Abstract
In the free-living nematode Caenorhabditis elegans, cilia are found on the dendritic endings of sensory neurons. C. elegans cilia are classified as `primary' or `sensory' according to the `9+0' axonemal ultrastiucture (nine doublet outer microtubules with no central microtubule pair) and lack of motility, characteristics of `9+2' cilia. The C. elegans ciliated nervous system allows the animal to perceive environmental stimuli and make appropriate developmental, physiological, and behavioral decisions. In vertebrates, the biological significance of primary cilia had been largely neglected. Recent findings have placed primary/sensory cilia in the center of cellular signaling and developmental processes. Studies using genetic model organisms such as C. elegans identified the link between ciliary dysfunction and human ciliopathies. Future studies in the worm will address important basic questions regarding ciliary development, morphogenesis, specialization, and signaling functions.
Keywords: Sensory, Cilia, C. elegans, Receptor Trafficking, Primary Cilia, Neuron, Behavior, Autosomal dominant polycystic kidney disease, Ciliopathy, Review
2. INTRODUCTION
In the nematode C. elegans, cilia are found on dendritic endings of sensory neurons. Ciliated sensory neurons located in the head and tail sense an extensive variety of extracellular and internal signals and mediate a wide spectrum of behaviors. In the wild, C. elegans lives at the water-soil interface, and must be able to navigate a complex environmental milieu. For example, animals must chemotax to attractive food sources while avoiding toxic substances. C. elegans cilia are sensory and nonmotile, with varieties in structure reflecting diversified sensory functions. Of the 302 neurons in the hermaphrodite, 60 have dendritic endings that terminate in cilia (1–5). The male possesses an additional 48 ciliated neurons (6). Interestingly, many of the genes required for the formation, maintenance, morphogenesis, or function of C. elegans cilia have human counterparts which, when mutated, cause diseases that present with cystic kidneys, including autosomal dominant polycystic kidney disease (ADPKD), Bardet-Biedl Syndrome (BBS), Meckel Gruber Syndrome (MKS), and Nephronophthisis (NPHP) (Table 1, (7, 8)).
Table 1.
C. elegans homologs of human ciliary disease genes are expressed in sensory neurons, localize to the cilium or transition zone, and have mutant phenotypes consistent with defects in ciliogenesis or sensory transduction
| Expression/Function | ADPKD | BBS | NPHP |
|---|---|---|---|
| GFP expression pattern in ciliated nervous system: | Subset: Male only | All/most | All/most |
|
| |||
| Protein localization in cilium: | tz, cm | tz, ax | tz |
|
| |||
| Mutant defects: | |||
|
| |||
| Osmotic Avoidance (Osm) | WT | √ | √ |
|
| |||
| Olfaction (Odr) | WT | √ | √ |
|
| |||
| Mating: Response (Rsp) | √ | WT | √ |
| Location of vulva (Lov) | √ | WT | WT |
|
| |||
| GFP-tagged PKD-2 ciliary localization | √ | WT | √ |
|
| |||
| Lipid Storage | N.D. | √ | N.D. |
tz, transition zone; ax, ciliary axoneme; cm, ciliary membrane; N.D., not determined; WT, wild type; √, defective, ADPKD homologs are PKD1 = lov-1; PKD2 = pkd-2; MKS homologs are MKS1 = mks-1 orxbx-7 (Efimenko et al., 2005); MKS3 = mks-3 (cosmid number F35D2.4); BBS homologs are BBS1 = bbs-1 (Y105E8A.5); BBS2 = bbs-2 (F20D12.3); BBS3 = bbs-3 (C38D4.8); BBS4 = bbs-4 (F58A4.14); BBS5 = bbs-5 (R01H10.6); BBS7 = bbs-7 (Y75B8A.12); BBS8 = bbs-8 (T25F10.5); BBS9 = bbs-9 (C48B6.8); NPHP gene homologs are NPHP1 = nphp-1; NPHP2/inversin = Y32G9A.6, NPHP4 = nphp-4. The C. elegans genome does not possess homologs to all human ciliopathy genes. For example, there is no C. elegans counterpart of the human ARPKD gene product fibrocystin. For a comprehensive list of references, readers are directed to these reviews (7, 8).
Vertebrate primary cilia are best known for their sensory roles. In the visual system, the connecting cilium between the outer segment and the cell body in rod and cone cell is a modified primary cilium. Olfactory neurons possess primary cilia that are endowed with G protein coupled receptors (GPCRs) and signaling molecules. In the inner ear, the microtubule based kinocilium is connected to actin-based stereocilia via tip links. Primary cilia are also located on many non-dividing cells, and very recently have been shown to have important roles in physiology and development (9, 10). The wide range of primary cilia function is reflected by diversity in morphology and molecular components of each cilium type. While the same basic intraflagellar transport (IFT) machinery constructs all cilia, the mechanisms contributing to ciliary diversity are poorly understood.
3. ANATOMY OF THE C. ELEGANS CILIATED NERVOUS SYSTEM
The majority of the ciliated sensory neurons are concentrated in the head of the worm. From the nerve ring, dendrites lead to the nose, where cilia reside (Figure 1). Some cilia are exposed to the environment whereas the others are embedded in structural cells or the cuticle. Based on ultrastructural anatomy, the former are putative chemosensors while the latter likely function in a mechanosensory capacity.
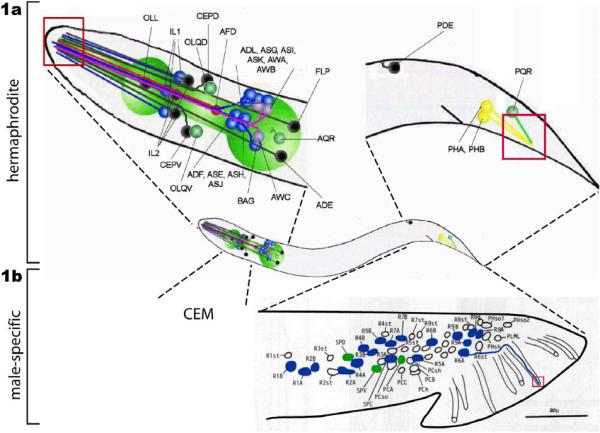
Figure 1.
C. elegans ciliated sensory neurons. la. Ciliated sensory neurons are concentrated in the hermaphrodite head. This image is a lateral view from the left side. The core (non-sex specific) neuronal cell bodies are indicated with the names. The locations of ciliary endings are indicated as red box. ADL, ASG, ASI, ASK, ADF, ASE, ASH, and ASJ are amphid channel neurons. AWA, AWB, and AWC are amphid wing neurons. ADE, anterior deirid; AED, amphid finger; CEPD, cephalic dorsal; CEPV, cephalic ventral; IL1 and IL2, inner labial 1 and 2; OLL, outer labial lateral; OLQD, outer labial quadrant dorsal; OLQV, OLQ ventral; PDE, posterior deirid; PHA/PHB, phasmid A/B. This cartoon is adapted from the Wormbook chapter (Inglis et al.: The sensory cilia of C. elegans, (2007)) with permission, 1b. The male possesses additional ciliated CEMs neurons in the head (not shown). The rest of male-specific ciliated sensory neurons are located in the tail: ray RnA/RnB (n=1–9, blue), spicule (SPV and SPD, green), p.c.s (PCA, green), and hook HOA/HOB (not shown). In this figure, the positions of left nuclei are shown. R6B dendritic process is drawn (blue line) from the cell body to the ray tip, where the cilium resides (red box). CEM, cephalic male; PCA/PCB/PCC, postcloacal; PCso; postcloacal socket; PCh, postcloacal hypodermal; PCsh; postcloacal sheath; PLML, posterior lateral microtubule left, RnA/RnB, ray neuron A/B (n=1–9); Rnst; ray structural; SPC/SPD/SPV; spicule neurons; The schematics of other dendrites are similar. Reproduced from (6) Copyright (1980), with permission from Elsevier.
