SUMMARY
Glycogen is a branched polymer of glucose that serves as an energy store. Phosphate, a trace constituent of glycogen, has profound effects on glycogen structure and phosphate hyperaccumulation is linked to Lafora disease, a fatal progressive myoclonus epilepsy that can be caused by mutations of laforin, a glycogen phosphatase. However, little is known about the metabolism of glycogen phosphate. We demonstrate here that the biosynthetic enzyme glycogen synthase, which normally adds glucose residues to glycogen, is capable of incorporating the β-phosphate of its substrate UDP-glucose at a rate of one phosphate per approximately 10,000 glucoses, in what may be considered a catalytic error. We show that the phosphate in glycogen is present as C2 and C3 phosphomonoesters. Since hyperphosphorylation of glycogen causes Lafora disease, phosphate removal by laforin may thus be considered a repair or damage control mechanism.
Keywords: glycogen, glycogen synthase, laforin, Lafora disease, glycogen phosphorylation, glycogen phosphatase
INTRODUCTION
Glycogen is a branched polymer of glucose that serves as a critical energy reserve in many cells from bacteria to mammals (Roach, 2002). It consists of chains of glucose residues connected by α-1,4-glycosidic linkages with α-1,6-glycosidic linkages forming branch points. Glycogen synthase catalyzes the formation of the primary polymerizing α-1,4-glycosidic linkages by transferring a glucosyl moiety from UDP-glucose to a non-reducing end of a glycogen molecule with the release of UDP (Roach, 2002). Our recent research has focused on the importance of covalently linked phosphate, a minor constituent of glycogen, of uncertain origin and function (Fontana, 1980; Lomako et al., 1994; Lomako et al., 1993). Muscle glycogen, for example, contains one phosphate per 600 – 1500 glucose residues, depending on the species (Tagliabracci et al., 2008; Tagliabracci et al., 2007). Abnormal glycogen phosphorylation is associated with Lafora disease, an autosomal recessive progressive myoclonus epilepsy with onset in the teenage years followed by gradual worsening of neurological symptoms and death usually within ten years (Andrade et al., 2007; Delgado-Escueta, 2007; Gentry et al., 2009). A characteristic of Lafora disease is the formation of Lafora bodies, deposits containing sparsely branched, insoluble glycogen-like polymers in many tissues, including neurons. About 50% of Lafora patients have mutations in the EPM2A gene that encodes laforin (Minassian et al., 1998; Serratosa et al., 1999), a phosphatase of the dual specificity family, with a carbohydrate binding module. Laforin is capable of releasing the phosphate from glycogen (Tagliabracci et al., 2007). Glycogen from Lafora patients has elevated phosphate (Sakai et al., 1970; Schnabel and Seitelberger, 1968) as does glycogen from a mouse model of Lafora disease in which the laforin gene is deleted (Tagliabracci et al., 2008; Tagliabracci et al., 2007). In mice, the hyperphosphorylation of glycogen is associated with progressive deterioration in glycogen structure as judged by its physico-chemical properties, reduced branching frequency and abnormal appearance by electron microscopy (Tagliabracci et al., 2008). Therefore, knowing the molecular details of phosphate metabolism in glycogen is critical to understanding Lafora body formation and Lafora disease. Prior to initiating this work, we knew only that the glycogen phosphate could be released by laforin and that excessive phosphate affected glycogen structure and solubility. Important unanswered questions were how the phosphate was introduced and where it was attached to glycogen. In this study, we identify a possible origin of the phosphate, an enzyme capable of its introduction, the chemistry of the phosphate linkage and first indications of the substrate specificity of laforin.
RESULTS
Introduction of phosphate into glycogen by glycogen synthase
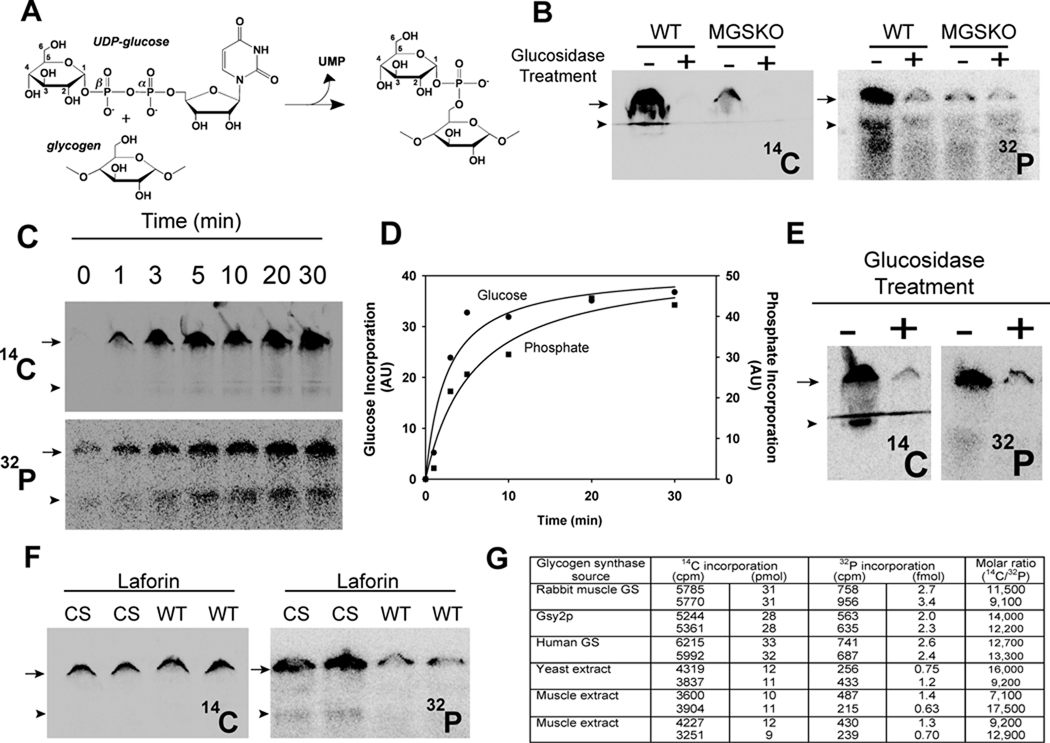
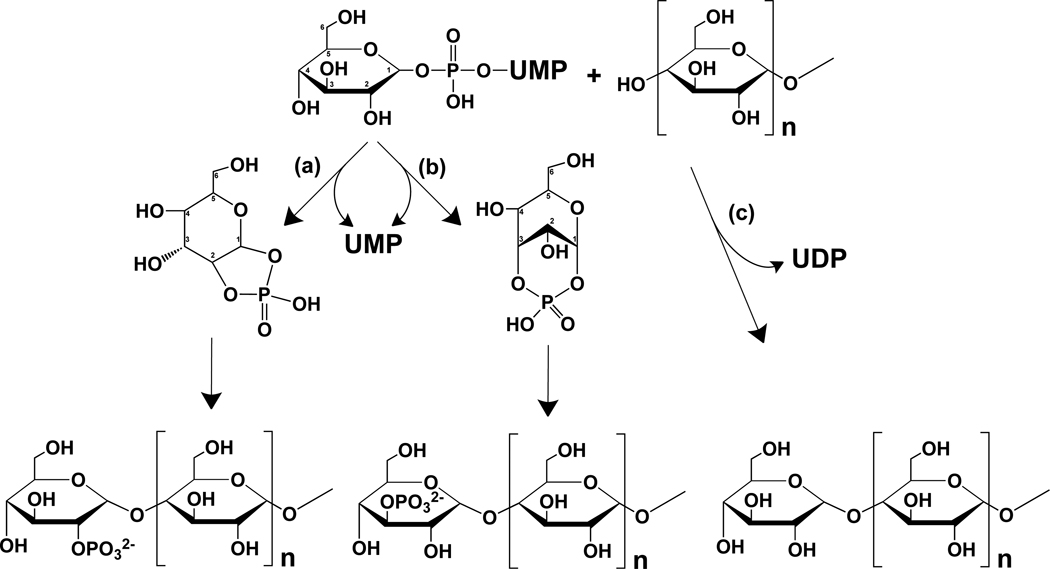
Lomako et al. (Lomako et al., 1994; Lomako et al., 1993) proposed that the phosphate in glycogen was present as a C-6 monoester and as a C1–C6 bridging phosphodiester, and that a distinct, but uncharacterized, glucose-1-phosphate transferase was responsible for the formation of the phosphodiester by transfer of the β-phosphate of UDP-glucose with release of UMP (Fig. 1A). To pursue the latter idea, we synthesized [β-32P]UDP-glucose to assay and purify the proposed glucose-1-phosphate transferase. A mouse muscle extract was indeed able to transfer 32P from [β-32P]UDP-glucose to glycogen, consistent with the previous study (Lomako et al., 1993)(Fig. 1B). A parallel incubation with UDP-[14C]glucose monitored the normal incorporation of glucose into glycogen by glycogen synthase (Fig. 1B). Treatment of the synthesized glycogen by a combination of the enzymes α-amylase and amyloglucosidase degrades the polysaccharide by hydrolyzing α-1,4- and α-1,6-glycosidic linkages so that it would no longer accumulate at the top of the gel. Both the 14C and 32P signals were reduced to background by glucosidase treatment, confirming that the radioactivity was associated with a glucose polysaccharide (Fig. 1B).
