Abstract
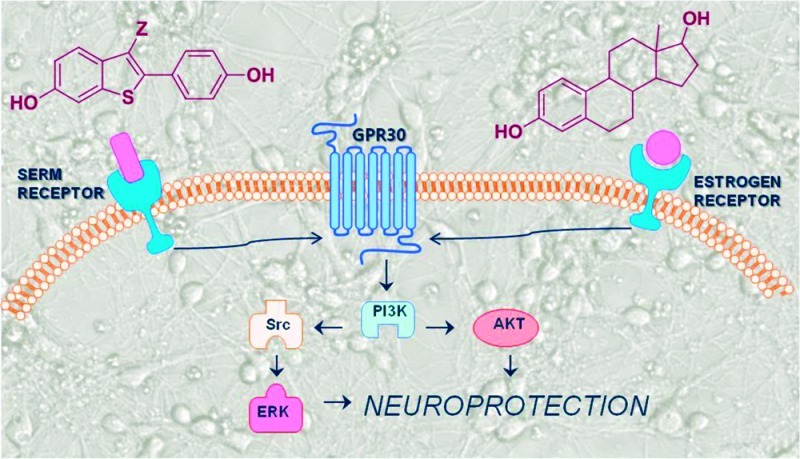
The clinical benzothiophene SERM (BT-SERM) raloxifene was compared with estrogens in protection of primary rat neurons against oxygen-glucose deprivation (OGD). Structure−activity relationships for neuroprotection were determined for a family of BT-SERMs displaying a spectrum of ERα and ERβ binding affinity and agonist/antagonist activity, leading to discovery of a neuroprotective pharmacophore, present in the clinically relevant SERMS, raloxifene, and desmethylarzoxifene (DMA), for which submicromolar potency was observed for neuroprotection. BT-SERM neuroprotection did not correlate with binding to ER or classical ER activity; however, both the neuroprotective SERMs and estrogens were shown, using pharmacological probes, to activate the same kinase signaling cascades. The antiestrogen ICI 182,780 inhibited the actions of estrogens but not those of BT-SERMs, whereas antagonism of the G-protein coupled receptor, GPR30, was effective for both SERMs and estrogens. Since SERMs have antioxidant activity, ER-independent mechanisms were studied using the classical phenolic antioxidants, BHT and Trolox, and the Nrf2-dependent cytoprotective electrophile, sulforaphane. However, neuroprotection by these agents was not sensitive to GPR30 antagonism. Collectively, these data indicate that the activity of neuroprotective BT-SERMs is GPR30-dependent and ER-independent and not mediated by antioxidant effects. Comparison of novel BT-SERM derivatives and analogues identified a neuroprotective pharmacophore of potential use in design of novel neuroprotective agents with a spectrum of ER activity.
Keywords: estrogen, SERM, GPCR, GPR30, neuroprotection, ischemic stroke
Menopause has been associated with numerous adverse effects in women including osteoporosis, dementia, depression, and anxiety.1−3 The management of such effects with safe and effective hormone therapies is a largely unmet need in women’s health. Women entering menopause report memory and concentration difficulties.4,5 In Alzheimer’s disease (AD), the age-adjusted mortality rate was shown to be 21.7% higher for women than for men.6 Estrogen replacement therapy (ERT) has been reported to improve or maintain levels of cognitive function in postmenopausal women and to reduce the risk of AD, observations that are in accord with proposed roles for estrogen in the brain in protection, growth, and differentiation of neurons.7−9 The Women’s Health Initiative Memory Study (WHIMS) was designed to investigate the impact of ERT in women over age 65.10,11 However, WHIMS was an ancillary study to the extensive Women’s Health Initiative (WHI) study that was terminated because of increased risks with ERT that are reported to include breast cancer,12 lung cancer,13 thrombosis, and stroke.14
The WHI and WHIMS observations highlight the need for further study on estrogens in neuroprotection and cognition and also point toward selective estrogen receptor modulators (SERMs) as a safer therapeutic approach toward postmenopausal neurodegenerative disorders. Raloxifene (Evista), a nonsteroidal benzothiophene (BT) SERM, is used for treatment of postmenopausal osteoporosis. The Study of Tamoxifen and Raloxifene (STAR) trial reported that raloxifene was as effective as the archetypal SERM, tamoxifen, in reducing the risk of invasive breast cancer and had a lower risk of thromboembolic events.15 Unlike tamoxifen, raloxifene does not stimulate uterine and endometrial growth.16,17 Raloxifene was reported, at twice the clinical dose, significantly to reduce the risk of cognitive impairment in postmenopausal women.18 The average age of study participants was too young to show significance for lower risk of AD, although a trend was observed.18,19 In a more recent study of healthy women aged 70 years and older, 60 mg/day raloxifene treatment for 1 year gave significant improved verbal memory compared to placebo.20 Interestingly, raloxifene was also reported in healthy elderly males after 3 months of treatment to enhance brain activation, spanning a number of different cognitive domains, compatible with cortical stimulation.21,22
Despite the positive safety and efficacy data, raloxifene has poor bioavailability,23 leading to the development of arzoxifene, a BT-SERM with greatly improved pharmacokinetics.24 In vivo, arzoxifene is metabolized to the potent SERM desmethylarzoxifene (DMA).25 The objective of this work was to compare the neuroprotective properties of estrogen with raloxifene, arzoxifene, and a series of novel DMA analogues, some of which have been the subject of previous reports from our laboratories on estrogen receptor (ER)-dependent and ER-independent activity, and on metabolism and bioavailability (Figure 1).26−29 Neuroprotection in response to oxygen-glucose deprivation (OGD) was used as a primary end point, revealing well-defined similarities and differences between estrogens and BT-SERMs, indicating a new receptor target for neuroprotective agents, and providing a pharmacophore scaffold for drug discovery of novel neuroprotective SERMs.
Figure 1.
Structures of BT-SERMs, and BTC derivatives used in this study with ER binding and ER agonist (EC50) or antagonist (IC50) activity data: [a] From radioligand binding assay using full-length ER. [b] From alkaline phosphatase reporter assays in Ishikawa endometrial cancer cells: n.a. = not active. [c] Selectivity for ERβ over ERα binding from radioligand binding assays. Data are either taken from refs (26 and 29) or were obtained using identical procedures.
Results and Discussion
Neuroprotection Assay
Exposure of primary cortical neurons to OGD is known to induce apoptosis and cell death and to provide a model for neuronal loss from ischemia-reperfusion injury and excitotoxicity.30−32 Drugs were applied to primary neuronal cultures in glucose free deoxygenated media immediately prior to initiation of ischemia and again 2 h later when media was replaced and O2 pressure restored. Measurements of cell viability by MTT assay and cell death by LDH assay were conducted under all experimental conditions, giving complementary results under all drug treatments used in this study, to ensure that cell death was being measured rather than artifacts associated with MTT reduction or cell wall disruption. Where not shown, LDH assay data is provided in the Supporting Information.
OGD in primary neurons, simulating ischemia for 2 h, followed by “reperfusion” and recovery for 24 h, led to 30−40% greater cell death in the vehicle treated group than the E2 treated group. The extent of cell death obtained under these conditions was reproducible, delivered significant data, and was therefore ideal for differentiating the variety of agents used in this study. Initially, two cell-permeable estrogens, E2 and EE (10 nM), one cell-impermeable estrogen, E2-BSA (10 ng/mL), and eleven BT-SERMs (100 nM) were applied to cells. E2 was neuroprotective, as predicted; therefore, cell death (LDH) was normalized to vehicle (100%), and cell survival (MTT) was normalized to vehicle (0%) and E2 (100%).