C. elegans sensilla are comprised of glia-like structural cells (sheath and socket) and ciliated neurons. Both the sheath and socket cell encapsulate the dendrite (s) of ciliated neurons, but the socket cell lies more distally and often has an opening to the exterior (1, 11). The sheath and socket cells are responsible for dendrite extension to nose tip, dendritic pathfinding, tubulogenesis, and axon guidance (12–14).
The major sensory organs in the head are amphid sensilla which are comprised of a left and right pair of 12 neurons that can be divided into three different subtypes. The amphid channel neurons, of which there are eight, possess a simple `rod like' ciliary structure and deliver chemosensory and mechanosensory functions (Figure 2a, details discussed below). The three pairs of amphid wing neurons (AWA, AWB, AWC) display an elaborate structure and are embedded in the sheath cell. The amphid wing neurons constitute the olfactory organ that sense many volatile odorants. One pair of amphid finger (AFD) neurons detects temperature and has a short cilium with many finger-like microvilli.
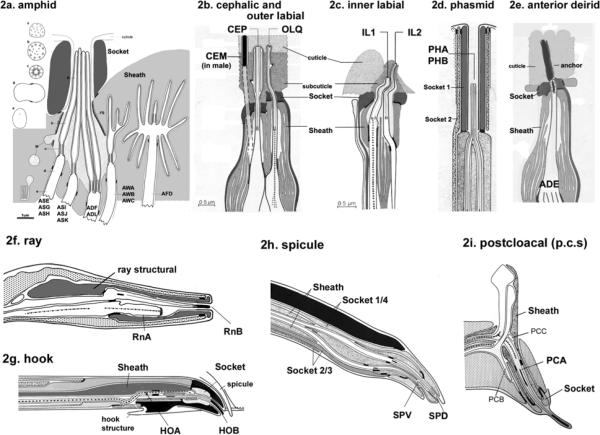
Figure 2.
Ultrastiucture of C. elegans cilia. 2a. Cilia in the amphid sensillum exhibit a variety of morphologies. The rod-like channel cilia are found in ASE, ASG, ASH, ASI, ASJ, ASK, ADF, and ADL neurons. ADF and ADL possess two cilia each, while the other cells possess a single cilium. These cilia are exposed to the environment through the cuticle. The amphid wing neurons (AWA, AWB, AWC) have complex ciliary structures. The AFD neuron possesses multiple villi. The sheath and socket cell encapsulate the amphid channel cilia, whereas the sheath cell encloses the amphid wing (AWA/AWB/AWC) and AFD cilia. Cross section views are shown on the left (a–e). This is modified from (4), Copyright (1986), with permission from Elsevier. . 2b. The cephalic and outer labial sensillum. In the male, the CEM neuron is also located in the cephalic sensillum. 2c. The inner labial sensillium contains IL1 and IL2 neurons. IL2 is embedded within subcuticular structure, while IL1 is exposed to the exterior. 2b and 2c were modified from (1) Copyright (1975, Ward et al) with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. 2d. The phasmid sensillum in the tail. The PHA and PHB cilia extend in parallel to each other. Two phasmid socket cells (socket 1 and 2) surround the sensillum. 2e. The anterior deidrid ADE cilia. The image was provided by Sam Ward. 2f. Each ray sensillum contains a pair of cilia: RnA and RnB. RnA cilia are embedded whereas RnB (except R6B) cilia are exposed. The ray structural cell functions as both socket and sheath cell. 2g. The hook sensillum encloses HOA (embedded) and HOB (exposed). 2h. The spicule sensillum has SPV and SPD ciliated neurons, four syncytial socket cells, and two syncytial sheath cells. 2i. The postcloacal sensillum (p.c.s.) possesses one ciliated neuron (PCA), which ciliary tip ends within the cuticle. 2d and 2f–2i. Reproduced from (6) Copyright (1980), with permission from Elsevier.
The cephalic (CEP) and outer labial quadrant (OLQ) sensilla exist in four sets in the head, constituting largely mechanosensory capacity. The paired outer labial lateral (OLL) sensilla possess a single cilium. Cilia of CEP, OLQ, and OLL reside beneath the cuticle (Figure 2b). At the apex of nose-tip, there are six inner labial sensilla each containing two inner labial neurons (IL1 and IL2). The IL1 cilia are embedded and involved in mechanosensation while IL2 cilia are exposed and proposed to be chemosensory (Figure 2c). Lastly, there are two other ciliated mechanosensory neurons BAG and FLP on the lateral tips of the head. In the tail, two phasmid neurons (PHA and PHB) on each side (left and right) have exposed cilia that are similar in structure to the single rod-like amphid channel cilia (Figure 2d). Most of amphid channel and phasmid neurons fill with fluorescent dye (Dye-filling, Figure 4a, detailed discussion later). When the worm encounters a chemical repellent such as SDS, phasmid and amphid neurons act antagonistically to regulate reversals required for escape behaviors (15). A subcuticular mechanosensary cilium is found on each of four lateral deirid ADE and PDE neurons (Figure 2e). In AQR and PQR neurons, a rudimentary cilium is exposed to the pseudocoelomic body fluid and involved in oxygen sensation and social feeding behavior (16).
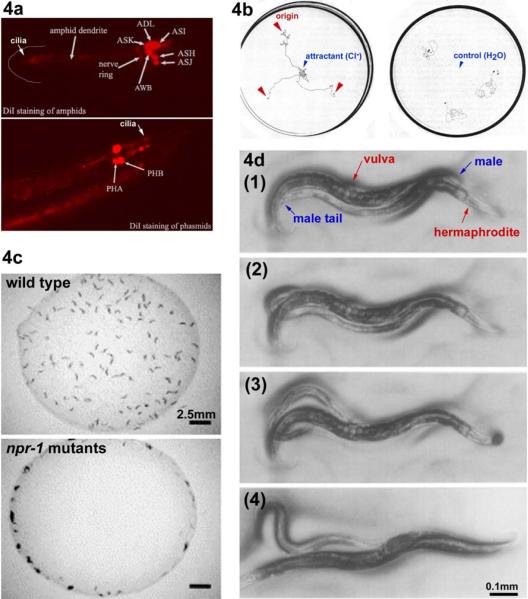
Figure 4.
Assays for C. elegans ciliated neurons. 4a. Dye-filling assay. When worms are soaked in a lipophilic dye such as Dil, FITC, and DiO, certain ciliated sensory neurons fill with the dye. In this picture, Dil-filling in amphid and phasmid neurons are shown. This is adapted from Wormbook chapter (Shaham, Methods in Cell Biology (2005)) with permission. The original image is provided by Zeynep Altun, www.wormatlas.org. 4b. Chemotaxis. Worms are attracted to the Cl− gradient towards the center of the plate. The worm tracks from the three origins indicated by the red arrowheads (left panel). When water is placed in the center as control, worms do not exhibit chemoattraction behavior (right panel). These images are modified from Ward et al, (1973). 4c. Social feeding. The laboratory standard strain N2 is a solitary feeder (top panel), whereas a natural isolate CB4856 and npr-1 N2 mutants are social feeders (bottom panel). Social feeders also aggregate at the border of a bacterial lawn. These images are modified (61) Copyright (1998), with permission from Elsevier. 4d. Male Mating. (1) A male exhibits `response' behavior by starting a backward movement with his ventral tail on the hermaphrodite body. (2) While continuing backing, he turns at the end of the body. In search of the vulva, his tail scans along the hermaphrodite. (3, 4) At the vulva, he stops backing and adjusts the precise location by fine back and forth movements to insert spicules (vulva location and spicule insertion). These images are reproduced from (20) Copyright (1995), with permission from Elsevier.
