Abstract
Background
Nuclear factor-κB (NF-κB) is a key transcription factor that regulates both innate and adaptive immunity as well as ectodermal development. Mutations in the coding region of the IκB kinase γ/NF-κB essential modifier (NEMO) gene cause X-linked ectodermal dysplasia with immunodeficiency.
Objective
To determine the genetic cause of recurrent sinopulmonary infections and dysgammaglobulinemia in a patient with a normal NEMO coding sequence and his affected brother.
Methods
TNF-α and IFN-α production in response to Toll-like receptor (TLR) stimulation was analyzed by ELISA, NEMO mRNA levels were measured by quantitative PCR, and NEMO protein expression was measured by Western blotting. NF-κB activation was assessed by nuclear translocation of p65 and luciferase reporter gene assays.
Results
TLR-induced TNF-α and IFN-α production by PBMCs was impaired in the patient and his brother. Sequencing of the patient’s NEMO gene revealed a novel mutation in the 5′ untranslated region, which was also present in the brother, resulting in abnormally spliced transcripts and a 4-fold reduction in mRNA levels. NEMO protein levels in EBV transformed B cells and fibroblasts from the index patient were 8-fold lower than normal controls. NF-κB p65 nuclear translocation in the patient’s EBV B cells after TLR7 ligation was defective. NF-κB–dependent luciferase gene expression in IL-1–stimulated fibroblasts from the patient was impaired.
Conclusion
This is the first description of immune deficiency resulting from low expression of a normal NEMO protein.
Keywords: NEMO, immune deficiency, recurrent infections, 5′ untranslated region mutation
The transcription factor nuclear factor-κB (NF-κB) is required for normal development and function of the immune system. Proper functioning of the immune system requires a tightly regulated inflammatory response, which is dependent on activation of NF-κB.1,2 In the resting state, NF-κB proteins are retained in the cytoplasm by the inhibitor of NF-κB (IκB) proteins, which include IκBα. Activation of numerous cell receptors, including proinflammatory cytokines (IL-1, TNF-α), CD40, and Toll-like receptors, causes activation of the IκB kinase complex (IKK), which leads to phosphorylation of IκB proteins. Phosphorylated IκB proteins are subsequently ubiquitinated and degraded, allowing nuclear translocation of NF-κB and activation of gene transcription.3,4
Proper function of the IKK complex is dependent on IKKγ/NF-κB essential modifier (NEMO), which is encoded by a gene (IKBKG) located on the X chromosome. NEMO functions as a scaffolding protein and links upstream signaling pathways to activation of the IKK complex.3 Numerous mutations in NEMO that result in the production of a dysfunctional NEMO protein have been described in male patients with the syndrome of ectodermal dysplasia associated with immune deficiency (EDID). EDID arises because normal ectodermal development (hair, teeth, and sweat glands), as well as effective innate and adaptive immune responses, require NEMO-dependent NF-κB activation downstream of both the ectodysplasin A receptor and several receptors of the immune system.1,2,5 Mutations in NEMO that result in EDID are termed hypomorphic because they result in reduced, but not absent, function of NEMO. Absence of NEMO function (amorphic mutations) in males is lethal in utero, whereas heterozygosity for null NEMO mutations results in incontinentia pigmenti in females.6,7
Impaired NF-κB activation is detrimental to both innate and adaptive immune function. Toll-like receptors (TLRs), nucleotide-binding oligomerization domain–like receptors, and retinoic acid–inducible gene I—like helicases are pathogen recognition receptors that signal through NF-κB to indicate detection of invading pathogens, including bacteria, mycobacteria, fungi, and viruses.2,8 Therefore, defects in NF-κB activation can result in impaired inflammatory responses to invading pathogens, resulting in decreased production of proinflammatory cytokines and type 1 IFNs.1,2 Because T-cell and B-cell receptors also signal through NF-κB, impaired NF-κB function can result in defective antigen-specific immunity.3 As a result, patients with EDID can be susceptible to a wide variety of bacterial, mycobacterial, viral, and fungal infections. Immunologic evaluation of these patients commonly reveals hypogammaglobulinemia with variably increased IgM and IgA levels, variable defects in specific antibody responses to protein and polysaccharide antigens, and variably impaired T-cell proliferation to antigens.6,7,9,10 Some immunodeficient patients with mutations in the NEMO gene have normal ectodermal development, suggesting a less stringent requirement for NEMO-dependent NF-κB activation for normal ectodermal development.10–13
In this report, we describe an 11-year-old boy with immune deficiency but not ectodermal dysplasia, and a normal NEMO coding sequence but low levels of NEMO mRNA and protein. Sequencing of the 5′ untranslated region (UTR) of the patient’s NEMO gene revealed a G to T mutation at position +1 of the donor splice site of the untranslated exon 1B. This results in destruction of the normal exon 1B to exon 2 splice site, generation of 2 abnormally sized NEMO mRNA species with intact coding sequences, reduced levels of NEMO mRNA overall, and production of 8-fold less NEMO protein relative to normal controls. A brother of the patient with similar clinical manifestations and low NEMO expression also had an identical mutation in the 5′ UTR of the NEMO gene.
