Abstract
Recurrent Respiratory Papillomatosis (RRP) is caused by HPV-6 or -11. Specific HLA-DR haplotypes, DRB1*01:02 and DRB1*03:01 are associated with the development of RRP, disease severity, and TH2-like responses to HPV early proteins. TH1-like responses to HPV proteins have been shown to be protective in animal models. Therefore, we investigated the hypothesis that RRP patients have dysfunctional TH1-like HPV-specific T-cell responses. Using MHC class II tetramers, we identified immunogenic peptides within HPV-11 early proteins. Two distinct peptides (E6113-132 and E21-20) contained DRB1*01:02 or DRB1*03:01 restricted epitopes respectively. An additional peptide (E2281-300) contained an epitope presented by both alleles. Peptide binding, tetramer, and proliferation assays identified minimal epitopes within these peptides. These epitopes elicited E2/E6 specific CD4+ T-cell responses in RRP patients and healthy controls, allowing the isolation of HPV-specific T-cell lines using tetramers. The cytokine profiles and STAT signaling of these tetramer-positive T-cells were measured to compare the polarization and responsiveness of HPV-specific T-cells from patients with RRP and healthy subjects. HPV-specific IFN-γ secretion was substantially lower in T-cells from RRP patients. HPV-specific IL-13 secretion was seen at modest levels in T-cells from RRP patients and absent in T-cells from healthy controls. HPV-specific T-cells from RRP patients exhibited reduced STAT-5 phosphorylation and reduced IL-2 secretion, suggesting anergy. Levels of STAT-5 phosphorylation and IFN-γ secretion could be improved through addition of IL-2 to HPV-specific T-cell lines from RRP patients. Therapeutic vaccination or interventions aimed at restoring TH1-like cytokine responses to HPV proteins and reversing anergy could improve clinical outcomes for RRP patients.
Keywords: Human, MHC, T-cells, Antigens/Peptides/Epitopes, human papillomavirus
Introduction
Human papillomaviruses (HPV2) are ubiquitous viruses which are typically held in check by a competent immune system, as evidenced by the presence of HPV-specific memory T-cells in most individuals (1–2). While the majority of HPV infections are short-lived and asymptomatic, persistent infections may progress to pre-cancerous lesions or invasive cancers (3). In addition, a small subset of HPV-exposed individuals develop recurrent respiratory papillomatosis (RRP3), a condition in which benign tumors of the larynx and upper respiratory tract result in significant morbidity, and in occasional mortality (4–5). RRP is caused by HPV-6 and -11 infection (6), is more common in children than adults (4.3 and 1.8 cases per 100,000 respectively), and in children, is typically associated with vertical transmission from an HPV-6/11-infected mother during vaginal delivery (7). There is significant clinical variability between patients, with some requiring surgical procedures as often as every 2 weeks to maintain a patent airway, while others have no recurrence after their first presentation (8).
HPV-induced diseases, including RRP, are characterized by the absence of HPV-specific, cytotoxic T-lymphocytes and the absence of CD4+ TH1 cells that secrete IFN-γ, IL-2, and TNF-α (9–11). These cell types are critical in generating effective anti-viral T-cell adaptive immunity (12). In particular, TH1-like responses to E2 and E6 protein were clearly protective in animal models (13–15). Responses to E2 and E6 proteins may also contribute to effective HPV-specific immunity in human subjects (16), but at present this has not been directly shown. The specific immunologic mechanisms which predispose to RRP and result in the development of disease are not well characterized, however select class II haplotypes (DRB1*01:02, DRB1*03:01, and DQB1*02:01) are associated with the development of RRP and the expression of IL-10 and IL-4 by effector T-cells (17). Based on other disease models, the absence of effective CD4+ T-cell immunity against HPV could be a consequence of functional exhaustion, caused by the persistent presence of antigen, or by anergy, caused by incomplete T-cell activation in an environment deficient in co-stimulation or high in co-inhibition (18–19).
The objective of the present study was to investigate mechanisms of immune dysfunction in RRP patients by comparing the HPV-specific CD4+ T-cell responses of RRP patients and healthy subjects. After identifying prevalent DR01024 and DR03015 restricted epitopes within the HPV E2 and E6 proteins, HPV-specific polyclonal T cells (T-cell lines) were isolated using tetramers and characterized on the basis of their cytokine profiles and STAT signaling. Our findings support a paradigm in which persistent HPV infection leads to increased TH2 polarization, altered signaling, and impaired CD4+ TH1-like T-cell immunity.
Materials and Methods
Human Subjects
Samples for this study were obtained from healthy volunteers at Benaroya Research Institute and from individuals with RRP at Long Island Jewish Medical Center. All subjects were recruited with informed consent obtained following approval by the North Shore-LIJ Institutional Review Board. RRP subjects had mild-moderate or severe disease, defined by a disease severity score ≤0.06 (mild-moderate) or >0.06 (severe) at the time of direct endoscopic surgical removal, or having tracheal, bronchial, or pulmonary extension of disease (severe) (17). All subjects were confirmed by high-resolution HLA class II genotyping to have HLA DRB1*01:02 or HLA DRB1*03:01 haplotypes (17).
Peptides, MHC Class II Protein, and Tetramer Assembly
Panels of overlapping 20-mer peptides with sequences based on HPV E2 and E6 protein sequences and positive control peptides – influenza A hemagglutinin (HA6) 306–318 and sperm whale myoglobin (myo7) 137–148 – were synthesized on polyethylene pins with 9-fluorenylmethoxycarbonyl chemistry by MIMOTOPES (Clayton, Australia) with a 12 amino acid overlap. Each peptide was dissolved in DMSO at 20 mg/ml and subsequently diluted as needed for various assays. To produce recombinant DR0102 and DR0301 protein, soluble protein was purified from insect cell culture supernatants by affinity chromatography and dialyzed against phosphate storage buffer, pH 6.0 as previously described (20). To assemble MHC class II tetramers, DR0102 or DR0301 protein was biotinylated at a sequence-specific site using biotin ligase (Avidity, Denver, CO) prior to dialysis into phosphate storage buffer. The biotinylated monomer was loaded with 0.2 mg/ml of peptide by incubating at 37°C for 72 hours in the presence of 2.5 mg/ml n-octyl-β-D-glucopyranoside and 1 mM Pefabloc SC (Sigma-Aldrich, St. Louis, MO). Peptide loaded monomers were subsequently conjugated as tetramers using R-PE streptavidin (Biosource International, Camarillo, CA) at a molar ratio of 8 to 1 (20).