Figure 1.
Glycogen synthase incorporates phosphate into glycogen from UDP-glucose. (A) UDP-glucose structure and the glucose-1-phosphate transferase reaction proposed by Whelan (see text) (B) Incorporation of [U-14C]glucose (left) and 32P (right) into glycogen (6.67 mg/mL) catalyzed by muscle extracts from wild type (WT) mice or mice lacking muscle glycogen synthase (MGSKO) using UDP-[U-14C]glucose and [β-32P]UDP-glucose, respectively, as glucose donors. Reaction products were separated by SDS-PAGE and radioactivity detected by phosphorimaging. The arrow indicates the bottom of the well where glycogen accumulates and the arrowhead the top of the stacking gel where smaller polysaccharides migrate. Some samples were also treated with α-amylase and amyloglucosidase, as indicated, prior to SDS-PAGE. Loss of radioactivity indicates association with polysaccharide. (C) Time dependent incorporation of [U-14C]glucose (upper) and 32P (lower) into glycogen (6.67 mg/mL) by purified rabbit muscle glycogen synthase (1 µg/mL). (D) Quantitation of the phosphorimager data in C. Different gels were analyzed for 32P and 14C incorporation and the arbitrary units are not comparable. Incorporation of radioactivity was quantitated in absolute terms by excision of the relevant gel areas and scintillation counting. (E) Labeled glycogen, prepared as in C, was purified by ethanol precipitation, subjected to treatment with α-amylase and amyloglucosidase, as indicated, and analyzed as in B. (F) Glycogen, prepared as in C, was purified by ethanol precipitation and subjected to treatment with either catalytically inactive C266S laforin (CS) or active laforin (WT). (G) Quantitation of 14C and 32P incorporation into glycogen. Glycogen was synthesized with the indicated source of glycogen synthase and duplicate measurements quantitated as described under Experimental Procedures. See also Figure S1.
In an attempt to demonstrate that the activity was distinct from glycogen synthase, we also analyzed an extract from the muscle of a genetically modified MGSKO mouse lacking muscle glycogen synthase (Pederson et al., 2004). The MGSKO extract transferred little 14C into glycogen, as expected, but unexpectedly also little 32P (Figs. 1B and S1A). Experiments with extracts of wild type Saccharomyces cerevisiae and a gsy1 gsy2 mutant strain that lacks both yeast glycogen synthase genes gave similar results, with glycogen phosphorylation comparable to that observed with mouse muscle and reduced to background in the absence of glycogen synthase (Fig. S1A). We therefore concluded that the principal activity in cell extracts responsible for 32P-incorporation into glycogen was glycogen synthase.
To address this hypothesis, we first tested glycogen synthase purified from rabbit muscle (DePaoli-Roach et al., 2003). We found that glycogen synthase catalyzed the time-dependent introduction of phosphate from UDP-glucose into glycogen at a constant relative rate with respect to glucose incorporation (Figs. 1C & D and S1B). Again, treatment of the radioactive product with glucosidases confirmed that the 32P was associated with glycogen (Fig. 1E). We repeated the experiment with recombinant Gsy2p, a yeast glycogen synthase ortholog produced in Escherischia coli, and recombinant human glycogen synthase produced by baculoviral expression. The results were very similar to what was observed with the rabbit enzyme (Fig. S1B). By quantitating the incorporated radioactivity and knowing the specific radioactivities of the labeled UDP-glucoses, we determined the relative molar proportions of phosphate and glucose. From such calculations, one phosphate was introduced for approximately 10,000 glucose residues by all three purified glycogen synthases (Fig. 1G). A similar phosphorylation stoichiometry was observed with extracts of yeast or mouse muscle extracts (Fig. 1G). This consistency might suggest that the frequency of phosphate introduction is linked to the properties of glycogen synthase itself.
The phosphate incorporated by glycogen synthase could be released after purification of the 32P-labelled glycogen and treatment with recombinant laforin (Fig. 1F). The catalytically inactive C266S mutant was ineffective. As would be expected, laforin had no effect on the 14C-content of the synthesized glycogen. If laforin can remove the phosphate from the glycogen, its presence during synthesis might be expected to reduce phosphorylation. Therefore, we compared 32P incorporation by extracts of muscle from wild type mice and mice with the laforin gene disrupted (Epm2a−/−) (Fig. S1A). Phosphate incorporation into glycogen was not increased in the knockout extracts (Fig. S1A). This result suggests that laforin does not act during the synthesis of glycogen in such experiments, a result consistent with the observation that relative phosphate incorporation by yeast extracts, which also lack laforin, was similar to that of muscle. In vivo, either conditions differ such that laforin can act during synthesis or alternatively it acts during glycogen degradation.