METHODS
Reagents
Toll-like receptor ligands used in this study were as previously described.14 Antiphospho-IκBα and antiphospho–p38 mitogen-activated protein kinase were from Cell Signaling (Danvers, Mass); anti-IκBα and anti-NEMO/IKKγ were from Santa Cruz Biotechnology (Santa Cruz, Calif). Recombinant human IL-1β and the ELISA kits for human TNF-α and human IFN-α were obtained from Biosource (Camarillo, Calif).
Cell isolation and stimulation
Informed consent for blood and dermal biopsy samples was obtained from the patient and healthy control subjects in accord with the institutional review board at Children’s Hospital Boston. PBMCs were isolated by centrifugation through Ficoll-Paque PLUS (Amersham Biosciences, Uppsala, Sweden). PBMCs were cultured in RPMI plus 10% FCS with L-glutamine and penicillin/streptomycin (Invitrogen, Carlsbad, Calif). Cell stimulations (4 × 105 PBMCs/condition)were performed in 96-well plates in a volume of 200 µL medium with the following concentrations of TLR ligands: PAM3CSK4 (0.1 µg/mL), poly I:C (50 µg/mL), LPS (0.1 µg/mL), flagellin (1 µg/mL), 3M-002 and 3M-013 (20 µmol/L), and CpG ODN2216 (5 µmol/L). PBMCs were also stimulated with phorbol 12-myristate 13-acetate (7.5 ng/mL) plus ionomycin (7.5 ng/mL), and IFN-β (1 × 105 U/mL) as positive controls. TNF-α and IFN-α were measured after 24 hours of stimulation by ELISA.
Western blotting
Fibroblasts, EBV-transformed B cells, or PBMCs from patients and controls were lysed in sample buffer (62.5 mmol/L TRIS, pH 6.8, 2% SDS, 10% glycerol, 2% b-mercaptoethanol, 0.01% bromophenol blue). Fibroblasts (40,000/condition) or PBMCs (5 × 105/condition) were stimulated with IL-1β (10 ng/mL) for the indicated times before lysis. Nuclear and cytoplasmic fractions were isolated by using a kit from Active Motif (Carlsbad, Calif). Proteins were resolved by 10% SDS-PAGE (Bio-rad, Hercules, Calif) and transferred to nitrocellulose membranes (Invitrogen). Western blotting was performed according to the manufacturer’s recommendations.
NEMO sequencing and expression analysis
Sequencing of genomic DNA was performed at the Children’s Hospital core facility. PCR primers and sequencing primers are available in this article’s Methods in the Online Repository at www.jacionline.org. The PCR products were cloned into the pCR2.1-TOPO vector (Invitrogen) for ease in sequencing. RNA was isolated from fibroblast lines or EBV lines and reverse-transcribed by using iScript (Bio-rad). NEMO cDNA using exons 1A, 1B, and 1C were amplified and cloned from cDNA isolated from normal fibroblasts (Methods, Online Repository). The misspliced 1B isoforms were amplified from cDNA isolated from patient fibroblasts. The PCR products were also cloned into the pCMV-Tag4a vector (Invitrogen) for expression studies. TaqMan gene expression assays were performed by using human NEMO (Hs99999905_m1) and GAPDH (Hs00175318_m1) probes from Applied Biosystems (Roche, Branchburg, NJ).
NF-κB reporter assays
Nuclear factor-κB–luciferase reporter plasmids containing 4 NF-κB binding sites in the promoter, and Renilla control plasmids were both kindly provided by Dr Laurie Glimcher, Harvard Medical School, Boston, Mass. The plasmids were transfected into patient and normal fibroblasts by using Lipofectamine LTX with Plus reagent (Invitrogen). After 24 hours, the cells were stimulated for 6 hours with 10 to 15 ng/mL recombinant IL-1β. The cells were lysed in passive lysis buffer, and luciferase activity was analyzed by using the Dual Luciferase Reporter Assay System (Promega, Madison, Wis).
