Abstract
Considering the latest researches, disruptions in the regulation of mitochondrial dynamics, low energy production, increased reactive oxygen species and mtDNA damage are relevant to human diseases, mainly in neurogenerative diseases and cancer. This article represents inner mitochondrial membrane as a natural superconductor giving also the corresponding mathematical model; nevertheless the creation of electric complexes into the inner mitochondrial membrane due to the unusual concentration of protons disrupts the normal flow of electrons and the production of ATP. Therefore, we propose the term ‘electric thromboses’ for the explanation of these inadequate electrons’ flow, presenting simultaneously a natural mechanism of this important and unique phenomenon.
Keywords: Mitochondrial dynamics, neurogenerative diseases, ‘electric thromboses’, superconductivity
Background
The number of mitochondria in a cell is regulated to match the cell's requirements for ATP, while fusions and fissions play a functional role in maintenance of proper inner membrane electrical potential. The main problems associated with mitochondrial disease, such as low energy, free radical production and lactic acidosis, can result in a variety of symptoms in many different parts of the body, including the nervous system, digestive tract, eyes, skeletal muscle, heart, liver, kidneys, and pancreas. After years of intense studies, a considerable number of scientific researches demonstrated the important role of mitochondrial dysfunction and oxidative stress to development of the more common neurogenerative diseases, like Alzheimer's disease, Parkinson's disease and Huntington's disease [1–3]. Recent studies have shown that mitochondria are significantly reduced in Alzheimer's disease (AD), supporting a topographic and probably temporal relationship between neuronal oxidative damage and mitochondrial abnormalities [4]. Scientists used brain tissue from cases with a diagnosis of AD [5, 6], as well as control cases with no clinical or pathological history of neurological disease, applying cytological in situ hybridization, immunocytochemistry and morphometry [4] techniques, showing that the area of intact mitochondria is significantly decreased in AD. While AD can be genetically classified as familiar or sporadic, researchers proposed that the case of sporadic AD is not caused by the accumulation of amyloid-β (Αβ), but instead is a consequence of a decline in mitochondrial function with age [7, 8]. Additionally, the overexpression of Αβ causes an alteration in the mitochondrial fission and fusion proteins resulting in mitochondrial dysfunction, mitochondrial fragmentation, increase in reactive oxygen species (ROS) and ATP production and reduced mitochondrial membrane potential [9]. In the brain, mitochondrial function declines with age and this functional decline associates with increased mitochondrial biogenesis. In various neurodegenerative disease states brain mitochondrial function declines even further, perhaps to the point where mitochondrial biogenesis can no longer compensate for functional declines. It should be taken into consideration that at some point neurons with impaired mitochondria may reach a point where mitochondrial biogenesis pathways deactivate [10]. Mitochondrial dynamics allows mitochondria to interact with each other; without such dynamics, the mitochondrial population consists of autonomous organelles that have impaired function [11]. In a wild-type cell, high rates of fusion and fission are independent events, which constantly change the identity of individual mitochondria. Therefore fusion and fission seems to be required to maintain mitochondrial function, as independent and different mechanisms. Fusion is likely to protect function by providing a chance for mitochondria to mix their contents, thus enabling protein complementation, mtDNA repair and equal distribution of metabolites, helping the isolation of damaged mitochondrial segments and promoting their autophagy [12]. In contrast, fission acts in order to facilitate equal segregation of mitochondria into daughter cells during cell division and to enhance distribution of mitochondria along cytoskeletal tracks. The failure in this biological machinery may also promote apoptosis [13]. A further two important aspects of mitochondrial dynamics beyond fusion and fission is the motility of mitochondria and mitophagy. It has been proved that perturbations in mitochondrial dynamics can lead to distinctive defects in neurons [14]. Even though, these four processes are independent, it is clear that any interactions will be critically important in neurons. For example defects in both fusion and fission have been shown to decrease mitochondrial movement. The large tangle of highly interconnected mitochondria in fission-deficient cells prevents efficient movement, especially into small pathways such as neuronal processes [15, 16]. While mitophagy denotes the degradation of mitochondria through autophagy, recent findings indicate that mitophagy can selectively degrade defective mitochondria [17].
While it is not possible to have a direct measure of mitochondrial current, we are able to express the electric potential across the inner membrane in terms of radioactive 42K+ ions, using the Nernst equation (see equation 1 in supplementary material). We can also calculate the proton motive force (pmf) in millivolts using equation 2 (see supplementary material). By taken into consideration the significant voltage gradient across the mitochondrial membrane we shall move towards to support in mathematical terms our hypothesis, that inner mitochondrial membrane is a natural superconductor.
Methodology
See supplementary material for detailed description.
