Abstract
Background
The effect of tobacco smoking on chronic rhinosinusitis (CRS) is not yet well delineated. The purpose of this investigation was to evaluate the overall effect of smoking on post-operative outcomes (endoscopic score and quality-of-life) after endoscopic sinus surgery (ESS) for CRS and determine if volume of daily smoking impacts outcomes.
Methods
A total of 784 patients with CRS were prospectively enrolled between January, 2001 and April, 2009 after electing ESS from one of three academic tertiary care centers. Follow-up longer than 6 months was available on 39 smoking patients. Smoking volume (cigarettes/day) analysis was performed by dichotomizing patients into either light (< 20 cigarettes per day) or heavy (≥ 20 cigarettes per day) daily smoking sub-groups. Primary outcomes were Lund-Kennedy endoscopy scores and two disease-specific health-related QoL (HRQoL) instruments: the Rhinosinusitis Disability Index (RSDI) and Chronic Sinusitis Survey (CSS).
Results
Smokers and non-smokers experienced similar improvement in HRQoL following surgery (RSDI p=0.792 and CSS p=0.117). No difference in HRQoL improvements between light and heavy smokers was identified. While overall changes in endoscopy scores did not differ between smokers and non-smokers, there was a significant difference in the prevalence of worsening post-operative endoscopy scores between heavy, light, and non-smokers (100%, 33%, and 20%, respectively; p=0.002).
Conclusion
Active smoking status does not alter post-operative improvement in HRQoL after ESS. Although limited by a small sample size, increasing smoking volume may contribute to worse post-operative endoscopy scores.
Keywords: Smoking, endoscopy, sinusitis, quality of life, tobacco
Introduction
Tobacco smoke has long been known as an etiology of lower airway disease, with several deleterious effects, such as ciliary dysfunction,1 carcinogenesis,2,3 and immunosuppression.4,5 Despite knowledge that the upper airway is affected in a similar manner as the lower airway,6 the debate over smoking’s effect on chronic rhinosinusitis (CRS) continues.
Smoking, as a predisposing factor, has been linked to CRS7, nasal polyposis,8 and olfactory dysfunction.9,10 Although the negative effects of smoking on sinonasal mucosa are generally accepted, the literature regarding endoscopic sinus surgery (ESS) outcomes in smokers is conflicting. Historically, active smoking status has been considered a contra-indication to ESS by some because of a concern for or perception of poor surgical outcomes. A long-term follow-up study on ESS clinical outcomes by Senior et al. demonstrated a higher rate of revision sinus surgery in smoking patients with CRS.11 In 2004, Briggs et al. demonstrated that smokers have less overall health-related quality-of-life (HRQoL) improvement after ESS.12 As a result, there has been general reluctance to perform ESS on smoking patients with medically recalcitrant CRS.
The paradigm of avoiding ESS in smoking patients has been challenged by several recent prospective studies. Although the prevalence of smokers was low in a 2005 study by Smith et al., they demonstrated that smokers had similar endoscopy scores and experienced similar HRQoL improvement after ESS compared to non-smokers with CRS.13 A recent long-term follow-up study by Das et al. demonstrated that the short-term improvements in HRQoL in smokers after ESS were stable after four years.14 These studies suggest that smoking status should not be considered a contra-indication to ESS, however there remains a debate based on the conflicting literature. An important question is whether or not volume of smoked cigarettes has an impact on outcomes. Intuitively, the higher volume of smoking would create a larger degree of deleterious effects such as ciliary dysfunction and immunosuppression. A study by Houser et al.8 demonstrated that the volume of smoking affected the rate of nasal polyposis in patients with CRS. The correlation between volume of smoking and ESS outcomes has not been evaluated.
The purpose of this study was to evaluate endoscopy and HRQoL outcomes after ESS in smokers and non-smokers with CRS. Additionally, we evaluated if the volume of daily smoking influenced outcomes. Our hypothesis was that smokers would experience similar endoscopic score and HRQoL improvement after ESS compared to non-smokers. Additionally, we hypothesized that higher volume of daily smoking would decrease both endoscopic and HRQoL improvements after ESS.