RESULTS
Case report
The index patient is an 11-year-old boy who was healthy until 3 years of age, when he began experiencing recurrent upper and lower respiratory infections (otitis requiring placement of tympanostomy tubes, lymphadenitis, bronchitis/bronchopneumonia), recurrent diarrhea, and hematuria. Causative pathogens for the diarrhea and hematuria were not identified, and these conditions have resolved. A chest computed tomography scan revealed bronchiectasis. There was no hepatosplenomegaly. The patient has no features of ectodermal dysplasia (see this article’s Fig E1 in the Online Repository at www.jacionline.org). His immune evaluation at 4 years of age revealed normal total white blood count, lymphocyte count, and T-cell and B-cell subsets, but a low percentage of CD27+IgD−IgM− switched memory B cells (see this article’s Table E1 in the Online Repository at www.jacionline.org). The patient had an elevated IgA level (901 mg/dL) with normal IgG (653 mg/dL) and low IgM (31 mg/dL). IgM levels have remained low (see this article’s Table E2 in the Online Repository at www.jacionline.org). Specific antibodies against tetanus toxoid, rubella, and pneumococccal polysaccharide antigens were detected, but the patient had rapidly waning antibody titers to tetanus and pneumococcus over time. There was no specific antibody response to hepatitis B virus, measles, or mumps immunization (see this article’s Table E3 in the Online Repository at www.jacionline.org). He had normal in vitro T-cell proliferation responses to phytohemagglutinin, pokeweed mitogen, and anti-CD3, but decreased responses to tetanus toxoid antigen (see this article’s Table E4 in the Online Repository at www.jacionline.org). The patient was started on intravenous immunoglobulin therapy at the age of 10 years. He is currently healthy, with improved and stable pulmonary status and no active gastrointestinal complaints. The patient’s younger brother, age 5 years, also had recurrent respiratory infections and had no features of ectodermal dysplasia. His immunologic analysis revealed normal lymphocyte counts, normal T-cell subset distribution, decreased percentage of CD27+IgD−IgM− switched memory B cells (3.8% of CD19+ cells, normal 10th to 90th percentile range, 5% to 12.3%), an IgG of 653 mg/dL (normal range, 441–1135 mg/dL), an IgA of 65 mg/dL (normal range, 22–159 mg/dL), a borderline IgM of 47 mg/dL (normal range, 47–200 mg/dL), and failure to respond to immunization with pneumococcus vaccine (preimmunization titer, 3 mg/L; postimmunization titer, 3 mg/L; normal response, >60 mg/L).
Family history is also significant for an older male maternal cousin of the patient who presented with recurrent upper respiratory infections (pharyngitis, tonsillitis) and hypogammaglobulinemia from early childhood. He did not have ectodermal dysplasia and was diagnosed with common variable immune deficiency. In his late teens he was diagnosed with widespread Mycobacterium avium intracellulare infection and had multiple pneumonias, chronic diarrhea, and malnutrition. He died of infection at the age of 19 years.
