Abstract
Polyphenols are bioactive natural products that appear to act against a wide range of pathologies. Mechanisms of activity have not been established, but recent studies have suggested that some polyphenols bind to membranes. We examined the interaction between lipid bilayers and three structurally diverse polyphenols. We hypothesized that features of the polyphenols such as polarity, molecular size, molecular geometry, and number and arrangement of phenol hydroxyl groups would determine the tendency to interact with the bilayer. We examined a mixed polyphenol, (−) epigallocatechin gallate (EGCg); a proanthocyanidin trimer comprising catechin-(4→8)-catechin-(4→8)-catechin (cat3); and a hydrolysable tannin, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG). These polyphenols were incorporated at different levels into 2H labeled 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) multi-lamellar vesicles (MLVs). 31P and 2H solid-state NMR experiments were performed to determine the dynamics of the headgroup region and the hydrophobic acyl chain region of the lipid bilayer upon addition of polyphenols. The chemical shift anisotropy (CSA) width of the 31P NMR spectra decreased upon addition of polyphenols. Addition of PGG induces a dramatic reduction on the CSA width compared with the control lipid bilayer sample, while addition of cat3 barely reduces the CSA width. The 2H quadupolar splitting of the lipids also decreased upon addition of polyphenols. At the same concentration, PGG substantially reduced the quadrupolar splitting while cat3 barely reduced it when compared with the control sample. By calculating the order parameters of the acyl chain region of the lipid bilayer, we concluded that the hydrophobic part of the lipid bilayer was perturbed by PGG while cat3 did not cause large perturbations. The data suggest that the polarity of the polyphenols affects the interaction between tannins and membranes. The interactions may relate to the biological activities of polyphenols.
Introduction
Polymeric polyphenols (tannins) are natural products found in virtually all dicotyledonous higher plants. The hallmark of this diverse group of compounds is their ability to interact strongly with proteins, yielding very stable and often insoluble complexes (1). Polyphenols are divided into three classes based on their monomer units: proanthocyanidins are comprised of flavan-3-ols, which may or may not be 3-galloylated, polymerized via C-C bonds; hydrolysable tannins are gallic acid esters of glucose; and the galloylated catechins are gallate esters of simple flavan-3-ols (2). Recent interest in polyphenols has been focused on their diverse bioactivities and their potential benefits as phytonutrients or pharmacological agents (3–5).
Neither the avid protein binding (1) nor the potent antioxidant activities of polyphenols (6) adequately account for all of the bioactivities noted for polyphenols. Some recent studies of the galloylated catechins have suggested that this class of polyphenols may interact with membranes (7–17), and that those interactions may be the basis for some polyphenol bioactivities. For example, it has been suggested that the anti-bacterial effect of catechin and its derivatives may be a consequence of increased membrane fluidity induced by the polyphenols (13). Catechin and its derivatives may adsorb to membrane surfaces and insert into the lipid bilayer causing fatal perturbations to membrane structure (13). Proanthocyanidins and hydrolysable tannins have bioactivity profiles similar to the galloyated catechins, but the interactions of these classes of polyphenols with membranes have not been explored in detail. One goal of our study was to evaluate the interactions between a model membrane system and a representative compound from each of the three main classes of polyphenols.
Interactions with the lipid bilayer are well established for the galloyated catechins such as EGCg, with variation related to the structural properties of the polyphenol (7, 14). Sirk and coworkers proposed that polyphenols form hydrogen bonds with membranes, with phenolic hydroxyl groups serving as the hydrogen bond donors and oxygen atoms on the phospholipid as the hydrogen bond acceptors (8). They concluded that the strength of the interaction depends on the number of hydrogen bonds formed, and thus on both the degree of hydroxylation and the stereochemical features of polyphenols. For instance, EGCg and (−)-gallocatechin-3-gallate (GCg) are epimers that are cis (epigallocatechin) and trans (gallocatechin) at C2 and C3. EGCg interacts with the lipid more strongly than GCg, perhaps because the parallel arrangement of ring B and ring D in EGCg promotes hydrogen bond formation.
Solid-state NMR has been used to examine the interaction between [4-2H] EGCg and multi-lamellar vesicles (MLVs) (14, 15). This work established that EGCg alters the dynamics of the lipid bilayer. The data suggested that the B and D rings of EGCg insert into the bilayer and contact the inner hydrophobic region. In order to interact with membrane, the polyphenol must be favorably oriented with the hydrophobic domains (rings B and D) in direct contact with the membrane. This model of interaction does not invoke hydrogen bonding between the polyphenol and the membrane. These two contrasting models for polyphenol-membrane interactions have not been resolved to date. One goal of our study was to compare the interactions between membranes and several polyphenols that have very different polarities in order to further evaluate the relative importance of hydrogen bonding and hydrophobic interactions.
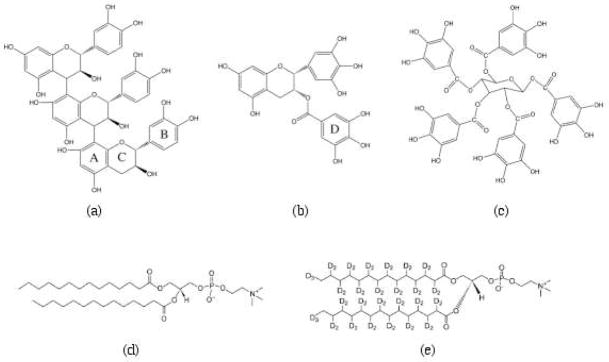
We have examined representatives of the three classes of polyphenols: a proanthocyanidin trimer comprising catechin-(4→8)-catechin-(4→8)-catechin (cat3),; a hydrolysable tannin, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), and a galloylated catechin, (−) epigallocatechin gallate (EGCg) (Figure 1). All three compounds are highly hydroxylated (cat3, 12 phenolic hydroxyls; PGG, 15; EGCg, 8), but their polarities are quite different. Cat3 is quite polar, with an octanol-water partition coefficient (Kow) of 0.12, EGCg somewhat less polar (Kow 12) and PGG is extremely hydrophobic (Kow 100) (1, 18). Studying the interactions of these three representative polyphenols with membranes should provide new mechanistic insights into possible modes of bioactivity for polyphenols.
