Abstract
Context
High levels of the phosphate regulating hormone, fibroblast growth factor 23 (FGF23), associate with mortality in patients with end-stage renal disease (ESRD), but little is known about its relationship with adverse outcomes in the much larger population of patients with earlier stages of chronic kidney disease (CKD).
Objective
Evaluate FGF23 as a risk factor for adverse outcomes in patients with CKD.
Design, Setting and Participants
A prospective study of 3,879 participants with CKD stages 2 – 4 who enrolled in the Chronic Renal Insufficiency Cohort between June 2003 and September 2008.
Main Outcome Measures
All-cause mortality and ESRD.
Results
At enrollment, mean estimated glomerular filtration rate (eGFR) was 42.8 ± 13.5 ml/min/1.73m2, and median FGF23 was 145 (interquartile range [IQR] 96 – 239) reference units/ml (RU/ml). During a median follow-up of 3.5 (IQR 2.5 – 4.4) years, 266 participants died (20.3/1000 person-years) and 410 reached ESRD (33.0/1000 person-years). Higher FGF23 levels independently associated with a greater risk of death in adjusted analyses of FGF23 on a continuous scale (hazard ratio [HR] per SD of lnFGF23, 1.5; 95%CI 1.3 – 1.7) or in quartiles (quartile 1, reference; quartile 2, HR 1.3; 95%CI 0.8 – 2.2; quartile 3, HR 2.0; 95%CI 1.2 – 3.3; quartile 4, HR 3.0; 95%CI 1.8 – 5.1). FGF23 was not independently associated with ESRD in adjusted analyses of the entire cohort, however, the effect was modified by eGFR (P for interaction = 0.005), which was the strongest predictor for ESRD. FGF23 independently associated with significantly greater risk of ESRD among participants with eGFR 30 – 44 (HR 1.3 per SD of lnFGF23; 95%CI 1.04 – 1.6) and ≥ 45 (HR 1.7; 95%CI 1.1 – 2.4), but not < 30 ml/min/1.73m2.
Conclusion
Elevated FGF23 is an independent risk factor for ESRD in patients with relatively preserved kidney function and for mortality across the spectrum of CKD.
Introduction
Fibroblast growth factor 23 (FGF23) is an endocrine hormone that regulates phosphorus metabolism. FGF23 levels increase progressively as kidney function declines, eventually reaching concentrations in patients with chronic kidney disease (CKD) that are among the highest in any condition commonly encountered in clinical practice.1 FGF23 levels increase in CKD as an appropriate physiological adaptation to maintain neutral phosphate balance and normal serum phosphate levels in the setting of reduced renal capacity for phosphate excretion. Preventing increases in serum phosphate is likely beneficial in CKD given reports of greater risk of adverse renal and cardiovascular outcomes in association with overt hyperphosphatemia and even modest elevations in serum phosphate within the normal range.2, 3 However, small studies suggest that elevated FGF23 is an independent risk factor for mortality in dialysis patients,4, 5 and for more rapid loss of kidney function in earlier stages of CKD.6, 7 Whether FGF23 is associated with greater risks of mortality and end-stage renal disease (ESRD) in the much larger population of patients with earlier stages of CKD is unknown. We tested the hypothesis that elevated FGF23 is an independent risk factor for death and ESRD in a large, racially and ethnically diverse, prospective cohort study of individuals with CKD stages 2 through 4.
Methods
Study Population
The Chronic Renal Insufficiency Cohort (CRIC) Study is a multi-center, prospective observational study of risk factors for cardiovascular disease, progression of CKD, and mortality.8 Individuals aged 21 to 74 years with an eGFR of 20 – 70 ml/min/1.73m2 were enrolled between June 2003 – March 2007 (n = 3,612). Since CKD and its associated adverse outcomes are more common in minorities,9 self-reported blacks were oversampled, and the ancillary Hispanic CRIC (HCRIC) study enrolled 327 additional self-reported Hispanic participants through September 2008. Exclusion criteria included inability to consent, institutionalization, enrollment in other studies, pregnancy, New York Heart Association class III–IV heart failure, HIV, cirrhosis, myeloma, polycystic kidney disease, renal cancer, recent chemotherapy or immunosuppressive therapy, organ transplantation, or prior treatment with dialysis for > 1 month. The protocol was approved by the institutional review board at each study site (eMethods), and participants provided written informed consent.
Exposure and Outcomes
The primary exposure was plasma FGF23, which was measured in baseline samples from 3,879 of the 3,939 total CRIC and HCRIC participants after a single thaw. The central CRIC laboratory used a second generation C-terminal assay (Immutopics, San Clemente, CA) to measure FGF23 in duplicate with a mean intra-assay coefficient of variation < 5%. Results from C-terminal and intact FGF23 assays are highly correlated,4 and biologically active FGF23 is accurately measured by either assay.10 There was no significant difference in eGFR between the study population and the 60 participants who were excluded because they had inadequate sample volumes for assay of FGF23 (45.0 ± 13.5 vs. 42.8 ± 13.5 ml/min/1.73m2). Clinical data were collected at the baseline visit by interview and questionnaire, and additional laboratory tests of blood and urine were measured centrally using standard assays (eMethods). Outcomes included all-cause mortality and ESRD, defined as initiation of dialysis or kidney transplantation. Participants were followed until the occurrence of death, voluntary withdrawal from the study, loss to follow-up, or until December 31, 2008, when the database was locked for analysis. During the longitudinal observation period, there was > 90% retention of participants.