Peptide binding competition assay
Various concentrations of each test peptide were incubated in competition with 0.01 mM biotinylated reference peptide – HA306-318 (PKYVKQNTLKLAT) for DR0102 and myo137-148 (LFRKDIAAKYKE) for DR0301 – in wells coated with DR0102 or DR0301 protein as previously described (21). After washing, the residual biotin-peptide was labeled using europium-conjugated streptavidin (Perkin Elmer) and quantified using a Victor2 D time resolved fluorometer (Perkin Elmer). Peptide binding curves were simulated by non-linear regression with Prism software (Version 4.03, GraphPad Software Inc.) using a sigmoidal dose response curve. IC50 binding values were calculated from the resulting curves as the peptide concentration needed for 50% inhibition of reference peptide binding.
Tetramer-based T-cell Assays
Tetramer Guided Epitope Mapping was conducted as previously described to define epitopes within the E2 and E6 proteins restricted by DR0102 and DR0301 (22, 23). Peripheral Blood Mononuclear Cells (PBMC) were isolated from the blood of healthy subjects and individuals with RRP by Ficoll® (GE healthcare) underlay and CD4+ T-cells isolated using the Miltenyi CD4+ T-cell isolation kit. Cells from the CD4-negative fraction were incubated in 48 well plates (2.5× 106 cells per well) for 1 hour and then washed, leaving adherent cells as antigen presenting cells. After adding ~2 million CD4+ T-cells per well, each well was stimulated with a pool of five consecutive HPV peptides (20 amino acids long with a 12 residue overlap). After 14 days, 75–100 μl of resuspended cells were stained with pooled peptide PE-conjugated tetramers for 60 min at 37°C. Subsequently, cells were stained with CD4-APC, CD3-PerCP and CD25-FITC mAbs (eBioscience) and analyzed by flow cytometry. Cells from pools that gave positive staining were analyzed again using the corresponding individual peptide tetramers. T-cell responses to single peptides were assayed in a similar fashion except that each well was stimulated with a single HPV peptide and stained after 14 days using a single peptide-loaded tetramer.
T-cell sorting and proliferation assays
CD4+ tetramer-positive cells were isolated as previously described using a FACS Vantage (Becton Dickinson) into 96-well plates containing T-cell medium (RPMI 1640 with 10% human serum, 1 mM sodium pyruvate, 50 U/ml penicillin and 50 μg/ml streptomycin) and expanded by adding 2 μg/ml phytohemagglutinin and 200,000 irradiated PBMCs plus 40 U/ml IL-2 (20). Expanded cells were stained with tetramers and analyzed on a FACSCalibur (Becton Dickinson). To assess proliferation, 104 T-cells/well were plated in T-cell medium with 105 irradiated PBMCs from an HLA-matched donor (with DRB1*01:02 or DRB1*03:01 haplotype) and 0, 1 or 10 μg/ml peptide (in triplicate), incubated at 37°C for 48 hours, pulsed with [3H]thymidine (1 μCi/well) and harvested 18 hours later, and [3H]thymidine incorporation measured with a scintillation counter. For blocking experiments, anti-DR antibody (protein A purified from L243 supernatant) or anti-DQ antibody (protein A purified from SPVL3 supernatant) was added at 20 μg/ml.
Cytokine Assays
The secretion of IFN-γ, TNF-α, IL-5 and IL-10 by HPV specific T-cell lines was characterized using cytokine capture kits (Miltenyi Biotec) according to manufacturer’s instructions. Briefly, T-cells were activated by incubating with 10 μg/ml tetramers, 10 μg/ml anti-CD28, and 2 μg/ml anti-CD49d. After 4 hours of incubation at 37°C, cells were washed twice in PBS and labeled in 100 μl of medium on ice for 10 min with a bi-specific Ab-Ab conjugate directed against both CD45 and cytokine (IFN-γ, TNF-α, IL-5 or IL-10). Pre-warmed medium was then added to a final volume of 2 ml, and cells were incubated at 37°C for 45 min under gentle rotation to allow cell surface capture of secreted cytokines. Cells were washed once in PBS and then stained for 15 min on ice with a FITC or APC-conjugated antibody directed against the cytokine of interest, washed, and analyzed on a FACSCalibur instrument (BD Biosciences). For IL-2 rescue experiments, HPV specific cell lines were incubated overnight in T cell medium with or without exogenous IL-2 (40 U/ml) prior to cytokine analysis.
Production of IL-2 and IL-13 by HPV specific T-cell lines was characterized by intracellular cytokine staining. Briefly, T-cells were activated by incubating with 10 μg/ml tetramers, 10 μg/ml anti-CD28, and 2 μg/ml anti-CD49d at 37°C for 2 hours and then an additional 4 hours in the presence of 10 μg/ml monensin. Cells were then harvested, re-suspended in Fixation/Permeabilization solution (BD Biosciences), washed in Perm/Wash buffer (BD Biosciences), and stained with anti-IL2 APC (Miltenyi) or anti-IL-13 PE (BD biosciences) according to manufacturer’s instructions. Cells were then washed and immediately analyzed by flow cytometry.
Assessing STAT Signaling
CD4+ tetramer-positive T-cell lines were activated with 10 μg/ml tetramers, 10 μg/ml anti-CD28, and 2 μg/ml anti-CD49d for various time intervals and then characterized by phospho-specific flow as previously described (24). In brief, T-cells were fixed with BD Biosciences Phosflow Buffer I, permeabilized using BD Phosflow Buffer III, and then divided to allow for analysis of multiple phosphoproteins (anti-pSTAT4, anti-pSTAT5, and anti-pSTAT6) using the respective phospho-specific antibodies from BD Biosciences. Phosphospecific Abs were added simultaneously for 20–30 min, washed, resuspended in staining buffer and kept at 4°C before analysis on a FACSCalibur instrument (BD Biosciences).
Statistical Analysis
For tetramer, cytokine capture, and STAT signaling data experimental means were analyzed for statistical differences using the Student’s t test with Welch’s correction for unequal variances. ICS data was log10 transformed, determined to be normally distributed with comparable standard deviations between groups, and analyzed by a 2-tailed unpaired t-test and reported as means and standard deviations after back transformation. All analyses were performed using Prism software (Version 4.03, GraphPad Software Inc.)