Discussion
Mitochondria provide most of the ATP for cellular reactions. ATP production in mitochondria is coupled to an electron transport system in which the passage of electrons down the various electron carriers is associated with the transport of protons from the matrix into the inter membrane space. The majority of these protons re-enter the mitochondrial matrix by the ATP syntheses, thereby generating ATP. However, approximately 20% of mitochondrial oxygen consumption is not coupled to ATP production, and protons enter the matrix through the phospholipids’ bilayer and through uncoupling proteins, generating heat [18]. From a geometrical point of view, it is quite obvious that a number of (un) correlated factors can affect the mitochondrial shape. There is a more complex problem, as morphological changes in mitochondrial structure are associated with biological dysfunctionalities and electrophysiology problems. These effects are directly or indirectly correlated with human neurodegenerative diseases. While fusions and fissions contribute to the wide variety of mitochondrial morphologies, a discrete mitochondrion at one point in time will be changed at a later time by the addition of new mitochondrial material through fusion or by the removal of material through division. It is a logical consequence of high probability that after a certain period of successful events (fusions and fissions) the inner structure will totally lose its initial characteristics in a non-reversible way, restricting the inner space and reducing the corresponding area and energy. It is obvious that any failure in inner membrane mitochondrial fissions can easily generate unstable electric potential, effecting functionality and reduce voltage gradient.This inappropriate intra-structure is followed by high level transmembrane proton concentration, actually blocking the natural pathway of producing ATP. Due to the action of complexes during the flow of electrons, protons are unequally distributed on both sides of the inner mitochondrial membrane. Therefore, the mitochondrial matrix becomes poorer in protons. Due to the action of the respiratory chain complexes, the energy of electrons is temporarily stored in the form of electrochemical energy potential. This form of energy will be restored via the returning of protons through particular channels of the internal membrane in the matrix and will be used to cover energy needs. In other words, the electrochemical potential, or proton-stimulatory power, corresponds to the tendency of the protons to be restored to their initial locations, in order to achieve equilibrium and reset the imbalance of the protons. The size of this energy is proportional to the size of the difference between the concentrations of protons on both sides of the membrane. This phenomenon can be characterized as an ‘electric thromboses’ and the superconductivity in the flow of electrons will be cancelled (Figure 1). The superconductivity of electrons is destroyed and no pair of electrons are transferred when ‘electric thromboses’ occurs where the final reaction in the respiratory chain is given by equation 10 (see supplementary material). It is obvious that the existence of electric complexes can be either temporary or permanent, with adverse impacts on nerve cells.
Figure 1.
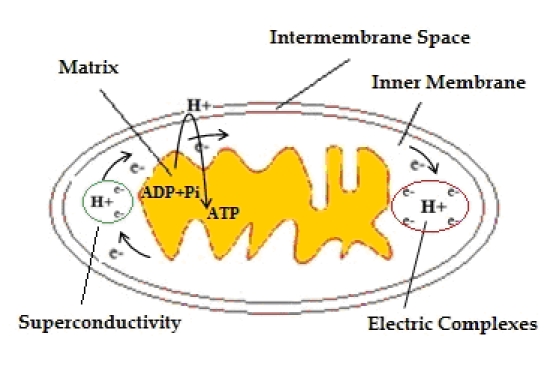
The phenomenon of ‘electric thromboses’, slow down the synthesis of ATP. While the reduction of O2 to H2O need 4e and 4H+, the existence of electric complexes prevent any pair of electrons to react with O2 and therefore any form of superconductivity in the membrane's electric potential expires.
Conclusion
Since many neurodegenerative diseases cause mitochondria to malfunction, it may be important to focus on developing methods to repair and restore mitochondria. Already a few strategies are being developed that open up ways for manipulating mitochondrial functions and may allow for the selective protection or eradication of neurons in the treatment of neurodegenerative diseases [10]. While recent studies have already prove the significant connection between mitochondrial dysfunction and human diseases and the disruptions of energy production due to inappropriate topological structure, a new model is presented in this paper concerning the formulation of the high energy concentration in mitochondrial inner membrane, characterizing the internal mitochondrial membrane as a natural superconductor, where electrical resistance of exactly zero occurs in certain temperature. Based on this model, a first solving approach should be the use of large electrical load or a larger potential difference to impel electrons into the transmembrane space. Future research will proceed to the statistical analysis and computational simulation providing clear experimental results. A complete and integrated picture of our hypothesis model requires the study of the critical point of local temperature T, where inner mitochondrial membrane's superconductivity occurs. These observations will definitely lead to alternative pharmacological manipulations of neurogenerative diseases.
Supplementary material
Footnotes
Citation:Alexiou et al Bioinformation 6(5): 173-175 (2011)
References
- 1.GM Enns. Mol Genet Metab. 2003;80:11. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.KA Jellinger. J Neural Transm. 2009;116:1111. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- 3.W Mandemakers, et al. J Cell Sci. 2007;120:1707. doi: 10.1242/jcs.03443. [DOI] [PubMed] [Google Scholar]
- 4.K Hirai, et al. J Neurosci. 2001;21:3017. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ZS Khachaturian. Arch Neurol. 1985;42:1097. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 6.SS Mirra, et al. Neurology. 1991;41:479. [Google Scholar]
- 7.RH Swerdlow, SM Khan. Med Hypotheses. 2004;63:8. [Google Scholar]
- 8.RH Swerdlow, SM Khan. Exp Neurol. 2009;218:308. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.X Wang, et al. Proc Natl Acad Sci U S A. 2008;105:19318. [Google Scholar]
- 10.Onyango IG, et al. Biochim Biophys Acta. 2010;1802:228. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.DC Chan. Annu Rev Cell Dev Biol. 2006;22:79. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 12.G Twig, et al. EMBO J. 2008;27:433. [Google Scholar]
- 13.DF Suen, et al. Genes Dev. 2008;22:1577. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.H Chen, DC Chan. Hum Mol Genet. 2009;18:R169. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.T Kanki, DJ Klionsky. J Biol Chem. 2008;283:32386. doi: 10.1074/jbc.M802403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.P Verstreken, et al. Neuron. 2005;47:365. [Google Scholar]
- 17.Z Li, et al. Cell. 2004;119:873. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.D Rolfe, G Brown. Physiol Rev. 1997;77:731. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