Statistical Analysis
We used descriptive statistics to compare clinical characteristics according to baseline FGF23 levels and examined Spearman correlations between FGF23, eGFR and other laboratory values. We used time to event analyses to examine risks of outcomes according to baseline FGF23. FGF23 was expressed as a continuous variable with hazard ratios (HR) calculated per standard deviation (SD) increment of natural log (ln) FGF23, and in quartiles, with the lowest quartile defined as the reference group. We used Cox proportional-hazards regression to examine unadjusted and multivariable-adjusted relationships between FGF23 and outcomes. We hierarchically adjusted for demographic factors (age, sex, race, and ethnicity), eGFR (based on the modified Modification of Diet in Renal Disease [MDRD] equation11) and other CKD-specific risk factors (urinary albumin to creatinine ratio [ACR], hemoglobin, and serum albumin), traditional cardiovascular risk factors and medications (systolic blood pressure [SBP], body mass index [BMI], diabetes, smoking, low density lipoprotein [LDL], prior history of coronary artery disease, congestive heart failure, stroke and peripheral vascular disease, and use of cardio- and renoprotective medications (aspirin, beta blockers, statins, and angiotensin converting enzyme inhibitors [ACEI] or angiotensin II receptor blockers [ARB]), and levels of mineral metabolites (serum calcium, phosphate and parathyroid hormone [PTH]). All adjusted models were stratified by study site to account for potential variability in baseline hazards across centers. We used Schoenfeld residuals to confirm the proportionality assumption.
Stratified and Sensitivity Analyses
Since reduced kidney function is an independent risk factor for ESRD and death,12, 13 we performed pre-specified stratified analyses by baseline eGFR and tested for interaction with FGF23. We also performed stratified analyses and tests for interaction with FGF23 for the individual CKD-specific risk factors, cystatin C, traditional cardiovascular risk factors, and other mineral metabolites. Since death precludes the occurrence of future ESRD, we used competing risk regression14 in a sensitivity analysis of ESRD. Twenty participants who died after reaching ESRD were included in the primary analysis of mortality, however, since incident ESRD increases the subsequent hazard of death, we performed an additional sensitivity analysis of mortality in which we censored at the time of ESRD. Phosphate binders and active vitamin D therapy can modulate FGF23 levels15, 16 and are associated with survival.17, 18 We adjusted for use of these medications and for use of vitamin D supplements, and performed a sensitivity analysis that excluded participants who were receiving these agents at enrollment. To test for potential confounding by vitamin D levels, we examined unadjusted, demographics- and vitamin D level-adjusted associations between lnFGF23 and outcomes in the 333 participants with 1,25-dihydroxyvitamin D and 25-dihydroxyvitamin D measurements at baseline, and the 1159 participants with these measurements at the 1-year follow up visit.
To determine if the results were robust to alternate measures of kidney function, we repeated the main analysis after substituting MDRD-based eGFR with cystatin C and direct iothalamate GFR measurements that were available in 1409 participants. To investigate if the relationship between FGF23 and mortality might represent more accurate capture of residual confounding introduced by imprecision in the measures of renal function, we assessed the specificity of the FGF23 results by also examining risk of death according to PTH, which correlated with eGFR to a similar extent as FGF23. Finally, since fractional excretion of phosphate (FePi = [urine phosphate × serum creatinine] / [serum phosphate × urine creatinine] × 100%) is a readily available clinical measure of the phosphaturic action of FGF23 (higher FGF23 causes increased FePi),19 we explored the utility of FePi as a surrogate measure of the effect of FGF23 by substituting it for FGF23 in the fully-adjusted mortality model. Analyses were performed with SAS 9.2 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided, and P values < 0.05 were considered significant.
Results
Baseline characteristics are presented in Table 1 for the overall population and according to FGF23 quartiles. The mean (± SD) eGFR at the baseline visit was 42.8 ± 13.5 ml/min/1.73m2. Although the mean serum phosphate was 3.7 ± 0.7 mg/dl, and 89% of participants had normal phosphate levels (<4.6 mg/dl), the median FGF23 was 145 RU/ml (interquartile range [IQR] 96 – 239), which is > 3-fold higher than the median of 43 RU/ml (IQR 29 –72) in a population with a low prevalence of CKD.20 FGF23 correlated with eGFR (r = − 0.52, P <0.001), cystatin C (r = 0.59, P <0.001), serum phosphate (r = 0.35, P <0.001), PTH (r = 0.37, P <0.001), FePi (r = 0.26, P <0.001), and hemoglobin (r = − 0.36, P <0.001). After FGF23, PTH was the next closest correlate of eGFR (r = –0.47, P<0.001).
Table 1.