Materials and Methods
Study Population and Data Collection
Adult study subjects were recruited from three tertiary rhinology clinics for a prospective, multi-institutional cohort study between January, 2001 and April, 2009. Comprehensive findings from this cohort have been previously reported elsewhere.13,15 Inclusion criteria consisted of: 1) age > 18 years, 2) CRS defined by the Task Force Criteria,16 and 3) sinonasal symptoms failed to resolve after medical therapy including, but not limited to, three or more weeks of culture-directed or broad-spectrum antibiotics and at least one trial of systemic corticosteroid therapy. Exclusion criteria included: 1) Follow-up < 6 months. We have found that improvements in postoperative QoL scores are stable between 6 and 20 months, therefore a minimum 6-month follow-up was necessary for inclusion.17 Patients were dichotomized into a smoking subgroup if they reported active use of tobacco cigarettes at the time of enrollment and day of ESS. During the subgroup analysis for daily smoking volume, patients were categorized into 2 groups based on volume of daily smoking: light smoking volume (< 20 cigarettes per day) and heavy smoking volume (≥20 cigarettes per day), whereby 20 cigarettes constitute a single pack. Institutional Review Boards at each enrollment site approved all study documents and the informed consent process.
The enrolling physician at each enrollment site performed all pre- and post-operative patient assessments. Computed tomography and endoscopy were scored using the Lund-Mackay and Lund-Kennedy scoring methods, respectively.18,19 The Lund-Mackay scoring method measures the severity of opacification evident in the maxillary, ethmoidal, sphenoidal, frontal, and ostiomeatal complex regions (score range: 0-24). The Lund-Kennedy scoring method quantifies pathologic states within the ethmoid and middle meatus sinus regions including nasal polyposis, mucosal discharge, edema, crusting, and scarring (score range: 0-20).
Quality of Life Evaluation
All study patients were asked to complete two disease-specific QoL surveys pre-operatively and at each post-operative visit for the duration of the study. The Rhinosinusitis Disability Index (RSDI) is a 30-question survey comprised of three individual subscales to measure the impact of sinus disease on the physical, functional, and emotional domains on a continuum (score range: 0-120).20 Higher RSDI total and subscale scores represent a higher impact of disease. The Chronic Sinusitis Survey (CSS) is a 6-question survey designed to measure sinusitis-specific symptoms and medication use within the preceding 8-week period (score range: 0-100).21 Lower total and subscale scores indicate a greater impact of CRS. A Research Coordinator assisted each patient in the completion of both QoL surveys and the enrolling physician was blinded to QoL responses for the study entirety. The two main HRQoL outcomes of interest were: 1) Overall pre- and post-operative total and subscale score comparisons between groups, and 2) Change in HRQoL scores (last post-operative score minus pre-operative score).
Statistical Analysis
Data were collected, transcribed, and manually scored after each clinic visit by a central Research Coordinator on clinical research forms. All data was deidentified and securely stored in a relational database during the collection period (Microsoft FoxPro; Microsoft Corp., Redmond, WA.). Statistical analysis was accomplished using SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL.). Descriptive statistics (means, standard deviations, ranges, and frequencies) and distributions were assessed for all patient cofactors, surgical procedures, and QoL outcome variables. Paired t-tests were used to test for significant improvement in mean endoscopy score and QoL measures between preoperative scores and follow-up responses over time. Two-tailed independent sample t-tests and Mann-Whitney U tests were used to assess differences in mean endoscopy score and QoL improvement between smoking and non-smoking patients, as well as light and heavy smoking volume patients. The proportion of subjects that experienced worsening of post-operative endoscopy scores (≥1 unit) were examined using a 2×3 contingency table and Chi-square testing and a continuity correction for zero cells. Without adjustment for multiple comparisons, a p-value <0.05 was considered statistically significant.
Results
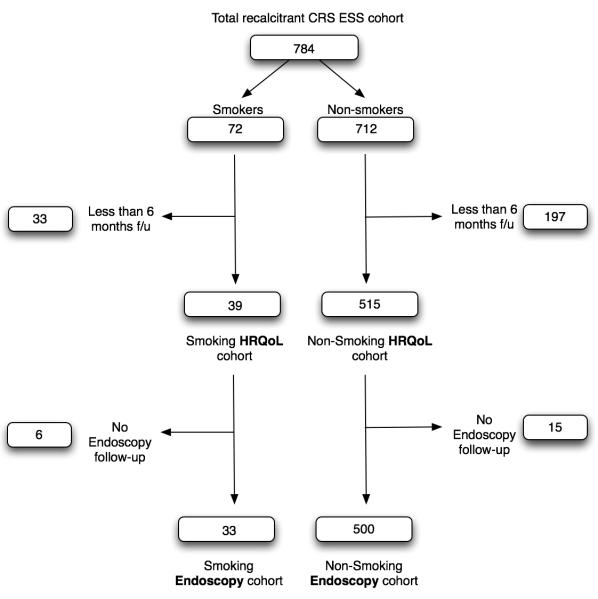
A total of 784 patients fulfilled eligibility requirements for study enrollment. Mean post-operative follow-up was 16.8 months and other baseline characteristics of 72 smoking and 712 non-smoking patients are outlined in Table 1. Long-term HRQoL information was available on 39 smoking and 515 non-smoking patients, while long-term endoscopy score data was available on 33 smoking and 500 non-smoking patients (Figure 1). During the smoking volume sub-group analysis, long-term HRQoL information in patients with detailed smoking information was available on 13 light and 5 heavy smoking patients, while long-term endoscopy score data was available in 12 light and 3 heavy smokers, who underwent ESS for recalcitrant CRS.
