Abstract
Alterations in the balance of functional activity within the serotonin (5-HT) system are hypothesized to underlie impulse control. Cocaine-dependent subjects consistently demonstrate greater impulsivity relative to non-drug using control subjects. Preclinical studies suggest that the 5-HT2A receptor (5-HT2AR) contributes to the regulation of impulsive behavior and also mediates some of the behavioral effects of cocaine. We hypothesized that the selective 5-HT2AR antagonist M100907 would reduce inherent levels of impulsivity and attenuate impulsive responding induced by cocaine in two animal models of impulsivity, the differential reinforcement of low rate (DRL) task and the one-choice serial reaction time (1-CSRT) task. M100907 reduced rates of responding in the DRL task and premature responding in the 1-CSRT task. Conversely, cocaine disrupted rates of responding in the DRL task and increased premature responding in the 1-CSRT task. M100907 attenuated cocaine-induced increases in specific markers of behavioral disinhibition in the DRL and 1-CSRT tasks. These results suggest that the 5-HT2AR regulates inherent impulsivity, and that blockade of the 5-HT2AR alleviates specific aspects of elevated levels of impulsivity induced by cocaine exposure. These data point to the 5-HT2AR as an important regulatory substrate in impulse control.
Keywords: cocaine, impulsivity, 5-HT2A receptor, behavioral disinhibition, differential reinforcement of low rate task, one-choice serial reaction time task, rat
INTRODUCTION
Impulsivity is a multidimensional personality trait characterized by poor inhibitory control over behavior in response to internal or external stimuli, the failure to consider the consequences of these reactions, and a preference for immediate rather than delayed reinforcement (Evenden 1999; Moeller et al. 2001a). Impulsive choice (or impulsive decision-making) and impulsive action (or behavioral disinhibition, i.e., the diminished ability to withhold inappropriate behavioral responses) are two primary dimensions of impulsivity that have been associated with addictive behaviors (e.g., binge eating, gambling, and drug abuse). Various aspects of drug abuse, including initial drug-taking, the transition from casual to compulsive drug use, the maintenance of drug-seeking behaviors as well as the penchant to reinstate drug-seeking behaviors in both humans (Moeller et al. 2001a, 2002, 2004; Coffey et al. 2003) and laboratory animals (Perry et al. 2005; Belin et al. 2008; Dalley et al. 2007; Diergaarde et al. 2008) appear to be correlated with the individual degree of impulsivity (Jentsch & Taylor 1999; de Wit & Richards 2004; Moeller et al. 2001b; Tarter et al. 2007; Belin et al. 2008). The behavioral and neurochemical underpinnings of impulsivity in relation to cocaine intoxication and dependence have received only limited attention to date.
Impulse control is linked with alterations in functional activity of the monoamine [serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA), norepinephrine (NE)] systems (for review, see Pattij & Vanderschuren 2008). Specifically, alterations in synaptic levels of either 5-HT, DA, or NE can disrupt the balance of the 5-HT:DA:NE interaction and may represent a neurobiological mechanism underlying impulsivity (Winstanley et al. 2003; Winstanley et al. 2006b). There is extensive evidence that serotonergic lesions, selective 5-HT reuptake inhibitors and other non-selective pharmacological manipulations of the 5-HT system alter performance in animal models of impulsivity (Winstanley et al. 2004a, b; Harrison et al. 1997; Koskinen et al. 2000; Koskinen & Sirvio 2001; Marek et al. 1989). In the past, studies on the role of 5-HT in animal models of impulsivity relied on nonselective pharmacological manipulation of the serotonergic system yielding mixed and sometimes complicated results, most likely due to the actions of 5-HT at multiple receptors (Winstanley et al. 2003, 2004a,b, 2006a; Harrison et al. 1997; Fletcher et al. 2007, 2009; Higgins et al. 2003; Robinson et al. 2008; Liao & Chang 2001) as well as within multiple neurotransmitter circuits, including DA and NE (Higgins et al. 2003; Winstanley et al. 2005; Bubar & Cunningham 2008). However, the development of compounds that act selectively at specific 5-HT receptors has enabled more defined analyses of 5-HT receptor involvement in impulsive behavior. Recent studies with antagonists selective for the 5-HT2AR (e.g., M100907) revealed that blockade of the 5-HT2AR decreased impulsive behaviors in animal models of impulsive action (Winstanley et al. 2003, 2004b; Marek et al. 2005; Higgins et al. 2003; Robinson et al. 2008), suggesting that tonic activation of the 5-HT2AR attunes the 5-HT:DA:NE balance (Bubar & Cunningham 2008) that regulates inherent impulsivity.
The psychoactive and behavioral effects of cocaine result from blockade of monoamine reuptake, enhancing the concentrations of 5-HT, DA, and NE in the synapse (Koe 1976) and subsequent activation of monoamine receptors within the limbic-corticostriatal pathway (Koob 1992). Neurotransmission through 5-HT2AR also regulates many of the behavioral and neurochemical effects of cocaine (Bubar & Cunningham 2008), including its locomotor stimulant (Fletcher et al. 2002; McMahon & Cunningham 2001), discriminative stimulus properties (McMahon & Cunningham 2001; Filip et al. 2006) as well as the incentive-motivational value of cocaine-associated cues (Nic Dhonnchadha et al. 2009; Burmeister et al. 2004; Filip 2005). Thus, the 5-HT2AR may be an important mediator in the neurobiological relationship between impulsivity and cocaine addiction.
Despite the development of multiple animal models of impulsivity, few attempts have been made to use more than one animal model within a single study to identify which dimensions of impulsive action are altered following pharmacological manipulations (Winstanley et al. 2004b; Fletcher et al. 2009). The purpose of this study was to employ two models of behavioral disinhibition [the differential reinforcement of low-rate (DRL) task and the 1-choice serial reaction time (1-CSRT) task] to measure the effect of 5-HT2AR antagonism on inherent and cocaine-evoked behavioral disinhibition.
The DRL task is an operant task which requires the rat to withhold a behavioral response until a certain time interval has elapsed in order to obtain a reinforcer. Responses made prior to the completion of the schedule are not reinforced, and the schedule clock is reset. Animals that exhibit high levels of impulsive-like behaviors tend to have higher rates of premature responding and, as a result, obtain fewer reinforcers (Stoffel & Cunningham 2008). This task was selected for various reasons. The DRL task has high face validity: an analogous model has been successfully utilized in humans to distinguish between impulsive and non-impulsive subjects (van den Broek et al. 1987). Second, DRL schedules in rodents have demonstrated robust sensitivity to the effects of psychostimulants (Sabol et al. 1995; Wang et al. 2001; Wenger & Wright 1990; Stoffel & Cunningham 2008) and serotonergic manipulations (Ardayfio et al. 2008; Jolly et al. 1999; Sokolowski & Seiden 1999; O’Donnell et al. 2005).
The 1-CSRT task is an operant task in which reinforcement is obtained for detecting and correctly responding to brief presentations of a visual target presented regularly in a spatially predictable location (Dalley et al. 2002). In the 1-CSRT task, a single stimulus light and nose poke hole is utilized; the rat needs only to predict when the stimulus will occur, then emit a nosepoke in the active (lit) aperture to earn a reinforcer. Thus, the 1-CSRT task provides a model of behavioral disinhibition independent from complex visuospatial attentional processes. The measure of anticipatory (premature) responding before presentation of the visual target provides the ability to study behavioral disinhibition. A variant of the 1-CSRT task is the 5-CSRT task that requires more demand on visuospatial attentional processes than the 1-CSRT task; the CSRT task in animals (Robbins 2002) is analogous to the continuous performance task in humans (Beck et al. 1956; Mirsky & Rosvold 1960; Robbins 2002), lending face validity to the use of CSRT tasks in preclinical studies of impulsivity. Premature responding in CSRT tasks is enhanced by the psychostimulant amphetamine (Cole & Robbins 1989; Loos et al. 2010) and is also highly sensitive to manipulations of serotonergic neurotransmission, e.g., blockade of 5-HT2AR (for reviews, see Pattij & Vanderschuren 2008; Robbins 2002). Furthermore, global serotonergic depletion within the rat forebrain enhances premature responding (i.e., behavioral disinhibition) in CSRT tasks (Winstanley et al. 2004a; Harrison et al. 1997; Fletcher et al. 2009).