Results
Identifying DR0102 and DR0301 Restricted E2/E6 Epitopes
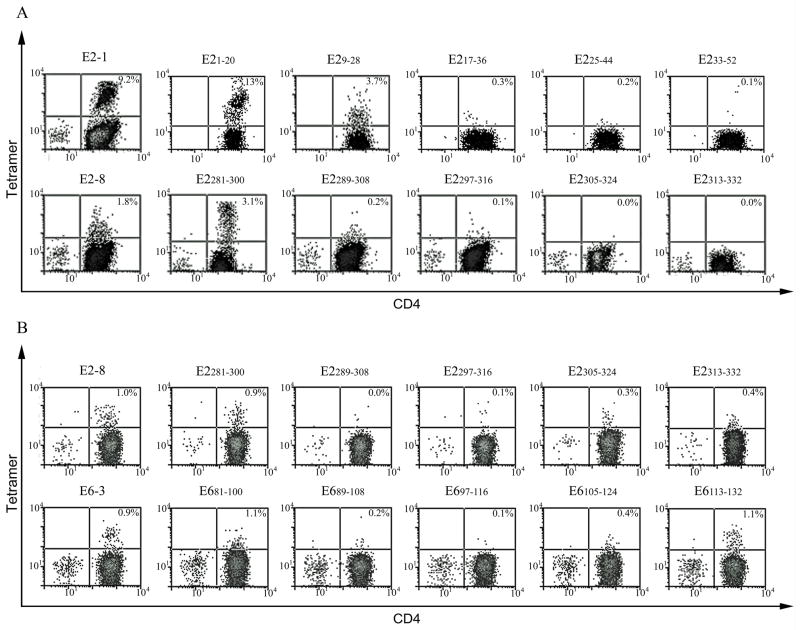
CD4+ T-cells from RRP patients and healthy subjects were stimulated with peptide pools spanning the HPV-11 E2 and E6 protein sequences. The design of these peptide sets is shown in Supplementary Table I. After fourteen days, cells were stained with pooled peptide tetramers (Supplementary Figure 1) and again using single peptide tetramers (Figure 1). As shown in Figure 1A, cells from a healthy subject with a DRB1*03:01 haplotype that were stained with E21-20, E2281-300 and the corresponding pools resulted in positive staining. All remaining pools were negative (Supplementary Figure 1A). As shown in Figure 1B, cells from a healthy subject with a DRB1*01:02 haplotype that were stained with E2281-300, E6113-132 and the corresponding pools gave positive staining. Staining with E681-100 was subsequently shown to be elevated background staining because staining irrelevant cells with this tetramer gave similar results (not shown). All remaining pools were negative (Supplementary Figure 1B).
Figure 1. Identifying E2/E6 CD4+ T-cell epitopes.
(A) CD4+ T-cells from a healthy subject with DRB1*03:01 haplotype were stimulated with peptide pools spanning the E2/E6 protein sequences. After fourteen days, cells were stained with DR0301 tetramers. Cells that were stained with E21-20, E2281-300 and the corresponding pools gave positive staining. All remaining pools were negative. (B) CD4+ T-cells from a healthy subject with DRB1*01:02 haplotype were stimulated with peptide pools spanning the E2/E6 protein sequences. After fourteen days, cells were stained with DR0102 tetramers. Cells that were stained with E2281-300, E6113-132 and the corresponding pools gave positive staining. Staining with E681-100 was subsequently shown to be background staining.
Defining minimal DR0102 and DR0301-restricted HPV epitopes
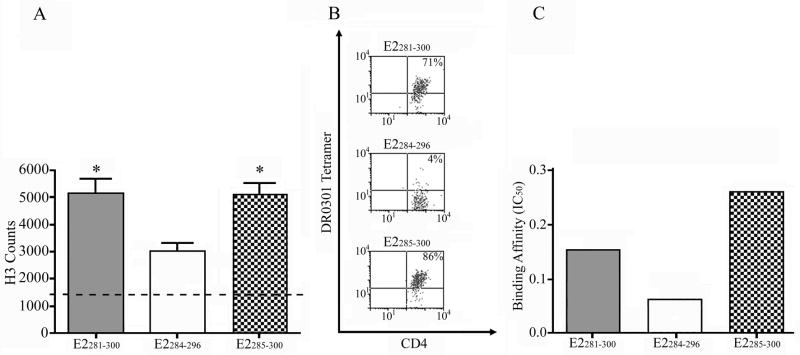
Complimentary proliferation, tetramer staining, and peptide binding experiments were conducted using truncated versions of each antigenic E2/E6 peptide to identify the core residues required for peptide presentation and T-cell activation. The sequences of these peptides are summarized in the bottom portion of Supplementary Table I. Some representative examples of these experiments are shown in Figure 2. As shown in Figure 2A, a DR0301-restricted E2281-300-specific T-cell line proliferated in response to E2285-300 but not E2284-296. This proliferative response was shown to depend on peptide presentation by HLA-DR and not HLA-DQ through the use of blocking antibodies (data not shown). The same T-cell line could be stained with tetramers loaded with E2285-300 but not E2284-296 (Fig 2B). While both peptides were able to bind to recombinant DR0301, E2285-300 bound with higher affinity (Fig 2C). Experiments using a DR0102-restricted E2281-300-specific T-cell line indicated that the same minimal epitope is presented and recognized in the context of DR0102. Similar experiments implicated E25-17 as the minimal epitope within E21-20 and E6115-130 as the minimal epitope within E6113-132. These minimal epitopes are summarized in the first two columns of Table I. In general these core antigenic regions are in agreement with the published binding motifs for DR0102 and DR0301 (25–26).
Figure 2. Identifying minimal epitopes within HPV E2/E6 peptides.
(A) Proliferation of an HPV-specific T-cell line in response to E2281-300, E2284-296, or E2285-300 peptide (dashed line indicates background proliferation). Asterisks denote proliferation that is at least 3-fold above background. (B) Staining of a T-cell line with DR0301 tetramer loaded with E2281-300, E2284-296, or E2285-300 peptide (quadrant boundary set based on the staining of an unloaded tetramer). (C) Relative binding affinity of E2281-300, E2284-296, or E2285-300 peptide to recombinant DR0301 protein in competition with the myo137-148 reference peptide.
Table I.
Summary of E2/E6 Epitopes
| E2/E6 residues | Amino Acid Sequence* | # of Patients | # of Healthy | |
|---|---|---|---|---|
| DR0102 | ||||
| E6113-132 | EIEKLKHILGKARFIKLNNQ | 2 of 2 | 2 of 3 | |
| E2281-300 | SAATPIVQLQGDSNCLKCFR | 2 of 2 | 3 of 3 | |
| DR0301 | ||||
| E21-20 | MEAIAKRLDACQDQLLELYE | 4 of 4 | 5 of 7 | |
| E2281-300 | SAATPIVQLQGDSNCLKCFR | 4 of 4 | 7 of 7 | |
Minimal stimulatory epitopes, as determined by proliferation and tetramer assays are underlined. The most likely core binding residues, based on assay results and binding predictions, are bolded.