Table 1.
Basic pre-operative demographic comparisons between smokers and non-smokers with CRS (n=784)
| Smokers (n=72) | Non-smokers (n=712) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Mean (SD) | [range] | n(%) | Mean (SD) | [range] | n(%) | p-value |
| Reported packs / day | 0.6 (0.5) | [0.05 - 2.0] | -- | -- | -- | ||
| Cigarettes/day | 13 (10.3) | [1 - 40] | -- | -- | -- | ||
| Follow-up (mo.) | 16.8 (7.0) | [5 - 40] | 17.7 (7.8) | [5 -62] | 0.459 | ||
| Age (years) | 43.2 (11.0) | [20 - 67] | 46.7 (14.0) | [18 -82] | 0.034 | ||
| Gender - male/female | 37 (51.4)/35 (48.6) | 337 (47.3)/375 (52.7) | 0.511 | ||||
| Previous Sinus Surgery | 37 (51.4) | 424 (59.6) | 0.180 | ||||
| Nasal polyposis | 22 (30.6) | 271 (38.1) | 0.210 | ||||
| Asthma | 12 (16.7) | 283 (39.7) | <0.001 | ||||
| ASA Intolerance | 4 (5.6) | 81 (11.4) | 0.163 | ||||
| Allergy | 14 (19.4) | 241 (33.8) | 0.013 | ||||
| Depression | 24 (33.3) | 107 (15.0) | <0.001 | ||||
| Lund-MacKay CT Score | 10.7 (7.0) | [0 - 24] | 12.4 (6.6) | [0 - 24] | 0.034 | ||
| Lund-Kennedy | |||||||
| Endoscopy Score | 6.9 (5.0) | [0 - 20] | 7.2 (4.9) | [0 - 20] | 0.533 | ||
CRS = chronic rhinosinusitis; SD = standard deviation; mo. = months; ASA = acetylsalicylic acid; CT = computed tomography
Figure 1.
Study patient inclusion flow chart
Mean pre-operative Lund-Kennedy endoscopy scores were similar between smoking and non-smoking patients (6.9 and 7.2, respectively; p=0.533). Long-term endoscopy score follow-up was available on 33 smoking and 500 non-smoking patients. There was no difference in mean post-operative endoscopy score or change over time between smoking and non-smoking patients (p=0.230 and p=0.222, respectively; Table 2).
Table 2.
Endoscopy score comparisons between smokers and non-smokers with CRS
| Smokers (n=72) | Non-smokers (n=712) | ||||
|---|---|---|---|---|---|
| Lund-Kennedy Endoscopy Score | Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | p-value |
| Pre-operative | 6.9 (5.0) | [5.8 , 8.1] | 7.2 (4.9) | [6.9 , 7.6] | 0.533 |
| Post-operative | 5.5 (4.7) | [3.8 , 7.1] | 4.6 (3.9) | [4.3 , 4.9] | 0.230 |
| Change over time | −1.9 (4.5) | [−3.5 , −0.3] | −2.9 (4.6) | [−3.3 , −2.5] | 0.222 |
CRS = chronic rhinosinusitis; SD = standard deviation; CI = confidence interval [lower limit, upper limit]
Both patient groups significantly improved in HRQoL scores over time (all p<0.001). Mean pre-operative HRQoL scores for the RSDI and CSS were similar between smoking and non-smoking patients, except for the RSDI physical and CSS symptom domains, where smokers appeared to have greater impact of disease (p=0.010 and p=0.035, respectively; Table 3). Following ESS, the mean post-operative scores for all HRQoL domains were similar between smokers and non-smokers, except for the CSS medication domain where smokers had a lower rate of post-operative medication usage (p=0.019; Table 3).