The use of two or more distinct behavioral disinhibition assays allows better validation of the neurochemical systems involved in individual aspects of impulsivity. Thus, we tested the hypotheses that the 5-HT2AR represents a neurobiological substrate of impulsive action and acts as a functional rheostat to maintain the balance of inherent levels of impulsivity as well as cocaine-disrupted impulsive action. Specifically, we hypothesized that the 5-HT2AR antagonist M100907 would reduce inherent behavioral disinhibition as well as attenuate cocaine-evoked behavioral disinhibition as measured with both the DRL and 1-CSRT tasks.
METHODS
Subjects
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing 250–275 g at the time of arrival were used (N=24). Rats were allowed to acclimate for 5–6 days to a colony room at a constant temperature (21–23°C) and humidity (40–50%) on a 12 hr light-dark cycle (lights on at 07:00 hr). Rats were housed two per cage during operant training. For DRL task training, food was freely available for the duration of all experiments; however, water intake was restricted to that available during operant training sessions, after sessions (10–15 min) and on weekends (approx 36 hrs). For 1-CSRT task training, rats were food restricted to 90% free-feeding weight for the duration of all experiments; water was freely available except during daily 30–60 min operant sessions. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Council, 1996) and with the approval of the UTMB Institutional Animal Care and Use Committee.
5-HT2AR regulation of inherent behavioral disinhibition and cocaine-evoked disruption of behavioral disinhibition in the DRL-20 sec task
Apparatus
DRL task procedures were carried out in eight two-lever operant chambers housed in sound-attenuating chambers (Lafayette Instruments, Lafayette, IN, or Med Associates, St. Albans, VT). The chambers contained two levers with a water dispenser centered between the two levers. Illumination was provided by a 28-V houselight. Ventilation and sound masking were provided by a fan mounted in the wall of the sound-attenuating cabinet. An interface (Med Associates) connected the chambers to a PC computer running Med PC for Windows software (Med Associates) that controlled and recorded all experimental events.
Training
After acclimation to the colony room, rats (n=8–16/group) were handled and weighed daily for 3–5 days after which DRL task training began. Procedures for the DRL task were performed as previously described with minor modifications (Stoffel & Cunningham 2008). All DRL task training and testing sessions were 60 min in length and were conducted between 10:00 and 15:00 hr on weekdays (Monday-Friday). Rats were placed into DRL task operant chambers following 23 hrs of water restriction. For all rats, the left lever was designated as the “active” lever and the right lever was the “inactive” lever. Rats were initially trained on a DRL 5-s schedule of reinforcement task. Each session began with illumination of the house light. Responses on the active lever that were at least 5 s apart resulted in the delivery of a reinforcer (water), and the house light was extinguished for a brief (2 sec) inter-trial interval (ITI). Responses on the active lever that were not at least 5 s apart initiated the ITI, and reset the interval timer to zero, resulting in an additional 5 s delay to the next available reinforcer. Responses on the inactive lever were recorded, but had no scheduled consequences. Once rats had obtained at least 100 reinforcers for two consecutive days, the DRL task interval requirement for the next session was increased to 20 s. Rats remained on the DRL 20-sschedule until stable responding was achieved; responding was considered stable when the standard error of the mean for the total response rate was less than 10% of the mean response rate for five consecutive sessions (Li et al. 1989).
After responding was stabilized, pretreatment with M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg, i.p.; synthesized by Kenner Rice, National Institute on Drug Abuse, Bethesda, MD) or vehicle (1% Tween 80 in saline, i.p.) occurred 15 min prior to an injection with saline (1 ml/kg, i.p.) or cocaine (10 mg/kg, i.p.; National Institute on Drug Abuse, Research Triangle, NC) immediately before the start of the DRL task session. Rats received saline or vehicle injections on Wednesdays and M100907 or cocaine injections on Thursdays; the order of M100907 and cocaine injections was randomized across all rats in a within-subjects design.
Data Analysis
During the DRL-20 s task sessions, the total number of responses per session (on both active and inactive levers), and total number of reinforcers obtained per session were recorded for each 60 min session. Once rats were well-trained, inactive lever responses consistently accounted for less than 5% of total responses during DRL-20 s task sessions. The response rate was calculated as total number of responses per min during the 60-min DRL-20 s task sessions. The total number of reinforcers obtained was used as a general indication of behavioral disinhibition in each rat; rats with high behavioral disinhibition (i.e., poor inhibitory control) earn fewer reinforcers (Stoffel & Cunningham 2008). Response rate and reinforcers obtained on test sessions (M100907 ± cocaine or saline) are presented as the percentage of values observed on the maintenance session immediately prior to the test session; prior to this maintenance session, rats are injected with vehicle+saline (control). Inter-response times (IRT) were calculated for DRL-20 s responding on the last session of stable baseline responding and on the session during which saline, cocaine or M100907 was administered; IRT frequencies were tabulated and converted to relative frequencies by dividing the frequency of each IRT value by the total number of responses made during the session. Relative frequencies were plotted as histograms (in 2-s bins), and the mean, median and mode were calculated for each IRT distribution. The frequency of burst responses represents an additional facet of behavioral disinhibition and was calculated based on the relative frequencies of responses made during the burst component of the IRT distribution (< 4 sec) (Jolly et al. 1999; Fletcher 1995; Pattij et al. 2003, 2004; Wogar et al. 1992). Modified peak shift analysis was performed by comparing the shift in the peak relative frequency for each drug treatment versus the control peak relative frequency (Richards et al. 1993).
The effects of administration of M100907 (pretreatment) in the presence or absence of cocaine administration (treatment) on DRL-20 s task response measures [response rate, number of reinforcers obtained, IRT mean, median, mode values, burst responding, peak shifts] were analyzed with a two-way repeated measures ANOVA [pretreatment, treatment] using the general linear model (GLM) procedure (Keppel 1973). A priori comparisons to analyze the effects of M100907 on inherent behavioral disinhibition or the effects of M100907 on cocaine-evoked behavioral disinhibition were defined before the start of experiment; therefore, one-way repeated measures ANOVA and preplanned comparisons of the treatment means versus control were made with Dunnett’s procedure. All statistical analyses were conducted with SAS for Windows version 8.2. The alpha level for all analyses was set at p<0.05. Graphic presentations of data are provided separately for the effects of M100907 in the absence (Fig. 1) and presence of cocaine (Fig. 2) to allow clear descriptions of effects on inherent and cocaine-evoked behavioral disinhibition, respectively.
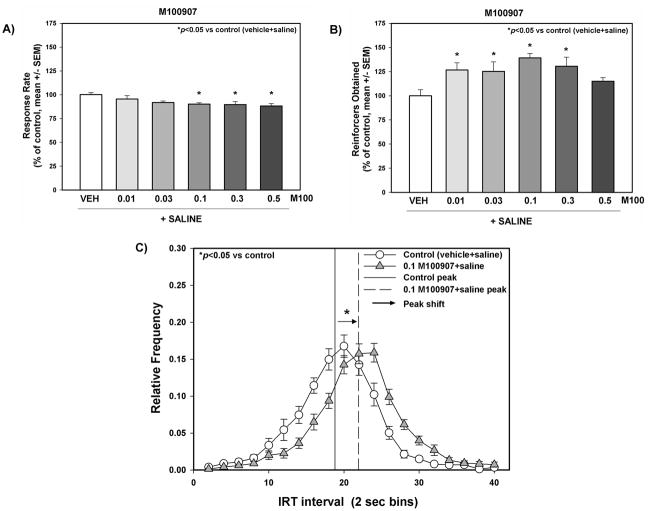
Figure 1. The effects of M100907 administration on behavioral disinhibition measured with the DRL-20 sec task.