Responsiveness of Healthy Subjects and RRP patients to HPV E2/E6 Peptides
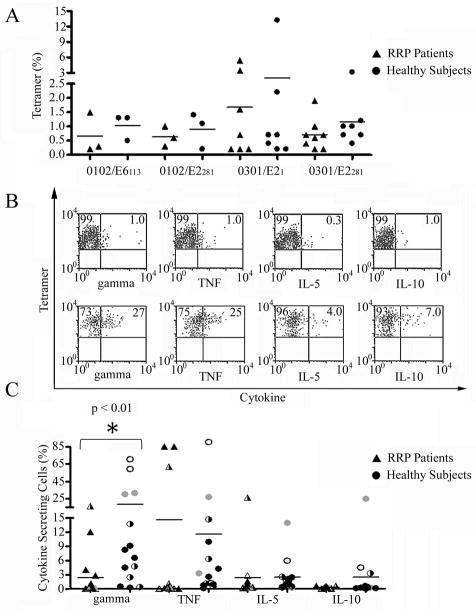
Using the peptides identified through initial epitope mapping experiments, T-cell responses to E2/E6 were assayed in healthy subjects and RRP patients by stimulating purified CD4+ T-cells with HPV peptide and staining with tetramers after two weeks of in vitro culture (Figure 3A). While the data suggested stronger T-cell expansion in certain healthy subjects, none of these differences were statistically significant. To assess functional responses to E2/E6 peptides, CD4+ T-cell lines were isolated from healthy subjects and RRP patients, activated using HPV peptide tetramers, and assayed for cytokine release using a capture assay. T-cell lines that were isolated from RRP subjects (Figure 3B) were typically deficient in their capacity to secrete cytokines, while most healthy subjects exhibited robust secretion of TH1 cytokines such as IFN-γ. As shown in Figure 3C, the deficit in IFN-γ secretion by RRP patients as compared to healthy subjects was statistically significant (p<0.01), while levels of secreted IL-5 and IL-10 were not significantly different. One subset of patients was deficient in TNF-α secretion while another had enhanced TNF-α secretion. These TNF-α producing cell lines had the highest IFN-γ secretion. Table II lists a summary of clinical and immune response data for each individual included in the study, including their cytokine responses. Among RRP subjects, there was a trend toward increased IFN-γ secretion in subjects with mild disease but this did not reach statistical significance (p=0.1). Both patients with significant TNF-α and IFN-γ secretion had mild disease and one had improved clinically in response to an experimental immunomodulator.
Figure 3. E2/E6 Specific T-cell responses and Cytokine Secretion.
(A) Percentage of E2/E6 tetramer positive T-cells after in vitro culture as measured in RRP patients and HLA matched healthy subjects. While many of the stronger responses were observed in healthy subjects, these differences were not statistically significant. (B) CD4+ T-cell lines from RRP patients (upper panels) and healthy subjects (lower panels) were activated using HPV peptide tetramers (plus CD28/CD49d) and assayed using a multiplex cytokine capture assay. As shown in these representative plots (gated for tetramer positive cells), T-cell lines isolated from RRP subjects were typically deficient in their capacity to secrete cytokines such as IFN-γ. (C) Cytokine secretion by DR0102/E6113-132 (gray symbols), DR0102/E2281-300 (open symbols), DR0301/E21-20 (half-filled symbols), and DR0301/E2281-300 (black symbols) specific T-cell lines measured by cytokine capture for 16 cell lines isolated from RRP patients (triangles) and 14 cell lines isolated from HLA matched healthy subjects (circles). The data indicates a significant deficit in IFN-γ secretion by RRP patients as compared to healthy subjects (p<0.01). One subset of patients was deficient in TNF-α secretion while another had enhanced TNF-α secretion.
Table II.
Summary of Clinical and Immune Response Data
| Subject ID | HLA | Age | Onset | Disease Score | IFN-γ‡ | STAT-5§ |
|---|---|---|---|---|---|---|
| RRP 1 | DR0102 | 63 | Adult | Mild/Moderate (0.018)† | 0.2 | 1.8 |
| RRP 2 | DR0301 | 53 | Adult | Mild/Moderate (0.0)* | 4.0 | 1.2 |
| RRP 3 | DR0301 | 30 | Adult | Severe (0.075)† | 0.6 | 3.9 |
| RRP 4 | DR0301 | 49 | Adult | Severe (0.068)† | 1.6 | 0.1 |
| RRP 5 | DR0102 | 46 | Adult | Severe (0.088)† | 0.2 | 8.3 |
| RRP 6 | DR0301 | 26 | Juvenile | Mild/Moderate (0.005)† | 6.0 | 0.6 |
| Cont 1 | DR0102 | NA | None | None | 49.4 | 5.6 |
| Cont 2 | DR0102 | 47 | None | None | 43.2 | 7.0 |
| Cont 3 | DR0301 | 38 | None | None | ND | 2.5 |
| Cont 4 | DR0301 | 55 | None | None | ND | ND |
| Cont 5 | DR0301 | 25 | None | None | 5.6 | 4.6 |
| Cont 6 | DR0301 | 28 | None | None | 10.2 | 14.1 |
| Cont 7 | DR0301 | 48 | None | None | 3.4 | ND |
| Cont 8 | DR0301 | 33 | None | None | 2.5 | ND |
Subject improved clinically (severe to mild/moderate) in response to an experimental immunomodulator as part of a pre-clinical trial which concluded immediately before these data were obtained.