Impaired cytokine production in response to TLR ligands
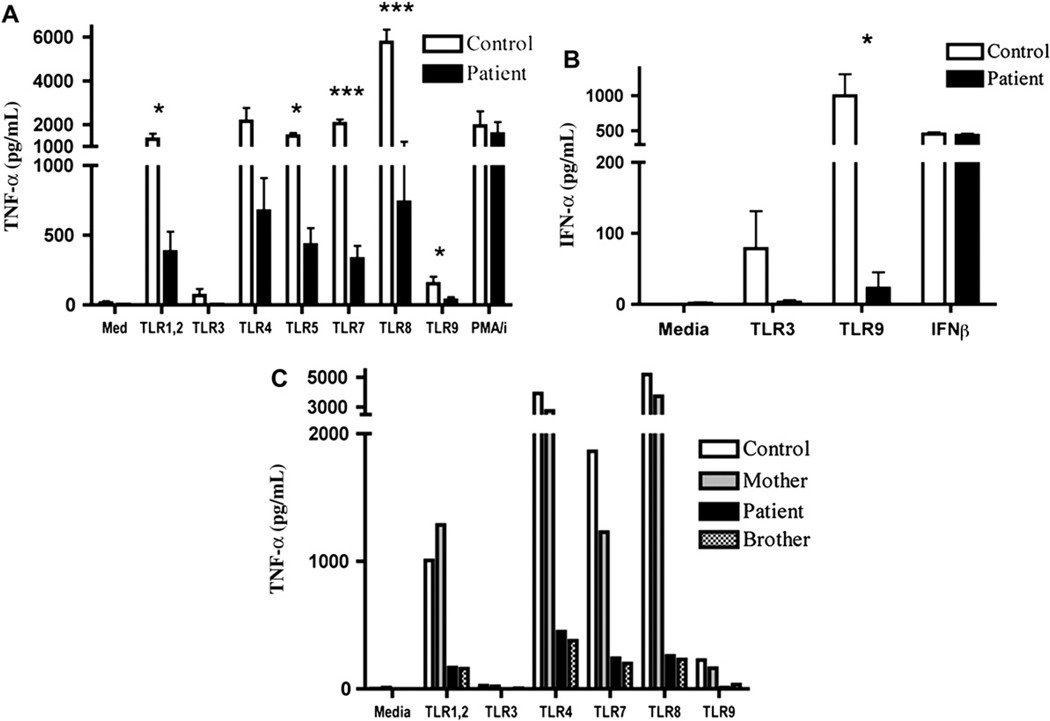
The index patient’s history of recurrent infections, low IgM, elevated IgA, and a family history of a maternal cousin with recurrent infections, including infection with a poorly virulent mycobacterium, was consistent with impaired NF-κB activation caused by a defect in NEMO. To test NF-κB function, we measured cytokine production by PBMCs in response to TLR ligands. Stimulation of the index patient’s PBMCs with PAM3CSK4 (TLR1, 2), poly I:C (TLR3), LPS (TLR4), flagellin (TLR5), 3M-013 (TLR7), 3M-002 (TLR8), and ODN2216 (TLR9) revealed significant impairment of TNF-α production compared with the mean of 4 normal healthy controls (Fig 1, A). Stimulation of the index patient’s PBMCs with TLR3 and 9 ligands also revealed significant impairment of IFN-α production compared with the mean of 4 normal healthy controls (Fig 1, B). TLR-induced TNF-α production by the patient’s affected younger brother was similarly impaired (Fig 1, C). In contrast, TLR-induced cytokine production in PBMCs from the patient’s mother was normal (Fig 1, C). Studies on the mother and brother of the index patient were performed only once because of limited availability of blood from them. Technical reasons precluded measurement of IFN-α production in response to TLR stimulation of their PBMCs.
FIG 1.
Impaired TLR-induced NF-κB–dependent cytokine production by patient PBMCs. PBMCs were incubated with medium or TLR ligands for 24 hours, and TNF-α (A) and IFN-α (B) were quantified. Graphs show the mean and SD of 4 independent experiments using PBMCs from the index patient and healthy controls (n = 4). *P < .05; ***P < .001. C, TNF-α production in PBMCs from the patient’s affected brother. PMA, Phorbol 12-myristate 13-acetate.
Levels of NEMO protein and mRNA are significantly decreased in the patient
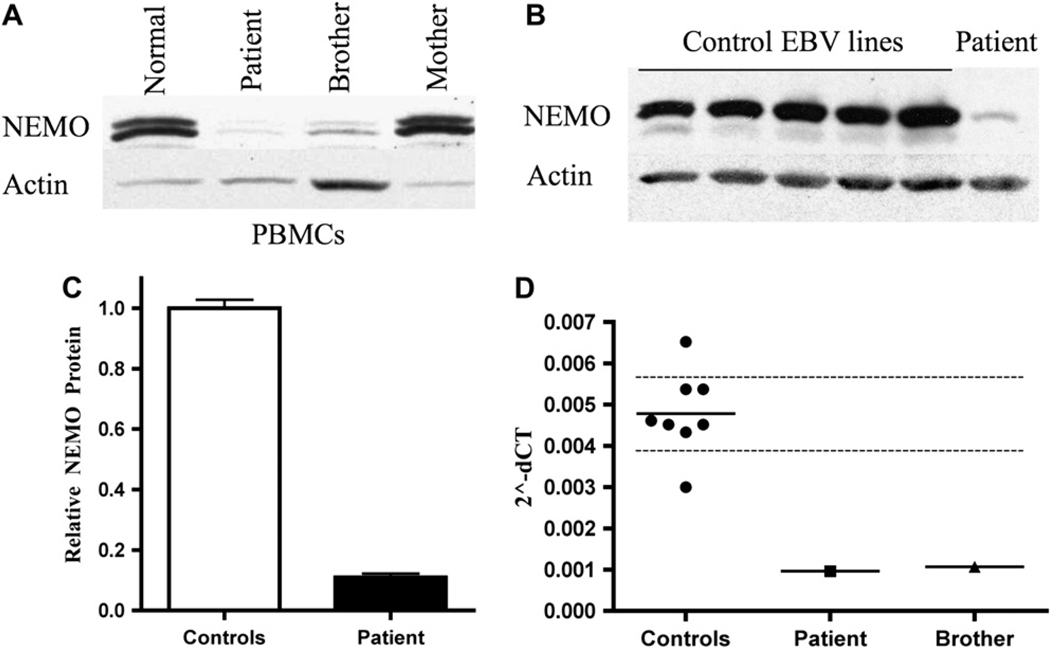
Impaired TLR functions in the patient and his brother are consistent with a defect in IKKγ/NEMO. A Western blot of lysates of PBMCs from the patient and his brother showed severely reduced NEMO protein levels compared with a normal control (Fig 2, A). In contrast, the NEMO protein level in the mother’s PBMCs was comparable to that of the normal control. Decreased NEMO protein levels in the patient were confirmed by comparing NEMO levels in lysates from EBV-transformed B cells from the patient and 5 healthy controls (Fig 2, B). Scanning densitometry revealed an 8-fold decrease in relative NEMO protein levels (normalized to actin) compared with the mean of 5 normal controls (Fig 2, C). This decrease was confirmed in fibroblasts from the index patient, which exhibited an 8-fold decrease in NEMO protein levels compared with the mean of fibroblasts from 4 normal controls (data not shown).