Response rate (A) and number of reinforcers (0.25 sec water deliveries) obtained (B) on the DRL-20 s task test day are presented as % of control (vehicle+saline) (mean ± SEM). (C) The relative frequency (frequency of inter-response time/total number of responses ± SEM) of a given inter-response interval for control and M100907 (0.1 mg.kg) is represented as a single point for each 2-s time bin. M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg) was administered 15 min prior to saline injections; vehicle (1 mL/kg saline or 1% Tween 80) was administered immediately before DRL-20 sec task test sessions in a counterbalanced within-subjects design. “→” Represents the peak shift for 0.1 mg/kg M100907 versus the control peak relative frequency. Statistical comparisons of descriptive statistics for the inter-response time distributions can be found in Table 1. *p<0.05 vs. control ^p<0.05 vs. vehicle+cocaine (one way repeated measures ANOVA with Dunnett’s procedure)
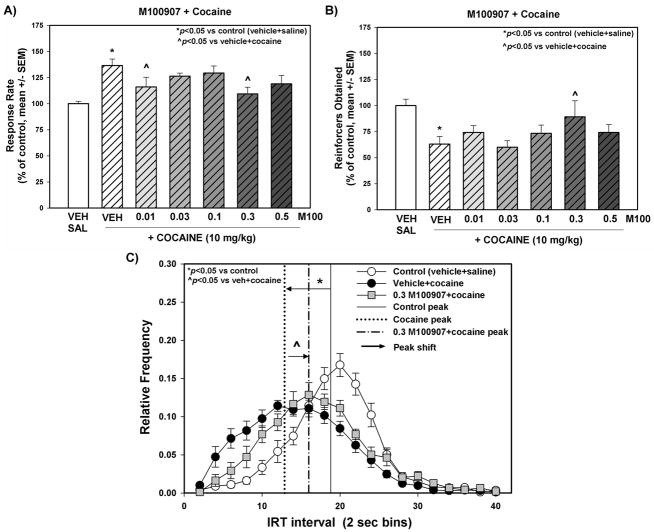
Figure 2. The effects of M100907 in combination with cocaine administration on behavioral disinhibition measured with the DRL-20 sec task.
Response rate (A) and number of reinforcers (0.25 sec water deliveries) obtained (B) on the DRL-20 s task test day are presented as % of control (vehicle+saline) treatment (mean ± SEM). (C) The relative frequency (frequency of inter-response time/total number of responses ± SEM) of a given inter-response interval for control and cocaine alone or M100907 (0.3 mg/kg) + cocaine is represented as a single point for each 2-s time bin. M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg) was administered 15 min prior to vehicle or cocaine injections; vehicle (1 mL/kg saline or 1% Tween 80) or cocaine (10 mg/kg) was administered immediately before DRL-20 sec task test sessions in a counterbalanced within-subjects design. “←” Represents the peak shift for cocaine versus the control peak relative frequency; “→” represents the peak shift for 0.3 mg/kg M100907 + cocaine versus cocaine peak relative frequency. Statistical comparisons of descriptive statistics for the inter-response time distributions can be found in Table 2. *p<0.05 vs. control ^p<0.05 vs. vehicle+cocaine (one way repeated measures ANOVA with Dunnett’s procedure)
5-HT2AR regulation of inherent behavioral disinhibition and cocaine-evoked disruption of behavioral disinhibition in the 1-CSRT task
Apparatus
1-CSRT task procedures took place in eight standard five-hole nosepoke operant chambers (Med Associates, Roanoke, VA) contained within a ventilated and sound-attenuating chamber. Each chamber was fitted with an array of five evenly-spaced response apertures (2.5 × 2.5 × 2.2 cm) positioned 2 cm above a bar floor, and each aperture contained a stimulus light. Nosepoke responses into these apertures were detected by a horizontally-positioned infrared beam located 1 cm from the entrance to each hole. The chambers are also fitted on the opposite wall with a houselight, food tray, and an external pellet dispenser capable of delivering 45 mg pellets (Dustless Precision pelletsR, Bio-Serv, Frenchtown, NJ) to the food tray. The chambers were connected via an interface (Med Associates) to a PC computer running Med PC for Windows software (Med Associates) that controlled and recorded all experimental events.
Training
Rats (n=8) were allowed one week to acclimate to the colony after which food restriction began. Rats were weighed daily to ensure that body weights were maintained at 90% of free-feeding levels during an initial seven days of food restriction prior to initiation of 1-CSRT task training. Training began with a pre-training stage (PT-1) in which the rat was habituated to the test chamber and introduced to nosepoke responding for food pellets. During PT-1, all responses made in the correctly lit (center) hole resulted in the illumination of the magazine light and presentation of a single food pellet. Retrieval of the food pellet from the food magazine resulted in termination of the magazine light, and after a 2-s intertrial interval (ITI) the next trial began with the stimulus (center) hole illumination. After reaching a criteria of 30 correct trials (i.e., reinforced trials) within the 60-min daily session, rats advanced to pre-training stage 2 (PT-2) which required a correct response in a given timeframe to receive a reinforcer. During PT-2, the stimulus light was illuminated for 60 s (stimulus duration); subsequently, the stimulus light was extinguished for 60 s (limited hold). Any correct response made during either time period (i.e., stimulus duration or limited hold) resulted in presentation of the reinforcer; incorrect responses (nosepoke in any hole other than the center hole) and/or omitted responses were recorded and now resulted in a 5-s time out period in which the house light was illuminated and no reinforcer delivered. In PT-2, premature responses were recorded but did not result in a time out period. The training stages thereafter were each comprised of daily sessions of 100 trials to be completed in a maximum of 30 min; each training stage involved incrementally lowering the stimulus duration with a 5-s limited hold and an ITI of 5 s. Thus, a maximum of 100 correct responses in a session would result in a maximum of 100 reinforcers delivered; incorrect or premature responses or omissions resulted in a 5-s time out period and a reduction in potential reinforcers obtained. Rats were required to meet an acquisition criteria of a minimum of 50 correct responses, >80% accuracy [correct responses/(correct + incorrect) × 100] and <20% omissions (omitted responses/trials completed × 100) to move from one training stage to the next.
Pharmacological test sessions commenced after animals met the acquisition criteria of >80% accuracy and <20% omissions for at least five consecutive training stages on the final training stage (0.5 s stimulus duration, 5 s limited hold, 5 s ITI) with less than 15% variability across training days. Pretreatment with M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg, i.p) or vehicle (1% Tween 80 in saline, i.p.) occurred 15 min prior to an injection with saline (1 ml/kg, i.p.) or cocaine (10 mg/kg, i.p) immediately before the start of the 1-CSRT task session. Rats received saline or vehicle injections on Mondays and Thursdays and M100907 or cocaine injections on Tuesdays and Fridays; the order of M100907 and cocaine injections was randomized across all rats in a within-subjects design.
Data Analysis
During the 1-CSRT task, the total number of responses (correct, incorrect, omissions, and premature) as well as the latency to start the task (sec) and time to finish (min) were recorded. As rats were well-trained upon achieving the task criterion, incorrect responses consistently accounted for less than 5% of total responses during 1-CSRT task test sessions. Percent accuracy [(correct responses/correct + incorrect) × 100] was used as a general indication of the attentional capacity of each rat. Percent omissions (omitted responses/trials completed × 100) during the 1-CSRT task were indicative of the motivation level of each rat to perform the task. Responses during the ITI (premature responses) and total number of reinforcers obtained (correct responses) during the 1-CSRT task session were used to assess behavioral disinhibition. Premature responses and total number of reinforcers obtained on test sessions (M100907 ± cocaine or saline) are presented as the percentage of values observed on the maintenance session immediately prior to the test session; prior to this maintenance session, rats are injected with vehicle+saline (control).
Statistical analyses (two-way repeated measures ANOVA) of the effects of M100907 (pretreatment) alone or in presence of cocaine (treatment) administration on 1-CSRT task measures [% accuracy, % omissions, premature responses, reinforcers obtained, latency to start, time to finish] were conducted as described above for the DRL-20 sec task data analysis. Graphic presentations of data are provided separately for the effects of M100907 in the absence (Fig. 3) and presence of cocaine (Fig. 4) to allow clear descriptions of effects on inherent and cocaine-evoked behavioral disinhibition, respectively.