Figure 1.
Structural formulas of (a) cat3, (b) EGCg, (c) PGG, (d) DMPC and (e) DMPC-d54.
We chose to use solid-state NMR spectroscopy to study the structural and dynamic properties of molecules incorporated into membrane lipid bilayers (19). MLVs, which are comprised of many stacked layers of lipid bilayer, are a useful model system for examining the interactions between the membrane and membrane proteins or other molecules with NMR (20, 21). 31P NMR is commonly used to study the headgroup motion and structure of the membrane lipid bilayer (19, 22). 2H NMR spectroscopy provides information on the dynamics of the hydrophobic regions of the membrane, by incorporating phospholipids labeled with 2H on the acyl chain (23–28).
In this study, model compounds representing each of the three kinds of polyphenols were investigated with the well-defined 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) MLV membrane model (18, 19). 31P and 2H solid-state NMR spectroscopic techniques were employed to reveal the dynamics of the lipid bilayer upon addition of the polyphenols to 2H labeled MLVs. We hypothesized that the interaction between the polyphenols and the lipid bilayers would be governed by a combination of structural features including polarity, molecular size, molecular geometry and number and arrangement of phenolic hydroxyl groups.
Materials
The proanthocyanidin trimer cat3 (Figure 1a) was a generous gift from H. H. Kolodziej (29) and the galloylated catechin EGCg (Figure 1b) was a generous gift from Douglas Balentine (Lipton Tea, Englewood Cliffs, NJ). The hydrolysable tannin PGG (Figure 1c) was prepared by methanolysis from commercial tannic acid (30). The polyphenols were kept in a −20 °C freezer before use. The lipids DMPC (Figure 1d) and 1,2-dimyristoyl (d54)-sn-glycero-3-phosphocholine (DMPC-d54) (Figure 1e) were purchased from Avanti Polar Lipids (Alabaster, AL) and were dissolved in chloroform at 10 mg/mL and 50 mg/mL respectively. N-[2-hydroxyethyl]piperazine-N′-2-ethane sulfonic acid (HEPES) was obtained from Sigma-Aldrich (St. Louis, MO), deuterium-depleted water from Cambridge Isotope Laboratories, Inc. (Andover, MA) and reagent grade sodium chloride and methanol were purchased from Fisher Scientific (Pittsburgh, PA).
NMR Sample Preparation
Polyphenols were incorporated into the lipid bilayers by the method of Rigby and co-workers (23). 2H-labeled MLVs were prepared by mixing DMPC and DMPC-d54 at a molar ratio of 9:1, using 0.045 mmol of DMPC and 0.005 mmol of DMPC-d54. Polyphenols were dissolved in minimal volume of methanol and then mixed with the lipids to reach the desired concentration (2.5, 5 or 10 mol% relative to the lipids). The lipids and the dissolved polyphenols were placed into a 12 mm × 75 mm test tube, the solvent was removed with a steady stream of N2 gas, and any residual solvent was removed by transferring the test tube into a vacuum desiccator and drying overnight. The lipid and polyphenol mixture was rehydrated by the addition of 47.5μL of pH 6.0 HEPES buffer (30 mM HEPES, 20 mM NaCl in deuterium-depleted water), dissolved by four to five freeze-thaw cycles to achieve homogeneity of MLV (21) and then transferred into a 4 mm solid-state NMR rotor.
Solid-state NMR Spectroscopy
A Bruker Avance 500-MHz solid-state NMR spectrometer operating at 202.4 MHz with a Bruker 4-mm double resonance CP-MAS probe was used to record the 31P NMR spectra. 1024 transients were averaged for each spectrum and the line broadening was set to 200 Hz. The spectral width was set to 150 ppm. The 31P NMR spectra were collected with the spin echo pulse sequence (90°-τ1-180°-τ2-acquire) with 1H decoupling. The 90° pulse was 4.2 μs and the first and second echo delay τ1 and τ2 were 20 μs and 14 μs respectively. The recycle delay was 5 s.
2H NMR spectra were recorded at 76.77 MHz with the same spectrometer and probe. The quadrupolar echo pulse sequence was employed using quadrature detection with complete phase cycling of the pulse pairs. The 90° pulse length was 3 μs, the interpulse delay was 40 μs, and the recycle delay was 0.3 s. The spectral width was 100 kHz and line broadening was set to 200 Hz. A total of 40960 transients were averaged for each spectrum. All the samples were equilibrated at 35 °C for at least 10 min before signal acquisition.
NMR data analysis
DMFIT software was used to simulate the 31P NMR spectra (31). The spectra can be fit to a sum of lines that correspond to a minimum number of species that contribute to the 31P NMR lineshape. The chemical shift anisotropy (CSA) width is obtained from the simulation.
2H NMR spectra were deconvoluted (dePaked) according to the algorithm of McCabe and Wassall (32, 33) so that the lipid bilayer normal was perpendicular to the direction of the static magnetic field. The order parameters can be calculated from the quadrupolar splitting which can be measured from the dePaked spectra according to the following expression (28, 34):
ΔνiQ is the quadrupolar splitting of a deuteron attached to the ith carbon. e2qQ/h is the quadrupolar splitting constant (168 KHz for the deuterons in C-2H bonds). SiCD is the chain order parameter of a deuteron attached to the ith carbon of the acyl chain of DMPC. The 2H nuclei attached to the terminal methyl carbons were designated carbon number 14 (carboxylate carbon is number 1). The remaining 2H nuclei were assigned in decreasing order along the phospholipid acyl chain. The quadrupolar splittings of the dePaked 2H spectra reveal the order parameters of the C-D methylene groups and the terminal methyl groups of the acyl chain. The quadrupolar splitting of the CD3 methyl groups at the end of the acyl chains is the smallest and closest to 0 kHz since they rotate at the fastest frequency. The next smallest quadrupolar splitting was assigned to the 2H attached to C-13 and so forth along the acyl chain (27). The order parameters calculated for the CD3 quadrupolar splitting were multiplied by 3 according to the literature (18, 21).