The C. elegans males possess 87 additional male-specific neurons (17), of which 48 are ultrastructurally confirmed to be ciliated (Figure 1b). The four CEMs are the only male-specific neurons in the head, terminating in exposed cilia at the nose-tip. The CEMs neurons are responsible for chemo-attraction toward hermaphrodite-and female-driven cues (18, 19). With the exception of the CEM head neurons, these male-specific sensory neurons are located in the tail, which mediates male copulatory behaviors. In male tail, each of nine bilateral ray sensilla (numbered 1–9 from anterior to posterior) has a pair of cilia: RnA and RnB (n=number of the ray). The encapsulated A-type cilium lays side-by-side to the exposed (except for R6B) B-type cilium (Figure 2f). Ray neurons are required for a male to respond to contact with a potential mate and to turn at the end of the mate's body (20). A similar pairing of two cilia is observed in the hook sensillum, composed of HOA and HOB neurons. Similarly, HOB cilia are exposed whereas HOA are not (Figure 2g). Ablation of either hook neuron specifically abrogates location of vulva behavior during copulation, resulting in the location of vulva (Lov) defective phenotype.
The SPV and SPD spicule neurons are ciliated, exposed to the environment and proposed to sense sensory cues from the mate's uterus (Figure 2h, (20, 21)). The postcloacal sensilla (p.c.s.) are comprised of a left-right arrangement of three neurons, of which one (PCA) pair is ciliated (Figure 2i). The p.c.s. acts in concert with the spicule neurons to regulate vulva prodding, spicule insertion, and sperm transfer into mate's uterus (20, 22).
Previous research on C. elegans anatomy, invariant cell lineage, and neuronal circuits has provided valuable tool sets for studying neuronal development and function. However, this `simple' nervous system is capable of generating sophisticated behaviors. For example, the C. elegans nervous system exhibits plasticity as well as gender-specific differences between the hermaphrodite and male. The male wiring project (S.W. Emmons, M.Xu, and D.H.Hall, personal communication), which is actively in progress, will provide insights into how the core and male-specific neurons are specified and connected to govern diverse behaviors.
4. CILIA DEVELOPMENT – INTRAFLAGELLAR TRANSPORT
C. elegans cilia are built by an evolutionary conserved mechanism called Intraflagellar transport (IFT) (Figure 3). The IFT machinery is driven by the anterograde motor Kinesin-2 and the retrograde motor cytoplasmic dynein-2 (23). Associated with the IFT machinery are IFT subcomplex A, IFT-subcomplex B, IFT regulators, and cargos. First identified in the green algae Chlamydomonas, IFT process is essential for ciliogenesis in every organism examined to date. C. elegans has been particularly useful for studying IFT using in vivo time lapse video microscopy with green fluorescent protein (GFP)-tagged IFT components in combination with powerful genetic tools (24).
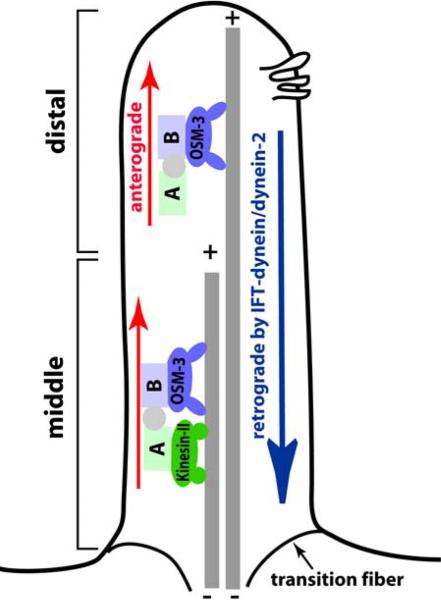
Figure 3.
Intraflagellar transport (IFT) builds cilia. A simplified cartoon of IFT process in C. elegans amphid channel cilia. Two anterograde motors (OSM-3 and Kinesin-II) move IFT complexes on the microtubule doublets in the 4 um-long middle segment. In the 2 um-long distal segment, OSM-3 motor acts alone. The cytoplasmic dynein-2 (IFT-dynein) brings the IFT complex back to the base of the cilium. The six transmembrane protein in the ciliary membrane is a representative of sensory receptors in the cilium.
In C. elegans, the amphid channel cilia are divided into three ultrastructurally distinct segments: proximal, middle, and distal (Figure 3, (4)). The proximal segment, or “transition zone” is a modified basal body approximately 1 um in length and the place where IFT proteins accumulate for transport into the cilium proper (25). The 4 um-long middle segment of the cilium contains doublets of microtubules, whereas the 2 um long distal segment encloses singlets. In amphid channel neurons, two types of Kinesin-2 motors (homodimeric OSM-3 and heterotrimeric Kinesin-II) function redundantly to construct the middle segment of the cilium, while OSM-3 acts alone to build the distal segment. Recent studies using C. elegans genetics and comparative genomics have identified a myriad of components acting in the IFT process (26). For an extensive summary on IFT and cilia, readers are directed to recent reviews (24, 27).
Although the IFT process builds every cilium, specialization is essential for both ciliary structure and function. Specialization of C. elegans sensory cilia occurs through a number of ways, including modulation of the IFT process (28–31), development of different ciliary structures (compare rod-like channel and finger-like wing cilia in Figure 1, (4)), and expression of a distinct set of sensory receptors and signaling molecules (32–35). C. elegans sensory cilia provide a unique and valuable tool to understand how ciliogenesis diverges from the core IFT process to produce functional diversity within the nervous system.
5. C. ELEGANS SENSORY ROLES OF CILIA
C. elegans continuously perceives its surroundings and adjusts its behaviors in order to survive its natural environment. In the laboratory, C. elegans exhibits a variety of sensory behaviors, including the drive for food, attraction/repulsion to chemicals, pursuit of mates, search for comfortable temperature, and entry or exit from the alternative dauer developmental stage. These sensory behaviors are mediated by cilia. Mutants with severe ciliary formation defects exhibit defects in most, if not all, sensory behaviors. One of the advantages of using C. elegans is genetic amenability that includes the hermaphroditic life cycle and ease in which to perform forward genetic screens (36–39). Genetics combined with the complete wiring diagram of the animal, electrophysiology, and in vivo optical imaging of calcium transients enables dissection of neural circuitries that control sensory behaviors (3, 40, 41). This approach yielded many genes acting in ciliary development and sensory neuron function. In this section, we will discuss how C. elegans interprets its surroundings into behaviors.
5.1. Chemosensation
The simple soil living nematode C. elegans developed a fine taste of likes and dislikes among chemicals. Approximately 5% of the C. elegans genome is devoted to chemical recognition (42, 43). Some chemicals, whether soluble or volatile, are attractive while others are repulsive. When there is a gradient of an attractant on a plate, worms move towards the source (Figure 4b). Chemical cues also elicit escape behaviors, changes in locomotion, and trigger both developmental and physiological process.
Genetic screens identified different types of chemosensory mutants including che (chemotaxis towards Na+ and Cl− defective), daf (dauer formation defective), osm (osmotic avoidance defective), odr (odorant response defective), and tax (general chemotaxis defective). A subset of amphid and phasmid ciliated neurons fills with lipophilic fluorescent dyes such as FITC and Dil (Figure 4a). Using this assay, Dyf (dye-filling defective) mutants were isolated (4,26,44). Although the exact mechanism of dye-filling is unknown, it is generally assumed that the exposed cilium takes up the dye from the outside and stains the entire neuron. Most of Dyf mutants exhibit defects both in general ciliogenesis and chemotaxis. However, it is worth noting that worms expressing a constitutively active form of GPA-3 (a G alpha subunit) are Dyf without apparent ciliogenesis defects (45). In contrast, Kinesin-II kap-1 and klp-11 mutants are non-Dyf but have defects in IFT and ciliary morphogenesis (28–30, 46). Using relatively simple assays (such as dye-filling, chemotaxis, and osmotic avoidance), C. elegans geneticists have identified a wealth of mutants with defects in ciliary structure and function ((4, 26, 44), J. Hu and M.M.Barr personal communication). From these screens, there remain many uncloned loci, hinting that far more molecular mechanisms in ciliated sensory neurons remain to be discovered.