Glycogen contains C2 and C3 phosphomonoesters of glucose
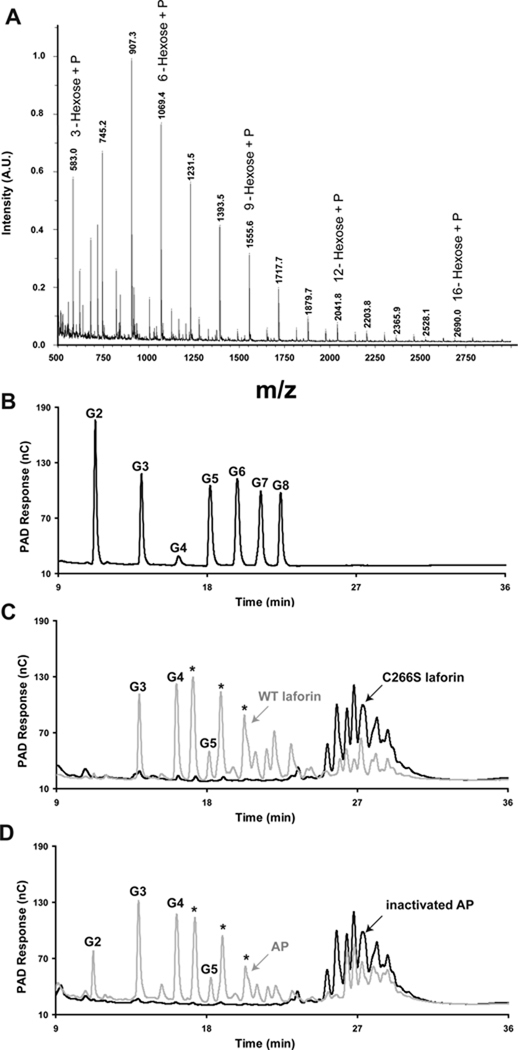
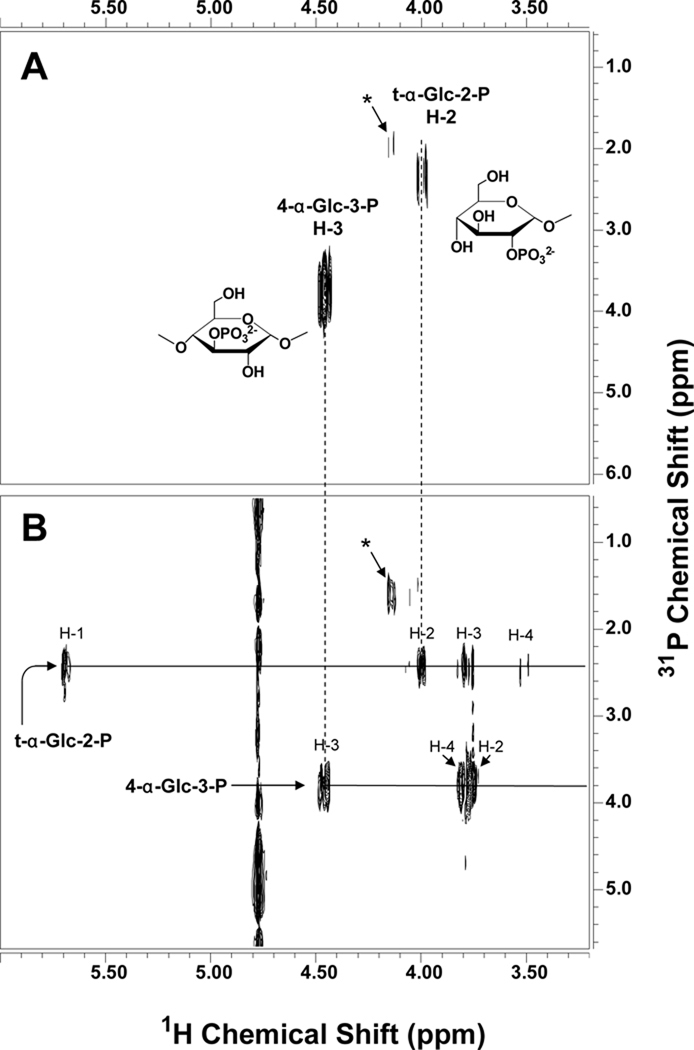
To explore the chemical nature of the glycogen phosphate, we analyzed glycogen that was purified from rabbit skeletal muscle by procedures designed to retain phosphoesters. Such glycogen was previously shown to be a substrate for laforin (Tagliabracci et al., 2007). Glycogen was digested with α-amylase and amyloglucosidase and the resulting phospho-oligosaccharides purified by anion exchange chromatography, with recovery of ~70% of the phosphate (Fig. S2). MALDI TOF mass spectra were dominated by signals consistent with the presence of phospho-oligosaccharides, varying from 3 hexoses plus a phosphate up to 16 hexoses plus a phosphate (Figs. 2A and S2). By high performance anion exchange chromatography (HPAEC), the phospho-oligosaccharides eluted as a complex cluster of species (Figs. 2C and S2) significantly later than maltooctaose (G8), the longest oligosaccharide standard (Fig. 2B), indicating the absence of linear neutral glucose oligomers smaller than G8. Although the phospho-oligosaccharide mixture is heterogeneous in terms of polymerization state, it is homogeneous in the sense that it consists of glucose and glucose-phosphate residues. It was therefore amenable to analysis by NMR spectroscopy. Several spin systems were detected by 1H-1H COSY and TOCSY and 1H-13C HSQC (see Fig. S3 and Table 1). The spectra were dominated by a group of similar spin systems belonging to 4-α-Glc-(1→4) residues. Other spin systems present included those of 4-α-Glc-(1→6), terminal α-Glc-(1→4), terminal α-Glc-(1→6), and α and β reducing end residues that appeared to be mostly 4,6-linked (i.e. a branching residue glycosylated in both the 4- and the 6-positions), judging by their H-1 chemical shifts (van Leeuwen et al., 2008). In addition, two spin systems were identified, each with one proton resonance uncharacteristically far downfield for glucose, suggesting substitution by phosphate (Fig. S3 and Table S1). On the basis of the connectivities established by the COSY, TOCSY, and ROESY experiments and the carbon chemical shifts determined by the HSQC experiment, one of these downfield signals appeared to be H-2 of a terminal glucose residue and the other H-3 of a 4-linked glucose. To confirm substitution by phosphate, we acquired a 1H-31P gHMQC spectrum, which showed two peaks, indicating substitution at the 2-OH and the 3-OH positions of glucose by phosphate (Fig. 3A). We also acquired a 1H-31P HMQC-TOCSY spectrum to ascertain that these two protons belong to the spin systems previously assigned. This spectrum showed the expected correlations of each of the peaks detected in gHMQC with their neighboring protons (Fig. 3B). From these experiments, therefore, the phosphate present in muscle glycogen appears to be in the form of C2 and C3 monoesters. We found no evidence for the presence of a phosphodiester or a C6 phosphate as had been previously postulated.
