative to normal tissues. These results were validated by stem loop real-time PCR analysis. Comparison of microRNA microarray data with clinicopathological parameters revealed a negative correlation (r = −0.9499) between let-7b expression and cortisol levels in PPNAD patients. Integration of microRNA microarray with serial analysis of gene expression (SAGE) data together with bioinformatic algorithm predictions revealed nine microRNA-gene target pairs with potential role in adrenal pathogenesis. Using a PPNAD cellular system we validated that miR-449 was up-regulated and identified that targeted directly WISP2 expression. Furthermore, pharmacological inhibition of PKA resulted in suppression of miR-449 expression leading to up-regulation of its target gene WISP2. Overall, we investigated for the first time the microRNA profile and its clinical significance in PPNAD; these data also suggest that PKA through microRNA regulation affects the Wnt signaling pathway, which has been identified by SAGE and other studies as a primary mediator of PRKAR1A-related tumorigenesis.
Keywords: Cushing's Syndrome, adrenocortical tumors, primary pigmented adrenal hyperplasia, miR-449, WISP2
Introduction
Primary pigmented nodular adrenocortical disease (PPNAD) is a rare form of bilateral adrenal hyperplasia that is linked to Carney Complex (CNC); an autosomal dominant disease predisposing to a variety of lesions (1). PPNAD is the most frequent endocrine tumor in patients with CNC and it presents with excess cortisol secretion and symptoms of Cushing syndrome in early life (2). PPNAD is caused by inactivating mutations of the gene coding for the regulatory (R) subunit 1A of the protein kinase A (PKA) enzyme (PRKAR1A). According to biochemical studies, PPNAD tumors exhibit increased PKA activity upon cAMP stimulation (3) mainly attributed to overexpression of other regulatory subunits, deficient control of the catalytic subunit, and consequently dysregulation of the PKA holoenzyme (4). Recently, the gene expression profile was studied in PPNAD, using serial analysis of gene expression (SAGE) and revealed the differential expression of several important genes implicated in steroidogenesis and cell proliferation signaling pathways, between PPNAD and normal adrenal (5).
MicroRNAs are 21–25 nucleotide-long sequences that form a new class of gene regulators, specifically in the post-transcriptional level. MicroRNAs exert their actions while incorporated into the RNA-induced silencing complex (RISC) and result in mRNA degradation or translational repression depending on the degree of complementarity with the target gene (6). MicroRNA expression has been studied extensively in the past few years; hundreds of samples have been analyzed, establishing microRNA signatures in several types of cancer (7–9). In the present study, we identified a microRNA gene signature for PPNAD; furthermore, we found that let-7b was highly associated with midnight cortisol levels, an index of clinical severity of the Cushing syndrome caused by PPNAD tmors. In addition, using a PPNAD cell line, we identified inhibition of miR-449 and up-regulation of its target gene WISP2. These results suggest that PKA through microRNA regulation affects Wnt signaling pathway, which several other studies have shown is important in the regulation of PRKAR1A-related tumorigenesis and in adrenocortical oncogenesis in general.
Materials and Methods
Subjects
Patients were seen at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under the protocol 95-CH-0059, approved by the Institutional Review Board. All patients gave their informed consent and signed the related forms. We studied 10 patients diagnosed with Cushing syndrome caused by CNC and/or PPNAD (Supplementary Table 1). Adrenal samples were collected during bilateral adrenalectomy, dissected and kept in −80 °C until use. Four normal adrenal samples were used as controls; three of them were commercially available RNA adrenal samples (Ambion, Biochain); a single normal adrenal tissue sample obtained from a cadaver through the National Development and Research Institutes, Inc. (NDRI, New York, NY).
MicroRNA Expression Analyses
The RNA isolation was performed using the mirVana miRNA isolation Kit (Ambion, Inc, TX, USA) according to the manufacturer's instructions. TaqMan microRNA array assays were used in order to study the expression levels of 365 microRNAs as previously described (10). We validated our results with real time (RT) polymerase chain reaction (PCR), using the mirvana qRT-PCR miRNA detection kit and qRT-PCR primer sets, according to the manufacturer's instructions (Ambion, Inc, TX, USA). We used the U6 small nuclear RNA as internal control.
Real-Time PCR Analysis
We performed reverse transcriptase PCR using the AMV kit (Roche, Indianapolis, USA) and subsequently RT-PCR in the Light Cycler Instrument (Roche Molecular Systems, Alameda, CA). The oligonucleotide primers used for WISP2 were 5'-CACGCTGCCTGGTCTGTCTGGATC-3' (forward) and 5'-CACGCATAGGCTTGTATTC AGGAAC-3' (reverse). All the samples were processed in triplicates. The average value was used for the measurements and the results were normalized using the expression levels of the housekeeping gene GAPDH. Real-time PCR primers for FOSB, CCND2, INHA, STK19, CHGA and GPR107 genes have been described previously (5).