Neuroprotection by Estrogens and SERMs
Extensive work has reported the neuroprotective activity of estrogens.33−38 Contributions to estrogenic neuroprotection include regulation of antiapoptotic Bcl-2 family proteins, evidence for which has been provided from in vivo studies.8 Promotion of mitochondrial viability by estrogens has been proposed to be regulated by mitochondrial ERβ39 and via ER-independent membrane antioxidant mechanisms.37 ER-dependent neuroprotective pathways include classical, genomic ER signaling via ERα and ERβ, as supported by the use of selective ERα and ERβ agonists in hippocampal neurons,40,41 and also include nonclassical, signaling via extranuclear ER.42 In general, these ER-mediated pathways are subject to inhibition by the antiestrogen ICI 182 780 (ICI) that represents a universally applied tool to define signaling via ER.43,44
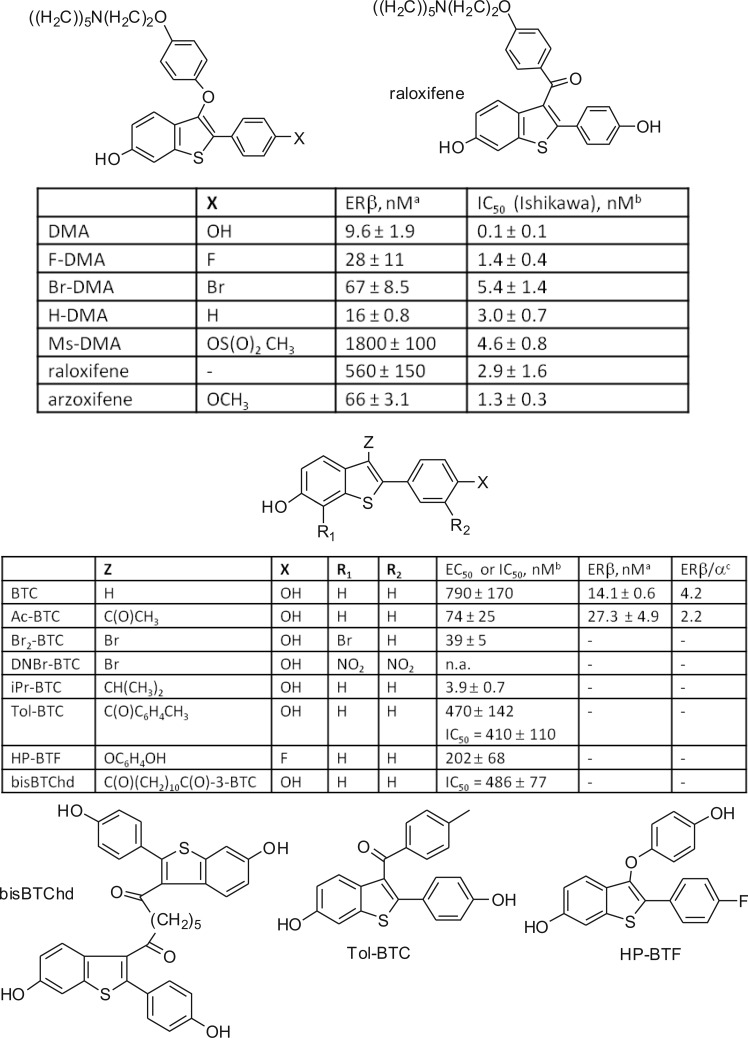
All estrogens studied were neuroprotective. The BT-SERMs were differentiated into a subset that was at least as protective as E2 itself or conversely devoid of neuroprotective activity at the concentration studied (Figure 2A). These observations were recapitulated with LDH measurements (Figure 2B). The concentration dependence of neuroprotection was measured for the clinically important BT-SERMs, Ral and DMA, that were both observed to be neuroprotective in the initial screen (Figure 2C,D), yielding estimations of EC50 (Ral = 19 nM; DMA = 11 nM) and equivalence to E2 (10 nM) at Ral (68 nM) and DMA (28 nM) using MTT cell viability assay (Figure 2C).
Figure 2.
Estrogens and a structural subset of BT-SERMs are neuroprotective toward OGD induced cell death in primary neuronal cultures; and neuroprotection by these BT-SERMs is concentration dependent. At 10−12 days, primary cultured cortical neurons were treated with test compounds followed immediately by exposure to 2 h OGD and subsequent reperfusion for 24 h. (A,B) Test compounds used: BT-SERMs (100 nM), estrogens (10 nM), or E2-BSA (10 ng/mL). (C,D) Concentration dependence for BT-SERMs. Cell death and cell survival were measured by MTT and LDH assays, respectively. LDH data were normalized to vehicle control as 100% cell death; MTT data was normalized to vehicle control as 0% and E2 as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test.
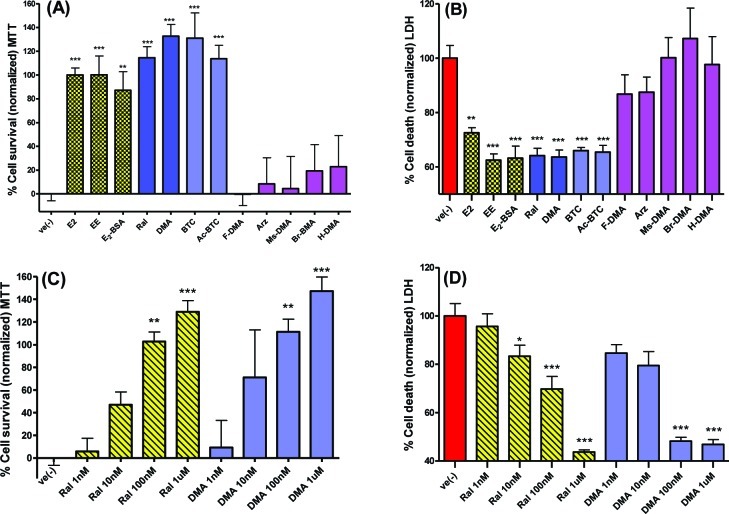
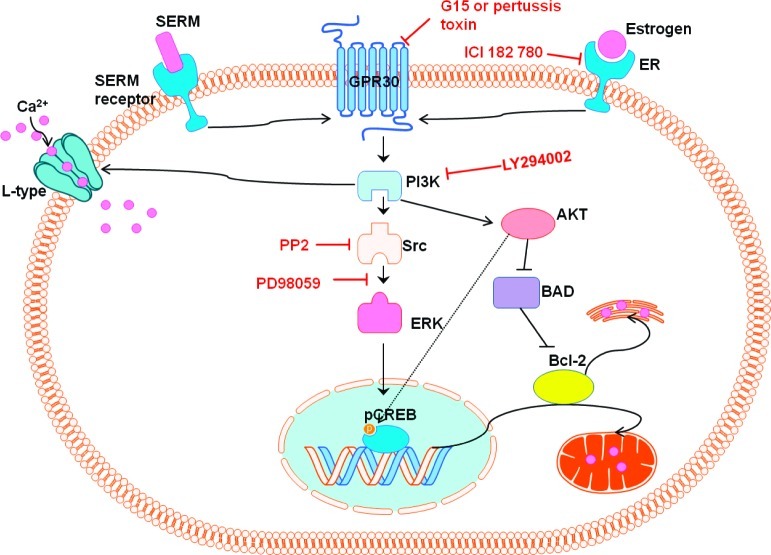
In contrast to SERMs that are tissue-dependent ER agonists and antagonists, ICI is a pure antiestrogen that has proven utility in defining signaling via classical ER pathways and nonclassical, extranuclear ER. Such extranuclear ER-mediated pathways are known to involve G-protein coupled receptors (GPCR) leading to activation of secondary messenger cascades, which may be blocked by pertussis toxin that inhibits binding of G-protein to GPCRs.45 Therefore, drug effects were studied in cells treated with ICI (1 μM) or pertussis toxin (100 ng/mL) 45 min prior to OGD (Figure 3). The clinically relevant BT-SERMs, raloxifene, DMA, and arzoxifene were studied, with the latter being inactive in the initial screen. BTC and Ac-BTC were included, since these were also active compounds in the initial screen. ICI was observed to block E2-mediated neuroprotection but not to attenuate neuroprotection elicited by the BT-SERMs (Figure 3A,B). In contrast to ICI, pertussis toxin inhibited the neuroprotective actions of estrogen and the BT-SERMs. After pertussis treatment, drug effects were not significant relative to vehicle control (p > 0.05) (Figure 3C, D).
Figure 3.
Estrogen neuroprotection is attenuated by ER antagonist ICI, whereas inhibition of GPCR by pertussis toxin attenuates the actions of estrogens and neuroprotective BT-SERMs. OGD induced cell death in 10−12 day primary neuronal cultures measured by MTT or LDH assays after pretreatment with antagonists (−45 min) and treatment with test compounds (SERMs 100nM; E2 10nM) immediately before OGD. (A,B) ER antagonist ICI 182 780 (1 μM). (C,D) GPCR antagonist pertussis toxin (100 ng/mL). LDH data was normalized to vehicle control as 100% cell death; MTT data was normalized to vehicle control as 0% and E2 (in the absence of antagonist) as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 or not significant compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test.