Figure 2.
Purification and analysis of phosphorylated oligosaccharides from rabbit muscle glycogen. (A) MALDI-TOF mass spectrometry of phosphorylated oligosaccharides (fraction V; see Fig. S1A) from rabbit muscle glycogen. The spectrum displays a series of species differing by 162.1 m/z indicative of a mixture of phosphorylated oligosaccharides ranging from 3 hexoses + phosphate to 16 hexoses + phosphate. (B) HPAEC analysis of malto-oligosaccharide standards. G1, glucose, G2, maltose, G3, maltotriose, G4, maltotetraose, G5, maltopentaose, G6, maltohexaose, G7, maltoheptaose, G8, maltooctaose. (C) Phosphorylated oligosaccharides (fraction V) were treated with either catalytically inactive laforin (C266S, black) of active laforin (WT, grey) and analyzed by HPAEC. (D) Phosphorylated oligosaccharides were treated with either heat denatured alkaline phosphatase (inactivated AP, black) or active alkaline phosphatase (AP, grey) and analyzed by HPAEC. The asterisks mark species generated by laforin or alkaline phosphatase treatment that do not align with any of the linear oligosaccharide standards (panel B) and which are likely branched oligosaccharides. See also Figure S2.
Table 1.
Chemical shift assignments of phosphorylated oligosaccharides. Data from Figs. 3 and S3 are collated in the table. See also Table S1.
| Residue | 1 | 2 | 3 | 4 | 5 | 6 | NOE | ||
|---|---|---|---|---|---|---|---|---|---|
| I | t-α-Glc-2-P-(1→?) | 1H | 5.69 | 4.00 | 3.76 | 3.50 | 3.69 | 3.83/3.77 | |
| 13C | 100.0 | 77.3 | 74.8 | 72.0 | 75.0 | 63.3 | |||
| II | t-α-Glc-(1→4) | 1H | 5.59 | 3.58 | 3.65 | 3.43 | 3.66 | 3.83/3.77 | IV-4 |
| 13C | 101.3 | 74.2 | 75.4 | 72.1 | 75.4 | 63.3 | |||
| III | 4-α-Glc-(1→4) | 1H | 5.40 | 3.60 | 3.97 | 3.64 | 3.84 | 3.83/3.77 | IIIn-1-4a |
| 13C | 102.2 | 74.1 | 76.0 | 79.7 | 73.8 | 63.3 | |||
| IV | 4-α-Glc-3-P-(1→4) | 1H | 5.35 | 3.75 | 4.46 | 3.81 | 3.87 | n.d.b | III-4, VII-4, or VIII-4 |
| 13C | 102.4 | 74.6 | 79.9 | 76.4 | 73.8 | n.d. | |||
| V | 4,6-α-Glcred | 1H | 5.22 | 3.55 | 3.96 | 3.64 | 3.93 | 3.98/3.83 | |
| 13C | 94.6 | 74.2 | 76.0 | 79.7 | 72.7 | 69.7 | |||
| VI | t-α-Glc-(1→6) | 1H | 4.99 | 3.54 | 3.74 | 3.44 | 3.86 | n.d. | |
| 13C | 101.5 | 74.7 | 74.2 | 72.1 | 73.8 | n.d. | |||
| VII | 4-α-Glc-(1→6) | 1H | 4.95 | 3.59 | 4.01 | 3.63 | 3.85 | n.d. | |
| 13C | 100.7 | 74.1 | 73.0 | 80.6 | 74.0 | n.d. | |||
| VIII | 4,6-β-Glcred | 1H | 4.65 | 3.25 | 3.77 | 3.62 | 3.60 | 3.97/3.73 | |
| 13C | 98.5 | 76.7 | 78.9 | 80.6 | 77.3 | 68.8 |
the next residue in the chain
not determined
Figure 3.
1H-31P correlated NMR spectra of phosphorylated oligosaccharides. The spectra were acquired at 25 °C and 11.7 T with a 1-s relaxation delay and 3.5 kHz spectral width in F2 (A) The 1H-31P gHMQC spectrum was acquired with an 8.0 kHz spectral width in F1, 0.4 s acquisition time, 32 increments, and 360 transients per increment. (B) The 1H-31P HMQC-TOCSY spectrum was acquired with a 3.0 kHz spectral width in F1, 0.3 s acquisition time, 40 ms spinlock time, 24 increments, and 400 transients per increment. Phosphorus decoupling was turned on during acquisition using the GARP-1 decoupling sequence. The signal marked with an asterisk is probably from a terminal glucose-2-phosphate residue linked in a different position but could not be clearly identified due to its low abundance. See also Figure S3.
Substrate specificity of laforin
Laforin was able to dephosphorylate phospho-oligosaccharides generated from glycogen. HPAEC analysis of laforin reaction products indicated the generation of maltotriose, maltotetraose, and maltopentaose (Figs. 2B & C) as well as three other prominent species (asterisks) which are likely branched oligosaccharides. The release of phosphate by laforin is consistent with the presence of phosphomonoesters since there is no evidence in the literature that laforin has phosphodiesterase activity and it is inactive towards p-nitrophenylphosphocholine or p-nitrophenyl phenylphosphonate, two common phosphodiesterase substrates (Tagliabracci and Roach, unpublished observations). Alkaline phosphatase treatment yielded similar results to laforin treatment with the important exception that significant amounts of maltose were also produced (Fig. 2D). The results further argue that phosphate is in a monoester linkage and also demonstrate the presence of maltose-P, maltotriose-P, maltotetraose-P and maltopentaose-P in the mixture. In separate experiments, we have been unable to demonstrate dephosphorylation of glucose-1-P, glucose-2-P or glucose-6-P by laforin (data not shown). These results provide the first insight into laforin’s selectivity for oligosaccharide substrates; laforin can hydrolyse C2 and C3 phosphomonoesters of glucose in oligosaccharides with a minimum requirement of a trisaccharide phosphate for activity.