Baseline characteristics in all participants and according to quartiles of FGF23
| Fibroblast growth factor 23, RU/ml | All Participants N=3879 145.5 |
Quartile 1 N = 969 < 95.8 |
Quartile 2 N = 970 95.8 – 145.4 |
Quartile 3 N = 970 145.5 – 239.1 |
Quartile 4 N = 970 ≥239.2 |
|---|---|---|---|---|---|
| Age, years | 58.2 ± 11.0 | 56.6 ± 11.3 | 58.7 ± 10.9 | 59.0 ± 10.8 | 58.3 ± 10.9 |
| Female, No. (%) | 1738 (44.8) | 350 (36.1) | 385 (39.7) | 455 (46.9) | 548 (56.5) |
| Black, No. (%) | 1620 (41.8) | 404 (41.7) | 374 (38.6) | 386 (39.8) | 456 (47.0) |
| Hispanic, No. (%) | 495 (12.8) | 72 (7.4) | 138 (14.2) | 138 (14.2) | 147 (15.1) |
| Hypertension, No. (%) | 3339 (86.1) | 731 (75.4) | 843 (87.0) | 872 (89.9) | 893 (92.1) |
| Diabetes, No. (%) | 1879 (48.4) | 291 (30.0) | 443 (45.7) | 552 (56.9) | 593 (61.1) |
| Coronary artery disease, No. (%) | 853 (22.0) | 146 (15.1) | 209 (21.5) | 237 (24.4) | 261 (26.9) |
| Congestive heart failure, No. (%) | 376 (9.7) | 33 (3.4) | 66 (6.8) | 100 (10.3) | 177 (18.2) |
| Stroke, No. (%) | 385 (9.9) | 77 (7.9) | 79 (8.1) | 117 (12.1) | 112 (11.5) |
| Peripheral vascular disease, No. (%) | 258 (6.6) | 33 (3.4) | 41 (4.2) | 74 (7.6) | 110 (11.3) |
| Current smoking, No. (%) | 508 (13.1) | 76 (7.8) | 97 (10.0) | 132 (13.6) | 203 (20.9) |
| Body mass index, kg/m2 | 32.1 ± 7.8 | 30.6 ± 6.7 | 31.5 ± 7.0 | 32.4 ± 7.8 | 33.9 ± 9.1 |
| Systolic blood pressure, mm Hg | 128.6 ± 22.2 | 123.6 ± 19.5 | 126.9 ± 21.6 | 131.8 ± 23.1 | 132.0 ± 23.3 |
| Medication use, No. (%) | |||||
| Aspirin | 1651 (42.9) | 367 (38.2) | 424 (44.1) | 427 (44.4) | 433 (44.8) |
| Beta blockers | 1899 (49.3) | 346 (36.0) | 447 (46.5) | 516 (53.6) | 590 (61.0) |
| Statins | 2127 (55.2) | 432 (44.9) | 537 (55.9) | 592 (61.5) | 566 (58.5) |
| ACE Inhibitors or ARBs | 2650 (68.8) | 581 (60.5) | 690 (71.8) | 707 (73.5) | 672 (69.5) |
| Phosphate binders | 270 (7.0) | 49 (5.1) | 59 (6.1) | 66 (6.9) | 96 (9.9) |
| Active vitamin D | 124 (3.2) | 14 (1.5) | 14 (1.5) | 36 (3.7) | 60 (6.2) |
| Nutritional vitamin D | 394 (10.2) | 95 (9.9) | 110 (11.4) | 87 (9.0) | 102 (10.5) |
| Laboratory Results | |||||
| Creatinine, mg/dl | 1.8 ± 0.6 | 1.5 ± 0.4 | 1.7 ± 0.5 | 1.9 ± 0.6 | 2.2 ± 0.8 |
| eGFR, ml/min/1.73m2 | 42.8 ± 13.5 | 52.1 ± 12.1 | 45.5 ± 11.7 | 39.2 ± 10.9 | 34.3 ± 12.2 |
| eGFR > 60 ml/min/1.73m2, No. (%) | 401 (10.3) | (23.5) | (12.0) | (2.8) | (3.1) |
| eGFR 30 – 60 ml/min/1.73m2, No. (%) | 2720 (70.1) | (73.4) | (79.2) | (73.4) | (54.5) |
| eGFR < 30 ml/min/1.73m2, No. (%) | 758 (19.5) | (3.0) | (8.9) | (23.8) | (42.4) |
| Urine albumin/creatinine ratio, mg/g | 52.1 (8.7 – 460.9) | 14.5 (5.0 – 121.7) | 32.2 (6.5 – 275.0) | 102.9 (15.0 – 713.2) | 212.7 (28.4 – 1277.3) |
| Albumin, g/dl | 3.9 ± 0.5 | 4.0 ± 0.4 | 4.0 ± 0.4 | 3.9 ± 0.5 | 3.8 ± 0.5 |
| Cystatin C, mg/l | 1.5 ± 0.5 | 1.1 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.4 | 2.0 ± 0.6 |
| Calcium, mg/dl | 9.2 ± 0.5 | 9.2 ± 0.4 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.1 ± 0.6 |
| Phosphate, mg/dl | 3.7 ± 0.7 | 3.4 ± 0.5 | 3.6 ± 0.6 | 3.8 ± 0.6 | 4.1 ± 0.8 |
| Fractional excretion of phosphate (%) | 26.5 (19.5 – 36.8) | 23.0 (17.8– 30.2) | 25.1 (18.7 – 33.9) | 27.8 (21.1 – 38.9) | 32.5 (22.8 – 44.4) |
| Parathyroid hormone, pg/ml | 54.0 (35.0 – 89.0) | 40.0 (29.0 – 59.0) | 47.4 (32.4 – 73.2) | 62.8 (40.0 – 105.0) | 82.4 (47.0 – 137.0) |
| 25-hydroxyvitamin D, ng/ml* | 21.3 ± 10.8 | 22.0 ± 10.1 | 24.0 ± 11.6 | 21.5 ± 10.8 | 16.6 ± 9.9 |
| 1,25-dihydroxyvitamin D, ng/ml* | 28.7 ± 10.1 | 31.9 ± 11.0 | 29.4 ± 9.6 | 25.6 ± 8.8 | 25.8 ± 9.2 |
| Hemoglobin, g/dl | 12.6 ± 1.8 | 13.5 ± 1.6 | 12.8 ± 1.7 | 12.4 ± 1.6 | 11.8 ± 1.8 |
| LDL, mg/dl | 102.6 ± 35.3 | 107.0 ± 33.1 | 102.1 ± 33.4 | 101.3 ± 36.3 | 99.8 ± 38.0 |
Abbreviations: ACE Inhibitors, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; LDL, low density lipoprotein. Values are No. (%), means ± standard deviation, medians (interquartile range).