Results
31P NMR Spectroscopy of Polyphenols in Phospholipid Bilayers
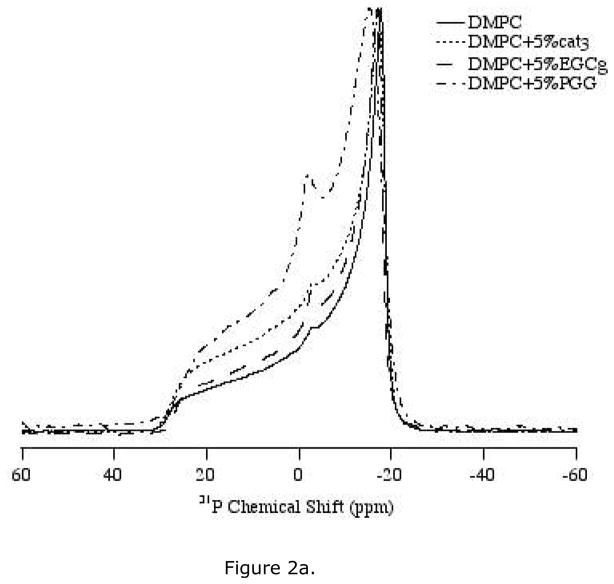
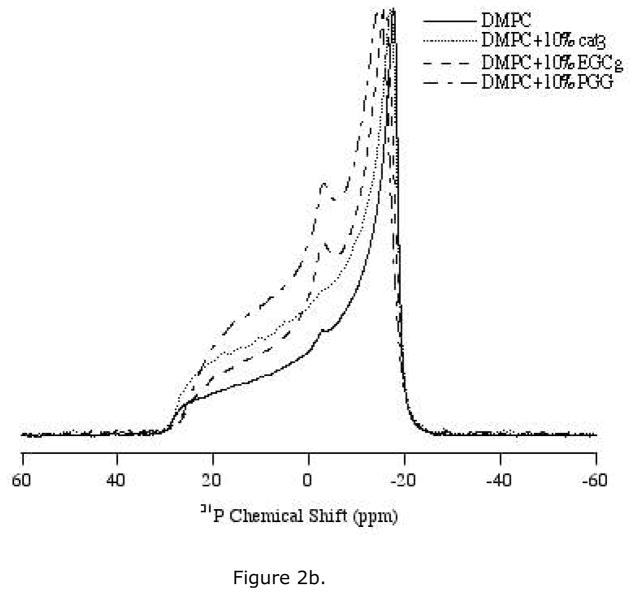
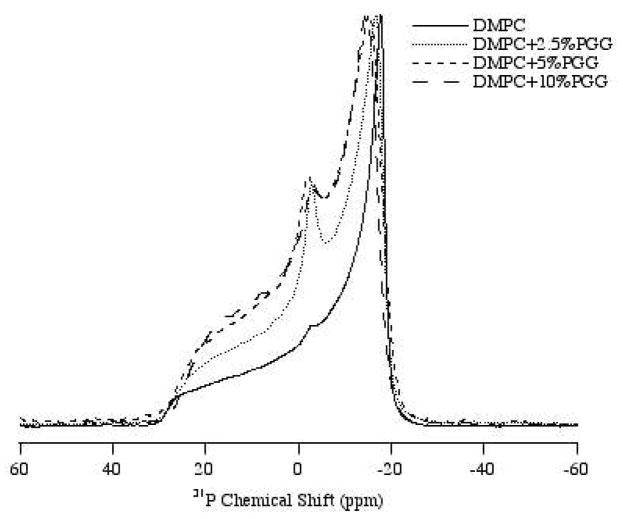
31P solid-state NMR spectra were collected to study the interaction between the head group region of the lipid bilayers and cat3, EGCg or PGG. The polyphenols were studied at different concentrations between 2.5 mol% and 10 mol% with respect to the lipid. The static 31P NMR spectra of the three polyphenols incorporated into DMPC/DMPC-d54 MLVs were recorded at 35 °C, which is near human physiological temperature and is higher than the phase transition (Lα) temperature of DMPC (23 °C) (Figure 2, 3). Thus, the MLVs were examined in the liquid-crystalline phase
Figure 2.
1H-decoupled 31P NMR powder spectra of MLV samples of DMPC/DMPC-d54 containing various amounts of the polyphenols. Spectra were collected using a Hahn-echo pulse sequence under static condition at 35 °C. (a) Bilayers with 5 mol% of three polyphenols: cat3, EGCg and PGG respectively. (b) Bilayers with 10 mol% of three polyphenols, cat3, EGCg and PGG respectively.
Figure 3.
1H-decoupled 31P NMR powder spectra of MLV samples of DMPC/DMPC-d54 and bilayers with 2.5 mol%, 5 mol% and 10 mol% of the polyphenol PGG. Spectra were collected using a Hahn-echo pulse sequence under static condition at 35 °C.
Cat3 has very little effect on the lipid bilayer mobility. When incorporated in the MLVs at either 5 mol% or 10 mol%, cat3 yielded 31P spectra with axially symmetric powder pattern lineshapes very similar to those of the control (Figure 2a, b). At 10 mol% cat3, the 31P chemical shift anisotropy width was slightly smaller than the CSA of the control sample or the 5 mol% cat3 sample (Table 1). The spectra indicate that the lipid bilayer remains in the Lα phase with respect to DMPC/DMPC-d54 with the addition of up to 10 mol% of cat3.