Surgical de-bulking performed
Average percentage of positive cells in interferon-γ capture assay
Average percentage of positive cells in phospho-specific STAT-5 assay
NA= Not available; ND= Not Done
STAT Signaling of HPV Specific T-cell lines
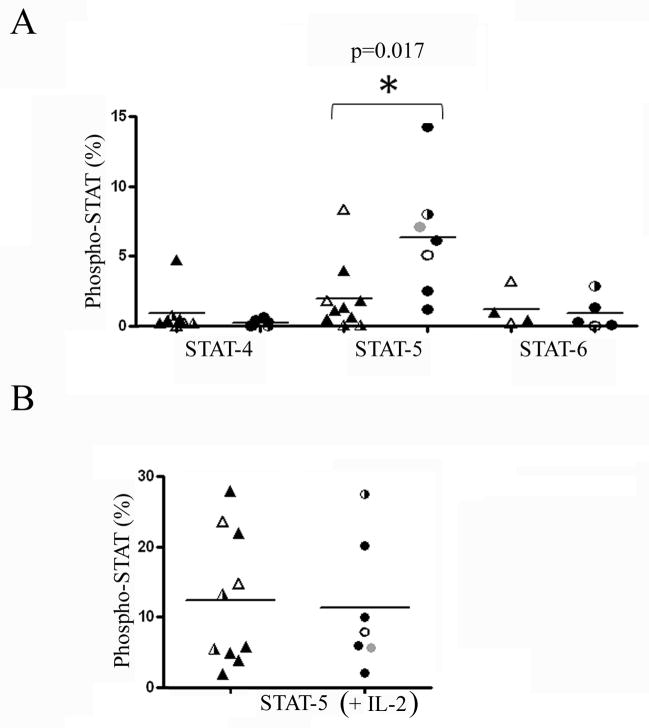
To address the underlying mechanism of deficient cytokine production in RRP, STAT-4, STAT-5, and STAT-6 signaling was measured in multiple CD4+ T-cell lines isolated from RRP patients and HLA matched healthy subjects. For these experiments tetramer stimulated E2/E6 specific T-cells lines were stained with phospho-specific STAT-4, STAT-5, and STAT-6 antibodies and analyzed by flow cytometry. As shown in Figure 4A, STAT-4 and STAT-6 signaling was comparable in RRP patients and healthy subjects. In contrast, STAT-5 signaling was significantly lower (p=0.017) in RRP patients than in healthy subjects. The observed difference in STAT-5 signaling appeared to be an HPV-specific phenomenon, since non-specific induction of STAT-5 using IL-2 (rather than tetramer stimulation) elicited similar signaling in RRP patients and healthy subjects (Figure 4B). STAT-5 signaling was reduced in patients regardless of specificity in that T cell lines with all three of the HLA/peptide restrictions tested showed low levels of phosphorylation.
Figure 4. STAT-5 Signaling of E2/E6 Specific T-cells is Altered in Patients with RRP and is Reversible with IL-2.
(A) STAT phosphorylation of tetramer stimulated DR0102/E6113-132 (gray symbols), DR0102/E2281-300 (open symbols), DR0301/E21-20 (half-filled symbols), and DR0301/E2281-300 (black symbols) specific T-cells as measured by phospho-specific flow cytometry in 10 cell lines isolated from RRP patients (triangles) and 7 cell lines isolated from HLA matched healthy subjects. STAT-4 signaling was elevated in one RRP patient, but virtually absent in all other subjects. No significant differences were observed between the two groups. STAT-5 signaling was significantly decreased in RRP subjects. STAT-6 signaling was unaltered between the two groups. (B) STAT-5 phosphorylation of IL-2 stimulated T-cells as measured by phospho-specific flow cytometry in RRP patients and HLA matched healthy subjects. Signaling was comparable in the two groups.
IL-2 and IL-13 production by HPV Specific T-cells
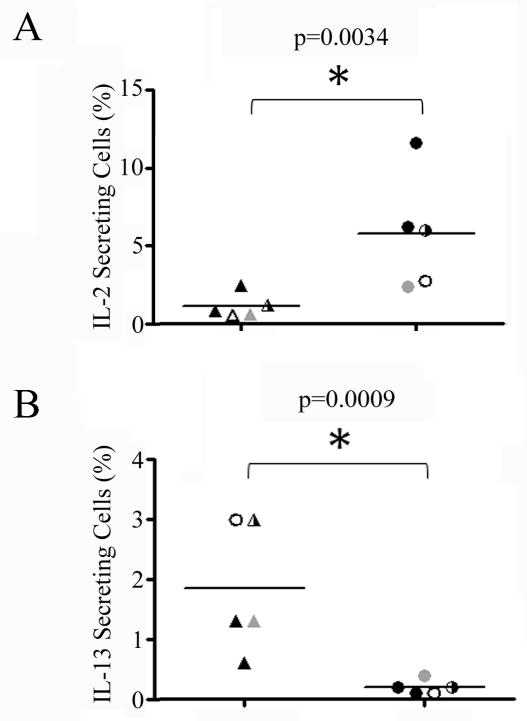
The observation of reduced STAT-5 accompanied by unaltered STAT-4 suggests a possible decrease in autocrine levels of STAT5-signaling cytokines such as IL-2. Therefore, we measured the IL-2 production of tetramer-stimulated E2/E6 specific T-cells by intracellular cytokine staining in multiple CD4+ T-cell lines isolated from RRP patients and HLA matched healthy subjects. As shown in Figure 5A, IL-2 production was significantly decreased (p=0.0034) in RRP subjects as compared with healthy control subjects. These results demonstrate a lack of IL-2 production by HPV specific T-cells. Interestingly, these HPV specific CD4+ T-cells did not express PD-1 or CTLA-4 (data not shown). As shown in Figure 5B, IL-13 production by HPV specific T-cells was modest, but significantly increased (p=0.0009) in RRP subjects as compared with healthy control subjects, suggesting a TH2-like polarization. However, these HPV specific cells rarely produced IL-5 (Figure 3) and did not secrete appreciable amounts of IL-4 (data not shown).
Figure 5. E2/E6 Specific T-cells show Decreased IL-2 and Enhanced IL-13 Production in Patients with RRP.
A) IL-2 production of tetramer stimulated DR0102/E6113-132 (gray symbols), DR0102/E2281-300 (open symbols), DR0301/E21-20 (half-filled symbols), and DR0301/E2281-300 (black symbols) specific T-cells as measured by intracellular cytokine staining for 5 cell lines isolated from RRP patients (triangles) and 5 cell lines isolated from HLA matched healthy subjects. IL-2 production was significantly decreased in RRP subjects. B) IL-13 production of tetramer stimulated E2/E6 specific T-cells as measured by intracellular cytokine staining in RRP patients and HLA matched healthy subjects. IL-13 production was increased in RRP subjects.
Effect of IL-2 Pretreatment on HPV Specific Cytokine Secretion
Given the lack of IL-2 production by HPV specific T-cells in RRP subjects, it seemed plausible that addition of exogenous IL-2 could restore the cytokine production of these cells. To test this possibility, we measured the cytokine secretion of HPV specific T cell lines with or without pre-incubation with exogenous IL-2. As shown in Figure 6, IL-2 treatment caused a significant (p=0.01) increase in IFN-γ secretion for cell lines from RRP patients. In contrast, IL-2 treatment caused no change in IFN-γ secretion for cell lines from healthy subjects.
Figure 6. Cytokine Secretion of HPV Specific T-cells is Increased in Patients with RRP after Pretreatment with IL-2.