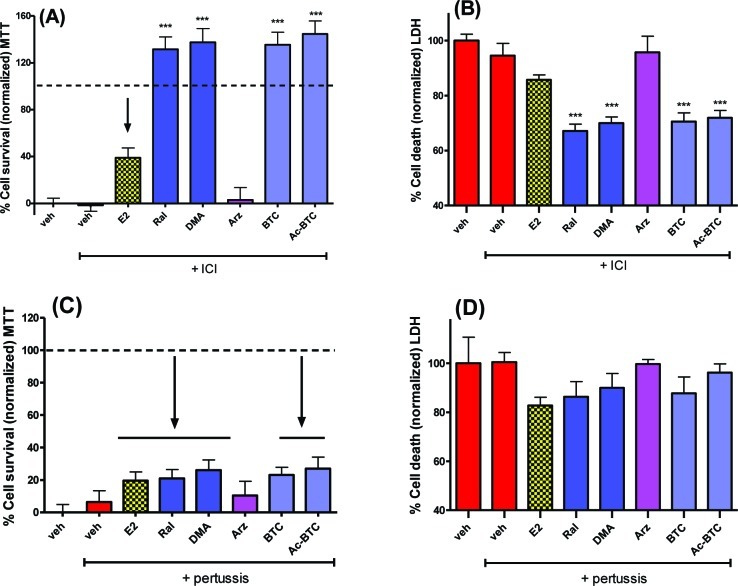
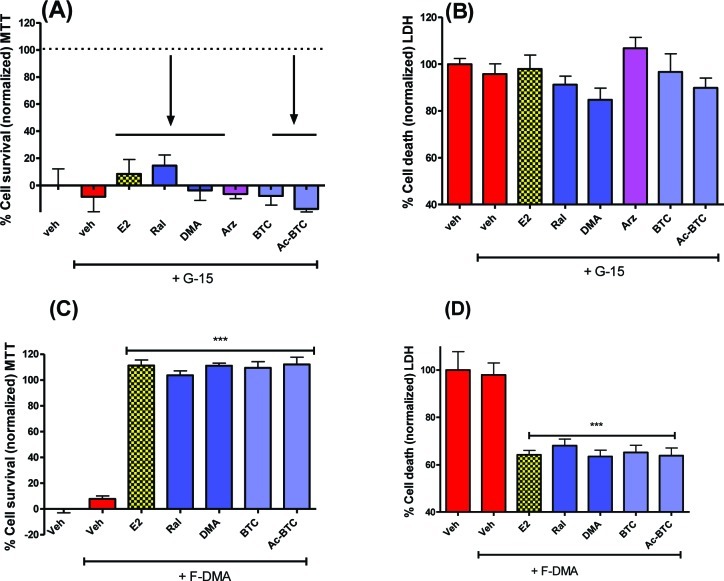
Evidence points toward the possibility of GPR30 being a primary GPCR contributing to the rapid extranuclear actions of E2.46 The presence of ERα, ERβ, and GPR30 in primary neurons was confirmed by Western blot (Supporting Information). Therefore, the GPR30 antagonist, G15 (100 nM), was applied to cells prior to OGD.47 G15 blocked the neuroprotective activity of all test compounds (Figure 4A,B). F-DMA is known to act as an antagonist of classical ER-mediated pathways27,28,48,49 and is inactive as a neuroprotectant against OGD-induced cell death (Figure 2); therefore, the possibility that F-DMA could act as an antagonist against neuroprotection elicited by estrogens or BT-SERMs required testing. F-DMA was observed to be without effect (Figure 4C,D).
Figure 4.
Both estrogen and BT-SERM neuroprotection is attenuated by antagonism of GPR30 using G-15 but not by the addition of the ER-antagonist F-DMA. OGD induced cell death in 10−12 day primary neuronal cultures measured by MTT or LDH assays after pretreatment with antagonists (−45 min) and treatment with test compounds (SERMs 100nM; E2 10nM) immediately before OGD. (A,B) GPR30 antagonist G15 (100 nM). (C,D) F-DMA (100 nM). LDH data was normalized to vehicle control as 100% cell death; MTT data was normalized to vehicle control as 0% and E2 (in the absence of antagonist) as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test.
Role of Kinase Cascades and NOS in Signaling Neuroprotection
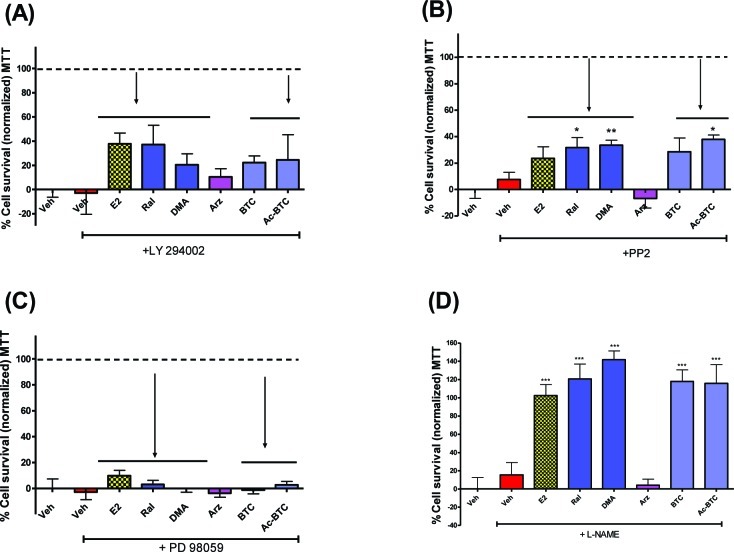
Estrogen has been demonstrated to activate the PI3K/Akt pathway through direct binding of activated ER to the p85 subunit of PI3K, a pathway that is implicated in estrogenic neuroprotection.50,51 To examine the role of this pathway, the PI3K inhibitor LY294002 (10 μM) was preincubated with primary neuronal cultures prior to OGD (Figure 5A); LY294002 reduced the neuroprotection afforded by the BT-SERMs and estrogen to equal extents, confirming that neuroprotection was mediated through a PI3K/Akt pathway. At the concentration used, PI3K inhibition alone did not enhance cell death.
Figure 5.
Estrogen neuroprotection is attenuated by inhibition of PI3K/AkT and Src/MEK signaling but not by inhibition of NOS. OGD induced cell death in 10−12 day primary neuronal cultures measured by MTT or LDH assays after pretreatment with antagonists (−45 min) and treatment with test compounds (SERMs 100nM; E2 10nM) immediately before OGD. (A) PI3K inhibitor LY294002 (10 μM). (B) SRC inhibitor PP2 (10 μM). (C) MEK inhibitor PD 98059 (10 μM). (D) NOS inhibitor L-NAME (100 μM). LDH data was normalized to vehicle control as 100% cell death; MTT data was normalized to vehicle control as 0% and E2 (in the absence of antagonist) as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test.
It has been reported that estrogen activates L-type Ca channels leading to increased intracellular Ca levels, activating a number of pathways including Src/ERK leading to activation of CREB and consequent upregulation of pro-survival proteins.36,52,53 Therefore, the effects of Src inhibitor PP2 (10 μM) and the MAPK/ERK kinase (MEK1) inhibitor PD 98059 (10 μM) were studied when applied to cells 45 min prior to OGD. Neuroprotection was strongly attenuated by PP2 (Figure 5B), although by MTT assay at the concentration of inhibitor used three BT-SERMs retained activity. Inhibition of the MAPK/ERK pathway by PD 98059 abolished neuroprotection by estrogen and the neuroprotective BT-SERMs (Figure 5C). These results taken together indicate that neuroprotection is mediated through the Src/ERK pathway.
It has been reported that both estrogen and raloxifene are able to activate eNOS via a PI3K/Akt pathway.54,55 Since neuroprotection observed in response to OGD was mediated by PI3K/Akt, the role of NOS was studied using L-NAME (100 μM), a commonly used prodrug of the NOS inhibitor NG-nitro-l-arginine (Ki eNOS = 0.04 − 0.2 μM; Ki iNOS = 4 μM; Ki nNOS = 0.02−0.2 μM).56−59 At the concentration used, L-NAME had no significant effect on neuroprotection either alone or in combination with estrogen and SERMs (Figure 5D), indicating that in this system NO does not mediate the neuroprotection elicited by estrogens and BT-SERMs.