Table 1.
Effects of three polyphenols: cat3, EGCg and PGG at both concentrations of 5 mol% and 10 mol% on the 31P CSA width (in ppm) of DMPC/DMPC-d54 bilayers using 31P solid-state NMR experiments under static condition at 35 °C.
| Sample DMPC/DMPC-d54 | 31P CSA(ppm) (±0.2) |
|---|---|
| Control | 47.0 |
| 5% cat3 | 46.5 |
| 10% cat3 | 45.8 |
| 5% EGCg | 45.1 |
| 10% EGCg | 43.6 |
| 2.5% PGG | 44.3 |
| 5% PGG | 43.1 |
| 10% PGG | 41.3 |
EGCg increases the mobility of the lipid bilayer in a concentration-dependent fashion. With the addition of EGCg, the CSA width of the 31P NMR spectra decreases relative to the control sample, with a 3.4 ppm decrease in CSA width for samples with 10 mol% EGCg (Table 1). Furthermore, an isotropic peak appears in the spectra upon the addition of EGCg, with more pronounced changes at the higher level of polyphenol (Figure 2a, b).
PGG affects bilayer structure more significantly than EGCg at the same concentration (Figure 2a, b). An isotropic peak at 0 ppm is observed at the lowest level of PGG tested (2.5 mol%) (Figure 3). The CSA width is decreased by PGG, with 5 mol% PGG changing the CSA as much as 10 mol% EGCg (Table 1). As little as 2.5 mol% of PGG reduces the CSA width of the spectrum by about 2.7 ppm when compared with the control lipid sample.
2H NMR Spectroscopy of Polyphenols in Phospholipid Bilayers
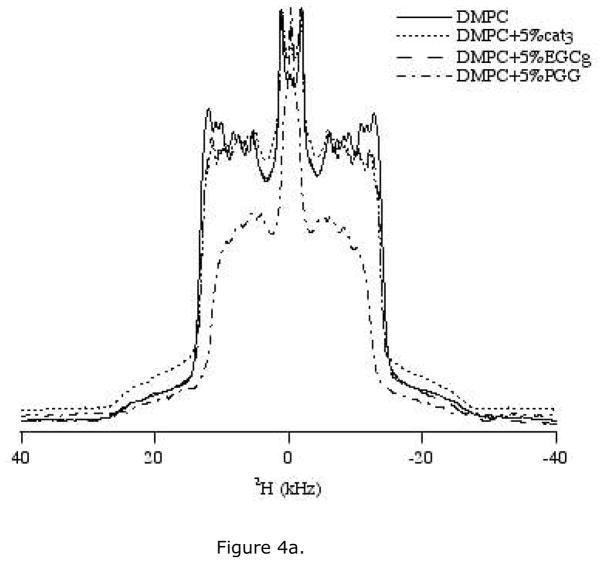
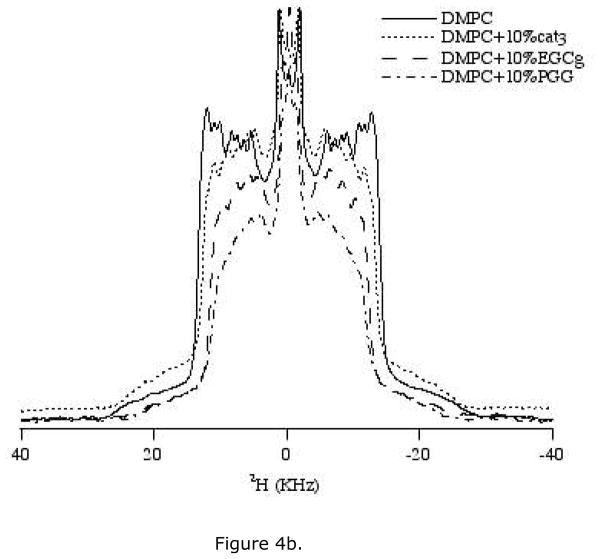
Incoporating DMPC-d54 (deuterated acyl chain) in the lipid bilayer reveals details of the order and dynamics of the acyl chains. We examined the effect of 5 mol% and 10 mol% polyphenols on 2H NMR spectra of DMPC/DMPC-d54 lipids (Figure 4a, b). The 2H quadrupolar splittings marginally decrease upon addition of polyphenols when compared to the control spectrum consisting of pure DMPC/DMPC-d54. The reduction in the quadrupolar splittings indicates that the acyl chains of the lipid are somewhat disordered by the addition of the polyphenols. At a given concentration, PGG reduced the quadrupolar splitting more than EGCg, while cat3 barely changed the splitting. For each type of polyphenol, the reduction in quadrupolar splitting is more noticeable at a higher concentration (Figure 4a, b). Isotropic components show up at around 0 ppm with addition of PGG at all three concentrations (2.5 mol%, 5 mol% and 10 mol%). Both 5 mol% and 10 mol% EGCg also induce isotropic components in 2H NMR spectra. This is consistent with the presence of an isotropic component in 31P NMR spectra of sample containing EGCg or PGG.
Figure 4.
2H NMR spectra of MLV samples of DMPC/DMPC-d54 containing various amounts of the three polyphenols. Spectra were collected using a quadrupolar echo pulse sequence under static condition at 35 °C. (a) Lipid bilayers with 5 mol% of three polyphenols: cat3, EGCg and PGG. (b) Lipid bilayers with 10 mol% of three polyphenols, cat3, EGCg and PGG.