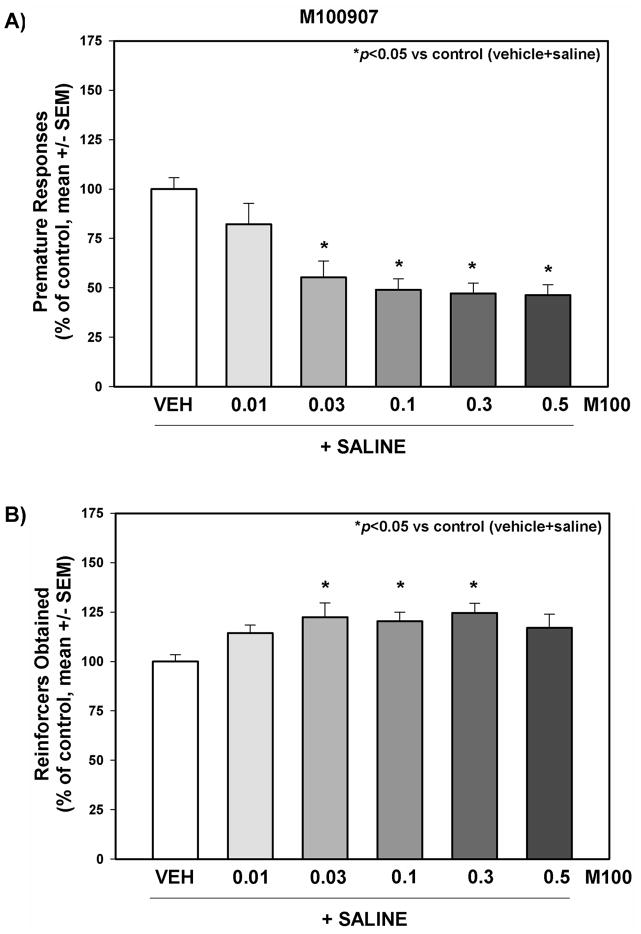
Figure 3. The effects of M100907 administration on behavioral disinhibition measured with the 1-CSRT task.
Premature responses (A) and reinforcers obtained (B) on the 1-CSRT task test day are presented as % of control (vehicle+saline) treatment (mean + SEM). M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg) was administered 15 min prior to saline injections; vehicle (1 mL/kg saline or 1% Tween 80) was administered immediately before 1-CSRT task test sessions in a counterbalanced within-subjects design. *p<0.05 vs. control ^p<0.05 vs. vehicle+cocaine (one way repeated measures ANOVA with Dunnett’s procedure)
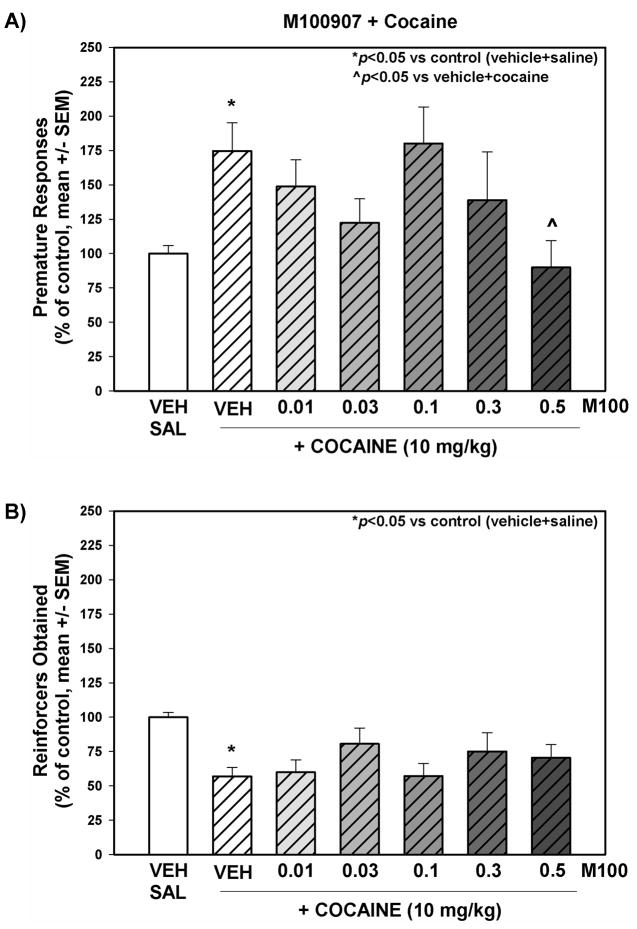
Figure 4. The effects of M100907 in combination with cocaine administration on behavioral disinhibition measured with the 1-CSRT task.
Premature responses (A) and reinforcers obtained (B) on the 1-CSRT task test day are presented as % of control (vehicle+saline) treatment (mean + SEM). M100907 (0.01, 0.03, 0.1, 0.3, 0.5 mg/kg) was administered 15 min prior to vehicle or cocaine injections; vehicle (1 mL/kg saline or 1% Tween 80) or cocaine (10 mg/kg) was administered immediately before 1-CSRT task test sessions in a counterbalanced within-subjects design. *p<0.05 vs. control ^p<0.05 vs. vehicle+cocaine (one way repeated measures ANOVA with Dunnett’s procedure)
RESULTS
5-HT2AR regulation of inherent behavioral disinhibition and cocaine-evoked disruption of behavioral disinhibition in the DRL-20 sec task
We evaluated rats injected with M100907 or vehicle (pretreatment) prior to saline or cocaine injection (treatment) in the DRL-20 s task to determine the effects of M100907 on inherent or cocaine-evoked behavioral disinhibition; the response rate, number of reinforcers obtained, IRT distribution, burst responding, and peak shift were the measures analyzed. Significant main effects of pretreatment (F5,58 = 2.46, p<0.05), treatment (F1,58 = 73.73, p<0.001) and pretreatment × treatment interaction (F5,58 = 3.85, p<0.01) on the response rate in the DRL-20 sec task were observed. There were significant main effects of pretreatment (F5,58 = 2.76, p<0.05) and treatment (F1,58 = 108.43, p<0.001), but no significant pretreatment × treatment interaction (F5,58 = 1.51, n.s.), on the number of reinforcers obtained in the DRL-20 s task. A significant main effect of treatment (F1,58 = 105.74, p<0.001) on the mean of the IRT distribution was observed, but the effect of pretreatment (F5,58 = 1.68, n.s.) and the pretreatment × treatment interaction (F5,58 = 1.56, n.s.) were nonsignificant. There was a trend toward a main effect of pretreatment (F5,58 = 2.18, p=0.06), a significant main effect of treatment (F1,58 = 137.22, p<0.001), but no significant pretreatment × treatment interaction (F5,58 = 1.31, n.s.) for the median of the IRT distribution. There was a significant main effect of treatment (F1,58 = 78.39, p<0.001), but no significant effect of pretreatment (F5,58 = 0.82, n.s.) or pretreatment × treatment interaction (F5,58 = 2.08, n.s.), for the mode of the IRT distribution. There was a significant main effect of treatment (F1,58 = 11.40, p<0.01), no significant main effect of pretreatment (F5,58 = 1.85, n.s), and a trend toward a pretreatment × treatment interaction (F5,58 = 2.23, p=0.06), on burst responding. There were significant main effects of treatment (F1,58 = 79.21, p<0.001) and of pretreatment (F5,58 = 5.03, p<0.001), and a trend toward a pretreatment × treatment interaction (F5,58 = 2.14, p=0.07), on the peak shift in the IRT distribution.
Effects of M100907 on inherent behavioral disinhibition in the DRL-20 sec task
We analyzed the data for rats pretreated with M100907 or vehicle prior to saline treatment in the DRL-20 s task to determine the effects of M100907 on inherent behavioral disinhibition. Figure 1 displays the response rate (Fig. 1A) and the number of reinforcers obtained (Fig. 1B) for vehicle and each dose of M100907; data are presented as a percentage of the response rate and number of reinforcers obtained on the vehicle+saline administration session (control) that preceded each dose evaluated. The average response rate/session across all control sessions (see Methods) was 3.13 ± 0.1 responses/min, while the number of reinforcers obtained/session was 50.51 ± 3.1. M100907 administration altered behavioral disinhibition in the DRL-20 sec task (Fig. 1). A significant main effect of pretreatment on response rate (F5,50 = 3.45, p<0.01) was observed; planned comparisons with Dunnett’s procedure showed that the doses of 0.1, 0.3 and 0.5 mg/kg M100907 decreased the response rate versus control (Fig. 1A). A significant main effect of pretreatment was also observed for number of reinforcers obtained (F5,58 = 5.55, p<0.001); planned comparisons with Dunnett’s procedure showed that the doses of 0.01, 0.03, 0.1, and 0.3 mg/kg M100907 increased the number of reinforcers obtained versus control (Fig. 1B).