25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels were available in 333 and 332 participants, respectively.
FGF23 and Risks of Clinical Outcomes
During a median follow-up of 3.5 years, 266 participants died (20.3/1000 person-years) and 410 reached ESRD (33.0/1000 person-years). Median FGF23 levels were significantly higher in those who died (234, IQR 142 – 419 RU/ml) or reached ESRD (236, IQR 160 – 372 RU/ml) compared with those who remained event-free (133, IQR 91 – 210 RU/ml; P<0.001 for each).
Mortality
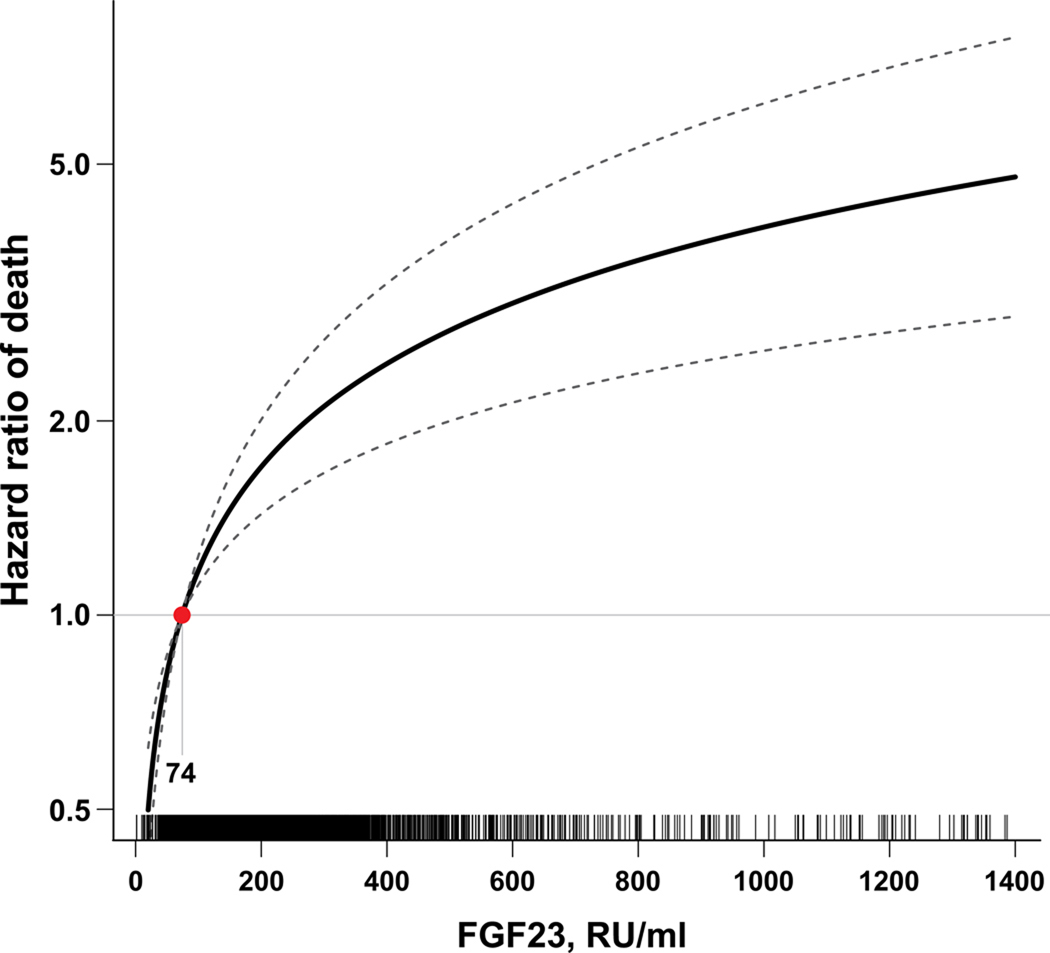
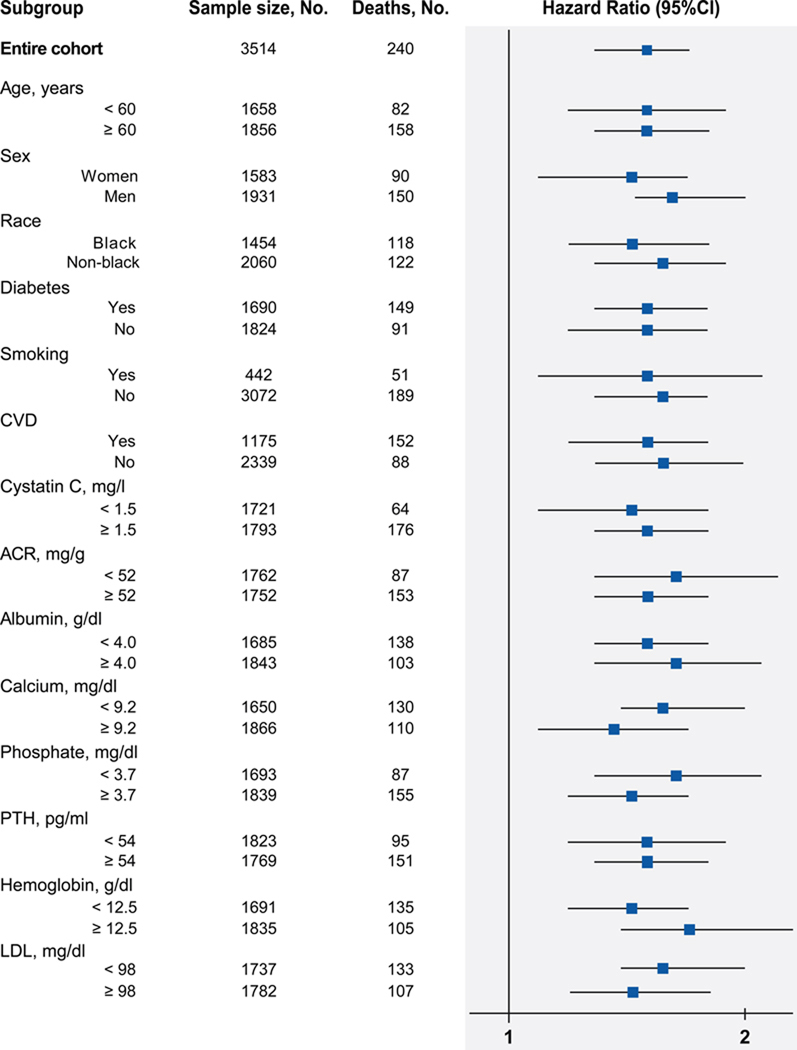
Unadjusted and multivariable-adjusted hazard ratios for mortality are presented in Table 2 according to baseline FGF23, expressed as a continuous variable and in quartiles. Adjusting for demographic characteristics, eGFR and other CKD-specific risk factors did not alter the relationship between elevated FGF23 and risk of death observed in unadjusted analyses. Participants in the highest versus the lowest quartile demonstrated a 4.3-fold greater risk of death, and the intermediate quartiles demonstrated intermediate risks. Further adjustment for traditional cardiovascular risk factors, use of cardio- and renoprotective medications, and serum calcium, phosphate and PTH levels minimally attenuated the relationship between FGF23 and risk of death. In the fully adjusted models, the graded increase in risk of death persisted across the spectrum of FGF23 levels (Figure 1), and the effects of eGFR and proteinuria on mortality were completely attenuated. In multivariable-adjusted stratified analyses, elevated FGF23 associated with homogenously greater risk of mortality (Figure 2).
Table 2.
Risks of adverse outcomes according to natural log transformed FGF23 and ascending quartiles of FGF23
| FGF23 Mean (Range) |
Rate per 1000 person-years |