Table 3.
Pre-operative and post-operative HRQoL scores between smokers and non-smokers with CRS
| Smokers (n=39) | Non-smokers (n=515) | ||||
|---|---|---|---|---|---|
| Outcome measures: | Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | p-value |
| Pre-operative: | |||||
| RSDI physical | 21.8 (7.3) | [19.4 , 24.1] | 18.6 (7.4) | [17.9 , 19.2] | 0.010 |
| RSDI functional | 15.8 (7.9) | [13.3 , 18.4] | 15.4 (7.1) | [14.8 , 16.1] | 0.747 |
| RSDI emotional | 15.2 (8.1) | [12.5 , 17.8] | 13.0 (8.0) | [12.3 , 13.7] | 0.107 |
| RSDI total | 52.7 (21.2) | [45.9 , 59.6] | 47.1 (20.0) | [45.3 , 48.8] | 0.088 |
| CSS symptom | 20.5 (22.9) | [13.1 , 27.9] | 29.6 (26.3) | [27.4 , 31.9] | 0.035 |
| CSS medication | 43.3 (25.2) | [35.2 , 51.5] | 44.0 (25.7) | [41.8 , 46.2] | 0.881 |
| CSS total | 31.9 (18.7) | [25.9 , 38.0] | 36.8 (19.8) | [35.1 , 38.5] | 0.136 |
| Post-operative: | |||||
| RSDI physical | 12.6 (8.5) | [9.8 , 15.4] | 11.4 (8.0) | [10.7 , 12.1] | 0.369 |
| RSDI functional | 9.9 (8.5) | [7.2 , 12.7] | 8.6 (7.4) | [8.0 , 9.3] | 0.303 |
| RSDI emotional | 10.1 (8.6) | [7.4 , 12.9] | 7.8 (7.5) | [7.1 , 8.4] | 0.064 |
| RSDI total | 32.6 (23.9) | [24.8 , 40.4] | 27.8 (21.4) | [25.9 , 29.6] | 0.178 |
| CSS symptom | 52.4 (30.2) | [42.6 , 62.1] | 60.1 (28.4) | [57.7 , 62.6] | 0.102 |
| CSS medication | 65.6 (31.9) | [55.2 , 76.0] | 55.8 (24.4) | [53.7 , 57.9] | 0.019 |
| CSS total | 58.9 (26.2) | [50.5 , 67.5] | 58.0 (20.7) | [56.2 , 59.8] | 0.772 |
HRQoL = health-related quality of life; CRS = chronic rhinosinusitis; SD = standard deviation; RSDI = Rhinosinusitis Disability Index; CSS = Chronic Sinusitis Survey; CI = confidence interval [lower limit, upper limit]
When evaluating the improvement in HRQoL scores, both smoking and non-smoking patients had similar long-term improvements, except for the CSS medication domain where smoking patients demonstrated a larger improvement equating to lower medication usage (p=0.029; Table 4).
Table 4.
Mean improvement in HRQoL scores between smokers and non-smokers with CRS
| Smokers (n=39) | Non-smokers (n=515) | ||||
|---|---|---|---|---|---|
| Outcome measures: | Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | p-value |
| RSDI physical | −9.2 (8.9) | [−12.1 , −6.3] | −7.2 (7.6) | [−7.9 , −6.5] | 0.123 |
| RSDI functional | −5.9 (8.3) | [−8.6 , −3.2] | −6.8 (7.4) | [−7.5 , −6.2] | 0.467 |
| RSDI emotional | −5.1 (6.9) | [−7.3 , −2.8] | −5.2 (7.4) | [−5.9 , −4.6] | 0.876 |
| RSDI total | −20.1 (21.9) | [−27.3 , −13.0] | −19.3 (19.7) | [−21.0 , −17.6] | 0.792 |
| CSS symptom | 31.8 (31.2) | [21.7 , 41.9] | 30.5 (31.2) | [27.8 , 33.2] | 0.792 |
| CSS medication | 22.2 (33.2) | [11.5 , 33.0] | 11.8 (28.3) | [9.3 , 14.2] | 0.029 |
| CSS total | 27.0 (25.2) | [18.9 , 35.2] | 21.3 (22.4) | [19.2 , 23.1] | 0.117 |
HRQoL = health-related quality of life; CRS = chronic rhinosinusitis; SD = standard deviation; RSDI = Rhinosinusitis Disability Index; CSS = Chronic Sinusitis Survey; CI = confidence interval [lower limit, upper limit]
When evaluating whether volume of daily smoking effects HRQoL and endoscopy score outcomes, there was no difference in HRQoL improvement between light and heavy smokers (Table 5). There was a statistically significant difference in the proportion of endoscopy scores that worsened after ESS between heavy, light, and non-smokers, 100%, 33%, and 20%, respectively (p=0.002; Table 6). In essence, all heavy smokers experienced a worsening of their endoscopy scores post-operatively.