The descriptive statistics for IRT distribution for each dose of M100907 administered are presented in Table 1. A significant main effect of pretreatment on the mean (F5,58 = 2.51, p<0.05) and median (F5,58 = 3.53, p<0.01), but not the mode (F5,58 = 1.69, n.s), of the IRT distribution were observed. Planned comparisons with Dunnett’s procedure showed that 0.1 mg/kg of M100907 increased the mean of the IRT distribution versus control; 0.1 and 0.5 mg/kg M100907 increased the median of the IRT distribution versus control; 0.1 mg/kg M100907 increased the mode of the IRT distribution versus control (Table 1). The IRT distributions for control and 0.1 mg/kg M100907 are shown across 2-s time bins (Fig. 1C). There was no significant main effect of pretreatment (F5,58 = 1.36, n.s.) on burst responding versus control. There was a significant main effect of pretreatment (F6,73 = 13.21, p<0.001) on the peak shift in the IRT distribution; planned comparisons with Dunnett’s procedure showed that M100907 at 0.1 (Fig. 1C) and 0.5 mg/kg (data not shown) shifted the IRT distribution right versus control.
Table 1.
IRT distribution descriptive statistics for M100907 administration.
| IRT descriptive statistics (mean ± SEM) | ||||
|---|---|---|---|---|
| M100907 Pretreatment | Treatment | Mean | Median | Mode |
| Vehicle | Saline | 19.06 ± 0.69 | 18.56 ± 0.64 | 18.81 ± 0.87 |
| 0.01 mg/kg | Saline | 20.58 ± 0.55 | 20.25 ± 0.53 | 20.50 ± 0.85 |
| 0.03 mg/kg | Saline | 21.50 ± 0.78 | 21.06 ± 0.75 | 20.63 ± 1.31 |
| 0.1 mg/kg | Saline | 22.48 ± 0.82* | 21.72 ± 0.49* | 21.94 ± 0.61* |
| 0.3 mg/kg | Saline | 20.52 ± 1.21 | 20.00 ± 0.91 | 19.88 ± 1.09 |
| 0.5 mg/kg | Saline | 22.54 ± 1.59 | 21.63 ± 1.16* | 20.50 ± 1.12 |
p<0.05 vs. control (vehicle+saline)
Effects of M100907 on cocaine-evoked disruption of behavioral disinhibition in the DRL-20 sec task
We analyzed the data for rats pretreated with M100907 or vehicle prior to cocaine treatment in the DRL-20 s task to determine the effects of M100907 on cocaine-evoked behavioral disinhibition. Figure 2 displays the response rate (Fig. 2A) and the number of reinforcers obtained (Fig. 2B) for vehicle and each dose of M100907 (pretreatment) in the presence of cocaine (treatment); data are presented as a percentage of the response rate and number of reinforcers obtained on the vehicle+saline administration session (control) that preceded each dose evaluated. The average response rate/session across all control sessions (see Methods) in vehicle+saline-treated animals was 3.13 ± 0.1 responses/min, while the number of reinforcers obtained/session was 50.51 ± 3.1. Cocaine administration altered behavioral disinhibition in the DRL-20 sec task (Fig. 2). A significant main effect of treatment on response rate (F6,72 = 5.66, p<0.001) was observed. Planned comparisons with Dunnett’s procedure showed that cocaine increased the response rate versus control (Fig. 2A, p<0.05); 0.01 and 0.3 mg/kg M100907 attenuated the cocaine-induced increases in response rate (Fig. 2A; p<0.05). Consistent with these changes in response rate, we also observed changes in the number of reinforcers obtained following pharmacological manipulations in the DRL-20 s task. A significant main effect of treatment on reinforcers obtained (F6,69 = 5.19, p<0.001) was observed. Planned comparisons with Dunnett’s procedure showed that cocaine decreased the number of reinforcers obtained versus control (Fig. 2B; p<0.05); and 0.3 mg/kg M100907 prevented the cocaine-induced decrease in number of reinforcers obtained (Fig. 2B; p<0.05).
The descriptive statistics for IRT distribution for each dose of M100907 in the presence of cocaine are presented in Table 2. Significant main effects of treatment on the mean (F6,73 = 2.73, p<0.05), median (F6,73 = 3.35, p<0.01), and the mode (F6,73 = 3.5, p<0.01), of the IRT distribution were observed. Planned comparisons with Dunnett’s procedure showed that cocaine decreased the mean (p<0.05), median (p<0.05), and mode (p<0.05) of the IRT distribution versus control (Table 2); M100907 did not significantly alter the cocaine-evoked IRT mean (n.s.), median (n.s.), or mode (n.s.) (Table 2). The IRT distributions for cocaine and a representative dose of M100907 (0.3 mg/kg) + cocaine (Fig. 2C) are shown across 2-s time bins. A significant main effect of treatment on burst responding (F6,69 = 2.60, p<0.05) was observed. Planned comparisons with Dunnett’s procedure showed that cocaine enhanced burst responding versus control (p<0.05); and M100907 at 0.01, 0.3 and 0.5 mg/kg attenuated the cocaine-induced increase in burst responding (p<0.05). A significant main effect of treatment on the peak shift in the IRT distribution (F6,71 = 5.04, p<0.001) was observed. Planned comparisons with Dunnett’s procedure showed that cocaine shifted the peak IRT distribution to the left versus control (Fig. 2C, p<0.05); and M100907 at 0.3 mg/kg shifted the cocaine-evoked peak IRT distribution right to control levels (Fig. 2C, p<0.05).
Table 2.
IRT distribution descriptive statistics for M100907 + Cocaine (10 mg/kg) administration.
| IRT descriptive statistics (mean ± SEM) | ||||
|---|---|---|---|---|
| M100907 Pretreatment | Treatment | Mean | Median | Mode |
| Vehicle | Saline | 19.06 ± 0.69 | 18.56 ± 0.64 | 18.81 ± 0.87 |
| Vehicle | Cocaine | 14.49 ± 0.72* | 13.81 ± 0.73* | 12.88 ± 1.58* |
| 0.01 mg/kg | Cocaine | 17.14 ± 2.04 | 15.50 ± 1.22 | 12.38 ± 1.69 |
| 0.03 mg/kg | Cocaine | 16.28 ± 0.85 | 16.25 ± 0.98 | 16.13 ± 1.43 |
| 0.1 mg/kg | Cocaine | 15.60 ± 0.88 | 14.94 ± 0.95 | 11.69 ± 1.52 |
| 0.3 mg/kg | Cocaine | 17.06 ± 0.97 | 16.50 ± 0.94 | 16.00 ± 0.82 |
| 0.5 mg/kg | Cocaine | 16.57 ± 1.25 | 15.88 ± 1.39 | 14.25 ± 2.05 |
p<0.05 vs. control (vehicle+saline)
5-HT2AR regulation of inherent behavioral disinhibition and cocaine-evoked disruption of behavioral disinhibition in the 1-CSRT Task
We analyzed the data for rats pretreated with M100907 or vehicle prior to saline or cocaine treatment in the 1-CSRT task to determine the effects of M100907 on inherent or cocaine-evoked behavioral disinhibition. Significant main effect of pretreatment (F5,42 = 4.57, p<0.01) and treatment (F1,42 = 46.58, p<0.001), but no significant pretreatment × treatment interaction (F5,42 = 1.10, n.s.), on premature responses in the 1-CSRT task were observed. Significant main effects of pretreatment (F5,42 = 2.53, p<0.05) and treatment (F1,42 = 93.88, p<0.001), but no significant pretreatment × treatment interaction (F5,42 = 0.55, n.s.), on reinforcers obtained in the 1-CSRT task were observed. The % accuracy measure in the 1-CSRT task exhibited no significant main effect of pretreatment (F5,42 = 1.16, n.s.), a significant main effect of treatment (F1,42 = 7.73, p<0.01) and no significant pretreatment × treatment interaction (F5,42 = 0.41, n.s.). The % omissions made in the 1-CSRT task exhibited no significant main effect of pretreatment (F5,42 = 0.63, n.s.), a significant main effect of treatment (F1,42 = 5.84, p<0.05) and no significant pretreatment × treatment interaction (F5,42 = 0.74, n.s.). Analysis of the latency to start the 1-CSRT task showed no significant main effect of pretreatment (F5,40 = 0.45, n.s.) or treatment (F1,40 = 0.04, n.s.) or pretreatment × treatment interaction (F5,40 = 0.9, n.s.). Analysis of time to finish the 1-CSRT task showed no significant effect of pretreatment (F5,42 = 0.5, n.s.), a significant main effect of treatment (F1,42 = 12.59, p<0.05) and no significant pretreatment × treatment interaction (F5,42 = 0.21, n.s.).