FIG 2.
Decreased NEMO protein and mRNA levels in the patient. Western blots from PBMC lysates from control, index patient, affected brother, and unaffected mother (A) and EBV B-cell lysates (B). C, Scanning densitometry of NEMO protein levels for each EBV B cell line, normalized to actin. Controls arbitrarily set to 1. D, qPCR analysis of NEMO mRNA levels, normalized to GAPDH, in EBV B-cell lines from index patient, brother, and 8 controls. Bar represents the mean. Dashed lines span the 95% CI.
Given the reduced NEMO protein levels in the patient and his brother, cDNA was generated from patient fibroblasts and sequenced. No mutations were found within the coding region. NEMO mRNA levels were then quantified by qPCR in EBV B cells from the patient, his brother, and 8 normal controls. GAPDH mRNA levels were used as an internal control. The patient’s and his brother’s EBV-transformed B-cell lines had 4-fold to 5-fold lower NEMO mRNA levels than EBV B-cell lines from healthy controls (Fig 2, D).
Sequencing reveals a splice site mutation in the 5′ UTR of the patient’s NEMO gene
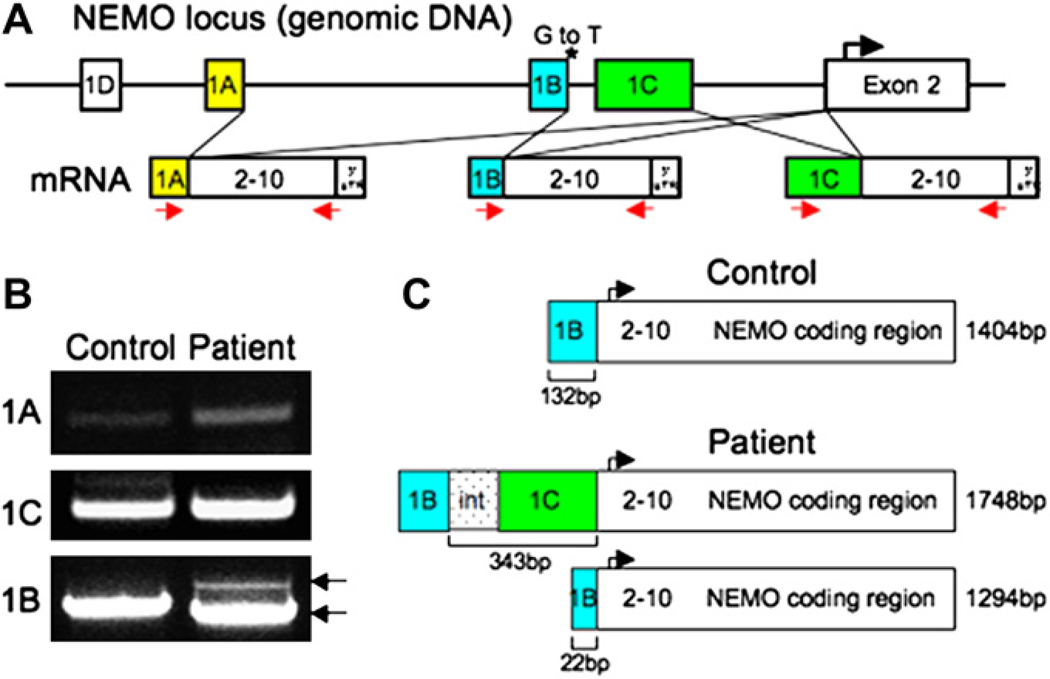
Because NEMO mRNA levels were significantly decreased in the patient, the 5′ and 3′ UTRs of his NEMO gene were analyzed. No mutations were detected in the 3′ UTR or in the polyA tail signal region. The 4 first exons (1A–D) of the NEMO gene are alternatively spliced to the ATG-containing second exon, resulting in mRNAs that are translated into an identical protein product (Fig 3, A). Lymphocytes express NEMO transcripts containing exons 1A, 1B, and 1C, but not 1D, spliced to exon 2. Exon 1B transcripts are much more abundant than transcripts starting at exon 1A or exon 1C.15 We found a similar pattern in normal fibroblasts (data not shown). We sequenced an approximately 20-kb region of genomic DNA upstream of the NEMO translation initiation site, including exons 1A, 1B, 1C, and 1D. Two previously described polymorphisms were found in the intron between exon 1A and 1B (−4875 bp and −4858 bp from the translation initiation site). A novel G to T mutation was found 4257 bp upstream of the translation initiation site, at position +1 of the donor splice-site of exon 1B (Fig 3, A). This mutation destroys the normal exon 1B to exon 2 splice site. The mutation was present in the patient’s affected younger brother. The patient’s mother and maternal aunt were confirmed to be carriers.