Table 5.
Absolute mean improvement in endoscopy and HRQoL scores between light smokers (<1 pack/day) and heavy smokers (≥1.0 pack/day) with CRS (n=18)
| Light Smokers (n=13) | Heavy smokers (n=5) | ||||
|---|---|---|---|---|---|
| Outcome measures: | Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | p-value |
| RSDI physical | −8.5 (8.3) | [−13.6 , −3.5] | −8.0 (12.3) | [−23.3 , 7.3] | >0.999 |
| RSDI functional | −8.0(7.1) | [−12.3 , −3.7] | −1.4 (9.0) | [−12.6 , 9.8] | 0.173 |
| RSDI emotional | −7.2 (5.8) | [−10.7 , −3.6] | −2.0 (4.1) | [−7.0 , 3.0] | 0.075 |
| RSDI total | −23.6 (17.7) | [−34.3 , −12.9] | −11.4 (22.9) | [−39.8 , 17.0] | 0.336 |
| CSS symptom | 25.6 (32.2) | [6.2 , 45.1] | 26.7 (33.5) | [−15.0 , 68.3] | >0.999 |
| CSS medication | 17.9 (36.3) | [−4.0 , 39.9] | 31.7 (14.9) | [13.2 , 50.2] | 0.246 |
| CSS total | 21.8 (29.2) | [4.2 , 39.4] | 29.2 (15.9) | [9.5 , 48.9] | 0.289 |
HRQoL = health-related quality of life; CRS = chronic rhinosinusitis; SD = standard deviation; RSDI = Rhinosinusitis Disability Index; CSS = Chronic Sinusitis Survey; CI = confidence interval [lower limit, upper limit]
Table 6.
Proportion of worse post-operative endoscopy scores by smoking volume
| Heavy smokers (≥ 1.0 ppd) |
Lighter smokers (0.1 - 0.9 ppd) |
Non-smokers | p-value | |
|---|---|---|---|---|
| Improved / No score change | 0.00% | 66.70% | 79.80% | |
| Worse (≥1 unit) score | 100.00% | 33.30% | 20.20% | 0.002 |
ppd = cigarette packs/day
Discussion
In this prospective study we demonstrated that, in general, smokers who underwent ESS for recalcitrant CRS experienced similar improvement in endoscopic scores and HRQoL compared to their non-smoking counterparts. To investigate the correlation between volume of daily smoking and ESS outcomes, we evaluated heavy and light smoking patient cohorts. Our results suggest that the volume of daily smoking does not affect HRQoL outcomes after ESS. However, there appears to be a correlation between smoking volume and post-operative endoscopic appearance, as higher smoking volume was more likely to result in worsening post-operative endoscopic scores. In fact, 100% of the heavy smoking volume cohort demonstrated worsening of endoscopy scores following ESS. These conflicting outcomes (improving HRQoL vs worsening endoscopy scores) may help explain the conflicting literature and expert opinions regarding the sensibility of offering surgery to smokers with CRS. Furthermore, we propose that smoking volume heterogeneity between previous study cohorts may explain the variation seen in study results and conclusions in prior studies.
Tobacco use continues to plague our society with preventable morbidity and mortality. Center for Disease Control (CDC) data confirms that the prevalence of cigarette smoking has declined 3.5% from 1998 (24.1%) to 2008 (20.6%), however, the decline is slowing with rates stabilizing around 20%.22 With attempts to drastically reduce tobacco-related morbidity, several smoking cessation initiatives have been developed, including the Healthy People 2010 Objective. Cessation strategies include public education, addiction counseling, and medical therapy support. A recent Cochrane review concluded that smoking cessation intervention can reduce post-operative morbidity,23 however, the ideal intervention is unknown.