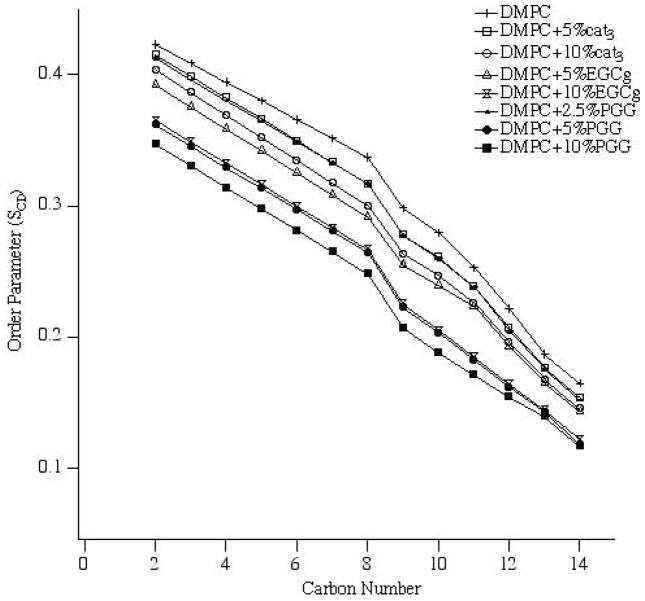
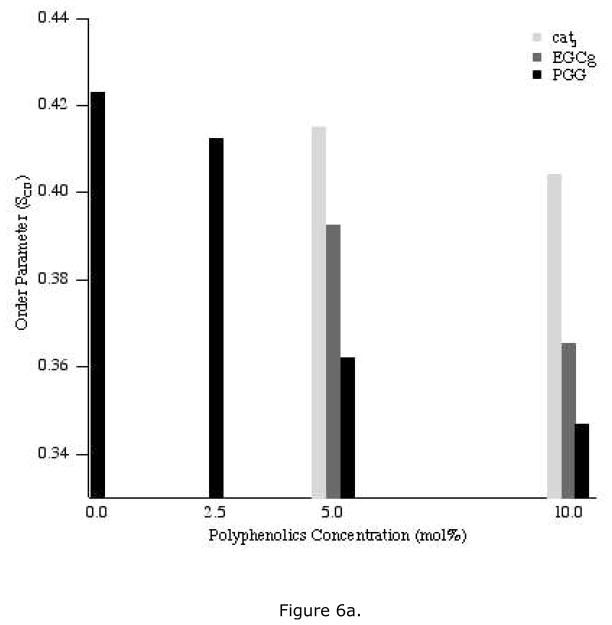
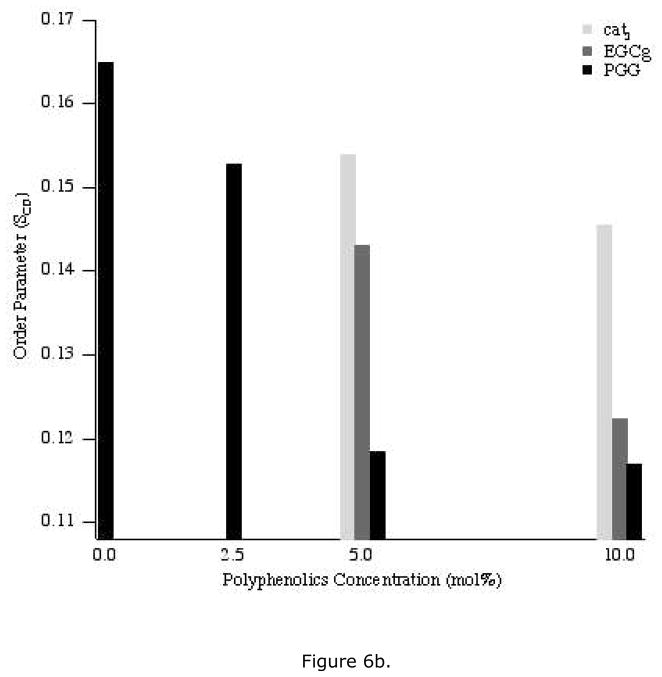
All of the 2H NMR spectra were deconvoluted (dePaked) to measure the dynamic changes in the lipids upon addition of polyphenols. The corresponding segmental C-D bond order parameters (SCD) were calculated from the dePaked powder spectra, and were plotted as a function of carbon number for each concentration of polyphenol (Figure 5). For control samples, the order parameter of the methylene near the head group is the largest, while the value decreases along the acyl chain and is smallest for the terminal methyl group (carbon number 14) (Figures 6a, b). Compared to the control, addition of polyphenol yields a similar pattern but the order parameters of the methylene groups are not decreased as much as the terminal methyl group is approached. For each polyphenol, a higher concentration decreased all of the order parameters more than a lower concentration. For the same concentration of polyphenolic, the order parameters were reduced the most by PGG and the least by cat3.
Figure 5.
Smoothed acyl chain (DMPC-d54) order parameter (SCD) calculated from the dePaked spectra of DMPC/DMPC-d54 and bilayers with three polyphenols, cat3, EGCg and PGG at concentrations of 5 mol% and 10 mol%.
Figure 6.
The order parameters of DMPC-d54 carbons two (a) or fourteen (b) upon addition of the three polyphenols: cat3, EGCg and PGG at different concentrations, 2.5 mol% (for PGG only), 5 mol% and 10 mol%.