Death Unadjusted |
Model 1a | HR (95% CI) Model 2b |
Model 3c | Model 4d | |
|---|---|---|---|---|---|---|---|
| Overall population | |||||||
| Per 1 SD lnFGF23 | 233 (<10 – 14318.9) |
20.3 (17.9 – 22.8) |
1.7 (1.6 – 1.9) |
1.8 (1.6 – 2.0) |
1.7 (1.5 – 1.9) |
1.5 (1.4 – 1.8) |
1.5 (1.3 – 1.7) |
| FGF23 Quartiles | |||||||
| 1 | 71.3 (<10 – 95.7) |
7.6 (4.9 – 11.1) |
Reference | Reference | Reference | Reference | Reference |
| 2 | 118.5 (95.8 – 145.4) |
12.6 (9.1 – 17.0) |
1.7 (1.0 – 2.7) |
1.5 (0.9 – 2.5) |
1.4 (0.8 – 2.3) |
1.3 (0.8 – 2.2) |
1.3 (0.8 – 2.2) |
| 3 | 184.7 (145.5 – 239.1) |
21.3 (16.6 – 26.9) |
2.8 (1.8 – 4.4) |
2.6 (1.6 – 4.1) |
2.3 (1.4 – 3.7) |
2.0 (1.2 – 3.3) |
2.0 (1.2 – 3.3) |
| 4 | 558.3 (239.2 – 14318.9) |
41.3 (34.5 – 49.1) |
5.5 (3.6 – 8.4) |
5.2 (3.4 – 8.0) |
4.3 (2.6 – 6.9) |
3.3 (2.0 – 5.4) |
3.0 (1.8 – 5.1) |
| FGF23 Quartiles |
FGF23 Mean (Range) |
Rate per 1000 person-years |
ESRD Unadjusted |
Model 1a |
HR (95% CI) Model 2b |
Model 3c | Model 4d |
| Overall population | |||||||
| Per 1 SD lnFGF23 | 233 (<10 – 14318.9) |
32.9 (29.8 – 36.3) |
1.9 (1.7 – 2.0) |
1.9 (1.7 – 2.0) |
1.1 (0.97 – 1.2) |
1.1 (0.97 – 1.2) |
1.0 (0.9 – 1.2) |
| FGF23 Quartiles | |||||||
| 1 | 71.3 (<10 – 95.7) |
7.6 (5.0 – 11.2) |
Reference | Reference | Reference | Reference | Reference |
| 2 | 118.5 (95.8 – 145.4) |
18.8 (14.4 – 24.2) |
2.5 (1.6 – 4.0) |
2.6 (1.6 – 4.1) |
1.2 (0.8 – 2.0) |
1.3 (0.8 – 2.1) |
1.2 (0.7 – 2.0) |
| 3 | 184.7 (145.5 – 239.1) |
40.7 (33.8 – 48.5) |
5.5 (3.6 – 8.4) |
5.9 (3.8 – 9.0) |
1.4 (0.9 – 2.2) |
1.4 (0.9 – 2.2) |
1.2 (0.8 – 2.0) |
| 4 | 558.3 (239.2 – 14318.9) |
72.7 (62.9 – 83.5) |
10.1 (6.7 – 15.2) |
10.6 (7.0 – 16.0) |
1.3 (0.8 – 2.1) |
1.3 (0.8 – 2.1) |
1.2 (0.7 – 1.9) |
Abbreviations: HR, hazard ratio; CI, confidence; SD, standard deviation; lnFGF23, natural log-transformed FGF23.