It is generally accepted that smoking impairs ciliary function24 and contributes to the pathogenesis of CRS.7 However, the literature is conflicting with regards to the effect smoking has on clinical outcomes following ESS for medically recalcitrant CRS. An overall evaluation of tobacco use on clinical outcomes of ESS reveals a breadth of clinical outcome measures and relatively small cohort studies (Table 7). Additionally, only 2 studies reported the volume of daily smoking in their smoking cohort. This makes definitive conclusions, based on the literature, challenging and has led to significant debate regarding the sensibility of offering ESS to smokers. However, five recent prospective studies all suggest that smoking does not adversely impact clinical outcomes after ESS. While the volume of smoking appears to contribute to the pathophysiology of CRS and nasal polyposis,8 we were not able to identify a study that evaluated the impact of daily smoking volume on ESS outcomes.
Table 7.
Literature summary of studies evaluating the effect of smoking on ESS outcomes for CRS
| Author | Year | Study type | Total No. CRS pts. |
Total no. of Smoking pts. |
Report Smoking Volume |
Mean F/U (years) |
Clinical outcome |
Results |
|---|---|---|---|---|---|---|---|---|
| Studies demonstrating worse ESS outcomes in smokers with CRS (n=5) | ||||||||
| Danielsen et al.26 |
1996 | Retrospective | 230 | 74 | No | 3.5 | Symptoms score rating 1 to 5 |
Smoking reduced post-op ESS symptom scores |
| Senior et al.11 | 1998 | Retrospective | 72 | 14 | No | 7.8 | Revision ESS | Higher number of smokers in revision ESS group (27% vs. 10%) |
| Sobol et al.27 | 1998 | Retrospective | 274 | 43 | No | 1 | Pt. reported outcome |
Smokers reported poorer outcome at 12 months (57.2% vs. 27.6%) |
| Sugiyama et al.28 | 2002 | Retrospective | 37 | 13 | No | Not stated |
Olfactory function |
Less olfactory improvement after ESS in smokers age > 40 |
| Briggs et al.12 | 2004 | Retrospective | 82 | 26 | Yes | 4.3 | HRQoL | Smoking is associated with reduced HRQoL improvement |
| Studies demonstrating no difference in ESS outcomes in smokers with CRS (n=6) | ||||||||
| Watelet et al.29 | 2004 | Prospective | 36 | 7 | No | 0.5 | Postop endoscopy |
No difference in Post-op endoscopy |
| Smith et al.13 | 2005 | Prospective | 119 | 11 | No | 1.4 | Postop endoscopy |
No difference in Post-op endoscopy |
| HRQoL | Smokers had the same HRQoL benefit as non- smokers |
|||||||
| Das et al.25 | 2007 | Prospective | 221 | 50 | No | 0.25 | Postop endoscopy |
No difference in Post-op endoscopy |
| HRQoL | Smokers experienced more HRQoL improvement at 3 months |
|||||||
| Danielides et al.30 |
2009 | Prospective | 116 | 44 | Yes | 0.5 | Olfactory function |
No difference in olfactory improvement rates between smokers (who quit post-ESS) and non- smokers |
| Das et al.14 | 2009 | Prospective | 116 | 26 | No | 3.6 | Postop endoscopy |
Smokers had stable endoscopic and HRQoL improvement at long-term follow-up |
| HRQoL | ||||||||
| Rudmik et al. (current study) |
2010 | Prospective | 712 | 39 | Yes | 1.4 | Postop endoscopy |
No difference in HRQoL outcomes between smokers and non-smokers |
| HRQoL | Heavy smokers demonstrated worsened endoscopic appearance |
|||||||
In 2004, a retrospective study by Briggs et al. demonstrated that patients who reported smoking at the time of their ESS experienced less improvement in HRQoL compared to non-smokers.12 These findings conflict with those of the recent prospective study by Das et al. which demonstrated that patients who smoked at the time of ESS experienced greater HRQoL improvements after ESS and this effect was stable after four years.14 One unique difference in the study by Briggs et al. was that they quantified the volume of smoking by sending out a smoking questionnaire, which demonstrated a high patient average of 23 cigarettes per day for 26 years. The study by Das et al. did not report the volume of smoking in their smoking patient cohort. According to the high daily smoking volume seen in the Briggs et al. study, there may have been a smoking recall bias, as periodic or light smokers might not report smoking on the questionnaire, thus their smoking cohort might have only consisted of heavy smokers. In contrast, a prospective study may more accurately identify the light smokers and include them into the smoking cohort, which would dilute the potentially negative effects seen in heavy smokers. Based on this postulation, we hypothesized that the difference in HRQoL outcomes between the two studies may in part be due to a difference in volume of daily tobacco use in the two smoking cohorts.