MicroRNA Target Prediction Methods
We used three databases to detect the putative microRNA gene-targets: miRBase (http//microrna.sanger.ac.uk), miRanda (http:/www. microrna.org) and Target scan version 4.2 (http://www.targetscan.org/index.html) databases. Then, we selected the commonly predicted microRNA targets from the three databases and those that were conserved in other species, aiming to the higher biological significance of our results. The data from this combined prediction analysis were compared with SAGE data that we have reported elsewhere (5). Knowing that microRNAs most commonly act by suppressing the expression of their target genes, we studied only the microRNA -gene target pairs with inversed correlation.
PPNAD Cell line
We used a cell line that was derived from the adrenocortical tissue of patient CAR 47.01 (Supplementary Table 1), as previously described (11, 12). The cell line has been frozen and regrown multiple times and continues to grow without any changes from the original report: PKA activity, in particular, in these cells remains high (12).
MicroRNA Transfection Experiments
PPNAD cells were seeded in 6-well plates and were transfected with 20 or 50 nM miR-449 (Ambion, Inc, TX, USA) using siPORT NeoFX transfection kit. siPORT NeoFX is a lipid transfection agent consisting of a mixture of lipid that spontaneously forms a complex of microRNAs and facilitates its transfer to target cells. Transfection with 50nM of scramble negative control microRNA was used as an internal control. No cell toxicity was detected due to the transfection agent (data not shown). RNA was extracted 24 and 48 hours after microRNA transfection and RT PCR analysis was performed as described above.
H89 treatment
PPNAD cells were treated with1uM of the PKA inhibitor H89; miR-449 expression was then evaluated by RT PCR analysis. WISP2 mRNA expression was evaluated 24h after H89 treatment by real-time PCR.
Luciferase reporter assay
HEK293 cells in 24-well plates were transfected using Fugene6 (Roche, Penzberg, Germany). Firefly luciferase reporter gene construct (WISP2 3'UTR in pEZX-MT01 vector) (200 ng) and 1 ng of the pRL-SV40 Renilla luciferase construct (for normalization) were co-transfected per well. Cell extracts were prepared 24h after transfection, and the luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega, WI, USA).
Results
MicroRNA Gene Signature in Primary Pigmented Nodular Adrenocortical disease (PPNAD)
We studied the expression of 365 microRNAs in 10 adrenal samples that we collected from PPNAD patients and compared them with four normal adrenal samples. We detected 44 differentially expressed microRNAs between PPNADs and normal samples. Specifically, 33 microRNAs were down-regulated, while 11 were up-regulated in PPNADs in comparison to normal samples (Figure 1). Real-time PCR analysis paralleled our microRNA array results and confirmed the differential expression of the 44 microRNAs in PPNAD compared to normal adrenal (Supplementary Figure 1). Among the highly down-regulated microRNAs, we detected miR-200c (−10.74 fold), miR-200b (−9.57 fold), miR-375 (−9.97 fold) and miR-449 (−8.42 fold). On the other hand, miR-594 (7.38 fold), miR-301 (7.57 fold) and miR-139 (5.33 fold) were the most highly up-regulated ones and the other microRNAs with increased expression had an increase of 1.84 to 3.94 fold.
Figure 1. PPNAD MicroRNA Gene Signature.
Heat map representation of 44 differentially expressed microRNAs between 10 PPNAD and 4 normal adrenal tissues. A color map is used to visualize the difference in expression; green, low expression; red, high expression. Thirty three microRNAs were down-regulated, while 11 were up-regulated in PPNAD relative to normal adrenal tissues.
Let-7b expression correlates with severity of Cushing syndrome due to PPNAD
We classified our patients on the basis of their midnight cortisol levels into three groups (Supplementary Table 1). The first group included the patients with midnight cortisol levels < 10μg/dl (patients 4, 6–8), the second had cortisol levels between 10–20 μg/dl (patients 1–3) and the third group included patients with cortisol levels >20μg/dl (patients 5, 9–10). Comparison between microRNA expression and midnight cortisol levels revealed a significant inverse correlation between let-7b expression and midnight cortisol levels (r = −0.9499) (Supplementary Figure 2).