Effects of M100907 on inherent behavioral disinhibition in the 1-CSRT task
The effects of M100907 on behavioral disinhibition indices in the 1-CSRT task were evaluated in rats pretreated with M100907 or vehicle prior to saline treatment in the 1-CSRT task. Figure 3 displays the number of premature responses (Fig. 3A) and reinforcers obtained (Fig. 3B) for vehicle and each dose of M100907 tested; data are presented as a percentage of the number of responses made or reinforcers obtained on the vehicle+saline administration session (control) that preceded each dose evaluated. The average number of premature responses/session across all control sessions (see Methods) in vehicle+saline-treated animals was 31.47 ± 1.8, while the number of reinforcers obtained/session was 61.94 ± 2.1. M100907 administration significantly altered behavioral disinhibition in the 1-CSRT task (Fig. 3). There was a significant main effect of M100907 on premature responses (F5,42 = 10.07, p<0.001); planned comparisons with Dunnett’s procedure showed 0.03, 0.1, 0.3 and 0.5 mg/kg M100907 decreased premature responses versus control (Fig. 3A). There was a significant main effect of M100907 on the number of reinforcers obtained (F5,42 = 2.72, p<0.05); planned comparisons with Dunnett’s procedure showed 0.03, 0.1, and 0.3 mg/kg M100907 increased the number of reinforcers obtained responses versus control (Fig. 3B).
Descriptive statistics for % accuracy, % omissions, latency to start and time to finish the 1-CSRT task for vehicle, cocaine and each dose of M100907 administered are presented in Table 3. There were no significant main effects of pretreatment on % accuracy (F5,42 = 0.63, n.s.), % omissions (F5,36 = 0.64, n.s.), latency to start (F5,40 = 0.72, n.s.) or time to finish (F5,42 = 1.05, n.s.) in the 1-CSRT task (Table 3).
Table 3.
1-CSRT descriptive statistics for M100907 administration.
| 1-CSRT task descriptive statistics (mean ± SEM) | |||||
|---|---|---|---|---|---|
| M100907 Pretreatment | Treatment | % Accuracy | % Omissions | Latency to Start (sec) | Time to Finish (sec) |
| Vehicle | Saline | 100 ± 0.27 | 4.31 ± 0.45 | 2.63 ± 0.79 | 926.60 ± 27.52 |
| 0.01 mg/kg | Saline | 100.82 ± 0.52 | 4.08 ± 1.32 | 0.63 ± 0.26 | 888.25 ± 22.91 |
| 0.03 mg/kg | Saline | 99.58 ± 0.55 | 4.95 ± 1.45 | 4.13 ± 3.70 | 1016.50 ± 109.59 |
| 0.1 mg/kg | Saline | 100.23 ± 1.05 | 7.05 ± 2.55 | 1.38 ± 0.84 | 990.38 ± 55.66 |
| 0.3 mg/kg | Saline | 99.98 ± 0.42 | 6.56 ± 1.72 | 1.50 ± 1.36 | 1077.00 ± 77.26 |
| 0.5 mg/kg | Saline | 100.83 ± 0.68 | 3.80 ± 1.65 | 0.25 ± 0.16 | 964.63 ± 55.56 |
Effects of M100907 on cocaine-evoked disruption of behavioral disinhibition in the 1-CSRT task
Figure 4 displays the number of premature responses (Fig. 4A) and reinforcers obtained (Fig. 4B) for each dose of M100907 in the presence of cocaine; data are presented as a percentage of the number of responses made or number of reinforcers obtained on the vehicle+saline administration session (control) that preceded each dose evaluated. The average number of premature responses/session across all control sessions (see Methods) in vehicle+saline-treated animals was 31.47 ± 1.8, while the number of reinforcers obtained/session was 61.94 ± 2.1. Cocaine administration altered behavioral disinhibition in the 1-CSRT task (Fig. 4). A significant main effect of treatment on premature responses (F6,48 = 2.36, p<0.05) was observed. Planned comparisons with Dunnett’s procedure showed that cocaine increased the number of premature responses versus control (Fig. 4A; p<0.05); and 0.5 mg/kg M100907 prevented the cocaine-induced increase in premature responses (Fig. 4A; p<0.05). There was a significant main effect of treatment on the number of reinforcers obtained (F6,49 = 2.75, p<0.05). Planned comparisons with Dunnett’s procedure showed that cocaine increased the number of premature responses versus control (Fig. 4B; p<0.05); M100907 did not alter the number of reinforcers obtained after 10 mg/kg cocaine treatment (Fig. 4B; n.s.).
Descriptive statistics for % accuracy, % omissions, latency to start and time to finish the 1-CSRT task for vehicle, cocaine and each dose of M100907 in combination with cocaine are presented in Table 4. There were no significant main effects of treatment on % accuracy (F6,49 = 1.01, n.s.), % omissions (F6,41 = 0.88, n.s.), latency to start (F6,48 = 0.55, n.s.) or time to finish (F6,49 = 0.74, n.s.) in the 1-CSRT task (Table 4). Cocaine did not alter % accuracy, % omissions, the latency to start or time to finish the 1-CSRT task versus control (Table 4; n.s.). M100907 did not alter % accuracy, % omissions, the latency to start or time to finish the 1-CSRT task versus vehicle+cocaine (Table 4; n.s.).
Table 4.
1-CSRT descriptive statistics for M100907 + Cocaine (10 mg/kg) administration.
| 1-CSRT task descriptive statistics (mean ± SEM) | |||||
|---|---|---|---|---|---|
| M100907 Pretreatment | Treatment | % Accuracy | % Omissions | Latency to Start (sec) | Time to Finish (sec) |
| Vehicle | Saline | 100 ± 0.27 | 4.45 ± 0.41 | 2.63 ± 0.79 | 926.60 ± 27.52 |
| Vehicle | Cocaine | 98.90 ± 0.54 | 9.65 ± 1.66 | 1.38 ± 1.12 | 1103.38 ± 126.54 |
| 0.01 mg/kg | Cocaine | 99.55 ± 1.19 | 12.13 ± 4.90 | 2.88 ± 1.48 | 1242.50 ± 278.08 |
| 0.03 mg/kg | Cocaine | 98.31 ± 0.87 | 6.08 ± 1.00 | 1.38 ± 0.53 | 1402.25 ± 202.26 |
| 0.1 mg/kg | Cocaine | 97.30 ± 1.87 | 9.05 ± 2.44 | 2.13 ± 1.43 | 1257.25 ± 254.97 |
| 0.3 mg/kg | Cocaine | 98.59 ± 1.00 | 5.12 ± 1.50 | 1.17 ± 0.65 | 1447.13 ± 315.85 |
| 0.5 mg/kg | Cocaine | 100.29 ± 0.68 | 14.85 ± 5.66 | 1.00 ± 0.50 | 1453.63 ± 247.75 |
DISCUSSION
This study replicates and extends previous work demonstrating a role for the 5-HT2AR in inherent behavioral disinhibition and demonstrates that cocaine enhances behavioral disinhibition in two distinct behavioral models of impulsive action. Treatment with the selective 5-HT2AR antagonist M100907 attenuated behavioral disinhibition in the DRL-20 sec task, as evidenced by a decrease in the response rate and concomitant increase in the number of reinforcers obtained as well as a rightward shift of the IRT distribution curve. Similarly, M100907 attenuated behavioral disinhibition in the 1-CSRT task, as shown by a decrease in the number of premature responses and increase in the number of reinforcers obtained. In contrast, cocaine administration increased behavioral disinhibition in both the DRL-20 sec task (increased response rate; decreased reinforcers obtained; increased burst responding; leftward shift in the IRT distribution curve) and the 1-CSRT task (increased premature responses; decreased reinforcers obtained). M100907 moderately attenuated the cocaine-induced disruption of behavioral disinhibition in the DRL-20 sec (response rate, burst responding, peak shift) and 1-CSRT (premature responses) tasks. Thus, the 5-HT2AR represents a neurobiological substrate which regulates differential facets of behavioral disinhibition as both inherent and cocaine-disrupted behavioral disinhibition are responsive to pharmacological antagonism of the 5-HT2AR.