FIG 3.
The patients’ NEMO mutation results in 2 aberrant mRNA products. A, Representation of normal NEMO splicing. *Patient’s mutation. mRNA splicing using exon 1A, 1B, or 1C is depicted; arrows represent PCR primers used. B, Agarose gel showing control and patient NEMO RT-PCR products containing exons 1A, 1C, and 1B. C, Schematic of aberrant NEMO exon 1B transcripts in the patient.
Splice site mutation results in 2 alternatively spliced mRNA products in the patient
The splice site mutation in the patient and his brother would be expected to result in aberrant splicing. To test this hypothesis, we amplified the 3 potential PCR products arising from the splicing of exons 1A, 1B, and 1C to exon 2. Oligonucleotides corresponding to the extreme 18 bp at the 5′ end of each of exons 1A, 1B, and 1C were used as forward primers. The common reverse primer corresponded to the last 25 bp before the stop codon in exon 10 (Fig 1, A). Transcripts from exon 1D are liver-specific15 and were not analyzed. PCR products were amplified from cDNA isolated from EBV B-cell lines from the patient and a healthy control. When the exon 1A and 1C–specific primers were used, both patient and control template cDNA produced the normal size transcripts (Fig 3, B). When the exon 1B specific primer was used, a normal size exon 1B containing transcript of 1404 bp was amplified from the control cDNA. However, the patient cDNA instead gave rise to 2 aberrantly spliced transcripts 1748 bp and 1294 bp in size, respectively (Fig 3, B). The larger 1748-bp transcript starts at exon 1B, reads through the 1B–1C intron and exon 1C, then splices to exon 2. The smaller 1294-bp transcript uses an alternative splice site within exon 1B that splices to exon 2, resulting in an mRNA product with an internal deletion (Fig 3, C). Similar results were obtained by using cDNA from fibroblasts (data not shown). Introduction in 293 T cells and NEMO−/− mouse embryonic fibroblasts of constructs that encoded cDNA corresponding to the 1748 bp and 1294 bp transcripts under the control of the pCMV promoter resulted in the expression of normal size NEMO protein (see this article’s Fig E2 in the Online Repository at www.jacionline.org), demonstrating that these transcripts were potentially translated in the patient’s cells.
Impaired phosphorylation and degradation of IκBα in response to IL-1 and reduced NF-κB activation in patient cells
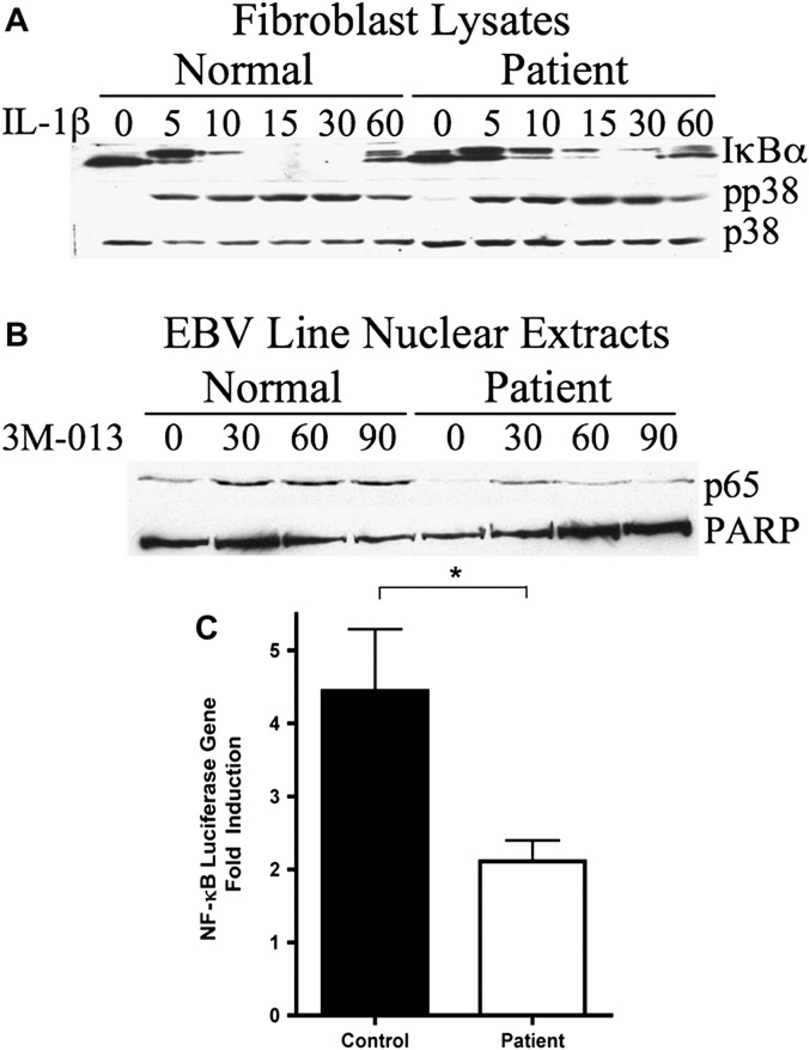
To assess the level of impairment of NF-κB signaling, primary fibroblasts from the patient were treated with IL-1β and IκBα phosphorylation and degradation were analyzed by Western blot (Fig 4, A). In normal fibroblasts, the majority of IκBα protein was phosphorylated after 5 minutes of IL-1β stimulation, and IκBα protein was completely degraded by 15 minutes. In contrast, in the patient’s fibroblasts, only about half of the IκBα was phosphorylated after 5 minutes of IL-1β stimulation, and there was still residual IκBα protein detected 15 and 30 minutes after stimulation. Western blotting with antiphospho–p38 mitogen-activated protein kinase demonstrates a comparable response of normal and patient fibroblasts to IL-1β stimulation.