Cloning of che, daf, osm, odr, and tax mutations have revealed two major categories of genes. First, genes required for general ciliogenesis such as the IFT machinery were identified. In this category, most, if not all, ciliogenesis is abrogated, resulting in stunted/malformed cilia that are functionally compromised. The second class includes genes encoding cell-type specific membrane receptors and downstream signaling molecules. Mutations in this class do not preclude general ciliogenesis, but cause specific cilium structural and/or sensory defects. For example, mutant phenotypes in Odr and some Daf mutants are restricted to a subset of chemosensory defects. This category will be discussed in a later section.
Extensive studies on molecular mechanisms of IFT regulation revealed functional modules acting in C. elegans amphid channel cilia (26). In addition to the core IFT-A and IFT-B that were originally isolated via biochemical approaches in Chlamydomonas (47–50), BBS (Bardet-Biedl syndrome) proteins (51), anterograde motor Kinesin-II and OSM-3 (52), the retrograde motor dynein-II complex, and linkers between modules were identified (46, 53–55). Furthermore, previously known chemotaxis mutants fall into one of the functional modules by genetic and phenotypic analysis of IFT in amphid cilia (26). In contrast, cell-type specific regulation of IFT is not well understood. It will be interesting to determine how cilia in sensory neurons in C. elegans are specialized for their function in terms of IFT regulation as well as receptor-signaling pathway aspects.
5. 2. Mcchanoscnsation
C. elegans responds to various types of mechanical stimuli, including nose touch, viscosity of the bacterial food lawn, and mating cues. Among these, light nose touch avoidance behavior requires ciliated neurons evoking backward movement upon encountering an obstacle (an eyelash in assays) at the nose tip.
Nose touch response is mediated by three sets of ciliated head neurons in the head: two ASHs, four OLQs, and two FLPs. Although these neurons act in parallel in nose touch response, each neuron mediates a fraction of the behavior: ASH 45%, FLP 29%, and OLQ 5% (56). The ASH, an amphid channel neuron, is a master nociceptive neuron in C. elegans. As a polymodal neuron, ASH has an exposed cilium and senses various repulsive signals, such as nose touch (56), high osmolarity (57), and noxious chemicals (32, 58). All of these stimuli evoke the same simple behavior: a rapid backward movement to reverse directions. Unlike ASH cilia, OLQ and FLP cilia are encased in the cuticle.
A worm slows down when entering a bacterial lawn. The basal slowing response requires four CEPs, two ADEs, and two PDE neurons (59). In these neurons, cilia are embedded in the cuticle: CEP cilia at the nose tip, ADE and PDE cilia in the anterior and posterior deirid sensilla along the body. Well-fed worms entering a bacterial lawn sense the changes in viscosity by these subcuticular cilia, resulting in the reduction of locomotory rate. This basal slowing response is dependent on dopamine. Consistent with this observation, CEP, ADE, and PDE are the only dopaminergic neurons in the hermaphroditic nervous system. C. elegans also exhibits a characteristic head movement, called foraging behavior. When encountered by an object during foraging, worms show a rapid head withdrawal reflex, which is mediated by four OLQ and six IL1 head neurons. Additionally, OLQ and IL1 neurons regulate the rate of spontaneous foraging (60).
5.3. Social Behavior
C. elegans social feeding behavior refers to aggregation on a bacterial lawn (Figure 4c). A natural isolate C. elegans strain CB4856 and neuropeptide receptor npr-1 mutant of the laboratory standard N2 strain exhibit social feeding behavior. The social feeders also accumulate at the edge of the bacterial lawn, which is referred as bordering (61). The social behavior is mediated by integration of information from nociceptive head neurons, oxygen levels, and food (16, 61–63). In ASH and ADL ciliated neurons, TRPV channels OSM-9 and OCR-2 sense the signals to promote social feeding and bordering. This activity, interestingly, is suppressed by other ciliated neurons, suggesting involvement of multiple sensory inputs (63).
Oxygen sensation by AQR, PQR (ciliated), and URX (non-ciliated) neurons is also required for social feeding and bordering (16, 62, 63). Supposedly, environmental oxygen levels are reflected in the pseudocoelomic body fluid, to which the AQR and PQR cilia are exposed. In these neurons, the soluble guanylate cyclase GCY-35 functions as an oxygen sensor by directly binding to molecular oxygen. The downstream cyclic GMP channels TAX-2/TAX-4 promote the AQR/PQR/URX neuronal activity, which is suppressed by NPR-1 (64).
5.4. Complex behaviors: male copulation
The C. elegans mating behavior is largely one-sided; males are obsessed with pursuing self-fertile and disinterested hermaphrodites. However, the adult hermaphrodite does provide chemical and mechanical cues to the male. Accordingly, the male-specific nervous system is dedicated to male sexual behaviors (6,20,65). Male mating is the most complex C. elegans behavior. A typical male mating ritual can be divided into sub-steps, including [1] attraction to long- and short-range hermaphrodite chemical cues, [2] response to contact with the hermaphrodite, [3] backing and turning along the mate's body, [4] location of vulva, [5] copulatory spicule insertion into the vulva, and [6] sperm transfer to the uterus (Figure 4d). During this copulatory process, the male utilizes male-specific ciliated neurons (Figure 1b) to perceive both chemical and mechanical signals from the hermaphrodite.
5.4.1. Sexual attraction
The adult hermaphrodite provides chemical cues to attract the males. In a 'holding assay' with hermaphrodite-conditioned agar plates, only adult males exhibit frequent backward movement and linger in the conditioned part of the plate (66). Intact cilia are required for detection of this hermaphrodite cue. In another assay that measures sex drive, adult males leave a food source in search of a mate at a higher frequency than hermaphrodites or larval males, which prefer to stay in bacterial lawns (67). This male 'leaving behavior' is suppressed by a hermaphrodite on the food source, suggesting that short range chemical and mechanical cues are provided by hermaphrodites.
The male may detect long-range chemical cues from potential mates using four CEM male-specific head neurons. A potent female-derived pheromone from female-male species of Caenorhabditis (C. remand and C. sp.) is reported to attract C. elegans males (18). Killing the CEM neurons significantly diminishes male chemotaxis toward the female-derived pheromone, indicating that the CEMs are required for detecting chemical cues. The male specific ciliary receptors PKD-2 and LOV-1 are required for chemotaxis to the female pheromone (18). These data suggest that PKD-2 and LOV-1 receptors in CEM neuronal cilia act as chemosensors of the female-derived pheromone, indicating a multiplicity of pheromone receptors acting in the male nervous system. This is in contrast to the 'holding assay' where pkd-2 and lov-1 are not required (65). It will be interesting to define the differences in content and potency in hermaphrodite and female-derived chemical cues.
5.4.2. Response
When a male comes in contact with a potential mate, he ceases forward locomotion, places the ventral side of his tail onto the mate's body, and starts backing. This process is called `response' and is dependent on bilateral ray neurons (1 to 9) in the tail. Each ray process consists of a glia-like structural cell and two ciliated neurons, RnA and RnB (n=1–9) (Figure 2f). These three cells are encapsulated in the cylindrical cuticle (6). Dorsally open rays (numbered 1, 5, and 7) are responsible for response to dorsal contact with a potential mate. Ventrally open rays (numbered 2, 4, and 8) mediate response to ventral contact (20). The requirement of mechanosensation for response behavior seems obvious; however, response may also require short-range chemical cues from hermaphrodites (J. Wang and M.M.B., personal communication).