Our prospective study was composed of primarily low volume smokers, with an average of 13 cigarettes per day. This supports our suggestion that prospective studies may more accurately identify lower smoking volume patients. The results from this study confirm the findings of Das et al. whereby patients who smoke receive a similar endoscopic and HRQoL improvement after ESS.14,25 Although the sample size is small, when we stratified the smoking patients based on volume of daily smoking, those who used more than 20 cigarettes per day (1 pack) received similar HRQoL improvement after ESS. However, smoking volume did appear to adversely impact the post-operative endoscopic appearance. There was a statistically significant difference in the proportion of post-operative endoscopy scores that worsened after ESS between heavy, light, and non-smokers (100%, 33%, and 20%, respectively; p=0.002). These results suggest that despite symptom improvement, otolaryngologists will more commonly encounter endoscopic exams that appear worse after ESS in patients who smoke higher volumes. The discrepancy between HRQoL outcomes and endoscopic scores, identified in this study, is consistent with other studies demonstrating a poor correlation between symptom scores and objective testing of CRS.26,27 Though we cannot make definitive conclusions on the volume of daily smoking data due to this low sample size, it introduces one plausible reason why there exists a paradox in the literature regarding ESS outcomes in patients who smoke. Larger studies that stratify patients based on volume of daily smoking will be required to confirm these findings. This will likely prove quite challenging given that the current study enrolled more than seven hundred patients and still achieved a relatively small sample of heavy smoking patients.
There are limitations of this study to consider when evaluating these findings: First, there was a moderate loss of long-term follow-up for the HRQoL and endoscopy score data (45% and 54%, respectively). However, this degree of drop-out from a prospective clinical outcome study is common in the tertiary care setting due to geographic, insurance, and other considerations. Furthermore, this loss to follow-up is comparable to the other reported prospective study.14 Secondly, the dichotomization of light smoking (<20 cigarettes per day) and heavy smoking (>20 cigarettes per day) is somewhat arbitrary but based upon the study by Briggs et al.12 Future studies incorporating larger sample sizes may want to evaluate several different smoking volume cut-points and integrate evaluations of smoking duration. Lastly, the sample size of smokers, even in a large multi-institutional study, is relatively small and not easily amenable to further subgroup analysis (e.g., light vs. heavy smoking). As a result of our findings, we have started to collect detailed smoking histories on all patients enrolled into prospective ESS clinical studies, with the attempt to improve sample sizes in future studies. Despite these possible limitations, we feel this study is strengthened through the use of stringent enrollment criteria, a prospective, multi-institutional design, and use of validated survey instruments.
Conclusion
In this prospective study we demonstrated that smokers who underwent ESS for medically recalcitrant CRS experience similar HRQoL improvements compared to their non-smoking counterparts. Although the sample size was limited, our results suggest that the volume of daily smoking does not impact HRQoL outcomes but may function to worsen post-operative endoscopic appearance. Smoking volume heterogeneity between retrospective and prospective trials may explain the paradox in the literature regarding ESS outcomes in smoking patients with CRS. The results confirm the conclusions from other recent prospective studies and suggest that active smoking status should not necessarily be considered a contra-indication for ESS in patients with recalcitrant CRS.
Acknowledgments
This investigation was made possible, in part, by a grant from the National Institute on Deafness and Other Communication Disorders (R01 DC005805), one of the National Institutes of Health, Bethesda, Maryland. Administrative support was provided by the Department of Otolaryngology – Head and Neck Surgery, Oregon Health & Science University, Portland, Oregon.
Supported by grant funding from the NIH/NIDCD R01 DC005805 (PI: Smith, TL)
Footnotes
The Institutional Review Board at Oregon Health & Science University provided approval and oversight for all investigational protocols and annual review.
Public clinical trial registration (http://www.clinicaltrials.gov) ID: NCT00799097
Conflict(s) of Interest: There is no conflict of interest or financial disclosure for Luke Rudmik, MD. Potential conflicts of interest exist as Timothy L. Smith, MD, MPH and Jess Mace, MPH, were funded by a grant from the NIH/NIDCD. Timothy L. Smith is also a consultant for Intersect and Entrigue which provided no financial support for this investigation.
Submitted for oral presentation at the annual Combined Otolaryngology Spring Meeting of the American Rhinologic Society on April 27-May 1, 2011, Chicago, IL.