Antioxidant and Nrf2-Mediated Neuroprotection
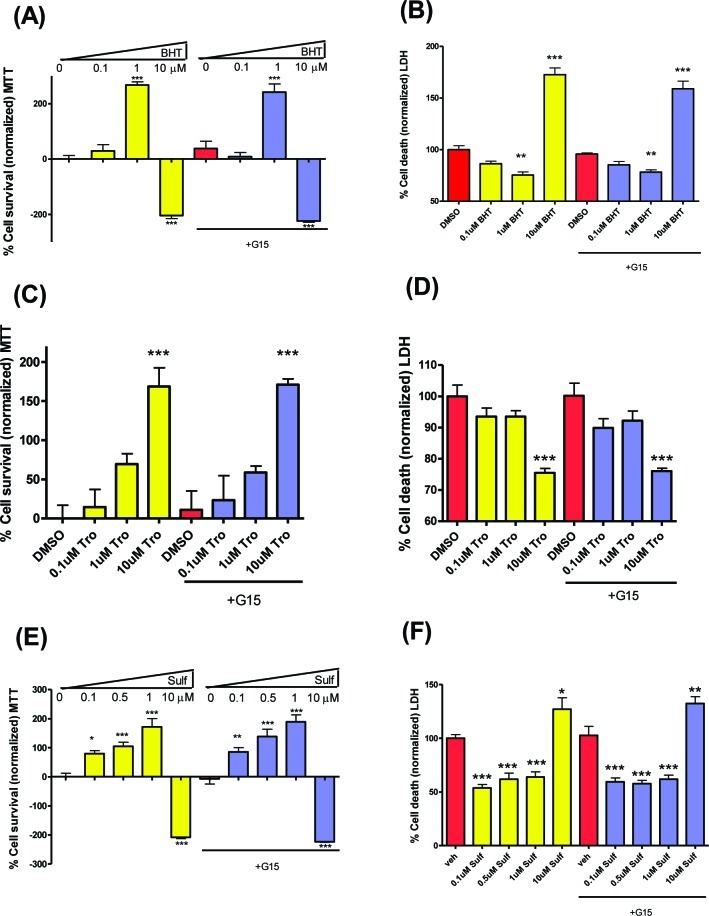
E2 has the ability to act as a classical phenolic antioxidant, and this activity has been argued to contribute to neuroprotection.60 Moreover, the extended conjugation of SERMs such 4-OHT weakens the phenolic O−H bond providing more efficient antioxidant activity. Potency for inhibition of lipid peroxidation in brain liposomes was reported to be 4-OHT > E2 > ICI.61 However, in cerebrocortical cultures, the concentration of 4-OHT required to elicit equivalent neuroprotection to that of E2 (10 nM) was 100-fold higher.62 Extended conjugation is also present in the BT-SERMs and derivatives studied herein. We have previously reported on the antioxidant activity of some of these BT-SERMs.29,48 Therefore, the phenolic antioxidants BHT and the water-soluble vitamin E analogue Trolox were tested. As anticipated, both BHT and Trolox were observed to be neuroprotective. At higher concentrations of BHT, neurotoxicity was observed (Figure 6A,B), whereas Trolox delivered concentration-dependent neuroprotection (Figure 6C,D).
Figure 6.
Classical phenolic antioxidants, BHT and Trolox, and the chemopreventive agent sulforaphane are neuroprotective; however, this activity is insensitive to antagonism by G15. OGD induced cell death in 10−12 day primary neuronal cultures measured by MTT or LDH assays after pretreatment with antagonist (−45 min) and treatment with test compounds (concentrations as indicated) immediately before OGD. (A,B) BHT was neuroprotective at 1 μM but neurotoxic at 10 μM. (C,D) Trolox showed concentration-dependent neuroprotection. (E,F) Sulforaphane was neuroprotective at concentrations 1 μM or below but neurotoxic at 10 μM. LDH data was normalized to vehicle control as 100% cell death. MTT data was normalized to vehicle control as 0% and E2 (in the absence of antagonist) as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test.
The quinoid oxidative metabolites of DMA, raloxifene, and BTC, are thiophilic electrophiles, an attribute that may contribute to biological activity mediated by nuclear factor (erythroid-derived 2)-like 2 (Nrf2).28,48 The natural product, sulforaphane, is a thiophilic electrophile, well studied as a chemopreventive agent, which has recently been reported to have Nrf2-dependent neuroprotective properties.63 In neurons subject to OGD, the behavior of sulforaphane was similar to that of BHT: neuroprotection at lower concentrations, and at higher concentration (10 μM) neurotoxicity (Figure 6E,F). The BTC pharmacophore of BT-SERMs provides both the potential for antioxidant and thiophilic electrophilic chemical reactivity; however, in contrast to neuroprotection by BT-SERMs, the GPR30 antagonist G15 did not inhibit neuroprotection by either BTC, Trolox, or sulforaphane (Figure 6). These results suggest that BT-SERMs are not acting via an antioxidant or sulforaphane-like mechanism.
Elucidation of the Neuroprotective Pharmacophore
Activation of antioxidant response element (ARE) by BT-SERMs in cell culture was observed to be ER-independent and correlated with the ability of SERMs to undergo oxidative bioactivation to a diquinone methide.26,29,48,49,64−66 This observation was compatible with the electrophilic reactivity of quinones toward thiols in sensor proteins and subsequent activation of ARE via Nrf2.48 In the preliminary screen of estrogens and BT-SERMs, neuroprotection was only observed for BT-SERMs containing the redox active 2-(4-hydroxyphenyl)-benzo[b]thiophen-6-ol core (BTC) pharmacophore (Figures 1, 2, and 7), raising the possibility that neuroprotection was elicited via covalent binding to a thiol sensor protein/receptor. Thus, one can envisage two modes of interaction: noncovalent receptor binding or covalent receptor activation via bioactivation to the quinone (Figure 7). In order to distinguish between these two mechanisms, preparation of further novel examples of BT-SERMs and structural analogues was necessary.
Figure 7.
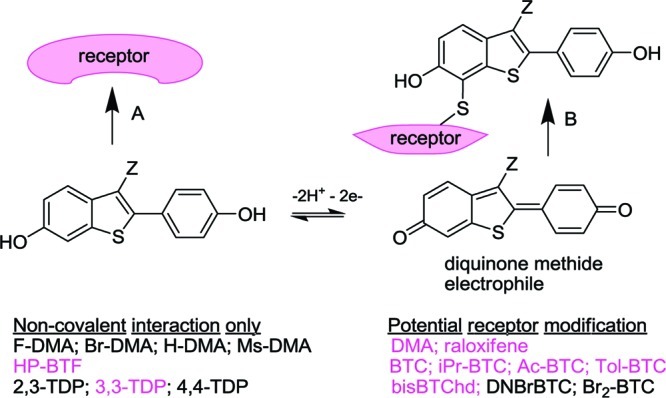
The BTC moiety is readily oxidized to a diquinone methide that acts as a Michael acceptor toward cysteine. The receptor mediating the neuroprotective activity of BT-SERMs could potentially be activated by Michael addition (B) or alternatively by simple noncovalent binding (A). Column 1 lists study compounds including BT-SERMs that cannot undergo bioactivation to diquinone methides. Column 2 lists redox reactive compounds able to form a diquinone methide. Study compounds shown in colored text were observed to deliver GPR30-dependent neuroprotection. The lack of correlation of activity with potential for bioactivation supports a noncovalent receptor binding mode.