Mutants with general ciliogenesis defects (osm-1, osm-5, osm-6, and che-3) exhibit response, location of vulva, and premature sperm transfer defects (34, 49). Specific sensory defects without abnormal ciliogenesis were found in pkd-2, lov-1, and klp-6 mutants (34, 68, 69). PKD-2 and LOV-1 are the C. elegans homologues of human PKD2 and PKD1, encoding polycystin-2 and polycystin-1 respectively (Table 1). Polycystin-1/LOV-1 and -2/PKD-2 are members of the TRPP (transient receptor potential-polycystin) channel family (70). The polycystins may form a mechanosensitive channel complex on ciliary membrane in human renal epithelial cells and C. elegans sensory neurons, klp-6 encodes a Kinesin-3 and regulates GFP-tagged PKD-2 localization. As expected by specific behavioral defects in pkd-2, lov-1 and klp-6 mutants, the genes are expressed in a subset of male-specific neurons: CEMs, RnBs (1–5, 7–9), and HOB. Additionally, klp-6 is expressed in the core IL2 neurons, which have no known function in the C. elegans nervous system.
The C. elegans homologs of the Bardet-Biedl Syndrome and Nephronophthisis ciliopathy genes are expressed in male ciliated neurons (71, 72). However, bbs-7, osm-12, nphp-1, and nphp-4 single mutant males exhibit wild-type response and vulva location behavior (Table 1, 34, 72). nphp-1; nphp-4 double mutant males have a slight response defect (72). Unlike the IFT polypeptide and motor mutations, the bbs and nphp mutations do not disrupt PKD-2∷GFP ciliary localization (28, 31), perhaps explaining why these mutant males mate normally.
5.4.3. Location of vulva
While backing, the male tail scans along the hermaphrodite to search for the vulva. The hook sensillum, which encloses the hook B (HOB) and hook A (HOA) neuron (Figure 2g), is required for the initial stopping in the approximate region of the vulva (20). Further adjustments by small back and forth movements allow precise location of the vulva. This latter step is mediated by p.c.s. and spicules neurons (20). Cilia are found in the majority of sensory neurons involved during vulva location. The HOB, SPV, and SPD cilia open to the exterior, whereas the HOA and PCA cilia are encapsulated in the cuticle (6). Based on the ciliary ultrastructure of the male-specific neurons, both mechanosensation and chemosensation regulate vulva location.
5.4.4. Spicule insertion and Sperm transfer
The spicules are inserted into the vulva, into the uterus, followed by sperm transfer. Like other male copulatory behavioral steps, spicule insertion involves ciliated neurons. The hook and p.c.s. neurons coordinate the initial prodding of spicules (22). The p.c.s. and spicule neurons regulate spicule penetration and the SPV ciliated neurons inhibit premature sperm transfer (20). A seven transmembrane receptor sra-1 (serpentine receptor a) is expressed in ciliated spicule neurons, suggesting sensory role in spicule insertion or sperm transfer (32). Sperm transfer defects were observed in osm-5 general ciliogenesis mutants, indicating sensory inputs from cilia regulate ejaculation (49).
5.5. Thermosensation
The C. elegans thermotaxis behavior is extremely sensitive, experience dependent, and mediated by ciliated neurons. Worms crawl to their cultured temperature in a spatial temperature gradient within a mere 0.1 Celsius degree (38). In addition, animals show short-term and long-term adaptation to the surrounding temperature. Two AFD amphid ringer neurons in the head govern the sensory and adaptive aspects of thermotactic behaviors (73). The elaborate finger-like villi on the AFD ciliated neuron are embedded in amphid sheath cells. When severed at the middle of the AFD dendrite, only the distal part containing the sensory cilium and finger villi retained calcium response to temperature stimuli, suggesting that AFD sensory cilia confer thermal detection and adaptation (74).
ttx (thermotaxis defective) mutants were isolated from a genetic screen looking for athermotactic mutants (38). ttx-1, a homolog of otd/Otx, specifies AFD neuronal fate and function (75). ttx-4 encodes a protein kinase C (nPKC-epsilon/eta) and negatively regulates thermosensation function of AFD neurons, ttx-4 also act in signaling pathways in nociceptive ASH neurons and olfactory AWA and AWC neurons (76). This data suggests that a signaling molecule such as PKC may have a distinct function in different sets of neurons to regulate diverse sensory behaviors.
5.6. Adaptation
C. elegans exhibits a reduced chemotatic behavior after a prolonged exposure to a chemical. This adaptation occurs in AWC, ASE, and ASH sensory neurons (77–82). The AWC adaptation requires components in G-protein coupled receptor (GPCR) pathways, including beta-arrestin ARR-1, which is responsible for internalization of activated GPCR (83) and G alpha protein GOA-1 (82). The TRPV gene osm-9 and an uncloned gene adp-1 act both in AWC and ASE adaptation, suggesting these two neurons share a common genetic pathway for odorant and salt adaptation (78, 80, 84). The G gamma protein GPA-1 is involved in ASE and ASH adaptation (81, 85).
6. NEUROENDOCRINE SIGNALING
In yet another example of evolutionary conservation of function, ciliated neuron function and obesity are linked in C. elegans, mice, and humans (86, 87). In both worms and mammals, mutations in the tubby gene result in increased fat storage. The C. elegans tub-1 homolog is expressed in the ciliated nervous system, and a TUB-1∷GFP fusion protein moves along dendrites and cilia, tub-1 and bbs-1, the C. elegans ortholog of Bardet-Biedl Syndrome gene 1, act in the same genetic pathway to control lipid homeostasis (87). bbs-1 acts in nine classes of ciliated neurons (ASG, ASI, ASJ, ASK, AWB, AWC, AQR, PQR, URX) to send neuroendocrine signals, which in turn regulate intestinal fat storage. In addition to a role in regulating body fat content, tub-1 also regulates lifespan via a distinct mechanism (86). C. elegans cilium structure mutants are long-lived and shorter compared to wild-type animals (88, 89), however what ciliated neurons mediate lifespan and body size are not known.
7. MECHANISMS FOR CILIARY RECEPTOR LOCALIZATION
Each C. elegans ciliated neuron gains its functional specialty by expressing a combination of multiple sensory receptors and signaling molecules. Receptors in ciliated neurons include seven transmembrane receptors (ODR-10, STR-2, SRD-1, SRG-2), TRP channels (OSM-9, OCR-2, PKD-2, LOV-1), and cyclic nucleotidegated channels (TAX-2), which function to initiate sensory transduction. How receptor proteins are targeted to, distributed in, and removed from the cilium is an interesting and relatively open question. Work in C. elegans has revealed mechanisms for ciliary localization involves both general and cell-type specific factors.
General mechanisms for ciliary localization of receptors include a vesicular transport system mediated by clathrin coated vesicle adaptor protein-1 (AP-1) (Table 3). In unc-101 (C. elegans AP-1 mul subunit) mutants, GFP-tagged ODR-10, STR-1, STR-2, OSM-9, and PKD-2 are similarly mislocalized to the entire neurons, as opposed to the normal restricted pattern in the cilium and cell body of vastly different sets of ciliated sensory neurons (28, 90). In C. elegans neurons, the AP-1 complex appears to be required for packaging ciliary receptors into dendritic vesicles (90) as in polarized cells where AP-1 complex involves in protein sorting at the trans-Golgi network (91, 92). The link between vesicular trafficking and ciliary targeting has recently been shown in mammalian systems. The phosphatidylinositol-4-phosphate adaptor protein-2 (FAPP2) is required for formation of a barrier between the ciliary membrane and the apical plasma membrane in the Madin-Darby canine kidney (MDCK) cells. In the absence of FAPP2, vesicles accumulated at the base of cilia and the ciliogenesis was abrogated (93, 94). It will be interesting to determine whether this pathway operates as part of the general ciliary trafficking system in C. elegans sensory neurons.