Model 1 is stratified by center and adjusts for age, sex, race, and ethnicity.
Model 2 adjusts for covariates in Model 1 plus estimated glomerular filtration rate, natural log-transformed urine albumin-to-creatinine ratio, serum albumin, and hemoglobin.
Model 3 adjusts for covariates in Model 2 plus systolic blood pressure, body mass index, diabetes, smoking, low density lipoprotein, history of coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease, and use of aspirin, beta-blockers, statins, and angiotensin converting enzyme inhibitors or angiotensin receptor blockers.
Model 4 adjusts for covariates in Model 3 plus calcium, phosphate and natural log-transformed parathyroid hormone.
Figure 1. Plot of the multivariable-adjusted hazard function for death according to measured (untransformed) levels of FGF23.
The median FGF23 level within the lowest FGF23 quartile (74 RU/ml) served as the referent value (hazard = 1). The model was stratified by center and adjusted for age, sex, race, ethnicity, estimated glomerular filtration rate, natural log-transformed urine albumin-to-creatinine ratio, hemoglobin, serum albumin, systolic blood pressure, body mass index, diabetes, smoking, low density lipoprotein, history of coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease, and use of aspirin, beta-blockers, statins, and angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and serum calcium, phosphate and natural log-transformed parathyroid hormone. Tick marks on the × axis indicate individual observations at corresponding levels of FGF23.
Figure 2. Stratified analyses of risk of death according to FGF23 levels.
The multivariable-adjusted hazard ratio of death per unit increment in standard deviation (SD) of natural log-transformed (ln) FGF23 is plotted for the entire cohort and according to strata of baseline covariates. Multivariable models were stratified by center and adjusted for age, sex, race, ethnicity, estimated glomerular filtration rate, natural log-transformed urine albumin-to-creatinine ratio, hemoglobin, serum albumin, systolic blood pressure, body mass index, diabetes, smoking, low density lipoprotein, history of coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease, and use of aspirin, beta-blockers, statins, and angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and serum calcium, phosphate and natural log-transformed parathyroid hormone. The number of participants (No.) and total number of deaths are presented for each stratum.
Abbreviations: CI, confidence; CVD, cardiovascular disease; ACR, urine albumin-to-creatinine ratio; PTH, parathyroid hormone, LDL, low density lipoprotein.
In sensitivity analyses, the results were qualitatively unchanged when we censored at the time of ESRD, when we substituted cystatin C (HR per SD of lnFGF23, 1.4; 95%CI 1.2 – 1.7) or iothalamate GFR instead of the MDRD eGFR, when we adjusted for vitamin D levels, when participants treated with phosphate binders, active vitamin D or vitamin D supplements were excluded, or when use of these medications was included in the multivariable models (eTable 1). Unlike FGF23, neither PTH (HR per SD of lnPTH, 1.1; 95%CI 0.9 – 1.3) nor FePi (HR per SD of lnFePi, 1.0; 95%CI 0.9 – 1.1) associated with mortality in fully adjusted models that excluded FGF23.
ESRD
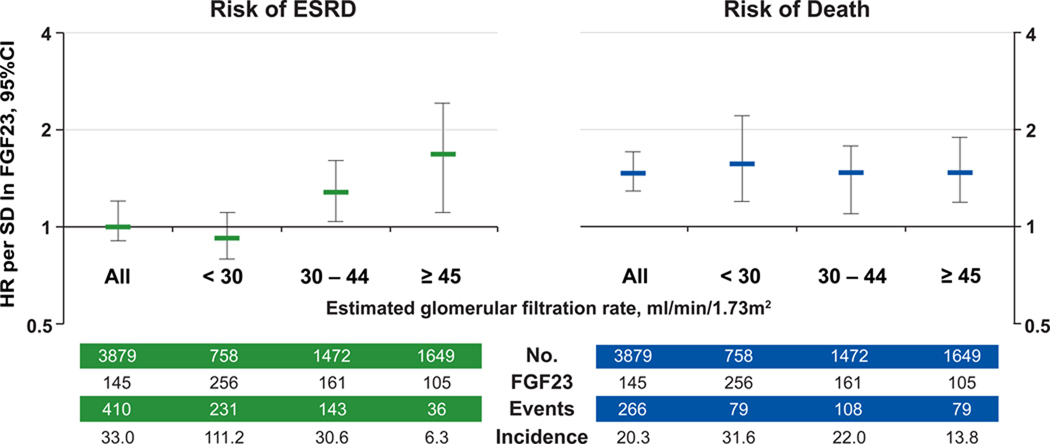
In contrast to mortality, adjustment for eGFR and CKD-specific risk factors attenuated the unadjusted association between FGF23 and risk of ESRD in the primary analysis (Table 2) and when death was treated as a competing risk (eTable 2). In the fully adjusted model, reduced eGFR was the strongest predictor of ESRD, but eGFR modified the relationship between FGF23 and risk of ESRD (P for lnFGF23 x eGFR = 0.005). Although median FGF23 was higher in more advanced CKD, elevated FGF23 independently associated with greater risk of ESRD in participants with baseline eGFR 30 – 45 and ≥ 45 ml/min/1.73m2, but not in those with eGFR < 30 ml/min/1.73m2 (Figure 3). In contrast, the risk of death according to FGF23 was homogenous across categories of eGFR (Figure 3).