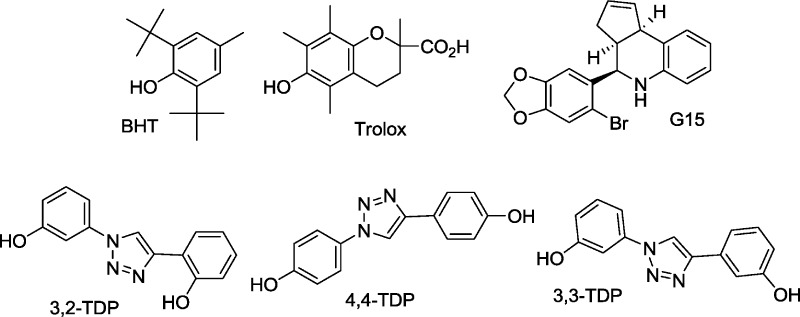
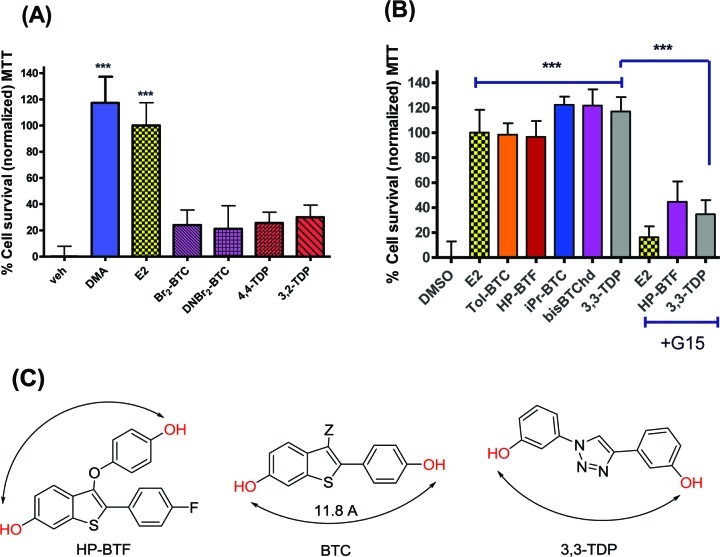
Electrophilic substitution of 3Br-BTC was used to synthesize two derivatives, Br2-BTC and DNBr-BTC, that retained the BTC core (Figure 1) but could be expected to show hindered binding to a putative “neuroprotective BTC receptor”. The presence of a nitro group at the 7- and 3′-positions of BTC would be expected to hinder binding to ERα, and this compound was devoid of estrogenic or antiestrogenic activity in the Ishikawa assay (Figure 1). However, it could also be argued that the electron-withdrawing bromo and nitro groups would inhibit oxidative bioactivation to a quinone. Therefore, a different class of compounds was prepared containing two phenol groups in simile with BTC, but linked via a triazole ring.67 These 1H-1,2,3-triazole-1,4-diyl-diphenol (TDP) derivatives cannot form diquinone methides (Scheme 1 and Figure 7). The first four molecules studied (4,4-TDP, 3,2-TDP, Br2-BTC, DNBr-BTC) showed no neuroprotective activity against OGD (Figure 8A), and only one of these, Br2-BTC, showed evidence for ER binding (Figure 1). However, of the second set of molecules assayed (iPr-BTC, Tol-BTC, the dimeric bisBTChd, 3,3-TDP, HP-BTF), all were neuroprotective (Figure 8B). Patterns of ER binding, obtained from assay in Ishikawa cell cultures, were highly diverse (Figure 1) and will be the subject of further studies. Neuroprotective compounds included those with agonist, antagonist, or no activity (TDP derivatives) in the Ishikawa assay. Furthermore, the neuroprotective activity of HP-BTF and 3,3-TDP was GPR30-dependent, strongly supporting the involvement of a diphenolic pharmacophore binding noncovalently to a receptor. The distance between the phenolic oxygens of BTC is approximately 11.8 Å, a distance that can be matched in conformers of both HP-BTF and 3,3-TDP, thus providing a minimal neuroprotective pharmacophore (Figure 8).
Scheme 1.
Figure 8.
Patterns of activity for neuroprotection by analogues and derivatives of BT-SERMs support a biphenolic pharmacophore signaling via G-protein coupled receptor. OGD induced cell death in 10−12 day primary neuronal cultures measured by MTT assay after pretreatment with antagonists (−45 min) and treatment with test compounds (all 100nM; except, E2 10nM) immediately before OGD. (A) Treatment with BT-SERM analogues and derivatives compared to DMA and E2. (B) Treatment with BT-SERM analogues and derivatives compared to DMA and E2 and influence of GPR30 antagonist G15 (100 nM). MTT data was normalized to vehicle control as 0% and E2 (in the absence of antagonist) as 100% cell survival. Data show mean and SEM (N = 6): ***P < 0.0001, **P < 0.001, *P < 0.01 compared to vehicle control using one-way ANOVA with Dunnett’s post hoc test. (C) Structures of diphenol pharmacophores common to neuroprotective BT-SERM analogues and derivatives in this study.
A Composite Signaling Model for Neuroprotection
Exposure of primary cortical neurons to OGD is known to induce apoptosis and cell death. In this assay, a structural subset of BT-SERMs, including DMA and raloxifene, and all estrogens, including the membrane impermeable BSA conjugate, were neuroprotective. The efficacy of E2 (10 nM) was equal to that of raloxifene at 62 nM and DMA at 28 nM. Estrogen and SERM mediated neuroprotection has been shown to be mediated via nongenomic, rapid signaling, including regulation of intracellular Ca2+ and phosphorylation of ERK.53,68 Extranuclear ER, linked to kinase signaling pathways, including PI3K/Akt and Src/ERK/CREB, has been shown to play a role in estrogenic neuroprotection.52,53 Both Akt and CREB activation provide direct links to antiapoptotic mechanisms of neuroprotection.69 In the present work, use of kinase inhibitors revealed that, in primary neurons subject to OGD, the activity of estrogens and neuroprotective BT-SERMs was mediated via PI3K, Src, and ERK dependent signaling pathways (Figure 9).
Figure 9.
Estrogen and BT-SERM neuroprotection is attenuated by inhibition of PI3K/AkT and Src/ERK signaling and also by inhibition of GPCR by pertussis toxin or antagonism of GPR30 by G-15, whereas only estrogenic neuroprotection is inhibited by ER antagonist ICI.
The antiestrogen, ICI, blocks activity mediated by both the nuclear and the extranuclear ER, and in combination with pertussis toxin has been used to demonstrate the coupling of extranuclear ER to G protein mediated signal transduction.70 GPR30 is a G-protein coupled receptor that is receiving considerable attention as a mediator of rapid estrogen signaling.71−73 Studies on GPR30 are facilitated by the availability of the selective antagonist G15 (Scheme 1).47 In immortalized hippocampal neurons, the neuroprotective effect of E2 (10−100 nM) against glutamate-induced cell death was abrogated by both ICI and G15.43 In the present study, G15 blocked neuroprotection against OGD by both E2 and BT-SERMs. The combined evidence from use of ICI, G15, and pertussis toxin implicates extranuclear ER binding coupled to GPR30 signal transduction via intracellular kinase cascades in the neuroprotective actions of E2 against OGD.
Neuroprotection by BT-SERMs is ER-Independent and G-Protein-Coupled
In addition to the lack of significant effect of ICI, the lack of correlation of neuroprotection with either ER binding or classical ER-mediated activity in cell cultures argues against a role for ERα or ERβ in the observed neuroprotective effects of BT-SERMs. DMA, F-DMA, and H-DMA are potent ER antagonists in breast and endometrial cancer cells, and they bind to ERα and ERβ with high affinity, yet DMA was neuroprotective against OGD, whereas F-DMA and H-DMA were inactive. Modification of the X-DMA 4′-substituent modulates ERα and ERβ binding and antiestrogenic activity in cell culture.26,29 Removal or truncation of the side chain at the 3-position, as in BTC and Ac-BTC, leads to ligands with selectivity for ERβ that deliver ER agonist activity in cell culture (Figure 1).74
Taken together, the evidence is supportive of BT-SERMs containing a bis-phenolic pharmacophore (Figure 8) as eliciting neuroprotection via G-protein coupled receptor binding. The tamoxifen derivative, STX, was reported in 2003 as a ligand for an as yet unidentified, G-protein coupled, neuronal membrane ER that was also activated by raloxifene.75 Rapid signaling mediated by STX was reported to be ER-dependent, being blocked by ICI. The response of astrocytes to STX, and selective ligands for ERα, ERβ, and GPR30 was recently compared.76 It was concluded that evidence exists for multiple membrane estrogen receptors and that ligands may bind directly to metabotropic glutamate receptors.
Prospects for Neuroprotective BT-SERMs
Substantial evidence has been reported for the neuroprotective activity of raloxifene in both ovariectomized female and male rats as assessed by histochemical and behavioral assay.77−79 In neuronal cell culture, raloxifene was found to be protective against a variety of toxic insults including glutamate, Aβ25−35, and H2O2.80 Interpretation of these results posited raloxifene as a partial ER agonist. The clinical potential for raloxifene in neurodegeneration and cognitive decline is shown by studies in elderly males21,22 and in postmenopausal women.18−20
The results presented herein provide a further neuroprotective mechanism for raloxifene and BT-SERMs. The conclusion from use of ICI and comparison with classical ER activity is that the actions of neuroprotective BT-SERMs are G-protein coupled and ER-independent. This observation is important, because it indicates that novel neuroprotective SERMs can be developed with modulated ER binding. The structure−activity relationship provided from assay of 15 BT-SERMs and 3 TDP analogues provided a neuroprotective pharmacophore. Further structural elaboration of this pharmacophore is anticipated to provide neuroprotective agents with modulated ER agonist and antagonist activity and neuroprotective agents devoid of ER binding. Agents with SERM activity have potential use in women’s health, whereas those devoid of ER binding may find general use in neurodegenerative disorders.