Table 3.
Both general and cell-type specific factors regulate ciliary localization of sensory receptors
| Ciliary Receptors | Cell-type specific | General |
|---|---|---|
| GPCR ODR-10 & STR-2 | A novel transmembrane protein/ODR-4 Uncloned gene odr-8 | AP-1/UNC-101 |
| TRPV OSM-9 | TRPV partner OCR-2 (in ASH and AWA) | AP-1/UNC-101 IFT components1 |
| TRPP PKD-2 | TRPP partner LOV-1, Kinesin-3 KLP-6, Casein kinase-22 and calcineurin TAX-62, Endosomal proteins STAM-12 and Hrs2 |
IFT involvement may be indirect and the requirement in GPCR localization is not determined
The requirement of these factors in downregulating other ciliary receptors is not determined. For reference, please see the text.
In addition to the general vesicular transport system, cell-type specific factors play essential roles in ciliary localization of receptors (Table 3). In amphid wing neurons, a novel membrane protein ODR-4 and an uncloned gene odr-8 are required for ciliary localization of two seven transmembrane odorant receptors ODR-10 and STR-2. In odr-4 and odr-8 mutants, GFP-tagged ODR-10 and STR-2 are retained in the cell bodies of AWA and AWC neurons, respectively, and do not localize to cilia as in wild-type animals (95). odr-4 and odr-8 act as specific factors for ODR-10 and STR-2 localization; other ciliary receptors such as OSM-9, TAX-2, SRD-1, and SRG-2 localize normally in odr-4 and odr-8 mutant backgrounds (95). When ectopically expressed in other neurons, GFP-tagged ODR-10 is only partially dependent on odr-4 and odr-8 for ciliary localization.
The requirement of cell-type specific factors extends to transient receptor potential (TRP) channel localization in C. elegans. In addition to forming functional complexes, TRP channel subunits influence subcellular localization of the partners. Two TRPV (TRP-vanilloid) channels OSM-9 and OCR-2 depend on each other for their ciliary targeting in the AWA and ASH neurons where these TRPVs function in nociception (35). Similarly, two TRPP (TRP-polycystin) proteins PKD-2 and LOV-1 facilitate each other's ciliary targeting in male specific neurons required for male mating behaviors (28). The pkd-2 expressing neurons contain unidentified cell-type specific localizing factors for GFP-tagged PKD-2: when ectopically expressed in other neurons, unlike ODR-10 in the AWB neuron that normally do not express odr-10, PKD-2 is retained in the cell bodies (28). These data suggest that cell-type specific localizing factors differ in their requirement stringency, depending on ciliary receptors and neuronal cell types.
Once targeted to the ciliary region of the neuron, receptors encounter multifaceted mechanisms that regulate trafficking into, within, and out of the cilium (Figure 5). Within the cilium, the IFT process transports a GFP-tagged OSM-9 but not PKD-2, suggesting PKD-2 may be distributed in the cilium by an alternative pathway (69, 96). However, a small proportion of Chlamydomonas PKD-2 does move in flagella in an IFT-dependent manner (97), indicating that the majority of PKD-2 is tethered or indeed localized in an IFT-independent manner (69). Increased levels of GFP-tagged PKD-2 were observed in a number of mutant backgrounds, implicating that multiple molecular pathways are involved in downregulation of PKD-2 from the cilium (Figure 5b). These pathways include the Kinesin-3 KLP-6, IFT components, endosomal proteins STAM-1 (signal transduction adaptor molecule) and Hrs (hepatocyte growth factor regulated tyrosine kinase substrate) (28, 49, 69, 98). Posttranslational modifications also affect PKD-2 ciliary levels. STAM-1 and Hrs sort ubiquitinated PKD-2 to a lysosomal degradation pathway (98). CK2 (casein kinase 2) and TAX- 6 (the calcineurin) act antagonistically in cilia to regulate ciliary localization of PKD-2 by affecting phosphorylation status of PKD-2 (99). It is largely unknown if removal of sensory receptors from cilia via ubiquitination and/or phosphorylation is a general mechanism acting in C. elegans sensory neurons or conserved in other ciliated organisms.
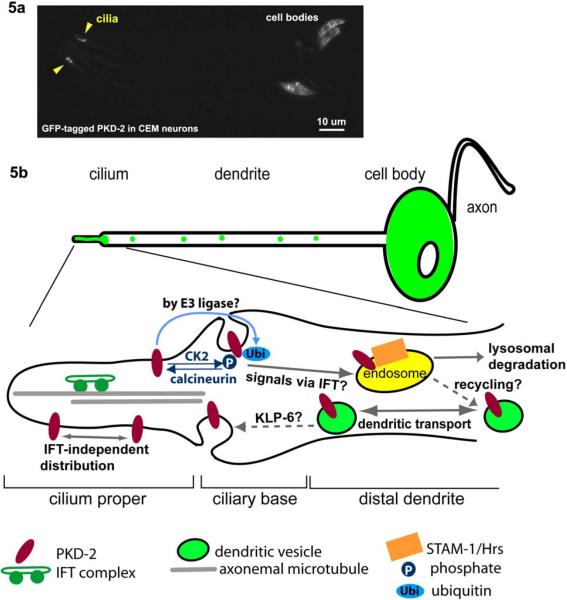
Figure 5.
Trafficking of ciliary receptors, a. A GFP-tagged PKD-2 localizes to ciliary endings and cell bodies of the male-specific CEM head neurons. Dendritic vesicles are not evident in this image due to the low exposure setting, b. A working model for PKD-2 trafficking at the ciliary region. PKD-2 containing vesicles are transported through the dendrite. At the ciliary base, PKD-2 is loaded onto the ciliary membrane followed by distribution by an IFT-independent manner. Phosphorylated and/or ubiquitinated PKD-2 proteins are readily removed from the cilium via endosomal STAM-1/Hrs complexes for lysosomal degradation. IFT-dependent signals and a Kinesin-3 KLP-6 may regulate PKD-2 levels in cilia, although the site of action is yet to be determined.
8. THE DAF-19 RFX TRANSCRIPTION FACTOR - THE MASTER REGULATOR OF CILIOGENESIS
daf-19 encodes a regulatory factor X (RFX)-type transcription factor that is expressed in all ciliated neurons (100). daf-19 mutants lack all cilia and are severely sensory defective (4). DAF-19 directly regulates ciliary gene expression via an X-box (XBX) promoter motif. A genome-wide search revealed 750 xbx gene candidates (101). Serial analysis of gene expression (SAGE), mRNA-tagging, and microarray methodologies identified numerous candidate ciliary genes (55, 102). Comparative genomics of the conserved X-box sequence of three different Caenorhabditis species (C. elegans, C. briggsae, and C. remand) identified 93 genes (103). DAF-19 targets include genes required for ciliary formation and morphogenesis, IFT, and sensory signaling, and orthologs of human ciliary disease genes, including the BBS, MKS (Meckel-Gruber Syndrome), and NPHP (Nephronophthisis) genes (55, 71, 101, 102, 104). daf-19 also indirectly regulates the transcription of the C. elegans ADPKD gene pkd-2 (105). RFX transcription factors also regulate ciliogenesis in Drosophila, zebrafish, and mice (106–109).