A vast literature links alterations in 5-HT function and impulsivity (for review, see Pattij & Vanderschuren 2008); however, the neurobiological mechanisms through which 5-HT regulates impulsivity are inconclusive, most likely due to the complex nature of the 5-HT system. The actions of 5-HT are mediated through 14 receptor family subtypes with its actions at the 5-HT2R family (e.g., 5-HT2AR, 5-HT2CR) being the most widely studied in relation to impulsive action. There is considerable evidence that inhibition of 5-HT2AR-mediated transmission through either pharmacological or gene deletion techniques attenuates inherent behavioral disinhibition (present study; Winstanley et al. 2003, 2004a,b, 2006a; Harrison et al. 1997; Higgins et al. 2003; Fletcher et al. 2007, 2009; Robinson et al. 2008). M100907 is one of the most selective 5-HT2AR antagonists available for preclinical studies (Bubar & Cunningham 2008) with 100-fold selectivity for the 5-HT2AR (Ki = 0.85 nM) over the homologous 5-HT2CR (Ki = 88 nM) (Kehne et al. 1996). Low doses of M100907 similar to those employed in this study have been shown to preferentially inhibit the effects of preferential 5-HT2AR agonists (McCreary et al. 2003; Hitchcock et al. 1997; Wettstein et al. 1999; Gresch et al. 2007), but not selective 5-HT2CR agonists (Dekeyne et al. 1999; Vickers et al. 2001), suggesting a selective, functional antagonism of the 5-HT2AR at the doses chosen here (Nic Dhonnchadha et al. 2009; Dekeyne et al. 1999; McCreary et al. 2003; Gresch et al. 2007; Hitchcock et al. 1997). In accordance with the aforementioned studies, M100907 potently diminished behavioral disinhibition (present study) in both the DRL-20 sec and 1-CSRT tasks, and this was not associated with alterations in additional measures of executive function (e.g., attention, motivation, the latency to start or time to finish the 1-CSRT task), indicating that M100907 does not alter the performance of the tasks and supporting a role for tonic activation of the 5-HT2AR to attune inherent levels of impulse control.
The limbic-corticostriatal circuit may be an important site of action for the 5-HT2AR to modulate impulsivity (Pompeiano et al. 1994; Bubar & Cunningham 2008). Extensive lines of evidence implicate serotonergic mechanisms within this circuit in behavioral disinhibition (Winstanley et al. 2006a; Pattij & Vanderschuren 2008; Robinson et al. 2008). A positive correlation between 5-HT release in the mPFC and increased premature responding on the 1-CSRT task has been reported (Dalley et al. 2002). In addition, intra-prefrontal cortex and intra-accumbens administration of M100907 decreased impulsive responding in the 5-CSRT task (Winstanley et al. 2003; Robinson et al. 2008). These data indicate that disruption of the tonic level of activation of the 5-HT2AR within the limbic-corticostriatal circuit plays a key role in impulsivity. The 5-HT2AR expressed within the limbic-corticostriatal circuit serves to modulate dopaminergic (Broderick et al. 2004; Auclair et al. 2004), noradrenergic (Millan et al. 2000) as well as glutamatergic neurotransmission (Santana et al. 2004; Carli et al. 2006). Levels of DA and its metabolite DOPAC have been shown to rise in the mPFC of rats during performance of the 1-CSRT task, while highly impulsive rats had higher DA turnover rates in the mPFC (Dalley et al. 2002). Further, inhibition of α1-noradrenoceptors, but not α2-noradrenoceptors abates enhanced premature responding in the 5-CSRT task induced by 5-HT2AR activation (Koskinen et al. 2003). Thus, 5-HT2AR modulation of serotonergic, dopaminergic, and/or noradrenergic neurotransmission within the limbic-corticostriatal circuits may be an important mechanism by which the 5-HT2AR influences impulsive behaviors (Winstanley et al. 2004b; Koskinen et al. 2003). Therefore, further exploration into the complex regulation of the 5-HT2AR over 5-HT:DA:NE balance within the corticolimbic circuit as it relates to impulsivity is imperative to elucidate the utility of 5-HT2AR ligands (e.g., antagonists, inverse agonists) as treatments for disorders characterized by impulse control deficits (e.g., drug abuse, eating disorders, etc.).
Impulsivity is a complex, non-unitary construct that is associated with cocaine abuse and dependence (Evenden 1999; Moeller et al. 2001b). In the current study, acute cocaine administration produced the hypothesized effects on behavioral disinhibition; that is, acute cocaine treatment enhanced behavioral disinhibition as measured by both the DRL-20 sec and 1-CSRT tasks. Acute cocaine treatment has previously been reported to increase impulsive action as measured in the Go/No-go task (Paine et al. 2003; Paine & Olmstead 2004), and premature responding in the DRL task (present study; Wenger & Wright 1990; Woolverton et al. 1978; Stoffel & Cunningham 2008; Wang et al. 2001). Further, acute administration of cocaine disrupted executive function as evidenced by a decrease in accuracy and increase in both omissions and premature responding in the 5-CSRT task (Winstanley et al. 2007). In the present study, cocaine did not alter accuracy or motivation in the 1-CSRT task, underscoring the utility of the 1-CSRT task to selectively measure behavioral disinhibition independent from effects on visuospatial processes (Dalley et al. 2002).
The apparent effectiveness of the selective 5-HT2AR antagonist M100907 to suppress inherent behavioral disinhibition is greater than its effectiveness to suppress cocaine-evoked behavioral disinhibition. The dose-dependent effects of M100907 to suppress behavioral disinhibition appear in a narrow dose range (0.01–0.1 mg/kg) and are limited to certain aspects of behavioral disinhibition. The diminished effects of M100907 at the higher doses may be due to a loss of selectivity for the 5-HT2AR and may reflect combined actions of M100907 at both the 5-HT2AR and 5-HT2CR, which tend to have oppositional effects upon steady-state and/or cocaine-evoked behaviors (Bubar & Cunningham 2008). The dose of cocaine (10 mg/kg) employed in this study was previously demonstrated to evoke maximal behavioral disinhibition in the DRL-20 sec task (Stoffel & Cunningham 2008), but did not produce maximal disruption of behavioral disinhibition in the 5-CSRT task (Winstanley et al. 2007). While the present findings support a role for the 5-HT2AR in cocaine-induced behavioral disinhibition, the effectiveness of M100907 is influenced by the manner in which cocaine impacts the neurochemistry involved in the generation of impulsivity. Serotonin, DA, and NE each have been implicated as mediators in multiple aspects of impulsivity (for review, see Pattij & Vanderschuren 2008). Given that this dose of cocaine would be expected to enhance synaptic levels of all three monoamines in the limbic-corticostriatal circuit (Koe 1976; Bradberry & Roth 1989; Kalivas & Duffy 1990; Broderick et al. 2004; Bubar et al. 2003), blockade of 5-HT2AR stimulation consequent to cocaine-evoked 5-HT release (Broderick et al. 2004) might be expected to modulate only the specific components of impulsivity related to the 5-HT2AR such as the response rate, burst responding, and/or the number of premature responses. Thus, additional aspects of enhanced impulsive behavior observed following cocaine administration are most likely mediated by monoamine neurotransmission that is not solely modulated by the 5-HT2AR and may likely be determined by the balance of function at multiple 5-HT2R subtypes (e.g., 5-HT2CR) (Fletcher et al. 2007), DA receptors (e.g., D1-like, D2-like) (van Gaalen et al. 2006; Koskinen & Sirvio 2001; Passetti et al. 2003), and/or NE receptors (e.g., α1 NE) (Koskinen et al. 2003).
From a translational perspective, cocaine-dependent subjects often present with both impulsivity (Moeller et al. 2001b, 2002, 2004) and high levels of reactivity to cocaine-associated cues (O’Brien et al. 1998; Carter & Tiffany 1999; Modesto-Lowe & Kranzler 1999), and studies in amphetamine-treated rats suggest that impulsivity and cue reactivity may be intertwined (Cardinal et al. 2000), thereby adding an additional layer of complexity to the relationship between cocaine exposure and impulsivity. Data from the current study and previous studies investigating the incentive motivational aspects of cocaine-associated cues (Nic Dhonnchadha et al. 2009) suggest that the 5-HT2AR serves as a functional rheostat over both impulsive behavior and cue reactivity. Thus, the 5-HT2AR may represent a promising pharmacotherapeutic for cocaine dependence in which impulsivity is a core trait (Moeller et al. 2001b) and a selective 5-HT2AR antagonist may help to prevent relapse and promote abstinence in cocaine-dependent individuals by reducing impulsivity and cue-reactivity.