Methods
Materials
All reagents, catalysts, and solvents were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Waltham, Massachusetts) and used as received, unless otherwise stated. All cell culture media and supplements were from Invitrogen (Carlsbad, CA). The Cytotox 96 assay kit was purchased from Promega (Madison, WI). The antibodies to ERα (MC-20), ERβ (H-150), and GPR30 (K-16) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Synthesis
Synthetic details and characterization are described in the Supporting Information.
Primary Cell Culture Preparation
Use of animals was approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago. Primary cultures of dissociated cortical neurons were performed as follows: briefly, cortices were dissected from the brains of embryonic day 16 (E16) Sprague−Dawley rats (Charles River Laboratories). The embryos were transferred to a plate with L15 medium (Leibovitz, Sigma L5520) and the cortices were disected. Neurons were mechanically dissociated in 80% basal medium Eagle supplemented with 10% horse serum, 10% fetal bovine serum, and 10% GGG (dextrose, l-glutamine 200 mM, and gentamicine) and plated at a concentration of 2 × 105 cells/cm2 on poly-l-lysine coated plates and maintained in a humidified incubator (37 °C with air and 5% CO2). After 24 h, the plating medium was replaced by neurobasal medium containing 1× B27 supplement minus AO and 0.5 mM l-glutamine. Cultures consisted of 98 ± 2% NeuN positive cells.81 After 3 days, half the growing media was replaced by fresh media, and the cells were left for a further 6−8 days.
OGD Experiment
Cells 10−12 DIV (days in vitro) were plated on 96-well plates. The cell medium was changed to phenol red free growing media 3 h before OGD prior to transfer to an airtight polycarbonate experimental hypoxia chamber (Coy lab, Grass Lake, MI, dimensions 41′′ L × 23′′ D × 23′′ H) controlled at 5% CO2 and 1% O2. The media was changed to a glucose free deoxygenated buffer solution (NaCl 116 mM, CaCl2 1.8 mM, MgSO4 0.8 mM, KCl 5.4 mM, NaHCO3 14.7 mM, NaHPO4 1 mM, HEPES 10 mM, pH = 7.4), and the drugs were then added to the cells. Inhibitors and antagonists were added 45 min before moving cells to the hypoxia chamber. The cells were exposed to OGD for 2 h and removed from the chamber, and the buffer was replaced by normal phenol red free growing media. The cells were maintained at 37 °C with air and 5% CO2 for 24 h after which LDH and MTT assays were performed to determine cell death and survival.
MTT Cell Viability Assay
Thiazolyl blue tetrazolium bromide was solubilized in PBS (5 mg/mL) and added to the cell culture media (20 μL of solution added to 200 μL of media in each well of the plate). The plate was incubated at 37 °C under 5% CO2 for 2−4 h. After the incubation period, the media was aspirated and replaced with MTT solubilizing solution (anhydrous isopropanol) with shaking. Spectrophotometric absorbance at λ = 570 nm using λ = 630 nm as a reference was measured on a Dynex MRX ll microplate spectrophotometer.
LDH Cell Injury Assay
The CytoTox 96 cytotoxicity assay kit was used. Briefly, 50 μL of supernatant from each well was transferred to a second plate, followed by 50 μL of substrate mix, out of direct light. The plate was left in room temperature for 30 min before adding stop solution and measurement at λ = 490 nm on a Dynex MRX ll microplate spectrophotometer.
Detection of ER and GPR30
Primary cells were grown on a 10 cm plate for 14 days, the media was removed, and then the cells were washed with PBS, scraped off the plate, collected into an Eppendorf tube using PBS as solvent, and centrifuged at 14 000 rpm to remove PBS. The cells were then lysed with RIPA lysis buffer in the presence of protease inhibitors and PMSF and then sonicated and left to recover on ice for 30 min. The supernatant was then extracted and boiled with SDS and 1% mercaptoethanol. Samples were then loaded to an SDS gel run at 150 V before transfer to a PVDF membrane, followed by blocking with 5% milk and application of primary antibodies for ERα (MC-20), ERβ (H-150), and GPR30 (K-16) at a concentration of 1:100. The membrane was then incubated with HRP-conjugated secondary antibody at a concentration of 1:2000, and results were visualized using the fluorChem HD2 imaging system (see the Supporting Information).
Acknowledgments
Johann Sohn is thanked for technical assistance with estrogenic assays.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- DMA
desmethylarzoxifene, ([6-hydroxy-3-[4-[2-(1-piperidinyl)-ethoxy]phenoxy]-2-(4-hydroxyphenyl)]benzo[b]thiophene
- 4-OHT
4-hydroxy-tamoxifen
- BHT
butylated hydroxtoluene
- E2
17β-estradiol
- EE
ethinylestradiol
- E2-BSA
bovine serum albumin conjugated estradiol
- ER
estrogen receptor
- ERT
estrogen replacement therapy
- ERE
estrogen response element
- SERM
selective estrogen receptor modulator
- BT-SERM
benzothiophene SERM
- L-NAME
l-nitro-arginine methyl ester
- eNOS
endothelial nitric oxide synthase
- LDH
lactate dehydrogenase
- G15
4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline
- MTT
3-(4,5dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- OGD
oxygen glucose deprivation
- GPCR
G protein coupled receptor
- ARE
antioxidant response element
- WHI
women’s health initiative
- WHIMS
women’s health initiative memory study
- MORE
multiple outcome of raloxifene evaluation
- STAR
study of tamoxifen and raloxifene
- RIPA
radioimmunoprecipitation assay buffer
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- TDP
(1H-1,2,3-triazole-1,4-diyl)diphenol
- Tol-BTC
(6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl)(p-tolyl)methanone
- bisBTChd
1,7-bis(6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl)heptane-1,7-dione
- iPr-BTC
2-(4-hydroxyphenyl)-3-isopropylbenzo[b]thiophen-6-ol
- HP-BTF
2-(4-fluorophenyl)-3-(4-hydroxyphenoxy)benzo[b]thiophen-6-ol.
Author Contributions
R.A., J.L., and L.V. conducted neuronal assays; I.K., B.M., V.A.L., I.S.T., T.G., and Z.Q. synthesized and characterized small molecule probes and drugs; P.Y. carried out ER assays; G.T. directed the research and wrote the paper using, in part, the PhD thesis of R.A.