9. C. ELEGANS MODELS FOR HUMAN DISEASES OF CILIA
Cystic kidneys are one of the most common inherited human pathologies, and shared among several genetic disorders including autosomal dominant and autosomal recessive polycystic kidney disease (ADPKD and ARPKD), BBS, MKS, and NPHP (110, 111). The ciliary hypothesis of cystic kidney disease posits that gene products that are implicated in cystic kidney disease localize to the cilium or basal body (112). Studies in C. elegans were the first to link ADPKD to cilia (34), followed by a series of papers demonstrating that primary cilium localization and function of the ADPKD gene products are evolutionarily conserved (113–115). Since these initial findings, the cilium has piqued the interest of both basic biologists and clinicians.
C. elegans is the ultimate model system to study the formation, morphogenesis, and sensory functions of primary cilia at the genetic, molecular, cellular, and biological networks levels. The C. elegans genome contains many of the human ciliopathy disease genes (Table 1, (7, 8). The C. elegans ADPKD genes lov-1 and pkd-2 are required for the sensory functions of a subset of male-specific ciliated neurons (34, 68). The BBS genes bbs-7 and bbs-8 appear regulate IFT and ciliogenesis in amphid channel and phasmid cilia (51, 116). The MKS genes mks-1/xbx-7 and mks-3 possess an X-box in their promoters (55, 101), suggesting a broad role in the ciliated nervous system although physiological functions of the worm MKS homologs have not been reported. Likewise, the NPHP homologs nphp-1, nphp-2, and nphp-4 are expressed in the ciliated nervous system of the male and hermaphrodite (55, 72, 104, 117). nphp-1 and nphp-4 appear to play cell type specific roles in cilia formation, ciliary length control, and sensory signal transduction (31, 72, 104). The human ciliary proteome is comprised of a daunting 1,000 candidates, some of which represent human disease genes (118). By studying orthologous ciliary genes in multicellular animals such as C. elegans, ascertaining function and defining molecular networks is experimentally tractable.
10. CONCLUDING REMARKS
Only recently has research on sensory and signaling function of primary cilia been pursued throughout the evolutionary ladder. However, many significant and intriguing questions remain unanswered. In mammals, how are cilia in different organs specified in terms of structure and function? How does the IFT process coordinate its general and cell-type specific functions? How does the ciliary membrane differ from the plasma membrane? What mechanisms regulate the ciliary localization of sensory receptors? How do sensory receptors gain entry/exit to/from cilia? Studies from the nematode C. elegans will continue to be a valuable source to understand basic cell biology of ciliogenesis as well as to identify new genes responsible for causing ciliopathies.
Table 2.
Sensory function of ciliated neurons in C. elegans
| Nervous system | Sensillum | Neuron | Function | Dye-filling |
|---|---|---|---|---|
| Core | Amphid | ASE | Water-soluble chemotaxis, avoidance | - |
|
| ||||
| ASG | Dauer formation, lifespan, chemotaxis | - | ||
|
| ||||
| ASH | Nociception (osmotic avoidance, nose touch, chemorepulsion), social feeding | FITC, DiI | ||
|
| ||||
| ASI | Dauer formation, chemotaxis, navigation | FITC, DiI | ||
|
| ||||
| ASJ | Dauer recovery, chemotaxis, lifespan | FITC, DiI | ||
|
| ||||
| ASK | Avoidance, chemotaxis, lifespan, navigation | FITC, DiI | ||
|
| ||||
| ADF | Dauer formation, chemotaxis | FITC | ||
|
| ||||
| ADL | Avoidance, social feeding | FITC, DiI | ||
|
| ||||
| AWA | Volatile chemotaxis, lifespan | - | ||
|
| ||||
| AWB | Volatile avoidance | DiI | ||
|
| ||||
| AWC | Volatile chemotaxis, lifespan, navigation | - | ||
|
| ||||
| AFD | Thermosenation | - | ||
|
| ||||
| Cephalic | CEP | Mechanosensation (basal slowing response) | FITC1 | |
|
| ||||
| Outer labial | OLQ | Mechanosensation (basal slowing response and nose touch) | - | |
|
| ||||
| OLL | Unknown (presumably mechanosensory) | - | ||
|
| ||||
| Inner labial | IL1 | Mechanosensation (nose touch) | - | |
|
| ||||
| IL2 | Unknown (putative chemosensory) | DiI, DiO | ||
|
| ||||
| Phasmid | PHA, PHB | chemorepulsion | FITC, DiI | |
|
| ||||
| Deirid | ADE, PDE | Mechanosensation (basal slowing response) | FITC1 | |
|
| ||||
| AQR, PQR | Oxygen sensation, social feeding | - | ||
|
| ||||
| BAG | Unknown | - | ||
|
| ||||
| FLP | Mechanosensation (nose touch) | - | ||
|
| ||||
| Male-specific | Cephalic | CEM | Sexual attraction to pheromone | - |
|
| ||||
| Ray | RnA, RnB | Response and turning during mating | - | |
|
| ||||
| Hook | HOA, HOB | Vulva location, spicule prodding | - | |
|
| ||||
| Spicule | SPV | Inhibition of ejaculation | - | |
| SPD | Sperm transfer | - | ||
|
| ||||
| Postcloacal | PCA | Spicule prodding and insertion, sperm transfer | - | |
Column 1 refers to the “core” nervous system that is present in males and hermaphrodites or the “male-specific” nervous system that is exclusive to the male. FITC: fluorescein isothiocyanate; DiI: 1,1'-Dioctadecyl 3,3,3',3'-Tetramethylindocarbocyanine Perchlorate
occasionally dye fills with FITC. Detailed reference lists are included in Table 1.
11. ACKNOWLEDGEMENTS
We thank members of the Barr laboratory for sharing unpublished data. This work is supported by NIH, PKD Foundation, and March of Dimes.