FIG 4.
NF-κB signaling is reduced in the patient. A, Western blot of lysates from fibroblasts stimulated with IL-1β. B, Western blot of p65 nuclear translocation in TLR7-stimulated EBV B cells. C, NF-αB–luciferase reporter assay in fibroblasts treated 6 hours with IL-1β. Fold activation represents mean and SD of 8 experiments using patient fibroblasts and 3 healthy controls.
Incomplete IκBα degradation in response to receptor stimulation would lead to reduced NF-κB nuclear translocation. To assess NF-κB nuclear translocation, patient and control EBV B-cell lines were stimulated with the TLR7 ligand 3M-013, and nuclear extracts were prepared 30, 60, and 90 minutes after stimulation and Western blotted with an anti-p65 antibody. Western blot with anti-poly (ADP-ribose) polymerase (PARP) was used as a protein loading control for nuclear extracts. The results demonstrated reduced p65 nuclear translocation in the patient’s EBV cells in response to stimulation with TLR7 ligand (Fig 4, B).
To measure NF-κB activity, NF-κB luciferase assays were performed on normal and patient fibroblasts. Fibroblasts were transfected with NF-κB luciferase reporter plasmids and control Renilla plasmids. Cells were lysed after a 6-hour stimulation with IL-1β. Patient fibroblasts had 2.4 times less NF-κB activity after IL-1β stimulation compared with control fibroblasts (P = .0126; Fig 4, C).
DISCUSSION
We present a boy with immunodeficiency without ectodermal dysplasia associated with a novel splice site mutation in the 5′ UTR of the NEMO transcript. This mutation results in abnormally spliced NEMO mRNA species, a 4-fold decreased level of NEMO mRNA levels, and an 8-fold lower expression level of NEMO protein than in normal controls. To our knowledge, this is the first description of an immunodeficiency caused by inadequate levels of a normal NEMO protein, as opposed to a mutation that results in an altered protein with hypomorphic function.
The low NEMO protein levels in the patient led to reduced IκBα phosphorylation and degradation, resulting in decreased NF-κB function after IL-1 stimulation. Although the reduced NEMO expression and reduced NF-κB activation allowed normal ectodermal development, they resulted in impaired innate and adaptive immune functions, including low IgM and high IgA, typical of patients with hypomorphic NEMO mutations.10,16 Antibody production to protein and polysaccharide antigens was variably impaired. Importantly, specific antibody titers waned rapidly over time. B-cell and T-cell total numbers were normal, although the absolute number of memory B cells was low. The patient’s younger brother is also affected and has the same NEMO mutation. In addition, a male cousin of the patient had recurrent infections and died from atypical mycobacterial pneumonia. Although his NEMO gene was never sequenced, his mother (the patient’s maternal aunt) is a carrier of the mutation in the 5′ UTR of NEMO. Thus, most likely he had the same mutation as the index patient and had NEMO deficiency.