Figure 3. FGF23 levels and risks of ESRD and death according to baseline kidney function.
Multivariable-adjusted risks of ESRD and death per unit increment in standard deviation (SD) of natural log-transformed (ln) FGF23 in all participants and according to categories of baseline estimated glomerular filtration rate (eGFR). Models were stratified by center and adjusted for age, sex, race, ethnicity, natural log-transformed urine albumin-to-creatinine ratio, hemoglobin, serum albumin, systolic blood pressure, body mass index, diabetes, smoking, low density lipoprotein, history of coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease, and use of aspirin, beta-blockers, statins, and angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and serum calcium, phosphate and natural log-transformed parathyroid hormone. Error-bars indicate 95% confidence intervals. The number of participants (No.), their median FGF23 levels, total number of events, and the unadjusted event rate, expressed per 1000 person-years, are presented for the categories of baseline eGFR.
Abbreviations: ESRD, end-stage renal disease; HR, hazard ratio; SD, standard deviation.
Comment
Elevated FGF23 is an independent risk factor for mortality in a referred population of patients with CKD stages 2 through 4. The effect was minimally confounded by other factors known to influence survival in CKD and was specific to FGF23 among the mineral metabolites we analyzed. These results are consistent with prior reports of greater risk of mortality in association with elevated FGF23 levels in patients undergoing hemodialysis,4, 5 kidney transplant recipients21 and patients with prior history of cardiovascular disease.20 Unexpectedly, FGF23 was more strongly associated with mortality than traditional cardiovascular and CKD-specific risk factors, most notably, reduced eGFR and proteinuria. These data emphasize the potential of FGF23 as a novel risk factor for mortality in CKD.
The mechanisms that underlie the association between elevated FGF23 and mortality are unclear. One possibility is that FGF23 is an excellent biomarker of toxicity of other factors in the family of disordered mineral metabolism, such as elevated serum phosphate and PTH. However, a recent meta-analysis demonstrated the poor relationship between PTH and mortality, and the modest effect of elevated serum phosphate.22 In the current study, similar to the few previous studies that performed direct comparisons,4, 5, 21 FGF23 was more strongly associated with mortality than other mineral metabolites, and its effect was neither confounded nor modified by phosphate or PTH. In addition, increased FePi, which is a measure of the physiological action of FGF23 on renal phosphate handling,19 was not associated with mortality. These findings suggest that mechanisms beyond mineral metabolism may underlie excess mortality in association with elevated FGF23.
Another possibility is that elevated FGF23 is a sensitive marker of the severity of kidney dysfunction that accurately conveys mortality risk associated with CKD but not directly attributable to FGF23. This also seems inadequate, however, given the previous report linking elevated FGF23 with mortality in a predominantly non-CKD cohort,20 and our observation of homogenous risk of mortality across the range of renal function regardless of whether it was quantified using the standard MDRD eGFR, iothalamate GFR or cystatin C. In addition, the specificity of the results for FGF23 relative to PTH, despite their similar correlation with eGFR, suggests that the effect of FGF23 on death may be acting through a mechanistic pathway that is at least partially independent of renal function. Finally, the observation that eGFR and proteinuria fell out as insignificant risk factors for mortality in multivariable models that included FGF23 suggests that rather than simply acting as a biomarker of CKD severity, elevated FGF23 may contribute to the excess mortality that was attributed to CKD in previous studies in which FGF23 levels were unavailable. Although postulating direct toxicity of FGF23 remains speculative,23 elevated FGF23 consistently associates with pathophysiologically plausible mechanisms of premature death including left ventricular hypertrophy, endothelial dysfunction, atherosclerosis and arterial calcification.24–28 Experimental or randomized human studies are needed to assign or refute a direct causal role for FGF23 in excess mortality.
The relationship between FGF23 and risk of ESRD was more complex. In the overall population, FGF23 associated with risk of ESRD until eGFR was added to the model. While this might suggest that elevated FGF23 is not a risk factor for ESRD, the relationship between FGF23 and ESRD was modified by eGFR, which was the most potent determinant of progression to ESRD. Unlike death, in which events were relatively balanced across the range of eGFR, analyses of ESRD in the overall population were driven primarily by participants with baseline eGFR < 30 ml/min/1.73m2. This group experienced the greatest number of ESRD events with incidence rates that were approximately 4-fold and 18-fold higher than those with eGFR of 30 – 45 and ≥ 45 ml/min/1.73m2, respectively (Figure 3). When the analyses were stratified by kidney function, higher FGF23 emerged as an independent risk factor for ESRD in those with eGFR > 30 ml/min/1.73m2, and the effect size grew with higher eGFR. While these results suggest that FGF23 testing might help stratify risk of progression to ESRD in the growing number of patients found to have modestly reduced eGFR, confirmation by future studies is needed.