Discussion
Polyphenols have been implicated as agents that provide protection from many chronic diseases including cardiovascular disease, cancer, diabetes and Alzheimer’s disease (3–5). Well-known activities of polyphenols that might be related to their beneficial bioactivities include their ability to bind protein (1) and their potent antioxidant activity (6). The ability of polyphenols to interact with the lipid bilayer is much more poorly understood, with mechanistic studies limited to those on the galloylated catechins such as EGCg (7–17). Interactions with the lipid bilayer are of interest in part because disruption or binding to membranes may mediate specific bioactivities (13), but also because solubility in lipids is a critical determinant of bioavailability. Here, we use a combination of 31P and 2H solid-state NMR spectroscopic techniques to study the dynamics of the lipid bilayer upon addition of representative compounds from the three classes of polyphenols: cat3, EGCg and PGG. The proanthocyanidin cat3 is a very polar polyphenol (Kow 0.12) with a molecular weight of 864 (1, 35). The slightly larger hydrolysable tannin PGG is very nonpolar (Kow 100, molecular weight 940) (1, 35). The mixed polyphenol EGCg has intermediate polarity (Kow 12) and is the smallest of the three compounds, with a molecular weight of 458 (1, 35). Differences in polarity reflect differences in the three dimensional structures of the compounds, with cat3 forming a mixture of extended conformers in solution (36) and PGG forming a hydrophobic sphere (37).
The reduction in quadrupolar splittings in the 2H NMR spectra upon the addition of PGG to the membrane indicates that PGG significantly disorders the acyl chains of the lipid bilayers. Smaller changes in the quadrupolar splittings obtained with EGCg and cat3 indicated that these compounds interact more weakly with the acyl chains. Our data indicates that the polarity of the polyphenol is inversely related to the strength of the interaction with the hydrophobic region of DMPC/DMPC-d54 lipid bilayer, suggesting an important role for hydrophobic forces in polyphenol-membrane interactions.
For DMPC/DMPC-d54 bilayers, the order parameters (SCD) decrease as the distance between the C-D bond and the glycerol backbone increases, because there is more motion near the end of the acyl chain than in the headgroup region. For any given methylene group in the acyl chain, the order parameter changes with added polyphenols follows: no polyphenol> 5 mol% cat3> 2.5 mol% PGG>10 mol% cat3 > 5 mol% EGCg > 10 mol% EGCg > 5 mol% PGG > 10 mol% PGG. Lower SCD values can be interpreted as increased fluidity of the acyl chain and the increased motion of the vesicles, presumably induced by interactions of polyphenols with the membrane. The order parameters that we obtained for three model polyphenols confirms that strength of interaction between the polyphenol and a bilayer is inversely related to the polarity of the polyphenol.
The 31P NMR spectra are most strongly affected by PGG while cat3 has little effect. The dramatically reduced CSA width in the 31P NMR spectra for MLVs containing PGG can be attributed to increased fluidity of the membrane surface or decreased size of the vesicles (19) in the presence of this polyphenol. The additional isotropic component that is observed in both 31P and 2H NMR spectra upon addition of EGCg and PGG at all concentrations provides further evidence for the presence of smaller vesicles (19). The isotropic peak induced by the 5 mol% and 10 mol% of EGCg and PGG suggests that those polyphenols that are sufficiently nonpolar to interact with the bilayer acyl chains may also fragment bigger vesicles into smaller vesicles. The spectral changes noted with addition of polyphenols indicate that these compounds do not aggregate the membrane vesicles, but increase fluidity or decrease size of the vesicles.
At the same concentration of polyphenols, the very nonpolar PGG increases the headgroup motion and disorders the acyl chain of the membrane lipid bilayer most significantly when compared with cat3 and EGCg. The most polar cat3 trimer disorders the lipid bilayer very little even at 10 mol%. The extent of interaction between the polyphenols and the membrane may depend part on the polyphenol polarity. Since PGG is the most nonpolar polyphenol, it is expected to interact with the nonpolar acyl chain region deep in the center of the lipid bilayer, and to affect the membrane more than the other two polyphenols. The significant decrease of the order parameter of the acyl chain according to the 2H solid-state NMR spectra reflects this interaction (Figure 5). Conversely, the very polar cat3 trimer has a small effect on order parameters consistent with low interaction with the nonpolar acyl chains. The 31P NMR spectra also confirm the model of hydrophobic interactions. The large perturbation to the membrane head group by PGG suggests interaction with the lipid acyl chains that disrupts the membrane overall. The very polar cat3 does not disturb the head groups, suggesting it is adsorbed on the membrane surface without disturbing it.
The naturally occurring polyphenol tannic acid (TA) has similar behavior to PGG (38, 39). TA is a mixture of galloyl glucoses varying from monogalloyl glucose to dodecagalloyl glucose, with a variable composition depending on source (40). TA may contain PGG, but it also contains other galloyl glucoses. A 2H powder pattern solid-state NMR study on TA suggested it moderately reduced the order parameter for the acyl chain near the head group and much more dramatically affected the terminal region (39). It was suggested that the first half of the acyl chain tilts cooperatively upon TA insertion, while the more mobile terminal region undergoes gauche conformational change, which induces the significant reduction in the order parameter (38, 39). TA induces a moderate reduction of SCD on the membrane surface and a dramatic reduction of SCD in the membrane hydrophobic core. At a similar concentration, PGG induces a larger SCD reduction throughout the lipid acyl chain when compared with TA. The isotropic component that appeared with PGG addition was not observed with TA addition. However, the effect of TA on the SCD of the membrane surface and the acyl chain terminal group is more obvious than that of PGG. Although PGG and TA are structurally similar, it is not surprising that they have different tendencies to interact with membrane. TA is a heterogeneous mixture, so while some components of TA are similar to PGG, and enter the hydrophobic part of bilayer, other components may only be adsorbed on the membrane surface (39). PGG is a small, very hydrophobic molecule that is able to interact with both the membrane surface and the hydrophobic core. It has been suggested that at low concentrations TA increases outer membrane surface area and decreases membrane thickness but that at higher concentrations the inner membrane cannot expand or the lipid and TA cannot exchange fast enough, so the bilayer breaks into smaller vesicles (39). The isotropic component we noted in both 31P and 2H spectra of the PGG incorporated into the lipid bilayer suggests a similar mechanism for PGG.