DISCUSSION
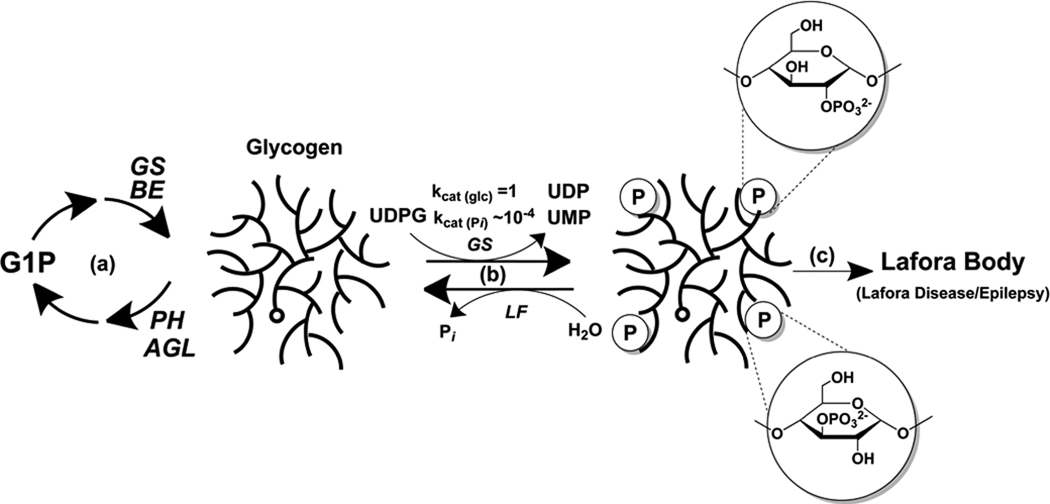
The results of this study expand our understanding of the metabolism of the phosphate in glycogen (Fig. 4). First, the phosphate is present as glucose C2- and C3-monoesters. Glucose C3 phosphate has been described in plant amylopectin (Blennow et al., 2002), a polymer chemically related to glycogen (Ball et al., 1996), but as best we can tell there are no confirmed descriptions of a glucose C2 phosphomonoester in nature. Some studies of amylopectin (for example, (Tabata and Hizukuri, 1971)) had raised the possibility of C2 phosphate in starch but without providing any formal proof. Of course, other modifications of the C2 position of glucose are known, as in glucosamine or N-acetyl-glucosamine, and phosphorylation at C2 in fructose occurs in fructose-2,6-bisphosphate. Secondly, we propose a potential origin of the phosphate in glycogen as the β-phosphate of UDP-glucose, introduced by the action of glycogen synthase in a rare but potentially important deviation from its presumed "normal" reaction. Though we cannot exclude the existence of other mechanisms for glycogen phosphorylation in vivo, the action of glycogen synthase has to be considered a strong candidate since very little phosphate is incorporated by muscle extracts lacking glycogen synthase. There are several examples of enzymes catalyzing reactions not considered to be their evolved function. For example, many phosphokinase enzymes catalyze hydrolysis of the co-substrate ATP (Knowles, 1980). Another instance is the glucosyltransferase glycogenin which is capable of hydrolyzing its substrate UDP-glucose to form UDP and glucose (Hurley et al., 2005). In these cases, the consequence is an effective waste of metabolic energy. For glycogen synthase, the alternative reaction would result in phosphorylated glycogen.
Figure 4.
Model for glycogen phosphate metabolism and Lafora Body formation. (a) Glycogen is synthesized by glycogen synthase (GS) and branching enzyme (BE), and degraded by glycogen phosphorylase (PH) and debranching enzyme (AGL). (b) Glycogen synthase infrequently (1 in ~10,000) incorporates phosphate residues into glycogen. The kcat values given simply denote the relative rates of glucose versus glucose phosphate incorporation. Excessive incorporation of phosphate as C2 or C3 phosphomonesters, which disrupts glycogen structure, is normally kept in check by the action of the laforin phosphatase (LF). (c) When laforin is defective, excessive phosphorylation results in the formation of Lafora bodies and Lafora disease.
With knowledge of the chemical nature of the phosphate present in glycogen, we can begin to formulate mechanisms by which glycogen synthase may mediate its introduction into the polysaccharide. Glycogen synthase belongs to the family of retaining glycosyltransferases (Lairson et al., 2008) and normally attaches the C1 of the donor glucose from UDP-glucose to the C4 of a non-reducing end of glycogen, with retention of the C1 stereochemistry and release of UDP as the leaving group (Fig. 5). Understanding the catalytic mechanism of this class of enzymes has been challenging and debate continues as to whether a two stage SN2 reaction or a concerted SNi mechanism is operative (Lairson et al., 2008). In either case, the mechanism involves reaction of the C1 of the donor monosaccharide moiety. Though little discussed in recent years, the formation of cyclic glucose C1 phosphodiesters is supported by a fairly extensive literature (Khorana et al., 1957; Kokesh et al., 1978; Kokesh et al., 1977; Nunez and Barker, 1976; Zmudzka and Shugar, 1964). For example, glucose-1,2-cyclic phosphate can form spontaneously from UDP-glucose under appropriate conditions (Paladini and Leloir, 1952). One potential explanation for the phosphorylation of glycogen by glycogen synthase would be if UDP-glucose infrequently breaks down in the enzyme active site to generate glucose-1,2-cyclic phosphate or glucose-1,3 cyclic phosphate, that then enters into the normal catalytic reaction, with attack at C1 now opening the ring to form a phosphoglucose moiety that is transferred to C4 of the acceptor (Fig. 5).
Figure 5.
Proposed mechanism for the introduction of phosphate into glycogen by glycogen synthase. Glycogen synthase normally transfers a glucose residue from UDP-glucose to form a new α-1,4-glycosidic linkage with the C4-hydroxyl at the reducing end of a glycogen chain (pathway c). The proposed phosphorylation mechanism would involve the formation, in the active site of glycogen synthase, of either glucose-1,2-cyclic phosphate (pathway a) or glucose-1,3-cyclic phosphate (pathway b). Reaction of the C1 of the cyclic phosphodiester would result in the transfer of either a glucose-2-phosphate or a glucose-3-phosphate moiety in α-1,4-glycosidic linkage to a reducing end of glycogen.
It is possible that the presence of a controlled amount of phosphate in glycogen reflects some as yet unappreciated metabolic, structural or functional purpose. Alternatively, phosphate incorporation into glycogen might result from a catalytic error by glycogen synthase. If so, it would resemble errors in the synthesis of other biopolymers like DNA (Kunkel and Bebenek, 2000), RNA (Thomas et al., 1998) and proteins (Zaher and Green, 2009). These examples involve mis-incorporation of a monomeric unit during polymer synthesis directed by a template, DNA or mRNA, and specialized repair systems have evolved to improve the fidelity of polymer production. Estimates of the error rates for single base substitutions by proof-reading deficient DNA polymerases range from 10−3 – 10−6, depending on the specific enzyme (Kunkel and Bebenek, 2000). Phosphate incorporation by glycogen synthase (~10−4) is in a similar range. Whereas an error in synthesis of an individual protein or RNA molecule would have limited impact, errors in DNA sequence are perpetuated and hence must be strictly suppressed. Similarly, without a mechanism for its removal, phosphate incorporated into a glycogen molecule would persist because each molecule undergoes many rounds of partial degradation and resynthesis. This turnover of glycogen explains the difference between the rate of phosphate incorporation into glycogen by glycogen synthase (1 in ~10,000 glucoses) and the higher level of basal phosphorylation of glycogen in tissues (~1 in 1,500 glucoses in mouse muscle). The latter is a function not only of the synthetic rates but also of the relative rates of glucose and phosphate removal from glycogen, mediated by glycogen phosphorylase/debranching enzyme and laforin, respectively.