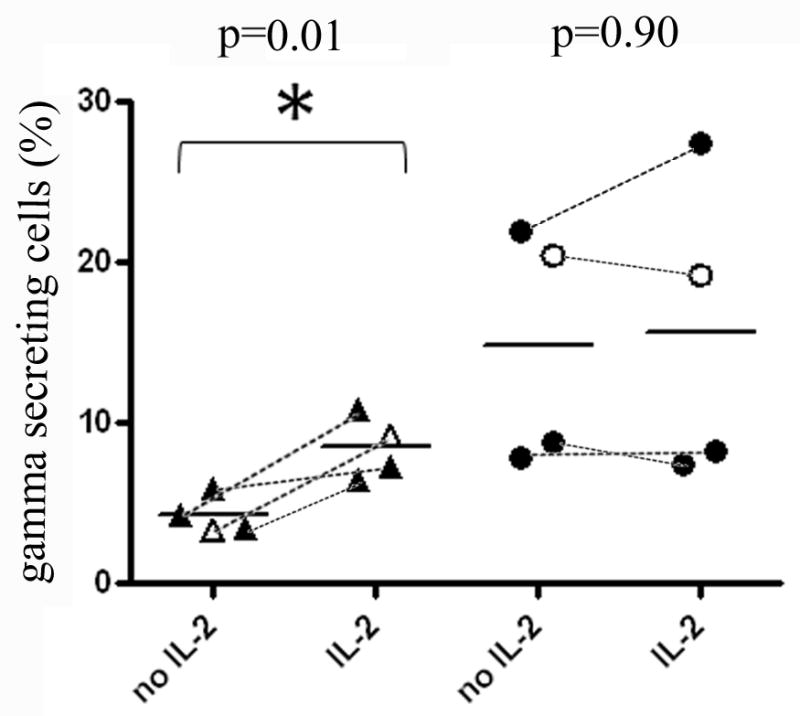
(A) IFN-γ secretion by DR0102/E2281-300 (open symbols) and DR0301/E2281-300 (black symbols) specific T-cell lines measured by cytokine capture for 4 cell lines isolated from RRP patients (triangles) and 4 cell lines isolated from HLA matched healthy subjects (circles) with or without pretreatment with exogenous IL-2 (identical cell lines connected by dashed lines). The data indicates a significant improvement in IFN-γ secretion for RRP patients (p=0.01) but not for healthy subjects (p=0.90). (B) Fold increase in IFN-γ secretion by HPV specific T cell lines from RRP patients and healthy subjects after pretreatment with exogenous IL-2. An average 1-fold increase (no change) was observed for healthy subjects and a 2-fold increase was observed for RRP patients, which represents a statistically significant difference (p=0.035).
Discussion
Recurrent respiratory papillomatosis (RRP) is a rare but serious disease that can significantly reduce the quality of life and cause mortality because chronic HPV infection in these patients leads to the formation of papillomas in the upper airway. HPV exposure is ubiquitous (virtually all individuals can be expected to have had exposure to at least one HPV strain) and memory T cell responses to HPV early proteins are commonly seen in the healthy population; however, symptomatic infections are uncommon (1–2). Approximately 5 percent of all individuals have evidence of HPV infection in the larynx without evidence of disease, but only a small fraction of these individuals develop RRP (27). In addition, only a subset of RRP patients develops an unrelenting and severe disease (8). RRP subjects exhibit chronic infection with HPV-6 or HPV-11 and can express serum antibodies (28). A study of the genetic background of RRP patients identified an enrichment of select HLA class II genes that correlated with reduced IFN-γ (17). These observations suggest that the immune mechanism that underlies RRP susceptibility involves a deficiency in protective CD4+ TH1-like T-cell responses against HPV. Indeed, previous studies have observed deficits in TH1 cytokine production and CD4+ T-cell immunity against HPV E2 and E6 proteins in the context of cervical cancer (29–30).
In this study we investigated CD4+ T-cell responses directed against the HPV-11 E2 and E6 proteins. Responses to these proteins have been previously implicated as being important in effective papillomavirus-specific immune responses (1, 9, 14–16). Using MHC class II tetramers, we identified multiple antigenic peptides within E2/E6 proteins that could be detected in RRP patients and healthy controls with DRB1*01:02 and DRB1*03:01 haplotypes. One of these peptides, E2281-300, could be presented by both DR0301 and DR0102. Notably, a closely related HPV-16 peptide was identified in a previous study as a DR1 restricted epitope (31). As summarized in Supplementary Table II, that antigenic region of the E2 protein is highly conserved among HPV strains. The N-terminal epitope within the leader sequence is much less conserved, but still is present in several strains. Therefore, these epitopes are likely to be relevant for studying various HPV-induced diseases.
For several reasons, it is likely that RRP patients exhibit HPV-specific immune dysregulation rather than global immune defects. Patients with RRP do not manifest other chronic infections and their peripheral CD4+ T-cell frequencies are comparable to those of unaffected controls (32–33). One possible mechanism for HPV-specific immune dysregulation is the induction of regulatory T-cells (Tregs) within papillomas or other HPV infected tissues. In preliminary studies we reported a two to seven fold enrichment of functional Tregs in papillomas compared to autologous blood from patients with RRP (33), while the frequency of Tregs in the peripheral blood of RRP patients was comparable to that found in control subjects. Enriched and functional Tregs in papillomas would likely suppress TH1-like responses to HPV in papilloma tissues, while having a lesser impact on responses in the periphery. In agreement with this notion, CD4+ T-cells specific for these E2/E6 epitopes were detectable in all of the subjects tested in our study. No statistically significant differences were observed between the E2/E6 specific T-cell responses of RRP patients and healthy subjects. However, it is possible that the in vitro culture methodology used could minimize differences in responsiveness between the two groups. In any case, these observations confirm that HPV-6/-11 infection is recognized by patients and controls and thus, is not a subliminal infection below the threshold of immune recognition as once thought (33–34). Thus, clonal ignorance (as suggested for certain CD8+ T-cell specificities) is not a viable explanation for why patients with RRP do not clear HPV-6/-11 infection (35), rather “low level immune tolerance” generated by immunosuppressive immunocytes responding to these HPVs would be more likely to be involved in the inability of RRP patients to clear this infection (33).