Previous studies have indicated that the B ring and the galloyl moiety of EGCg tend to interact with the membrane surface (9, 14, 15). Our work extends those previous studies by using MLVs instead of the smaller bicelles or unilamellar vesicles used earlier, and by using lower polyphenol concentrations to prevent aggregation of the lipid. Under our conditions EGCg interacted weakly with the acyl groups in the membrane, and had intermediate effects on the polar head groups. This is consistent with earlier studies (9, 14, 15), and like those studies suggests that EGCg bioavailability might be limited by its limited ability to penetrate the membrane.
In conclusion, structural features can affect the level of interaction between polyphenols and lipid bilayers. Compounds representing the three major types of naturally occurring polyphenols, cat3, EGCg and PGG, were investigated by solid state NMR methods. The most nonpolar compound PGG, perturbed the membrane most, supporting hydrophobic models for polyphenol-membrane interactions rather than hydrogen bonding models. Our data demonstrating limited interactions between cat3 and membranes suggest that the limited bioavailability of proanthocyanidins (41) may be in part due to their membrane insolubility. The bioavailability of PGG has not been established in vivo (4) but transport studies in Caco cells suggest that PGG uptake may involve a carrier protein (42). Our study with MLVs suggest high bioavailability for PGG and related nonpolar tannins based on their strong tendency to interact with membranes. Furthermore, our data suggest that attempts to increase natural product bioavailability by microencapsulation in membrane vesicles (“phytosomes”) (43) may have varying success depending largely on the hydrophobicity of the polyphenol and its tendency to interact with bilayers. Additional studies to examine the effect of proteins such as the salivary tannin-binding proteins (42) on the interaction between polyphenols and membrane bilayers may provide further insights into bioavailability and bioactivities of this widespread class of compounds.
Acknowledgments
This work was supported by the NIGMS/NIH grant GM60259-01 and the NSF (CHE-0645709 and MRI-0722403).
References
- 1.Hagerman AE, Rice ME, Ritchard NT. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin(16) (4→8) catechin (procyanidin) J Agric Food Chem. 1998;46:2590–2595. [Google Scholar]
- 2.Yoshida T, Hatano T, Ito H. High molecular weight plant polyphenols (tannins): Prospective functions. In: Romeo JT, editor. Chemical Ecology and Phytochemistry of Forest Ecosystems. Elsevier; San Diego, CA: 2005. pp. 163–190. [Google Scholar]
- 3.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JH, Li L, Kim SH, Hagerman AE, Lu JX. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res. 2009;26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 6.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Hung WC, Chen FY, Lee CC, Huang HW. Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys J. 2009;96:1026–1035. doi: 10.1016/j.bpj.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirk TW, Brown EF, Sum AK, Friedman M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J Agric Food Chem. 2008;56:7750–7758. doi: 10.1021/jf8013298. [DOI] [PubMed] [Google Scholar]
- 9.Uekusa Y, Kamihira M, Nakayama T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J Agric Food Chem. 2007;55:9986–9992. doi: 10.1021/jf0712402. [DOI] [PubMed] [Google Scholar]
- 10.Caturla N, Vera-Samper E, Villalain J, Mateo CR, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radical Biol Med. 2003;34:648–662. doi: 10.1016/s0891-5849(02)01366-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya H. Effects of green tea catechins on membrane fluidity. Pharmacology. 1999;59:34–44. doi: 10.1159/000028303. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Kumazawa S, Nanjo F, Hara Y, Nakayama T. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci, Biotechnol Biochem. 1999;63:2252–2255. doi: 10.1271/bbb.63.2252. [DOI] [PubMed] [Google Scholar]
- 13.Kajiya K, Hojo H, Suzuki M, Nanjo F, Kumazawa S, Nakayama T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J Agric Food Chem. 2004;52:1514–1519. doi: 10.1021/jf0350111. [DOI] [PubMed] [Google Scholar]
- 14.Kajiya K, Kumazawa S, Naito A, Nakayama T. Solid-state NMR analysis of the orientation and dynamics of epigallocatechin gallate, a green tea polyphenol, incorporated into lipid bilayers. Magn Reson Chem. 2008;46:174–177. doi: 10.1002/mrc.2157. [DOI] [PubMed] [Google Scholar]
- 15.Kumazawa S, Kajiya K, Naito A, Saito H, Tuzi S, Tanio M, Suzuki M, Nanjo F, Suzuki E, Nakayama T. Direct evidence of interaction of a green tea polyphenol, epigallocatechin gallate, with lipid bilayers by solid-state nuclear magnetic resonance. Biosci, Biotechnol Biochem. 2004;68:1743–1747. doi: 10.1271/bbb.68.1743. [DOI] [PubMed] [Google Scholar]
- 16.Tamba Y, Ohba S, Kubota M, Yoshioka H, Yamazaki M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys J. 2007;92:3178–3194. doi: 10.1529/biophysj.106.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka H, Haga H, Kubota M, Sakai Y. Interaction of (+)-catechin with a lipid bilayer studied by the spin probe method. Biosci, Biotechnol Biochem. 2006;70:395–400. doi: 10.1271/bbb.70.395. [DOI] [PubMed] [Google Scholar]
- 18.Dufourc EJ, Parish EJ, Chitrakorn S, Smith ICP. Structural and dynamical details of cholesterol lipid interaction as revealed by deuterium NMR. Biochemistry. 1984;23:6062–6071. [Google Scholar]