Whether or not glycogen phosphate has a function or is simply the result of a catalytic error, we do know that excess phosphate is detrimental to glycogen structure. Therefore, we propose that laforin serves to prevent overaccumulation of covalent phosphate in glycogen and can be considered part of a repair or damage control mechanism, simpler but conceptually not unlike DNA repair. In mice, phosphate introduced by glycogen synthase accumulates to a basal, tolerated level that remains constant from three to twelve months of age (Tagliabracci et al., 2008). When laforin is disabled in laforin knockout mice, however, excessive phosphorylation of glycogen occurs and is associated with a progressive deterioration in glycogen structure (Tagliabracci et al., 2008), leading ultimately to Lafora disease and its tragic consequences (Andrade et al., 2007; Delgado-Escueta, 2007; Gentry et al., 2009). It is interesting that, for the most part, laforin is restricted to vertebrates (Gentry et al., 2007), organisms with lifespans long enough for excessive phosphorylation of glycogen to have structural and metabolic consequences.
MGSKO mice are disrupted for the GYS1 gene (Pederson et al., 2004), which encodes the glycogen synthase isoform expressed in skeletal muscle, heart, brain and several other tissues. Some 90% of MGSKO mice die perinatally, most likely due to defects in embryonic cardiac development caused by the absence of heart glycogen (Pederson et al., 2004). However, surviving MGSKO mice, which totally lack brain glycogen, appear normal, have similar exercise capacity as wild type animals, and if anything have improved ability to dispose of glucose (Pederson et al., 2005a; Pederson et al., 2005b). Relevant to this discussion, the MGSKO mice display no neurological symptoms up to 20 months of age (Pederson, unpublished results). Therefore, selective inhibition of brain glycogen synthase might provide a viable therapeutic intervention in Lafora disease, for which there is currently no effective treatment.
EXPERIMENTAL PROCEDURES
Enzymes
Rabbit muscle glycogen synthase and yeast Gsy2p were purified as previously described (Baskaran et al., 2010; DePaoli-Roach et al., 2003). Purified recombinant human glycogen synthase muscle isoform (GYS1) produced by baculoviral expression was the generous gift of Drs. May Khanna and Yuichiro Takagi.
Synthesis and purification of [β32P] UDP-glucose
[β32P]UDP-glucose was synthesized enzymatically and purified by a modification of the method of Dhugga and Ray (Dhugga and Ray, 1994) as described in Heyen et al. (Heyen et al., 2009). Briefly, 5 mCi of [γ-32P]ATP (3000 Ci/mmol) was converted enzymatically to [β32P]UDP-glucose and bound to activated charcoal to remove glucose-1P and glucose-6P. Differential elution from the charcoal separated the [β32P]UDP-glucose from unreacted [γ-32P]ATP. The eluate was dried in a Speed Vac, the [β32P]UDP-glucose resuspended in 100–200 µL of H2O and stored in aliquots at −80°C until use.
Phosphorylation of glycogen with glycogen synthase
A reaction mixture consisting of 50 mM Tris-HCl pH 7.6, 6.7 mg/mL deionized rabbit liver glycogen, 7.2 mM glucose-6P, 5 mM EDTA and 1 µM [β32P]UDP-glucose (~50,000–100,000 cpm/pmol) or 1 µM UDP-[U-14C]glucose (~500–2000 cpm/pmol) and 1 –1.2 µg/mL of rabbit muscle glycogen synthase, 1.9 µg/mL human glycogen synthase, or 0.6 µg/mL Gys2p was incubated for the indicated time or if not stated, 30 minutes, and terminated either by boiling in a 100°C H2O bath or by the addition of 2 volumes of ethanol. Immediately before termination of the reaction, KH2PO4 was added to both the 14C and 32P reactions to a final concentration of 50 mM to reduce background 32P. All reactions, whether terminated by boiling or ethanol precipitation, were placed at −20°C for a minimum of 1 hr in 66% ethanol containing 5–15 mM LiCl to precipitate the radiolabeled glycogen. The glycogen was recovered by centrifugation at 17,500 × g for 20 min at 4°C, dissolved in H2O to a final concentration of 6.67 mg/mL and SDS loading buffer added. After boiling, the glycogen (~200–250 µg) was subjected to SDS PAGE and the radioactivity visualized by autoradiography using a phosphorimager. For quantitation of 32P, the radioactive region was excised from the gel, minced and added to scintillation fluid. Background from blank reactions lacking glycogen synthase were subtracted. For quantitation of 14C, the purified glycogen was spotted onto filter paper, washed in 66% ethanol, dried, and added to scintillation fluid. Radioactivity was determined using a scintillation counter.
Initially, we attempted to adapt the standard glycogen synthase assay (Thomas et al., 1968) to measure 32P incorporation. In this assay, the reaction is transferred to filter paper, immersed in 66% ethanol to terminate the reaction and to remove unreacted UDP-[U-14C]glucose, leaving 14C-labeled glycogen trapped on the filter paper. However, we discovered that UDP binds significantly to the filter papers. With [β32P]UDP-glucose as substrate, the normal glycogen synthase reaction generates [β32P]UDP which binds to the filter and gives a false value for the incorporation of 32P.
For some experiments, the glycogen pellet was redissolved in H2O to a final concentration of ~8.3 mg/ml and diluted to 6.7 mg/mL by the addition of α-amylase and amyloglucosidase (final concentration of 300 µg/mL for each glucosidase and 20 mM sodium acetate pH 4.8) or laforin (see below). The glucosidase reaction was for a minimum of 1 hour at 37–40°C, and was terminated by the addition of SDS loading buffer.
Phosphorylation of glycogen using skeletal muscle or yeast extracts
Rabbit liver glycogen was incubated with skeletal muscle extracts from wild type or genetically modified mice. Frozen tissue samples (30 mg) were homogenized in 10 volumes (w/v) of a buffer containing 50 mM Tris HCl, pH 7.8, 10 mM EDTA, 2 mM EGTA, 100 mM NaF, 2 mM benzamidine, 0.1 mM Nα-p-tosyl-L-lysine chloromethyl ketone, 50 mM β-mercaptoethanol, 0.5 mM PMSF, 10 µg/ml leupeptin with a Tissue Tearer at maximal speed for 20–30 sec. Muscle homogenates were centrifuged at 3,600 × g and the supernatant (~100 µg of total protein) used to start the reaction as described above. S. cerevisiae (EG3281A or DH3 gsy1 gsy2 (Cheng et al., 1995)) were grown on YPD overnight, harvested by centrifugation and homogenized by agitation with glass beads in a homogenization buffer as above without EGTA. Low speed centrifugation yielded a cell extract used for the assay. After incubation at 30°C for 30 minutes the glycogen was purified, treated with or without glucosidases and visualized as above.