Supporting Information Available
Synthesis and characterization data, ER/GPR30 immunoassay, and LDH assay. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported in part by National Institutes of Health (NIH) Grant CA102590.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Maki P.; Hogervorst E. (2003) The menopause and HRT. HRT and cognitive decline. Best Pract. Res., Clin. Endocrinol. Metab. 17, 105–122. [DOI] [PubMed] [Google Scholar]
- Pines A.; Sturdee D. W.; Birkhauser M. H.; de Villiers T.; Naftolin F.; Gompel A.; Farmer R.; Barlow D.; Tan D.; Maki P.; Lobo R.; Hodis H. (2008) HRT in the early menopause: scientific evidence and common perceptions. Climacteric 11, 267–272. [DOI] [PubMed] [Google Scholar]
- Frye C. A. (2009) Steroids, reproductive endocrine function, and affect. A review. Minerva Ginecol. 61, 541–562. [PubMed] [Google Scholar]
- Carlson L. E.; Sherwin B. B. (1998) Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology 23, 583–603. [DOI] [PubMed] [Google Scholar]
- Resnick S. M.; Metter E. J.; Zonderman A. B. (1997) Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect?. Neurology 49, 1491–1497. [DOI] [PubMed] [Google Scholar]
- Steenland K.; MacNeil J.; Vega I.; Levey A. (2009) Recent trends in Alzheimer disease mortality in the United States. Alzheimer Dis. Assoc. Disord. 23, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal D. B.; Shughrue P. J.; Wilson M. E.; Merchenthaler I.; Wise P. M. (1999) Estradiol Modulates bcl-2 in Cerebral Ischemia: A Potential Role for Estrogen Receptors. J. Neurosci. 19, 6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.; Pandey S. C.; Cohen R. S. (2008) Estrogen affects levels of Bcl-2 protein and mRNA in medial amygdala of ovariectomized rats. J. Neurosci. Res. 86, 3655–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L. A. (2008) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev. 57, 332–341. [DOI] [PubMed] [Google Scholar]
- Shumaker S. A.; Legault C.; Kuller L.; Rapp S. R.; Thal L.; Lane D. S.; Fillit H.; Stefanick M. L.; Hendrix S. L.; Lewis C. E.; Masaki K.; Coker L. H. and for the Women’s Health Initiative Memory, S. (2004) Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women: Women’s Health Initiative Memory Study. JAMA 291, 2947−2958. [DOI] [PubMed] [Google Scholar]
- Coker L. H.; Espeland M. A.; Rapp S. R.; Legault C.; Resnick S. M.; Hogan P.; Gaussoin S.; Dailey M.; Shumaker S. A. (2010) Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS). J. Steroid Biochem. Mol. Biol. 118, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw J. E.; Anderson G. L.; Prentice R. L.; LaCroix A. Z.; Kooperberg C.; Stefanick M. L.; Jackson R. D.; Beresford S. A.; Howard B. V.; Johnson K. C.; Kotchen J. M.; Ockene J. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333. [DOI] [PubMed] [Google Scholar]
- Chlebowski R. T.; Schwartz A. G.; Wakelee H.; Anderson G. L.; Stefanick M. L.; Manson J. E.; Rodabough R. J.; Chien J. W.; Wactawski-Wende J.; Gass M.; Kotchen J. M.; Johnson K. C.; O’Sullivan M. J.; Ockene J. K.; Chen C.; Hubbell F. A. (2009) Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 374, 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker S. A.; Legault C.; Rapp S. R.; Thal L.; Wallace R. B.; Ockene J. K.; Hendrix S. L.; Jones B. N.; Assaf A. R.; Jackson R. D.; Kotchen J. M.; Wassertheil-Smoller S.; Wactawski-Wende J. (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: A Randomized controlled trial. JAMA 289, 2651–2662. [DOI] [PubMed] [Google Scholar]
- Vogel V. G.; Costantino J. P.; Wickerham D. L.; Cronin W. M.; Cecchini R. S.; Atkins J. N.; Bevers T. B.; Fehrenbacher L.; Pajon E. R. Jr.; Wade J. L. 3rd; Robidoux A.; Margolese R. G.; James J.; Lippman S. M.; Runowicz C. D.; Ganz P. A.; Reis S. E.; McCaskill-Stevens W.; Ford L. G.; Jordan V. C.; Wolmark N. (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295, 2727–2741. [DOI] [PubMed] [Google Scholar]
- Cohen F. J.; Watts S.; Shah A.; Akers R.; Plouffe L. E. O. Jr. (2000) Uterine Effects of 3-Year Raloxifene Therapy in Postmenopausal Women Younger Than Age 60. Obstet. Gynecol. 95, 104–110. [DOI] [PubMed] [Google Scholar]
- Goldstein S. R.; Scheele W. H.; Rajagopalan S. K.; Wilkie J. L.; Walsh B. W.; Parsons A. K. (2000) A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet. Gynecol. 95, 95–103. [DOI] [PubMed] [Google Scholar]
- Yaffe K.; Krueger K.; Cummings S. R.; Blackwell T.; Henderson V. W.; Sarkar S.; Ensrud K.; Grady D. (2005) Effect of Raloxifene on Prevention of Dementia and Cognitive Impairment in Older Women: The Multiple Outcomes of Raloxifene Evaluation (MORE) Randomized Trial. Am. J. Psychiatry 162, 683–690. [DOI] [PubMed] [Google Scholar]
- Agnusdei D.; Iori N. (2000) Raloxifene: results from the MORE study. J Musculoskeletal Neuronal Interact. 1, 127–132. [PubMed] [Google Scholar]
- Jacobsen D. E., Samson M. M., Emmelot-Vonk M. H. Verhaar H. J. (2009) Raloxifene improves verbal memory in late postmenopausal women: a randomized, double-blind, placebo-controlled trial. Menopause. [DOI] [PubMed] [Google Scholar]
- Goekoop R.; Duschek E. J.; Knol D. L.; Barkhof F.; Netelenbos C.; Scheltens P.; Rombouts S. A. (2005) Raloxifene exposure enhances brain activation during memory performance in healthy elderly males; its possible relevance to behavior. Neuroimage 25, 63–75. [DOI] [PubMed] [Google Scholar]
- Goekoop R.; Barkhof F.; Duschek E. J.; Netelenbos C.; Knol D. L.; Scheltens P.; Rombouts S. A. (2006) Raloxifene treatment enhances brain activation during recognition of familiar items: a pharmacological fMRI study in healthy elderly males. Neuropsychopharmacology 31, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Kemp D. C.; Fan P. W.; Stevens J. C. (2002) Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug Metab. Dispos. 30, 694–700. [DOI] [PubMed] [Google Scholar]
- Morello K. C.; Wurz G. T.; DeGregorio M. W. (2003) Pharmacokinetics of selective estrogen receptor modulators. Clin. Pharmacokinet. 42, 361–372. [DOI] [PubMed] [Google Scholar]
- Suh N.; Glasebrook A. L.; Palkowitz A. D.; Bryant H. U.; Burris L. L.; Starling J. J.; Pearce H. L.; Williams C.; Peer C.; Wang Y.; Sporn M. B. (2001) Arzoxifene, a new selective estrogen receptor modulator for chemoprevention of experimental breast cancer. Cancer Res. 61, 8412–8415. [PubMed] [Google Scholar]
- Overk C. R.; Peng K. W.; Asghodom R. T.; Kastrati I.; Lantvit D. D.; Qin Z.; Frasor J.; Bolton J. L.; Thatcher G. R. J. (2007) Structure-activity relationships for a family of benzothiophene selective estrogen receptor modulators including raloxifene and arzoxifene. ChemMedChem 2, 1520–1526. [DOI] [PubMed] [Google Scholar]
- Liu H.; Bolton J. L.; Thatcher G. R. J. (2006) Chemical modification modulates estrogenic activity, oxidative reactivity, and metabolic stability in 4′F-DMA, a new benzothiophene selective estrogen receptor modulator. Chem. Res. Toxicol. 19, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Liu J.; van Breemen R. B.; Thatcher G. R. J.; Bolton J. L. (2005) Bioactivation of the selective estrogen receptor modulator desmethylated arzoxifene to quinoids: 4′-fluoro substitution prevents quinoid formation. Chem. Res. Toxicol. 18, 162–173. [DOI] [PubMed] [Google Scholar]
- Qin Z.; Kastrati I.; Chandrasena R. E.; Liu H.; Yao P.; Petukhov P. A.; Bolton J. L.; Thatcher G. R. J. (2007) Benzothiophene selective estrogen receptor modulators with modulated oxidative activity and receptor affinity. J. Med. Chem. 50, 2682–2692. [DOI] [PubMed] [Google Scholar]
- De Keyser J.; Sulter G.; Luiten P. G. (1999) Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing?. Trends Neurosci. 22, 535–540. [DOI] [PubMed] [Google Scholar]
- Lipton P. (1999) Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568. [DOI] [PubMed] [Google Scholar]
- Cimarosti H.; Zamin L. L.; Frozza R.; Nassif M.; Horn A. P.; Tavares A.; Netto C. A.; Salbego C. (2005) Estradiol protects against oxygen and glucose deprivation in rat hippocampal organotypic cultures and activates Akt and inactivates GSK-3beta. Neurochem. Res. 30, 191–199. [DOI] [PubMed] [Google Scholar]
- Nilsen J.; Chen S.; Irwin R.; Iwamoto S.; Brinton R. (2006) Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Green P. S.; Simpkins J. W. (2001) Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 77, 804–811. [DOI] [PubMed] [Google Scholar]
- Goodenough S.; Schleusner D.; Pietrzik C.; Skutella T.; Behl C. (2005) Glycogen synthase kinase 3beta links neuroprotection by 17beta-estradiol to key Alzheimer processes. Neuroscience 132, 581–589. [DOI] [PubMed] [Google Scholar]
- Sarkar S. N.; Smith L. T.; Logan S. M.; Simpkins J. W. (2010) Estrogen-induced activation of extracellular signal-regulated kinase signaling triggers dendritic resident mRNA translation. Neuroscience 170, 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins J. W.; Dykens J. A. (2008) Mitochondrial mechanisms of estrogen neuroprotection. Brain Res. Rev. 57, 421–430. [DOI] [PubMed] [Google Scholar]
- Yi K. D.; Cai Z. Y.; Covey D. F.; Simpkins J. W. (2008) Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J. Pharmacol. Exp. Ther. 324, 1188–1195. [DOI] [PubMed] [Google Scholar]