In conclusion, M100907 attenuated inherent levels of behavioral disinhibition in two distinct animal models and is effective at reducing specific aspects of cocaine-evoked disruption of behavioral disinhibition in rats, indicating a key role for the 5-HT2AR in maintaining the balance of monoaminergic interactions underlying impulsivity. As a whole, the results from this study and others suggest that the loss of impulse control is most likely a combination of, and complex interaction between, the multiple phenotypic facets of impulsivity under the influence of the 5-HT2AR system. A greater understanding of the 5-HT:DA:NE etiology of impulse control disorders associated with cocaine use and the benefits of intervention strategies that normalize the balance within 5-HT2R systems may reveal ways to devise more personalized therapeutics based on levels of impulsivity in cocaine-dependent patients.
Acknowledgments
Funding: This work was supported by the National Institute on Drug Abuse grants DA006511 (KAC), DA000260 (KAC), DA020087 (KAC), DA07287 (KAC), DA000403 (FGM), the Peter F. McManus Charitable Trust (KAC), the Jeane B. Kempner Postdoctoral Scholar Award (NCA) and the Ruth L. Kirschstein National Research Service Award DA0121438 (ECS).
The research was supported by the National Institute on Drug Abuse grants DA006511 (KAC), DA000260 (KAC), DA020087 (KAC), DA07287 (KAC), DA00403 (FGM), the Peter F. McManus Charitable Trust (KAC), the Jeane B. Kempner Postdoctoral Scholar Award (NCA) and the Ruth L. Kirchstein National Research Service Award DA0121438 (ECS). A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
References
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. The 5-hydroxytryptamine2A receptor antagonist R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinem ethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Ther. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Beck LH, Bransome ED, Jr, Mirsky AF, Rosvold HE, Sarason I. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Roth RH. Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdiaysis. Neurosci Lett. 1989;103:97–102. doi: 10.1016/0304-3940(89)90492-8. [DOI] [PubMed] [Google Scholar]
- Broderick PA, Olabisi OA, Rahni DN, Zhou Y. Cocaine acts on accumbens monoamines and locomotor behavior via a 5-HT2A/2C receptor mechanism as shown by ketanserin: 24-h follow-up studies. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:547–557. doi: 10.1016/j.pnpbp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, McMahon LR, De Deurwaerdere P, Spampinato U, Cunningham KA. Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology. 2003;44:342–353. doi: 10.1016/s0028-3908(02)00381-7. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacol. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacol. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in Impulse Control Associated with Tonically-elevated Serotonergic Function in Rat Prefrontal Cortex. Neuropsychopharmacol. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60–0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de VW, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57:35–46. [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology. 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain Res. 1995;675:45–54. doi: 10.1016/0006-8993(95)00037-q. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacol. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chambers JW, Rizos Z, Chintoh AF. Effects of 5-HT depletion in the frontal cortex or nucleus accumbens on response inhibition measured in the 5-choice serial reaction time test and on a DRL schedule. Behav Brain Res. 2009;201:88–98. doi: 10.1016/j.bbr.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Lister S, Fischer TR, Wettstein JG. Disruption of latent inhibition in the rat by the 5-HT2 agonist DOI: effects of MDL 100,907, clozapine, risperidone and haloperidol. Behav Brain Res. 1997;88:43–49. doi: 10.1016/s0166-4328(97)02315-2. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jolly DC, Richards JB, Seiden LS. Serotonergic mediation of DRL 72s behavior: receptor subtype involvement in a behavioral screen for antidepressant drugs. Biol Psychiatry. 1999;45:1151–1162. doi: 10.1016/s0006-3223(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, Mccarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1973. [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Sirvio J. Studies on the involvement of the dopaminergic system in the 5-HT2 agonist (DOI)-induced premature responding in a five-choice serial reaction time task. Brain Res Bull. 2001;54:65–75. doi: 10.1016/s0361-9230(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Haapalinna A, Sirvio J. Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol. 2003;92:214–225. doi: 10.1034/j.1600-0773.2003.920504.x. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT, Sirvio J. Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology. 2000;39:471–481. doi: 10.1016/s0028-3908(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Li AA, Marek GJ, Vosmer G, Seiden LS. Long-term central 5-HT depletions resulting from repeated administration of MDMA enhances the effects of single administration of MDMA on schedule-controlled behavior of rats. Pharmacol Biochem Behav. 1989;33:641–664. doi: 10.1016/0091-3057(89)90402-4. [DOI] [PubMed] [Google Scholar]
- Liao RM, Chang YH. Different effects of 5-HT receptor agonists on operant response in rats under DRL 10-s and DRL 30-s schedules. Proc Natl Sci Counc Repub China B. 2001;25:223–232. [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Li AA, Seiden LS. Evidence for invovlement of 5-hydroxytryptamine receptors in antidepressant like drug effects on differential reinforcement of low rate 72 second behavior. J Pharmacol Exp Ther. 1989;250:60–71. [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacol. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Filip M, Cunningham KA. Discriminative stimulus properties of (+/-)-fenfluramine: the role of 5-HT2 receptor subtypes. Behav Neurosci. 2003;117:212–221. doi: 10.1037/0735-7044.117.2.212. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: relevance to the actions of antidepressant agents. J Psychopharmacol. 2000;14:114–138. doi: 10.1177/026988110001400202. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Rosvold HE. The use of psychoactive drugs as a neuropsychological tool in studies of attention in man. In: Uhr L, Miller JG, editors. Drugs and behavior. New York: Wiley; 1960. pp. 375–392. [Google Scholar]
- Modesto-Lowe V, Kranzler HR. Using cue reactivity to evaluate medications for treatment of cocaine dependence: a critical review. Addiction. 1999;94:1639–1651. doi: 10.1046/j.1360-0443.1999.941116393.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001a;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001b;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Fischer CJ, Dougherty DM, Reilly EL, Mathias CW, Swann AC. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–173. doi: 10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neurosci Biobehav Rev. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Paine TA, Olmstead MC. Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacology (Berl) 2004;175:443–50. doi: 10.1007/s00213-004-1845-3. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl) 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, van der LJ, Groenink L, van der GJ, Maes RA, Olivier B. Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behav Brain Res. 2003;141:137–145. doi: 10.1016/s0166-4328(02)00345-5. [DOI] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, Peter S, Olivier B. Impulsive-like behavior in differential-reinforcement-of-low-rate 36 s responding in mice depends on training history. Neurosci Lett. 2004;354:169–171. doi: 10.1016/j.neulet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacol. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Richards JB, Layton K, Seiden LS. Amphetamine analogs have differential effects on DRL 36-s schedule performance. Psychopharmacology (Berl) 1995;121:57–65. doi: 10.1007/BF02245591. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Seiden LS. The behavioral effects of sertraline, fluoxetine, and paroxetine differ on the differential-reinforcement-of-low-rate 72-second operant schedule in the rat. Psychopharmacology (Berl) 1999;147:153–161. doi: 10.1007/s002130051155. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Kirillova GP, Gavaler J, Giancola P, Vanyukov MM. Social dominance mediates the association of testosterone and neurobehavioral disinhibition with risk for substance use disorder. Psychol Addict Behav. 2007;21:462–468. doi: 10.1037/0893-164X.21.4.462. [DOI] [PubMed] [Google Scholar]
- van den Broek MD, Bradshaw CM, Szabadi E. Behaviour of ‘impulsive’ and ‘non-impulsive’ humans in a temporal differentiaiont schedule of reinforcement. Personality and Individual Differences. 1987;8:233–239. [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PFC, Schoffelmeer ANM, Vanderschuren LJMJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Simpao A, Sun L, Falk JL, Lau CE. Contribution of the active metabolite, norcocaine, to cocaine’s effects after intravenous and oral administration in rats: pharmacodynamics. Psychopharmacology (Berl) 2001;153:341–52. doi: 10.1007/s002130000568. [DOI] [PubMed] [Google Scholar]
- Wenger GR, Wright DW. Behavioral effects of cocaine and its interaction with d-amphetamine and morphine in rats. Pharmacol Biochem Behav. 1990;35:595–600. doi: 10.1016/0091-3057(90)90296-t. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacol. 2004a;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004b;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacol. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006a;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006b;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Laplant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Impaired acquisition of temporal differentiation performance following lesions of the ascending 5-hydroxytryptaminergic pathways. Psychopharmacology (Berl) 1992;107:373–378. doi: 10.1007/BF02245164. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Kandel D, Schuster CR. Effects of repeated administration of cocaine on schedule-controlled behavior of rats. Pharmacol Biochem Behav. 1978;9:327–37. doi: 10.1016/0091-3057(78)90293-9. [DOI] [PubMed] [Google Scholar]