Dephosphorylation of 32P-labeled glycogen with laforin
Phosphorylated glycogen (~320–350 µg) was incubated with 50 µg/mL active wild type laforin or catalytically inactive C266S laforin in phosphatase buffer (100 mM sodium acetate, 50 mM bis-Tris, 50 mM Tris HCl, 2 mM DTT, pH 6.5) in a final reaction volume of 50 µL for a minimum of 1.5 hrs at 37°C. The reaction was terminated by the addition of SDS loading buffer and subjected to SDS PAGE and the radiolabeled polysaccharide detected by a phosphorimager.
Purification of phosphorylated oligosaccharides from rabbit skeletal muscle glycogen
Rabbit skeletal muscle glycogen was purified as in Tagliabracci et al. (Tagliabracci et al., 2007). Approximately 250 mg of rabbit skeletal muscle glycogen was digested overnight at 37–40°C with 300 µg/mL amyloglucosidase and 300 µg/mL α-amylase containing 1 mM CaCl2, and 10 mM sodium acetate pH 4.8 in a 3 mL reaction volume. After digestion the insoluble glycogenin was removed by centrifugation at 5,000 × g for 5 min and the digest was placed in a boiling H2O bath for 10 minutes to denature the α-amylase and amyloglucosidase. After cooling, the protein was removed by centrifugation and the cleared supernatant added to 2 mL (bed volume) DEAE Sepharose resin equilibrated with 10 mM sodium acetate pH 4.8 and incubated overnight on a Nutator at 4°C. The resin was poured into a column and washed with 20 column volumes of H2O. The anionic species were eluted stepwise (flow rate of ~0.5 mL/min) with 10 mM, 50 mM, 100 mM, 500 mM, and 1 M ammonium bicarbonate (NH4HCO3). Four mL of each concentration was used and 1 mL fractions were collected. An aliquot (40–60 µL) of each fraction was used to measure covalent phosphate using the sensitive Malachite green method (Hess and Derr, 1975). The remainder was dried in a Speed Vac and redissolved in ~500 µL H2O, and dried further until there was no visible NH4HCO3. The fractions containing phosphate were dissolved in H2O to a final concentration of 1 mM phosphate and stored at −20°C until use.
Dephosphorylation of phosphorylated oligosaccharides purified from rabbit skeletal muscle glycogen
Phosphorylated oligosaccharides (10 µL, 1 mM with respect to phosphate) were treated with either wild type laforin (25 µg/mL) or C266S laforin (25 µg/mL) in 25 mM sodium acetate pH 5.5 in a final reaction volume of 20 µL overnight at 37°C to ensure completion. The reactions were terminated by boiling in a 100°C H2O bath for 10 minutes and the precipitated protein removed by centrifugation, filtered and analyzed as described below.
Analysis of purified phosphorylated oligosaccharides by high performance anion exchange chromatography (HPAEC)
Phosphorylated oligosaccharides purified from glycogen were analyzed by HPAEC using a Dionex ICS3000 with a PA1 column and detected by pulsed amperometric detection. All samples were filtered using a spin column before injection into a 25 µL injection loop. Eluent A consisted of 100 mM NaOH and Eluent B 100 mM NaOH containing 1 M sodium acetate. The fractions were eluted with a continuous gradient from 0–75% of Eluent B over 60 minutes with a flow rate of 0.25 mL/min. Five to 10 nmoles of phosphorylated oligosaccharides (based on phosphate concentration) were analyzed. Carbohydrate standards (250 pmoles-2.5 nmoles) were also analyzed.
Matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) analysis of phosphorylated oligosaccharides
An aliquot (2 µL, 1 mM phosphate) of purified phosphorylated oligosaccharides were analyzed by MALDI/TOF-MS. The matrix was 2’,4’,6’-trihydroxyacetophenone monohydrate (THAP); 0.5 M THAP in ethanol:0.1 M diammonium hydrogen citrate in H2O : 2:1 (v/v). The matrix solution (1 µL) was deposited first on the target followed by an equal volume of the sample. All spectra were obtained using a Microflex LRF (Bruker). MALDI/TOF-MS analysis was performed in the reflector negative ion mode.
Nuclear magnetic resonance (NMR) spectroscopy
The phosphorylated oligosaccharides were deuterium-exchanged by lyophilization from D2O, dissolved in D2O, and transferred to an NMR tube with magnetic susceptibility plugs matched to D2O (Shigemi). The pD of the sample was 5.9. Proton-proton and proton-carbon correlated spectra were acquired on a Varian Inova 600 MHz spectrometer equipped with a 3-mm cryoprobe or a Varian Inova-800 MHz spectrometer, equipped with a 5-mm cryoprobe. Proton-phosphorous correlated spectra were acquired on a Varian Inova 500 MHz spectrometer, equipped with an 8-mm XH room temperature probe. All spectra were taken at 25 °C. Chemical shifts were referenced to internal acetone [δ(1H) = 2.218 ppm, δ(13C) = 33.0 ppm] (Wishart et al., 1995). All experiments except the 1H-31P correlated spectra were acquired with standard Varian pulse sequences. For 1H-31P correlated experiments, the regular gHMQC and HMQC-TOCSY experiments were modified for 31P in the X channel, using a π pulse of 13 µs at a level of 60 dB, and a 3JH-P coupling constant of 7 Hz. Chemical shifts were referenced to external 85 % phosphoric acid [δ(31P) = 0.00 ppm]. Additional acquisition parameters are listed in Table S2.
Supplementary Material
ACKNOWLEDGMENTS
Supported in part by NIH grants DK27221 and NS56454 (to PJR), NIH/NCRR grant P41 RR018502 to the Complex Carbohydrate Research Center, University of Georgia, and the American Heart Association (to VST). We thank Drs. Zhong-Yin Zhang, James Maller and Nina Raben for comments regarding the manuscript.