Integration of microRNA with SAGE data together with bioinformatic algorithms predicts microRNA-gene target pairs in PPNAD
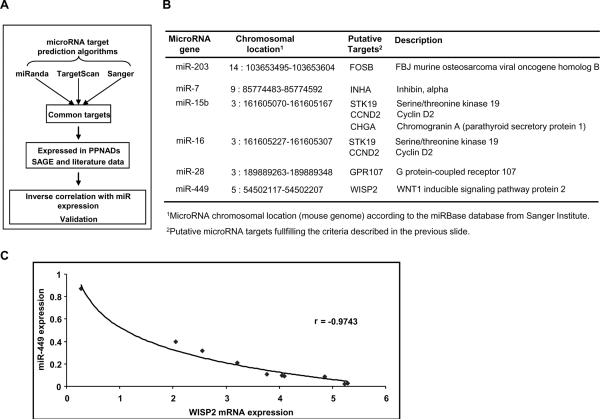
Since microRNA exert their biological functions through suppression of target genes, it is important to identify microRNA-gene target pairs. In order to validate our data we used experimental data; recently Huang et al, used microRNA with cDNA expression profiling data to identify human microRNA targets using Bayesian data analysis algorithm (13). To identify statistically significant microRNA gene targets we selected only the microRNA gene targets that were predicted by three different bioinformatic algorithms (Figure 2A). We then integrated our microRNA and SAGE data (5) and selected only the microRNA-gene target pairs that were expressed in PPNAD patients and were inversely correlated.
Figure 2. Prediction of microRNA- gene targets in PPNAD.
A, Strategy followed in order to identify microRNA-gene target pairs relevant to PPNAD: three main selection criteria were used; 1) to identify the common gene-targets predicted by miRanda, Targetscan and Sanger algorithms; 2) next, we selected microRNA gene targets that were expressed in PPNAD according to the previously published SAGE data; and 3) finally, since microRNAs are negative regulators of gene expression we selected only the gene targets that were inversely correlated with microRNA expression. B, MicroRNA-gene target pairs in PPNAD. The microRNA and its genomic location together with its gene targets are shown. C, Inverse correlation between miR-449 and WISP2 mRNA expression levels in PPNAD tissues (r = −0.9743).
We identified nine microRNA-gene target pairs that are potentially implicated in PPNAD pathogenesis (Figure 2B, Supplementary Figure 3). Specifically miR-203 potentially targets murine osteosarcoma viral oncogene homolog B (FOSB), while miR-7 potentially targets inhibin alpha (INHA). Both miR-15 and miR-16 potentially target serine/threonine kinase 19 (STK19) and cyclin D2 (CCND2); also miR-16 potentially regulates chromogranin A (CHGA) expression. Additionally, miR-7 potentially regulates G-protein coupled receptor 107 (GPR107) and miR-449 is predicted to regulate WNT1 inducible signaling pathway protein 2 (WISP2). Real-time PCR analysis of these nine microRNA-gene target pairs revealed that miR-449 – WISP2 pair was the highest inversely correlated in our PPNAD tissues (r=−0.9743) (Figure 2C, Supplementary Figure 4).
MiR-449 regulates WISP2 expression in PPNAD cellular system
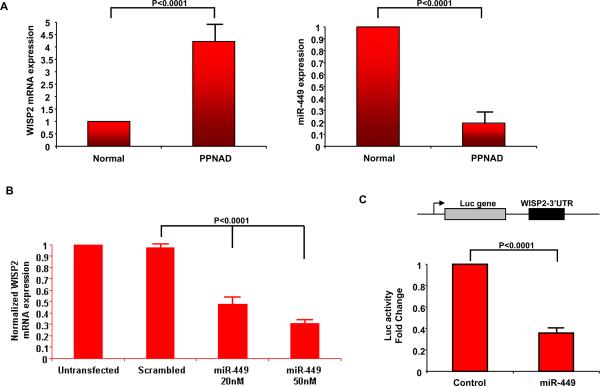
Due to the fact that miR-449 was one of the highest down-regulated microRNAs in PPNAD and predicted to regulate WISP2, an important player of Wnt signaling pathway, we further studied the role of miR-449 – WISP2 interaction in PPNAD pathogenesis using a cell line derived from a PPNAD patient. In these PPNAD cells we identified that WISP2 was highly expressed (3.6 fold) in comparison to normal adrenal (Figure 3A). On the other hand, miR-449 expression was down-regulated in PPNAD cells. The inverse correlation between miR-449 and WISP2 mRNA expression levels suggested that miR-449 regulates Wnt pathway in PPNAD. According to our integrative analysis we found that miR-449 targets directly WISP2 (Figure 2, Supplementary Figure 3). To test the potential interaction between miR-449 and WISP2, we overexpressed miR-449 in the same PPNAD cells and examined WISP2 mRNA expression. MiR-449 overexpression (50 nM) suppressed >70% WISP2 mRNA expression (Figure 3B). To identify if miR-449 regulated directly WISP2 through binding in the 3'UTR we performed luciferase assay which revealed that miR-449 inhibited >60% WISP2 3'UTR luciferase activity, suggesting that miR-449 regulates directly WISP2 expression (Figure 3C).