The mutation in the 5′ UTR of NEMO that we have described is unique because it results in the generation of abnormal size NEMO message, with low total NEMO mRNA, resulting in protein levels significantly lower than normal controls. Importantly, the reduction in NEMO protein levels appears greater than the reduction in the level of NEMO message, suggesting that translation of the abnormal size NEMO message may be relatively inefficient. The reduction in NEMO protein expression was associated with a 2.4-fold (60%) reduction in NF-κB activation, as measured by luciferase assay. This case demonstrates that a residual NF-κB activity of̃ 40% might be sufficient for ectodermal development; however, it results in both innate and adaptive immune dysfunction. This is consistent with the notion that immune function has a more stringent requirement for NF-κB function than does ectodermal development. We have demonstrated that NEMO expression is low in ectodermally derived cells (eg, fibroblasts) and mesenchymally derived cells (eg, PBMCs and EBV-transformed B cells) in our index patient. However, it is currently not known which NEMO transcripts are used during ectodermal development. It remains possible that other isoforms may be used that would circumvent the aberrant 1B transcripts and allow normal ectodermal development.
The 8-fold reduction in expression of a normal NEMO protein resulted in a reproducible, modestly reduced degradation of IκBα in IL-1–stimulated fibroblasts, likely because of inefficient activation of the IKK complex, relative to normal fibroblasts (Fig 4, A). The modestly reduced degradation of IκBα, however, resulted in significant impairment in TLR-induced TNF-α production (Fig 1). Although we did not examine the effects of reduced NEMO expression on IL-1–induced degradation of IκBβ and IκBε, degradation of these inhibitors of NF-κB would be expected to be similarly impaired. Therefore, the effect of reduced NEMO expression on NF-κB–dependent functions would be a result of impaired disinhibition of IκBα, IκBβ, and IκBε.
The case we present indicates that in male patients with dysgammaglobulinemia and recurrent sinopulmonary infections, but with normal ectodermal development and a normal NEMO coding sequence, evaluation of NEMO mRNA and protein levels and of NF-κB–dependent immune responses (eg, TLR function) is essential. Abnormal expression of NEMO should then be followed by analysis of the 5′ and 3′ untranslated regions.
Clinical implications: In male patients with a clinical presentation consistent with impaired NF-κB function but normal NEMO coding sequence, analysis of the untranslated regions of the NEMO gene may be informative.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grants AI076210 (to R.S.G.) and AI076625 (to D.R.M.). J.L.M. is supported by the Stern Family Fund at Children’s Hospital Boston.
We thank Dr Michel Masaad for useful discussions.
Abbreviations used
- EDID
Ectodermal dysplasia associated with immune deficiency
- IκB
Inhibitor of nuclear factor κB
- IKK
Inhibitor of nuclear factor-κB kinase complex
- NEMO
Nuclear factor-κB essential modifier
- NF-κB
Nuclear factor-κB
- TLR
Toll-like receptor
- UTR
Untranslated region
Footnotes
Disclosure of potential conflict of interest: R. S. Geha has received research support from the National Institutes of Health and the March of Dimes. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 3.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 5.Cui CY, Schlessinger D. EDA signaling and skin appendage development. Cell Cycle. 2006;5:2477–2483. doi: 10.4161/cc.5.21.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Curr Opin Immunol. 2004;16:34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, et al. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 9.Ku CL, Yang K, Bustamante J, Puel A, von Bernuth H, Santos OF, et al. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol Rev. 2005;203:10–20. doi: 10.1111/j.0105-2896.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niehues T, Reichenbach J, Neubert J, Gudowius S, Puel A, Horneff G, et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Puel A, Reichenbach J, Bustamante J, Ku CL, Feinberg J, Doffinger R, et al. The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation. Am J Hum Genet. 2006;78:691–701. doi: 10.1086/501532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orange JS, Levy O, Brodeur SR, Krzewski K, Roy RM, Niemela JE, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 14.McDonald DR, Brown D, Bonilla FA, Geha RS. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J Allergy Clin Immunol. 2006;118:1357–1362. doi: 10.1016/j.jaci.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Fusco F, Mercadante V, Miano MG, Ursini MV. Multiple regulatory regions and tissue-specific transcription initiation mediate the expression of NEMO/IKK-gamma gene. Gene. 2006;383:99–107. doi: 10.1016/j.gene.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2003;203:21–37. doi: 10.1111/j.0105-2896.2005.00221.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.