REFERENCES
- Andrade DM, Turnbull J, Minassian BA. Lafora disease, seizures and sugars. Acta Myol. 2007;26:83–86. [PMC free article] [PubMed] [Google Scholar]
- Ball S, Guan HP, James M, Myers A, Keeling P, Mouille G, Buleon A, Colonna P, Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. doi: 10.1016/s0092-8674(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Baskaran S, Roach PJ, DePaoli-Roach AA, Hurley TD. Structural basis for glucose-6-phosphate activation of glycogen synthase. Proc Natl Acad Sci U S A. 2010;107:17563–17568. doi: 10.1073/pnas.1006340107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow A, Nielsen TH, Baunsgaard L, Mikkelsen R, Engelsen SB. Starch phosphorylation: a new front line in starch research. Trends Plant Sci. 2002;7:445–450. doi: 10.1016/s1360-1385(02)02332-4. [DOI] [PubMed] [Google Scholar]
- Cheng C, Mu J, Farkas I, Huang D, Goebl MG, Roach PJ. Requirement of the self-glucosylating initiator proteins Glg1p and Glg2p for glycogen accumulation in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6632–6640. doi: 10.1128/mcb.15.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Escueta AV. Advances in lafora progressive myoclonus epilepsy. Curr Neurol Neurosci Rep. 2007;7:428–433. doi: 10.1007/s11910-007-0066-7. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach AA, Vilardo PG, Kim JH, Mavila N, Vemuri B, Roach PJ. Determination of mammalian glycogen synthase phosphatase activity. Methods Enzymol. 2003;366:17–34. doi: 10.1016/s0076-6879(03)66002-7. [DOI] [PubMed] [Google Scholar]
- Dhugga KS, Ray PM. Purification of 1,3-beta-D-glucan synthase activity from pea tissue. Two polypeptides of 55 kDa and 70 kDa copurify with enzyme activity. Eur J Biochem. 1994;220:943–953. doi: 10.1111/j.1432-1033.1994.tb18698.x. [DOI] [PubMed] [Google Scholar]
- Fontana JD. The presence of phosphate in glycogen. FEBS Lett. 1980;109:85–92. doi: 10.1016/0014-5793(80)81317-2. [DOI] [PubMed] [Google Scholar]
- Gentry MS, Dixon JE, Worby CA. Lafora disease: insights into neurodegeneration from plant metabolism. Trends Biochem Sci. 2009;34:628–639. doi: 10.1016/j.tibs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry MS, Dowen RH, 3rd, Worby CA, Mattoo S, Ecker JR, Dixon JE. The phosphatase laforin crosses evolutionary boundaries and links carbohydrate metabolism to neuronal disease. J Cell Biol. 2007;178:477–488. doi: 10.1083/jcb.200704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess HH, Derr JE. Assay of inorganic and organic phosphorus in the 0.1–5 nanomole range. Anal Biochem. 1975;63:607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Heyen CA, Tagliabracci VS, Zhai L, Roach PJ. Characterization of mouse UDP-glucose pyrophosphatase, a Nudix hydrolase encoded by the Nudt14 gene. Biochem Biophys Res Commun. 2009;390:1414–1418. doi: 10.1016/j.bbrc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley TD, Stout S, Miner E, Zhou J, Roach PJ. Requirements for catalysis in mammalian glycogenin. J Biol Chem. 2005;280:23892–23899. doi: 10.1074/jbc.M502344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana HG, Tener RS, Wright RS, Moffatt JG. Cyclic Phosphates. III. Some General Observations on the Formation and Properties of Five-, Six- and Seven-membered Cyclic Phosphate Esters. Journal American Chemical Society. 1957;79:430–436. [Google Scholar]
- Knowles JR. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Kokesh FC, Cameron DA, Kauda Y, Kuras PV. Hydrolysis of a- -glucopyranose 1,2-cyclic phosphate: the effect of pH and temperature on the product distribution, and the position of opening of the phosphate diester ring in formation of D-glucose 2-phosphate. Carbohydrate Research. 1978;62 [Google Scholar]
- Kokesh FC, Stephenson RK, Kakuda Y. Inhibition of potato starch phosphorylase by alpha-D-glucopyranose-1,2-cyclic phosphate. Biochim Biophys Acta. 1977;483:258–262. doi: 10.1016/0005-2744(77)90054-7. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- Lomako J, Lomako WM, Kirkman BR, Whelan WJ. The role of phosphate in muscle glycogen. Biofactors. 1994;4:167–171. [PubMed] [Google Scholar]
- Lomako J, Lomako WM, Whelan WJ, Marchase RB. Glycogen contains phosphodiester groups that can be introduced by UDPglucose: glycogen glucose 1-phosphotransferase. FEBS Lett. 1993;329:263–267. doi: 10.1016/0014-5793(93)80234-l. [DOI] [PubMed] [Google Scholar]
- Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, Dunham I, Gardner R, Fong CY, Carpenter S, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nature Genetics. 1998;20:171–174. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- Nunez HA, Barker R. The metal ion catalyzed decomposition of nucleoside diphosphate sugars. Biochemistry. 1976;15:3843–3847. doi: 10.1021/bi00662a030. [DOI] [PubMed] [Google Scholar]
- Paladini AC, Leloir LF. Studies on uridine-diphosphate-glucose. Biochem J. 1952;51:426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson BA, Chen H, Schroeder JM, Shou W, DePaoli-Roach AA, Roach PJ. Abnormal cardiac development in the absence of heart glycogen. Mol Cell Biol. 2004;24:7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL, DePaoli-Roach AA, Roach PJ. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem. 2005a;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- Pederson BA, Schroeder JM, Parker GE, Smith MW, A, D RA, Roach PJ. Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes. 2005b;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2:101–120. doi: 10.2174/1566524024605761. [DOI] [PubMed] [Google Scholar]
- Sakai M, Austin J, Witmer F, Trueb L. Studies in myoclonus epilepsy (Lafora body form) II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology. 1970;20:160–176. doi: 10.1212/wnl.20.2.160. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Seitelberger F. Histophysical and histochemical investigations of myoclonus bodies. Pathol Eur. 1968;3:218–226. [PubMed] [Google Scholar]
- Serratosa JM, Gomez-Garre P, Gallardo ME, Anta B, de Bernabe DB, Lindhout D, Augustijn PB, Tassinari CA, Malafosse RM, Topcu M, et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2) Hum Mol Genet. 1999;8:345–352. doi: 10.1093/hmg/8.2.345. [DOI] [PubMed] [Google Scholar]
- Tabata S, Hizukuri S. Studies on Starch Phosphate. Part 2. Isolation of Glucose 3-Phosphate and Maltose Phosphate by Acid Hydrolysis of Potato Starch. Starch-Stärke. 1971;23:267–272. [Google Scholar]
- Tagliabracci VS, Girard JM, Segvich D, Meyer C, Turnbull J, Zhao X, Minassian BA, Depaoli-Roach AA, Roach PJ. Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem. 2008;283:33816–33825. doi: 10.1074/jbc.M807428200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci VS, Turnbull J, Wang W, Girard JM, Zhao X, Skurat AV, Delgado-Escueta AV, Minassian BA, Depaoli-Roach AA, Roach PJ. Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo. Proc Natl Acad Sci U S A. 2007;104:19262–19266. doi: 10.1073/pnas.0707952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Schlender KK, Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968;25:486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Platas AA, Hawley DK. Transcriptional fidelity and proofreading by RNA polymerase II. Cell. 1998;93:627–637. doi: 10.1016/s0092-8674(00)81191-5. [DOI] [PubMed] [Google Scholar]
- van Leeuwen SS, Kralj S, van Geel-Schutten IH, Gerwig GJ, Dijkhuizen L, Kamerling JP. Structural analysis of the alpha-D-glucan (EPS35-5) produced by the Lactobacillus reuteri strain 35-5 glucansucrase GTFA enzyme. Carbohydr Res. 2008;343:1251–1265. doi: 10.1016/j.carres.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell. 2009;136:746–762. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka BS, Shugar D. Preparation and chemical and enzymic properties of cyclic phosphates of D-glucopyranose and synthesis of derivatives of N-(D-glucopyranosyl) pyridine. Acta Biochimica Polonica. 1964;11:509–525. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.