Figure 3. miR-449 regulates directly WISP2 expression.
A, Real-time PCR analysis of WISP2 and miR-449 expression levels in a PPNAD cell line. WISP2 is overexpressed while miR-449 is down-regulated in PPNAD cells relative to normal adrenal tissue. B, Transfection of miR-449 in our PPNAD cell line (20nM, 50nM) reduced WISP2 expression (50% and >70% suppression of mRNA expression respectively). C, Luciferase assay after transfection of miR-449 (50 nM) together with luciferase vactor harboring the 3'UTR of WISP2 gene. MiR-449 inhibited more than 60% of WISP2 3'UTR luciferase activity.
PKA activates Wnt pathway through miR-449 inhibition
In order to detect if miR-449 is regulated by PKA, we treated PPNAD cells with high PKA activity (13) with H89 (1uM) and tested miR-449 expression. Inhibition of PKA activity by H89 increased miR-449 expression (Figure 4A); moreover, the increase of miR-449 was followed by a decrease in WISP2 expression (Figure 4B). To further investigate if PKA affects WISP2 expression through miR-499, we transfected the same PPNAD cells with increasing concentrations of the inhibitor of miR-449 (as-miR-449) together with H89 (Figure 4C). H89, which up-regulated miR-449 expression, down-regulated by more than 60% WISP2 mRNA (lane 2), while treatment with as-miR-499 blocked the inhibitory effect of H89 (lanes 4–6). These data suggested that PKA activation blocks miR-449 expression and miR-449 inhibition allows up-regulation of its target gene WISP2.
Figure 4. PKA affectsWISP2expression through miR-449 regulation.
A, Inhibition of PKA activity using PKA inhibitor H89 (at 1uM) increased miR-449 expression; and B, decreased the expression of WISP2 mRNA expression. C, Real-time PCR analysis of WISP2 expression after H89 treatment together with increasing concentrations of as-miR-449 (20, 50, 100 nM). Tranfection with negative control (100 nM) microRNA was used as a transfection control.
Discussion
This is the first study of microRNAs in PPNAD and, indeed, in any form of adrenal hyperplasia. We detected 44 microRNAs which were differentially expressed in PPNAD versus normal adrenal tissue. The PPNAD microRNA signature revealed an aggressive phenotype that is similar to the microRNA profiles of various malignancies: it is characterized by significant down-regulation of microRNAs (33/44). According to previous reports, in carcinogenesis there is mainly suppression of miRNA expression (14). Several of the microRNAs identified down-regulated in PPNAD relative to normal adrenal tissues, have been implicated in tumorigenesis. Specifically, 4 members of let-7 family (let-7a, let-7b, let-7c, let-7g) were down-regulated in PPNADs suggesting that the aggressiveness and increased cell proliferation of PPNADs may be also due to let-7 down-regulation. Let-7 family is a very important microRNA family in cancer: in pre-cancerous conditions, let-7 members are found down-regulated. Let-7 seems to have a tumor suppressor role; its loss has been correlated with increased cell proliferation, activation of survival pathways and anti-apoptotic mechanisms (15, 16). Furthermore, miR-200b and miR-200c, both highly down-regulated microRNAs in PPNAD tissues, are important regulators in epithelial-mesenchymal transition (17). We also identified miR-449 as one of the highest down-regulated microRNAs in PPNAD; its function has not been studied previously. Additionally, we detected microRNAs with increased expression in PPNAD tissues. Among them, miR-106b has been found with altered expression in prostate cancer and targets the p21 and E2F1 molecules (18), while miR-210 has been found up-regulated in breast cancer and is regulated by the hypoxia inducible factor (HIF1A) (19).
To discover a putative association between microRNA expression levels and the severity of the disease we studied the correlation of different clinicopathological parameters such as diurnal cortisol levels, urinary free cortisol levels and 17-OH-steroid with the PPNAD microRNA signature. All these parameters are important diagnostic factors of PPNAD and previous studies have shown that midnight cortisol levels correlate with disease severity (20). Let-7b was found negatively correlated to the above clinical index.