- 19.Dave PC, Tiburu EK, Damodaran K, Lorigan GA. Investigating structural changes in the lipid bilayer upon insertion of the transmembrane domain of the membrane-bound protein phospholamban utilizing P-31 and H-2 solid-state NMR spectroscopy. Biophys J. 2004;86:1564–1573. doi: 10.1016/S0006-3495(04)74224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traikia M, Langlais DB, Cannarozzi GM, Devaux PF. High-resolution spectra of liposomes using MAS NMR. The case of intermediate-size vesicles. J Magn Reson. 1997;125:140–144. doi: 10.1006/jmre.1996.1068. [DOI] [PubMed] [Google Scholar]
- 21.Stockton GW, Polnaszek CF, Tulloch AP, Hasan F, Smith ICP. Molecular-motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures-deuterium nuclear magnetic-resonance study of specifically labeled lipids. Biochemistry. 1976;15:954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- 22.Chu SD, Hawes JW, Lorigan GA. Solid-state NMR spectroscopic studies on the interaction of sorbic acid with phospholipid membranes at different pH levels. Magn Reson Chem. 2009;47:651–657. doi: 10.1002/mrc.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigby AC, Barber KR, Shaw GS, Grant CWM. Transmembrane region of the epidermal growth factor receptor: Behavior and interactions via H-2 NMR. Biochemistry. 1996;35:12591–12601. doi: 10.1021/bi9611063. [DOI] [PubMed] [Google Scholar]
- 24.Seelig A, Seelig J. Dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic-resonance. Biochemistry. 1974;13:4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- 25.Lafleur M, Fine B, Sternin E, Cullis PR, Bloom M. Smoothed orientational order profile lipid bilayers by H-2 Nuclear Magnetic Resonance. Biophys J. 1989;56:1037–1041. doi: 10.1016/S0006-3495(89)82749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Baker S, Lu JX, Chu SD, Brinn CC, Makaroff CA, Lorigan GA. Side chain and backbone dynamics of phospholamban in phospholipid bilayers utilizing H-2 and N-15 solid-state NMR spectroscopy. Biochemistry. 2007;46:11695–11706. doi: 10.1021/bi700749q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huster D, Arnold K, Gawrisch K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 1998;37:17299–17308. doi: 10.1021/bi980078g. [DOI] [PubMed] [Google Scholar]
- 28.Huster D, Yao YL, Jakes K, Hong M. Conformational changes of colicin Ia channel-forming domain upon membrane binding: a solid-state NMR study. BBA-Biomembranes. 2002;1561:159–170. doi: 10.1016/s0005-2736(02)00340-1. [DOI] [PubMed] [Google Scholar]
- 29.Kolodziej H. Oligomeric flavan-3-ols from medicinal willow bark. Phytochemistry. 1990;29:955–960. [Google Scholar]
- 30.Chen Y, Hagerman AE. Characterization of soluble non-covalent complexes between bovine serum albumin and beta-1,2,3,4,6-penta-O-galloyl-D-glucopyranose by MALDI-TOF MS. J Agric Food Chem. 2004;52:4008–4011. doi: 10.1021/jf035536t. [DOI] [PubMed] [Google Scholar]
- 31.Massiot D, Fayon F, Capron M, King I, Le Calve S, Alonso B, Durand JO, Bujoli B, Gan ZH, Hoatson G. Modelling one- and two-dimensional solid-state NMR spectra. Magn Reson Chem. 2002;40:70–76. [Google Scholar]
- 32.McCabe MA, Wassall SR. Fast-Fourier-Transform DePaking. J Magn Reson Ser B. 1995;106:80–82. [Google Scholar]
- 33.McCabe MA, Wassall SR. Rapid deconvolution of NMR powder spectra by weighted fast Fourier transformation. Solid State Nucl Magn Reson. 1997;10:53–61. doi: 10.1016/s0926-2040(97)00024-6. [DOI] [PubMed] [Google Scholar]
- 34.Dave PC, Tiburu EK, Nusair NA, Lorigan GA. Calculating order parameter profiles utilizing magnetically aligned phospholipid bilayers for H-2 solid-state NMR studies. Solid State Nucl Magn Reson. 2003;24:137–149. doi: 10.1016/S0926-2040(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 35.Mueller-Harvey I, Mlambo V, Sikosana JLN, Smith T, Owen E, Brown RH. Octanol-water partition coefficients for predicting the effects of tannins in ruminant nutrition. J Agric Food Chem. 2007;55:5436–5444. doi: 10.1021/jf070308a. [DOI] [PubMed] [Google Scholar]
- 36.Tarascou I, Ducasse MA, Dufourc EJ, Moskau D, Fouquet E, Laguerre M, Pianet I. Structural and conformational analysis of two native procyanidin trimers. Magn Reson Chem. 2007;45:157–166. doi: 10.1002/mrc.1938. [DOI] [PubMed] [Google Scholar]
- 37.Feldman KS, Smith RS. Ellagitannin chemistry. First total synthesis of the 2,3- and 4,6-coupled ellagitannin pedunculagin. J Org Chem. 1996;61:2606–2612. doi: 10.1021/jo952130+. [DOI] [PubMed] [Google Scholar]
- 38.Huh NW, Porter NA, McIntosh TJ, Simon SA. The interaction of polyphenols with bilayers: conditions for increasing bilayer adhesion. Biophys J. 1996;71:3261–3277. doi: 10.1016/S0006-3495(96)79519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon SA, Disalvo EA, Gawrisch K, Borovyagin V, Toone E, Schiffman SS, Needham D, McIntosh TJ. Increased adhesion between neutral lipid bilayers - interbilayer bridges formed by tannic-acid. Biophys J. 1994;66:1943–1958. doi: 10.1016/S0006-3495(94)80988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romani A, Ieri F, Turchetti B, Mulinacci N, Vincieri FF, Buzzini P. Analysis of condensed and hydrolysable tannins from commercial plant extracts. J Pharm Biomed Anal. 2006;41:415–420. doi: 10.1016/j.jpba.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Shivashankara KS, Acharya SN. Bioavailability of dietary polyphenols and cardiovascular diseases. The Open Nutraceuticals J. 2010;3:227–241. [Google Scholar]
- 42.Cai KH, Hagerman AE, Minto RE, Bennick A. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochem Pharmacol. 2006;71:1570–1580. doi: 10.1016/j.bcp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]