Some evidence suggests that HPV-specific immune dysregulation may be directly modulated by viral proteins and/or by the tumor microenvironment (11, 36). It was previously demonstrated that allospecific CTL activity was reduced in PBMC that had been pre-treated with recombinant HPV-11 E6 protein (33). It is plausible that the functional responses of CD4+ T-cells would also be reduced by similar mechanisms. These effects would be likely to impact responses in the periphery. To directly address the question of immune dysregulation, we isolated T-cell lines from multiple RRP and healthy subjects and assessed their functional responses to E2/E6 peptides. Assaying these for cytokine release revealed robust IFN-γ secretion from healthy subjects and significantly reduced IFN-γ secretion from RRP subjects compared to healthy control subjects. With the exception of two patients (three cell lines) RPP subjects also tended to exhibit reduced TNF-α secretion. These patients with significant TNF-α secretion also secreted appreciable levels of IFN-γ and had mild disease. Interestingly, one of these subjects had improved clinically in response to an experimental immunomodulator, anecdotally suggesting a connection between immune phenotype and prognosis. While levels of secreted IL-5 were modest and unaltered, RRP subjects produced modest levels of IL-13 protein in response to tetramer stimulation, suggesting a TH2-like response polarization. We previously observed polarized expression of a TH2-like repertoire of cytokines by tumor-infiltrating lymphocytes in studies of the same patient cohort (11, 37). Taken together with our current data, this suggests that deficient protective TH1-like CD4+ T-cell immunity to HPV can be detected both in the relevant tissue and in peripheral blood.
In addition to results suggesting that there are differences in response polarization, RRP subjects exhibited a clear reduction in STAT-5 signaling in response to immunodominant HPV E2/E6 peptides. This reduction was HPV-specific and also reversible, as the deficiency in STAT-5 signaling in RRP patients was comparable to healthy subjects after the administration of IL-2. These results suggest an intrinsic defect within HPV-specific CD4+ T-cells in RRP. Taken together, these data suggest a lack of autocrine IL-2 production, directly resulting in reduced STAT-5 and consequently dampening cytokine responses. Loss of IL-2 production has been described as one of the first functional defects exhibited by exhausted T-cells, followed by the loss of other effector functions, and in some cases deletion (38). Progressive T-cell exhaustion is commonly observed in settings of chronic viral infection and is also a component of T-cell dysfunction in cancer (18). Therefore it would not be surprising to observe this phenomenon in association with a chronic condition such as RRP. However, while exhaustion is associated with persistently high antigenic loads, HPVs generally use non-preferred codons, resulting in decreased levels of viral protein synthesis and subsequent antigenic function (39). In addition, peripheral HPV specific CD4+ T-cells from RRP subjects did not express PD-1, a marker that has been strongly associated with exhaustion (40).
These factors appear to preclude exhaustion as the best explanation for reduced TH1-like CD4+ T-cell effector function in RRP. An alternative explanation for this functional deficit would be anergy. Anergy, which is induced by TCR ligation in the absence of adequate co-stimulation, is associated with impaired proliferative capacity and lack of IL-2 production (19). In the anergic state, phosphorylation and activation of selected signaling molecules in response to TCR stimulation are significantly impaired (41). Unlike the exhausted state, which is rescued by decreasing antigenic load, sustained levels of antigen are not required to maintain clonal anergy and it is reversed by stimulation with IL-2 (41). We observed improvement in HPV-specific CD4+ T-cell responses from RRP subjects in an assay that included the addition of exogenous IL-2. Specifically, addition of exogenous IL-2 resulted in a significant increase in IFN-γ secretion by T cell lines isolated from RRP subjects. These cells lacked CTLA-4 expression, which has been shown to be important in some models of anergy induction (42). However, it has also been shown in CTLA-4-deficient mice that T-cells can persist in a hypo-responsive state in the absence of CTLA-4 (43). Furthermore, induction of anergy has been shown to occur in the presence of antibodies that block the CTLA-4/B7 interaction (44). This concept that HPV-specific, CD4+ T-cells from RRP patients may be anergic and require exogenous IL-2 replacement to restore function is supported by previous reports of the reduction of RRP severity following IFN-γ therapy (45–47). This suggests that restoration of a TH1-like microenvironment in HPV-infected tissues can restore HPV-specific T-cell function in RRP.
In summary, our results demonstrate that RRP patients have only marginally reduced numbers of HPV-specific T-cells, but significantly reduced functional CD4+ T-cell responses to E2/E6 epitopes. These findings support a paradigm in which persistent HPV infection leads to increased TH2-like polarization, due to tissue and HPV-specific events, and induction of clonal anergy through inadequate costimulation in respiratory papillomas. Anergized T-cell populations have been shown to regain functional attributes through the effects of IL-2 and other common gamma chain cytokines. Therefore, further investigation should be aimed at therapeutic interventions designed to reverse clonal anergy and restore HPV-specific CD4+ T-cell effector function by the development of a therapeutic vaccine that enhances TH1-like, and prevents/blocks TH2-like responses to HPV proteins, and thereby improves clinical outcomes for RRP patients. To this end, the T-cell assays described in this study would be useful for evaluating responsiveness to therapeutic strategies that can restore the TH1-/TH2-like balance to these HPVs in patients with RRP.
Supplementary Material
Acknowledgments
This work is supported by the National Institute of Dental & Craniofacial Research/National Institutes of Health R01 DE017227 (Dr. V. R. Bonagura).
We thank Dr. K. Arumuganathan for technical assistance and Virginia Mullooly, RN for clinical coordination. We thank Dr. E. Wambre and D. A. Long for technical advice about cytokine and STAT signaling assays.