It is known that microRNAs exert their biological functions through suppression of their target genes. Several bioinformatic algorithms have been constructed in order to predict microRNA gene targets. Most of these algorithms search for sequence complementarity between the microRNA and the 3' UTR of the gene target. These algorithms predict hundreds of potential gene targets, which can not all be experimentally validated. Previous studies have tried to identify microRNA gene targets using cDNA microarray data (21). To clarify the biological effects of microRNAs in PPNAD, we identified their putative gene targets using the available bioinformatic algorithms and we integrated the computationally predicted target genes with expression data on PPNAD that we have previously published (5). Interestingly, the most significant correlation was found between miR-449 and WISP2 expression levels, supporting the potential involvement of Wnt signaling in PPNAD that has been suggested by other studies (5, 22). The Wnt signaling pathway regulates a vast range of cellular functions such as growth and differentiation and is critical during embryonic development (23). In familial adenomatous polyposis (FAP), mutations of the adenomatosis polyposis coli (APC) gene activate the Wnt pathway and lead to the formation of adrenal adenomas (24, 25). Interestingly, expression studies in both MMAD/AIMAH (26) and PPNAD (5) have indicated overexpression of genes involved in the Wnt pathway such as axin1 (AXIN1), WNT1-inducible signaling pathway protein 2 (WISP2), catenin- β1 (CTNNB1) and glycogen synthase kinase −3β (GSK3B).
Our findings here, propose that microRNAs potentially link PKA and Wnt signaling pathways in the context of PPNAD. The data suggest that microRNAs are a mechanism that might be involved in the formation of PPNAD nodules and the consequent activiation of the Wnt pathway shown by many studies (5, 22).
Supplementary Material
Acknowledgements
This work was supported by NIH intramural project Z01-HD-000642-04 to Dr. C.A. Stratakis and, in part, by the University of Crete, School of Medicine, Post-Graduate Program (to Drs. Bimpaki and Stratakis).
References
- 1.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–83. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin- independent Cushing syndrome. Endocr Dev. 2008;13:117–32. doi: 10.1159/000134829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 4.Boikos SA, Stratakis CA. Carney complex: pathology and molecular genetics. Neuroendocrinology. 2006;83:189–99. doi: 10.1159/000095527. [DOI] [PubMed] [Google Scholar]
- 5.Horvath A, Mathyakina L, Vong Q, et al. Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab. 2006;91:584–96. doi: 10.1210/jc.2005-1301. [DOI] [PubMed] [Google Scholar]
- 6.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 7.Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–5. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 9.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–5. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 10.Thum T, Galuppo P, Wolf C, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–67. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 11.Stratakis CA, Jenkins RB, Pras E, et al. Cytogenetic and microsatellite alterations in tumors from patients with the syndrome of myxomas, spotty skin pigmentation, and endocrine overactivity (Carney complex) J Clin Endocrinol Metab. 1996;81:3607–14. doi: 10.1210/jcem.81.10.8855810. [DOI] [PubMed] [Google Scholar]
- 12.Nesterova M, Bossis I, Wen F, Horvath A, Matyakhina L, Stratakis CA. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type Allele and other protein kinase A subunits. J Clin Endocrinol Metab. 2008;93:565–71. doi: 10.1210/jc.2007-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang JC, Babak T, Corson TW, et al. Using expression profiling data to identify human microRNA targets. Nat Methods. 2007;4:1045–9. doi: 10.1038/nmeth1130. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 16.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–9. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 18.Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–70. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin- independent Cushing syndrome) Endocr Dev. 2008;13:117–32. doi: 10.1159/000134829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang JC, Babak T, Corson TW, et al. Using expression profiling data to identify human microRNA targets. Nat Methods. 2007;4:1045–9. doi: 10.1038/nmeth1130. [DOI] [PubMed] [Google Scholar]
- 22.Stratakis CA. New genes and/or molecular pathways associated with adrenal hyperplasias and related adrenocortical tumors. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.11.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–91. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naylor EW, Gardner EJ. Adrenal adenomas in a patient with Gardner's syndrome. Clin Genet. 1981;20:67–73. doi: 10.1111/j.1399-0004.1981.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 25.Bertherat J, Groussin L, Bertagna X. Mechanisms of disease: adrenocortical tumors-molecular advances and clinical perspectives. Nat Clin Pract Endocrinol Metab. 2006;2:632–41. doi: 10.1038/ncpendmet0321. [DOI] [PubMed] [Google Scholar]
- 26.Bourdeau I, Antonini SR, Lacroix A, et al. Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene. 2004;23:1575–85. doi: 10.1038/sj.onc.1207277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.