- Simpkins J. W.; Yang S. H.; Sarkar S. N.; Pearce V. (2008) Estrogen actions on mitochondria--physiological and pathological implications. Mol. Cell. Endocrinol. 290, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Wu T. W.; Brinton R. D. (2004) Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 1010, 22–34. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Brinton R. D. (2007) Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 1172, 48–59. [DOI] [PubMed] [Google Scholar]
- Vasudevan N.; Pfaff D. W. (2007) Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr. Rev. 28, 1–19. [DOI] [PubMed] [Google Scholar]
- Gingerich S.; Kim G. L.; Chalmers J. A.; Koletar M. M.; Wang X.; Wang Y.; Belsham D. D. (2010) Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 170, 54–66. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Bosch M. A.; Rick E. A.; Kelly M. J.; Ronnekleiv O. K. (2009) 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J. Neurosci. 29, 10552–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff M. H.; Chambliss K. L.; Mineo C.; Yuhanna I. S.; Mendelsohn M. E.; Mumby S. M.; Shaul P. W. (2001) Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i). J. Biol. Chem. 276, 27071–27076. [DOI] [PubMed] [Google Scholar]
- Filardo E. J.; Quinn J. A.; Bland K. I.; Frackelton A. R. Jr. (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 14, 1649–1660. [DOI] [PubMed] [Google Scholar]
- Dennis M. K.; Burai R.; Ramesh C.; Petrie W. K.; Alcon S. N.; Nayak T. K.; Bologa C. G.; Leitao A.; Brailoiu E.; Deliu E.; Dun N. J.; Sklar L. A.; Hathaway H. J.; Arterburn J. B.; Oprea T. I.; Prossnitz E. R. (2009) In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 5, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Dietz B. M.; Dunlap T.; Kastrati I.; Lantvit D. D.; Overk C. R.; Yao P.; Qin Z.; Bolton J. L.; Thatcher G. R. J. (2007) Structural modulation of reactivity/activity in design of improved benzothiophene selective estrogen receptor modulators: induction of chemopreventive mechanisms. Mol. Cancer Ther. 6, 2418–2428. [DOI] [PubMed] [Google Scholar]
- Qin Z.; Kastrati I.; Ashgodom R. T.; Lantvit D. D.; Overk C. R.; Choi Y.; van Breemen R. B.; Bolton J. L.; Thatcher G. R. J. (2009) Structural modulation of oxidative metabolism in design of improved benzothiophene selective estrogen receptor modulators. Drug Metab. Dispos. 37, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella P.; Brinton R. D. (2006) Estrogen Receptor Protein Interaction with Phosphatidylinositol 3-Kinase Leads to Activation of Phosphorylated Akt and Extracellular Signal-Regulated Kinase 1/2 in the Same Population of Cortical Neurons: A Unified Mechanism of Estrogen Action. J. Neurosci. 26, 9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Mengual T., Miyawaki T., Latuszek A., Alborch E., Zukin R. S. Etgen A. M.. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 1321, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. W.; Wang J. M.; Chen S.; Brinton R. D. (2005) 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135, 59–72. [DOI] [PubMed] [Google Scholar]
- Nilsen J.; Chen S.; Brinton R. D. (2002) Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 930, 216–234. [DOI] [PubMed] [Google Scholar]
- Simoncini T.; Genazzani A. R. (2003) Non-genomic actions of sex steroid hormones. Eur. J. Endocrinol. 148, 281–292. [DOI] [PubMed] [Google Scholar]
- Simoncini T.; Genazzani A. R.; Liao J. K. (2002) Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation 105, 1368–1373. [DOI] [PubMed] [Google Scholar]
- Furfine E. S.; Harmon M. F.; Paith J. E.; Garvey E. P. (1993) Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry (Moscow) 32, 8512–8517. [DOI] [PubMed] [Google Scholar]
- Pollock J. S.; Forstermann U.; Mitchell J. A.; Warner T. D.; Schmidt H. H.; Nakane M.; Murad F. (1991) Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 88, 10480–10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P.; Schmidt K.; Brunner F.; Mayer B. (1994) Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J. Biol. Chem. 269, 1674–1680. [PubMed] [Google Scholar]
- Garvey E. P.; Tuttle J. V.; Covington K.; Merrill B. M.; Wood E. R.; Baylis S. A.; Charles I. G. (1994) Purification and characterization of the constitutive nitric oxide synthase from human placenta. Arch. Biochem. Biophys. 311, 235–241. [DOI] [PubMed] [Google Scholar]
- Prokai L.; Prokai-Tatrai K.; Perjesi P.; Zharikova A. D.; Perez E. J.; Liu R.; Simpkins J. W. (2003) Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Natl. Acad. Sci. U. .A. 100, 11741–11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman H. (1994) The antioxidant action of a pure antioestrogen: ability to inhibit lipid peroxidation compared to tamoxifen and 17 beta-oestradiol and relevance to its anticancer potential. Biochem. Pharmacol. 47, 493–498. [DOI] [PubMed] [Google Scholar]
- Lee E. S.; Yin Z.; Milatovic D.; Jiang H.; Aschner M. (2009) Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol. Sci. 110, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane L.; Li Dai W.; Fiskum G.; Bambrick L. L. (2010) Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 88, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Qin Z.; Wijewickrama G. T.; Edirisinghe P.; Bolton J. L.; Thatcher G. R. J. (2009) Comparative methods for analysis of protein covalent modification by electrophilic quinoids formed from xenobiotics. Bioconjugate Chem. 20, 728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Qin Z.; Thatcher G. R. J.; Bolton J. L. (2007) Uterine peroxidase-catalyzed formation of diquinone methides from the selective estrogen receptor modulators raloxifene and desmethylated arzoxifene. Chem. Res. Toxicol. 20, 1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowers T. S.; Qin Z. H.; Thatcher G. R. J.; Bolton J. L. (2006) Bioactivation of selective estrogen receptor modulators (SERMs). Chem. Res. Toxicol. 19, 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirali T.; Gatti S.; Di Brisco R.; Tacchi S.; Zaninetti R.; Brunelli E.; Massarotti A.; Sorba G.; Canonico P. L.; Moro L.; Genazzani A. A.; Tron G. C.; Billington R. A. (2007) Estrogenic analogues synthesized by click chemistry. ChemMedChem 2, 437–440. [DOI] [PubMed] [Google Scholar]
- Nilsen J.; Diaz Brinton R. (2003) Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc. Natl. Acad. Sci. U.S.A. 100, 2842–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcellier A.; Tintignac L. A.; Zhuravleva E.; Hemmings B. A. (2008) PKB and the mitochondria: AKTing on apoptosis. Cell. Signalling 20, 21–30. [DOI] [PubMed] [Google Scholar]
- Navarro C. E.; Saeed S. A.; Murdock C.; Martinez-Fuentes A. J.; Arora K. K.; Krsmanovic L. Z.; Catt K. J. (2003) Regulation of cyclic adenosine 3′,5′- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol. Endocrinol. 17, 1792–1804. [DOI] [PubMed] [Google Scholar]
- Prossnitz E. R.; Barton M. (2009) Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediators 89, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G.; Bader B.; Meoli L.; Isensee J.; Delbeck M.; Noppinger P. R.; Otto C. (2010) A critical review of fundamental controversies in the field of GPR30 research. Steroids 75, 603–610. [DOI] [PubMed] [Google Scholar]
- Levin E. R. (2011) Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol. Endocrinol 25, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer U.; Schoeffter P.; Bischoff S. F.; Nozulak J.; Feuerbach D.; Floersheim P. (2002) Toward selective ERbeta agonists for central nervous system disorders: synthesis and characterization of aryl benzthiophenes. J. Med. Chem. 45, 1399–1401. [DOI] [PubMed] [Google Scholar]
- Qiu J.; Bosch M. A.; Tobias S. C.; Grandy D. K.; Scanlan T. S.; Ronnekleiv O. K.; Kelly M. J. (2003) Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 23, 9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J.; Hamid N.; Bondar G.; Prossnitz E. R.; Micevych P. (2010) Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J. Neurosci. 30, 12950–12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Glinn M. A.; Ostrowski N. L.; Su Y.; Ni B.; Cole H. W.; Bryant H. U.; Paul S. M. (1999) Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 847, 98–104. [DOI] [PubMed] [Google Scholar]
- Kokiko O. N.; Murashov A. K.; Hoane M. R. (2006) Administration of raloxifene reduces sensorimotor and working memory deficits following traumatic brain injury. Behav. Brain Res. 170, 233–240. [DOI] [PubMed] [Google Scholar]
- Ciriza I.; Carrero P.; Azcoitia I.; Lundeen S. G.; Garcia-Segura L. M. (2004) Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J. Neurobiol. 61, 209–221. [DOI] [PubMed] [Google Scholar]
- O’Neill K.; Chen S.; Brinton R. D. (2004) Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer’s disease. Exp. Neurol. 185, 63–80. [DOI] [PubMed] [Google Scholar]
- Brewer G. J.; Torricelli J. R.; Evege E. K.; Price P. J. (1993) Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35, 567–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.