Limitations of the study include the lack of data on cause of death. Although greater risk of cardiovascular mortality is likely given previous reports of FGF23 and cardiovascular events,20 future studies should examine cause-specific mortality and risk of major cardiovascular events in CKD patients according to FGF23. Second, vitamin D levels were only available in subsets of participants. However, since adjustment for vitamin D levels or vitamin D treatment did not alter the point estimate for FGF23 similar to previous studies,4 it is unlikely that vitamin D confounds the relationship between FGF23 and mortality. Third, we did not study other biomarkers, such as troponin T and brain natriuretic peptide, which have been associated with adverse outcomes in CKD.13 Finally, the lack of a validated assay in CKD precluded us from measuring circulating levels of the soluble form of klotho, the FGF23 co-receptor that is expressed in the kidneys and parathyroid glands and that demonstrates anti-aging and vascular-protective effects.29, 30 Future studies should explore whether reduced expression of klotho due to CKD itself31 or secondary to increased FGF23 in CKD32 is a potential mediator of FGF23-associated mortality.
Previous reports of greater risk of mortality in association with elevated FGF23 levels among dialysis patients4, 5 established the possibility that FGF23 may be a novel predictor of adverse outcomes in patients with kidney disease. In the current study, we extend these results to the much larger population of patients with CKD stages 2 – 4 in whom treatment of disordered phosphorus metabolism is not recommended because their serum phosphate levels are usually normal. If the results of the current study are confirmed, and experimental studies support the hypothesis of direct toxicity of FGF23, future research should evaluate whether therapeutic or preventative strategies that lower FGF23 can improve outcomes.
Supplementary Material
Acknowledgements
Funding and Support: This CRIC ancillary study was supported by NIH grants R01DK081374 (MW), K23DK087858 (TI) and R01DK077128 (ML). The CRIC Study is supported by cooperative agreement project grants 5U01DK060990, 5U01DK060984, 5U01DK06102, 5U01DK061021, 5U01DK061028, 5U01DK60980, 5U01DK060963, and 5U01DK060902 from the National Institute of Diabetes and Digestive and Kidney Diseases, and by grants UL1RR024134, UL1RR025005, M01RR16500, UL1RR024989, M01RR000042, UL1RR024986, UL1RR029879, RR05096, and UL1RR024131 from the National Institutes of Health.
Role of the Sponsor: The National Institutes of Health (NIH) contributed to the design, development and steering (JWK) of the Chronic Renal Insufficiency Cohort study. For the current report, one NIH co-author (JWK) contributed to the design and conduct of the study, analysis and interpretation of the data, and review and approval of the manuscript.
Footnotes
Author Contributions: Dr. Isakova had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Dawei Xie and Wei Yang of the CRIC Scientific and Data Coordinating Center at the University of Pennsylvania performed an independent review and verification of the data analysis.
Study concept and design: Isakova, Hsu, Leonard, Feldman, Wolf
Acquisition of data: Anderson, Steigerwalt, He, Schwartz, Lo, Ojo, Sondheimer, Hsu, Lash, Leonard, Feldman, Wolf
Analysis and interpretation of data: Isakova, H. Xie, Yang, D. Xie, Anderson, Scialla, Gutiérrez Wahl, Kusek, Wolf
Drafting of the manuscript: Isakova, Wolf
Critical revision of the manuscript for important intellectual content: Isakova, H Xie, Yang, D Xie, Anderson, Scialla, Wahl, Gutiérrez, Steigerwalt, He, Schwartz, Lo, Ojo, Sondheimer, Hsu, Lash, Leonard, Kusek, Feldman, Wolf
Statistical analysis: Isakova, H. Xie, D. Xie, Yang, Anderson, Scialla, Gutiérrez, Lash, Wolf
Obtained funding: He, Hsu, Leonard, Feldman, Wolf
Administrative, technical or material support: Steigerwalt, Schwartz, Lo, Ojo, Sondheimer, Hsu
Study supervision: Hsu, Kusek, Feldman, Wolf
Disclosures
Dr. Wolf has served as a consultant or received honoraria from Abbott Laboratories, Amgen, Ardelyx, Baxter, Cytochroma, Genzyme, Lutipold, Novartis, Mitsubishi and Shire. Dr. Isakova has served as a consultant and received honoraria from Shire.
References
- 1.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21(9):1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16(2):520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 3.Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17(10):2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24(9):2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 6.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 7.Titan SM, Zatz R, Graciolli FG, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6(2):241–247. doi: 10.2215/CJN.04250510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7) Suppl 2:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 10.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.Landray MJ, Emberson JR, Blackwell L, et al. Prediction of ESRD and death among people with CKD: the Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am J Kidney Dis. 2010;56(6):1082–1094. doi: 10.1053/j.ajkd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 15.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5(2):286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesseling-Perry K, Pereira RC, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79(1):112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 17.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20(2):388–396. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 20.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22(5):956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305(11):1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 23.Juppner H, Wolf M, Salusky IB. FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25(10):2091–2097. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirza MA, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24(10):3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 26.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz MI, Sonmez A, Saglam M, et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78(7):679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 29.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 30.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77(3):232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 32.Isakova T, Wahl P, Vargas G, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011 doi: 10.1038/ki.2011.47. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.