References
- 1.Cohen D, Arai SF, Brain JD. Smoking impairs long-term dust clearance from the lung. Science. 1979;204:514–7. doi: 10.1126/science.432655. [DOI] [PubMed] [Google Scholar]
- 2.Homburger F, Bernfeld P. Cigarette smoke-induced laryngeal cancer: a model for bronchogenic carcinoma in humans. N Engl J Med. 1979;300:862. doi: 10.1056/NEJM197904123001517. [DOI] [PubMed] [Google Scholar]
- 3.Wynder EL, Taguchi KT, Baden V, et al. Tobacco carcinogenesis. IX. Effect of cigarette smoke on respiratory tract of mice after passive inhalation. Cancer. 1968;21:134–53. doi: 10.1002/1097-0142(196801)21:1<134::aid-cncr2820210122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Therriault MJ, Proulx LI, Castonguay A, et al. Immunomodulatory effects of the tobacco-specific carcinogen, NNK, on alveolar macrophages. Clin Exp Immunol. 2003;132:232–8. doi: 10.1046/j.1365-2249.2003.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. 2008;57:497–503. doi: 10.1007/s00011-008-8078-6. [DOI] [PubMed] [Google Scholar]
- 6.Spira A. Upper airway gene expression in smokers: the mouth as a “window to the soul” of lung carcinogenesis? Cancer Prev Res (Phila Pa) 2010;(3):255–8. doi: 10.1158/1940-6207.CAPR-10-0013. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy DW. Pathogenesis of chronic rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;193:6–9. doi: 10.1177/00034894041130s503. [DOI] [PubMed] [Google Scholar]
- 8.Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope. 2008;118:1521–7. doi: 10.1097/MLG.0b013e31817d01b8. [DOI] [PubMed] [Google Scholar]
- 9.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–6. [PubMed] [Google Scholar]
- 10.Katotomichelakis M, Balatsouras D, Tripsianis G, et al. The effect of smoking on the olfactory function. Rhinology. 2007;45:273–80. [PubMed] [Google Scholar]
- 11.Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151–7. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Briggs RD, Wright ST, Cordes S, et al. Smoking in chronic rhinosinusitis: a predictor of poor long-term outcome after endoscopic sinus surgery. Laryngoscope. 2004;114:126–8. doi: 10.1097/00005537-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Smith TL, Mendolia-Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Khichi SS, Perakis H, et al. Effects of smoking on quality of life following sinus surgery: 4-year follow-up. Laryngoscope. 2009;119:2284–7. doi: 10.1002/lary.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142:55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 17.Soler ZM, L. ST. Quality of life outcomes after endoscopic sinus surgery: How long is enough? Otolaryngol Head Neck Surg. 2010;143(5):621–5. doi: 10.1016/j.otohns.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 19.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–4. [PubMed] [Google Scholar]
- 20.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–9. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 21.Gliklich RE, Metson R. Effect of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117:12–7. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 22.Prevention CfDCa Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–32. [PubMed] [Google Scholar]
- 23.Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2010;7:CD002294. doi: 10.1002/14651858.CD002294.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol Allied Sci. 1998;23:227–30. doi: 10.1046/j.1365-2273.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 25.Das S, Becker AM, Perakis H, et al. The effects of smoking on short-term quality of life outcomes in sinus surgery. Laryngoscope. 2007;117:2229–32. doi: 10.1097/MLG.0b013e318145388f. [DOI] [PubMed] [Google Scholar]
- 26.Danielsen A, Olofsson J. Endoscopic endonasal sinus surgery. A long-term follow-up study. Acta Otolaryngol. 1996;116:611–9. doi: 10.3109/00016489609137898. [DOI] [PubMed] [Google Scholar]
- 27.Sobol SE, Wright ED, Frenkiel S. One-year outcome analysis of functional endoscopic sinus surgery for chronic sinusitis. J Otolaryngol. 1998;27:252–7. [PubMed] [Google Scholar]
- 28.Sugiyama K, Matsuda T, Kondo H, et al. Postoperative olfaction in chronic sinusitis: smokers versus nonsmokers. Ann Otol Rhinol Laryngol. 2002;111:1054–8. doi: 10.1177/000348940211101119. [DOI] [PubMed] [Google Scholar]
- 29.Watelet JB, Annicq B, van Cauwenberge P, et al. Objective outcome after functional endoscopic sinus surgery: prediction factors. Laryngoscope. 2004;114:1092–7. doi: 10.1097/00005537-200406000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Danielides V, Katotomichelakis M, Balatsouras D, et al. Improvement of olfaction after endoscopic sinus surgery in smokers and nonsmokers. Ann Otol Rhinol Laryngol. 2009;118:13–20. doi: 10.1177/000348940911800103. [DOI] [PubMed] [Google Scholar]