12. REFERENCES
- 1.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–37. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 2.Ware RW, Clark DV, Crossland K, Russell RL. The nerve ring of the nematode Caenorhabditis elegans: Sensory input and motor output. J. Comp. Neurol. 1975;162:71–110. [Google Scholar]
- 3.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans: the mind of a worm. Phil. Trans. R. Soc. Lond. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 4.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–87. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 5.Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–76. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 7.Barr MM. Caenorhabditis elegans as a model to study renal development and disease: sexy cilia. J Am Soc Nephrol. 2005;16:305–12. doi: 10.1681/ASN.2004080645. [DOI] [PubMed] [Google Scholar]
- 8.Inglis PN, Guangshuo O, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. In: Moerman J. M. K. a. D. G., editor. WormBook. The C. elegans Research Community; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–42. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Marshall WF, Nonaka S. Cilia: tuning in to the cell's antenna. Curr Biol. 2006;16:R604–14. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Shaham S. Glia-neuron interactions in the nervous system of Caenorhabditis elegans. Curr Opin Neurobiol. 2006;16:522–8. doi: 10.1016/j.conb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 13.Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8:893–906. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 15.Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002;12:730–4. doi: 10.1016/s0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- 16.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marietta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–22. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 17.Barr MM, Garcia LR. Male mating behavior. In: Jorgensen EM, Kaplan JM, editors. Wormbook. The C. elegans Research Community; 2006. [Google Scholar]
- 18.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Nail Acad Sci USA. 2007;104:6730–5. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The Sensory Circuitry for Sexual Attraction in C. elegans Males. Current Biology. 2007;17:1–11. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu KS. Biology. California Institute of Technology; 1995. Male Mating Behavior in Caenorhabditis elegans. [Google Scholar]
- 22.Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell. 2001;107:777–88. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum J. Intraflagellar transport. Curr Biol. 2002;12:R125. doi: 10.1016/s0960-9822(02)00703-0. [DOI] [PubMed] [Google Scholar]
- 24.Scholey JM, Ou G, Snow J, Gunnarson A. Intraflagellar transport motors in Caenorhabditis elegans neurons. Biochem Soc Trans. 2004;32:682–4. doi: 10.1042/BST0320682. [DOI] [PubMed] [Google Scholar]
- 25.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–90. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 26.Ou G, Koga M, Blacque OE, Murayama T, Ohshima Y, Schafer JC, Li C, Yoder BK, Leroux MR, Scholey JM. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell. 2007;18:1554–69. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole DG. Intraflagellar transport: keeping the motors coordinated. Curr Biol. 2005;15:R798–801. doi: 10.1016/j.cub.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–70. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 29.Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;111:663–9. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 2007;26:2966–80. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jauregui AR, Nguyen KCQ, Hall DH, Barr MM. C. elegans nephrocystin-1 and nephrocystin-4 modulate cilia development and morphogenesis. 2007 in revision. [Google Scholar]
- 32.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–18. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 34.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–9. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 35.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 36.Dusenbery DB, Sheridan RE, Russell RL. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics. 1975;80:297–309. doi: 10.1093/genetics/80.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis JA, Hodgkin JA. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J Comp Neurol. 1977;172:489–510. doi: 10.1002/cne.901720306. [DOI] [PubMed] [Google Scholar]
- 38.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1975;72:4061–5. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorgensen EM, Mango SE. The art and design of genetic screens: Caenorhabditis elegans. Nat Rev Genet. 2002;3:356–69. doi: 10.1038/nrg794. [DOI] [PubMed] [Google Scholar]
- 40.Goodman MB, Hall DH, Avery L, Lockery SR. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–72. doi: 10.1016/s0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 2000;26:583–94. doi: 10.1016/s0896-6273(00)81196-4. [DOI] [PubMed] [Google Scholar]
- 42.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–33. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 43.Bargmann CI. Chemosensation in C. elegans. In: Jorgensen E, editor. WormBook. The C. elegans Research Community; 2006. [Google Scholar]
- 44.Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW, Collet J, Thomas JH, Riddle DL. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–88. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–27. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 47.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol. 2001;11:457–61. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- 50.Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development. 2001;128:1493–505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- 51.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–42. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shakir MA, Fukushige T, Yasuda H, Miwa J, Siddiqui SS. C. elegans osm-3 gene mediating osmotic avoidance behaviour encodes a kinesin-like protein. Neuroreport. 1993;4:891–4. doi: 10.1097/00001756-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–30. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wicks SR, de Vries CJ, van Luenen HG, Plasterk RH. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- 55.Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, McKay SJ, Huang P, Swoboda P, Jones SJ, Marra MA, Baillie DL, Moerman DG, Shaham S, Leroux MR. Functional genomics of the cilium, a sensory organelle. Curr Biol. 2005;15:935–41. doi: 10.1016/j.cub.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1993;90:2227–31. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–38. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- 58.Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. Embo J. 2004;23:1101–11. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 60.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–5. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 61.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–89. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 62.Coates JC, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–9. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- 63.de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung BH, Arellano-Carbajal F, Rybicki I, de Bono M. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr Biol. 2004;14:1105–11. doi: 10.1016/j.cub.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 65.Hodgkin J. Male phenotypes and mating efficiency in Caenorhabditis elegans. Geneitcs. 1983;103:43–64. doi: 10.1093/genetics/103.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:1598–603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24:7427–34. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–6. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 69.Peden EM, Barr MM. The KLP-6 Kinesin Is Required for Male Mating Behaviors and Polycystin Localization in Caenorhabditis elegans. Current Biology. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 70.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. Faseb J. 2004;18:740–2. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 71.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 72.Jauregui AR, Barr MM. Functional characterization of the C. elegans nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp Cell Res. 2005;305:333–42. doi: 10.1016/j.yexcr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–8. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 74.Clark DA, Biron D, Sengupta P, Samuel AD. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26:7444–51. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, Sengupta P. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–56. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 76.Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–37. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA. 1973;70:817–21. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–12. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 79.Saeki S, Yamamoto M, lino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204:1757–64. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 80.Jansen G, Weinkove D, Plasterk RH. The G-protein gamma subunit gpc-1 of the nematode C. elegans is involved in taste adaptation. Embo J. 2002;21:986–94. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuki M, Kunitomo H, Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103:1112–7. doi: 10.1073/pnas.0506954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmitessa A, Hess HA, Bany IA, Kim YM, Koelle MR, Benovic JL. Caenorhabditis elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J Biol Chem. 2005;280:24649–62. doi: 10.1074/jbc.M502637200. [DOI] [PubMed] [Google Scholar]
- 84.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hukema RK, Rademakers S, Dekkers MP, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–22. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA. C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab. 2005;2:35–42. doi: 10.1016/j.cmet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Polygenic control of Caenorhabditis elegans fat storage. Nat Genet. 2006;38:363–8. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- 88.Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–9. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- 89.Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- 90.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mul clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–87. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 91.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–98. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 92.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 93.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–61. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiter JF, Mostov K. Vesicle transport, cilium formation, and membrane specialization: the origins of a sensory organelle. Proc Natl Acad Sci U S A. 2006;103:18383–4. doi: 10.1073/pnas.0609324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–66. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 96.Qin H, Burnette DT, Bae Y-K, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar Transport Is Required for the Vectorial Movement of TRPV Channels in the Ciliary Membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 97.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and Dynamics of PKD2 in Chlamydomonas flagella. Journal of Cell Biology. 2007;179:501–14. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu J, Wittekind SG, Barr MM. STAM and Hrs Down-Regulate Ciliary TRP Receptors. Mol Biol Cell. 2007;18:3277–89. doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu J, Bae YK, Knobel KM, Barr MM. Casein Kinase II and Calcineurin Modulate TRPP Function and Ciliary Localization. Mol Biol Cell. 2006;172:663–9. doi: 10.1091/mbc.E05-10-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;5:411–21. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 101.Efimenko E, Bubb K, Mak HY, Holzman T, Leroux MR, Ruvkun G, Thomas JH, Swoboda P. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–1934. doi: 10.1242/dev.01775. [DOI] [PubMed] [Google Scholar]
- 102.Kunitomo H, Uesugi H, Kohara Y, Iino Y. Identification of ciliated sensory neuron-expressed genes in Caenorhabditis elegans using targeted pull-down of poly (A) tails. Genome Biol. 2005;6:R17. doi: 10.1186/gb-2005-6-2-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen N, Mah A, Blacque OE, Chu J, Phgora K, Bakhoum MW, Newbury CR, Khattra J, Chan S, Go A, Efimenko E, Johnsen R, Phirke P, Swoboda P, Marra M, Moerman DG, Leroux MR, Baillie DL, Stein LD. Identification of ciliary and ciliopathy genes in Caenorhabditis elegans through comparative genomics. Genome Biol. 2006;7:R126. doi: 10.1186/gb-2006-7-12-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118:5575–87. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- 105.Yu H, Pretot RF, Burglin TR, Sternberg PW. Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development. 2003 doi: 10.1242/dev.00678. [DOI] [PubMed] [Google Scholar]
- 106.Dubruille R, Laurencon A, Vandaele C, Shishido E, Coulon-Bublex M, Swoboda P, Couble P, Keman M, Durand B. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development. 2002;129:5487–98. doi: 10.1242/dev.00148. [DOI] [PubMed] [Google Scholar]
- 107.Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol. 2004;24:4417–27. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma M, Jiang YJ. Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007;3:el8. doi: 10.1371/journal.pgen.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–22. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 110.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease. Nat Rev Genet. 2005;6:928–40. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 111.Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 112.Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–6. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- 113.Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–5. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 114.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. JAm Soc Nephrol. 2002;13:2508–16. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 115.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 116.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 117.Wolf MT, Lee J, Panther F, Otto EA, Guan KL, Hildebrandt F. Expression and phenotype analysis of the nephrocystin-1 and nephrocystin-4 homologs in Caenorhabditis elegans. J Am Soc Nephrol. 2005;16:676–87. doi: 10.1681/ASN.2003121025. [DOI] [PubMed] [Google Scholar]
- 118.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–2. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]