Footnotes
HPV: human papillomavirus
RRP: Recurrent Respiratory Papillomatosis
DR0102: DRA1/B1*01:02 protein
DR0301: DRA1/B1*03:01 protein
HA: influenza A hemagglutinin
Myo: sperm whale myoglobin
References
- 1.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, Franken KL, Drijfhout JW, Fleuren GJ, Kenter G, Melief CJ, Offringa R, van der Burg SH. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 2.Broker TR, Jin G, Croom-Rivers A, Bragg SM, Richardson M, Chow LT, Vermund SH, Alvarez RD, Pappas PG, Squires KE, Hoesley CJ. Viral latency--the papillomavirus model. Dev Biol (Basel) 2001;106:443–451. [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med. 2003;127:930–934. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 4.Zacharisen MC, Conley SF. Recurrent respiratory papillomatosis in children: masquerader of common respiratory diseases. Pediatrics. 2006;118:1925–1931. doi: 10.1542/peds.2006-1555. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong LR, Preston EJ, Reichert M, Phillips DL, Nisenbaum R, Todd NW, Jacobs IN, Inglis AF, Manning SC, Reeves WC. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31:107–109. doi: 10.1086/313914. [DOI] [PubMed] [Google Scholar]
- 6.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnürch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–1247. doi: 10.1097/MLG.0b013e31816a7135. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg BM, Gallagher T, Stoler M, Abramson AL. Persistence and expression of human papillomavirus during interferon therapy. Arch Otolaryngol Head Neck Surg. 1988;114:27–32. doi: 10.1001/archotol.1988.01860130031010. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 10.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 11.DeVoti JA, Steinberg BM, Rosenthal DW, Hatam L, Vambutas A, Abramson AL, Shikowitz MJ, Bonagura VR. Failure of gamma interferon but not interleukin-10 expression in response to human papillomavirus type 11 E6 protein in respiratory papillomatosis. Clin Diagn Lab Immunol. 2004;11:538–547. doi: 10.1128/CDLI.11.3.538-547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209–220. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, Zelterman D, Buonocore L. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J Virol. 2007;81:5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandsma JL, Shlyankevich M, Zhang L, Slade MD, Goodwin EC, Peh W, Deisseroth AB. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. J Virol. 2004;78:116–123. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, Dobson JA, Jack LC, St Clair Roberts JA, Offringa R, van der Burg SH, Hickling JK. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 16.Vambutas A, DeVoti J, Nouri M, Drijfhout JW, Lipford GB, Bonagura VR, van der Burg SH, Melief CJ. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23:5271–5280. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Bonagura VR, Vambutas A, DeVoti JA, Rosenthal DW, Steinberg BM, Abramson AL, Shikowitz MJ, Gjertson DW, Reed EF. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum Immunol. 2004;65:773–782. doi: 10.1016/j.humimm.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 20.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James EA, Moustakas AK, Berger D, Huston L, Papadopoulos GK, Kwok WW. Definition of the peptide binding motif within DRB1*1401 restricted epitopes by peptide competition and structural modeling. Mol Immunol. 2008;45:2651–2659. doi: 10.1016/j.molimm.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, Kwok WW. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001;166:6665–6670. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 23.James EA, Bui J, Berger D, Huston L, Roti M, Kwok WW. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int Immunol. 2007;19:1291–1301. doi: 10.1093/intimm/dxm099. [DOI] [PubMed] [Google Scholar]
- 24.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang ZY, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verreck FA, van de Poel A, Drijfhout JW, Amons R, Coligan JE, Konig F. Natural peptides isolated from Gly86/Val86-containing variants of HLA-DR1, -DR11, -DR13, and -DR52. Immunogenetics. 1996;43:392–397. doi: 10.1007/BF02199809. [DOI] [PubMed] [Google Scholar]
- 26.Geluk A, van Meijgaarden KE, Southwood S, Oseroff C, Drijfhout JW, de Vries RR, Ottenhoff TH, Sette A. HLA-DR3 molecules can bind peptides carrying two alternative specific submotifs. J Immunol. 1994;152:5742–5748. [PubMed] [Google Scholar]
- 27.Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope. 1987;97:678–685. doi: 10.1288/00005537-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Bonnez W, Kashima HK, Leventhal B, Mounts P, Rose RC, Reichman RC, Shah KV. Antibody response to human papillomavirus (HPV) type 11 in children with juvenile-onset recurrent respiratory papillomatosis (RRP) Virology. 1992;188:384–387. doi: 10.1016/0042-6822(92)90770-p. [DOI] [PubMed] [Google Scholar]
- 29.Seresini S, Origoni M, Lillo F, Caputo L, Paganoni AM, Vantini S, Longhi R, Taccagni G, Ferrari A, Doglioni C, Secchi P, Protti MP. IFN-gamma produced by human papilloma virus-18 E6-specific CD4+ T cells predicts the clinical outcome after surgery in patients with high-grade cervical lesions. J Immunol. 2007;179:7176–7183. doi: 10.4049/jimmunol.179.10.7176. [DOI] [PubMed] [Google Scholar]
- 30.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 31.de Jong A, van der Burg SH, Kwappenberg KM, van der Hulst JM, Franken KL, Geluk A, van Meijgaarden KE, Drijfhout JW, Kenter G, Vermeij P, Melief CJ, Offringa R. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62:472–479. [PubMed] [Google Scholar]
- 32.Bonagura VR, Siegal FP, Abramson AL, Santiago-Schwarz F, O’Reilly ME, Shah K, Drake D, Steinberg BM. Enriched HLA-DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol. 1994;1:357–360. doi: 10.1128/cdli.1.3.357-360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonagura VR, Hatam LJ, Rosenthal DW, de Voti JA, Lam F, Steinberg BM, Abramson AL. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118:455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109:S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Melero I, Singhal MC, McGowan P, Haugen HS, Blake J, Hellstrom KE, Yang G, Clegg CH, Chen L. Immunological ignorance of an E7-encoded cytolytic T-lymphocyte epitope in transgenic mice expressing the E7 and E6 oncogenes of human papillomavirus type 16. J Virol. 1997;71:3998–4004. doi: 10.1128/jvi.71.5.3998-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 37.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93:302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 38.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–81. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg T, Ohlschläger P, Sehr P, Osen W, Gissmann L. Modification of HPV 16 E7 genes: correlation between the level of protein expression and CTL response after immunization of C57BL/6 mice. Vaccine. 2005;23:1149–1157. doi: 10.1016/j.vaccine.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 41.Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson KM, Czinn SJ, Redline RW, Blanchard TG. Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. J Immunol. 2006;176:5306–5313. doi: 10.4049/jimmunol.176.9.5306. [DOI] [PubMed] [Google Scholar]
- 43.Inobe M, Schwartz RH. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J Immunol. 2004;173:7239–7248. doi: 10.4049/jimmunol.173.12.7239. [DOI] [PubMed] [Google Scholar]
- 44.Frauwirth KA, Alegre ML, Thompson CB. Induction of T Cell Anergy in the Absence of CTLA-4/B7 Interaction. J Immunol. 2000;164:2987–2993. doi: 10.4049/jimmunol.164.6.2987. [DOI] [PubMed] [Google Scholar]
- 45.Healy GB, Gelber RD, Trowbridge AL, Grundfast KM, Ruben RJ, Price KN. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med. 1988;319:401–407. doi: 10.1056/NEJM198808183190704. [DOI] [PubMed] [Google Scholar]
- 46.Mullooly VM, Abramson AL, Steinberg BM, Horowitz MS. Clinical effects of alpha-interferon dose variation on laryngeal papillomas. Laryngoscope. 1988;98:1324–1329. doi: 10.1288/00005537-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Leventhal BG, Kashima HK, Mounts P, Thurmond L, Chapman S, Buckley S, Wold D. Long-term response of recurrent respiratory papillomatosis to treatment with lymphoblastoid interferon alfa-N1. Papilloma Study Group. N Engl J Med. 1991;325:613–617. doi: 10.1056/NEJM199108293250904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.