Abstract
There are two isoforms of selenocysteine (Sec) tRNA[Ser]Sec that differ by a single methyl group, Um34. The non-Um34 isoform supports the synthesis of a subclass of selenoproteins, designated housekeeping, while the Um34 isoform supports the expression of another subclass, designated stress-related selenoproteins. Herein, we investigated the relationship between tRNA[Ser]Sec aminoacylation and Um34 synthesis which is the last step in the maturation of this tRNA. Mutation of the discriminator base at position 73 in tRNA[Ser]Sec dramatically reduced aminoacylation with serine, as did an inhibitor of seryl-tRNA synthetase, SB-217452. Although both the mutation and the inhibitor prevented Um34 synthesis, neither precluded the synthesis of any other of the known base modifications on tRNA[Ser]Sec following microinjection and incubation of the mutant tRNA[Ser]Sec transcript, or the wild type transcript along with inhibitor, in Xenopus oocytes. The data demonstrate that Sec tRNA[Ser]Sec must be aminoacylated for Um34 addition. The fact that selenium is required for Um34 methylation suggests that Sec must be attached to its tRNA for Um34 methylation. This would explain why selenium is essential for the function of Um34 methylase and provides further insights into the hierarchy of selenoprotein expression.
Keywords: Selenium status, selenocysteine, selenocysteine tRNA, selenoproteins, selenoprotein hierarchy, Um34 tRNA methylation
1. Introduction
The biosynthesis of selenocysteine (Sec) was recently established in eukaryotes and archaea, and unlike any other known amino acid in eukaryotes, Sec synthesis occurs on its tRNA (reviewed in [1]). Sec tRNA, designated tRNA[Ser]Sec, is initially aminoacylated with serine in the presence of seryl-tRNA synthetase (SerRS) that is in turn phosphorylated on the seryl moiety to form phosphoseryl-tRNA[Ser]Sec by phosphoseryl-tRNA[Ser]Sec kinase (PSTK) [2]. Phosphoseryl-tRNA[Ser]Sec serves as a substrate for Sec synthase (SecS), wherein the active selenium donor, monoselenophosphate, that is synthesized by selenophosphate synthetase 2 (SPS2) in eukaryotes, replaces the phosphate group in phosphoserine to yield selenocysteyl-tRNA[Ser]Sec [1].
There are two isoforms of tRNA[Ser]Sec that differ from each other by a single methyl group, Um34, and are designated 5-methoxycarbonylmethyluridine (mcm5U) and 5-methoxycarbonylmethyl-2’-O-methyluridine (mcm5Um) [3]. Addition of Um34 is a highly specialized step in the maturation of tRNA[Ser]Sec in that this methylation step is stringently dependent on an intact primary and tertiary structure [4] and requires selenium (5). When mammalian cells or tissues are deficient in selenium, the level of the mcm5U isoform is enriched and the mcm5Um isoform is reduced, while cells and tissues sufficient in selenium have the ratios of these two isoforms reversed [5,6]. Interestingly, the expression of stress-related selenoproteins, such as glutathione peroxidase 1 (GPx1), is dependent on the presence of selenium and their abundance correlates with the presence of mcm5Um [6,7]. On the other hand, the expression of housekeeping selenoproteins, such as the thioredoxin reductase 1 and 3 (TR1 and TR3), is less dependent on selenium status and their expression occurs in the presence of mcm5U [7–9]. As expected, mice and organs lacking the mcm5Um isoform synthesize only housekeeping selenoproteins [9,10]. In addition to the mcm5U modification at position 34, there are only three other modified bases in tRNA[Ser]Sec which are N6-isopentenyladenosine (i6A) at position 37, pseudouridine (ψU) at position 55 and 1-methyladenosine (m1A) at position 58 [3].
The purpose of the present study was to determine the relationship between synthesis of Um34 and aminoacylation of tRNA[Ser]Sec. To elucidate the aminoacylation status of tRNA[Ser]Sec prior to Um34 methylation, we introduced a mutation at position 73, the discriminator base, in the synthetic gene of tRNA[Ser]Sec. This discriminator base is essential in the recognition of tRNA by its corresponding aminoacyl-tRNA synthetase [11] and is, in fact, critical in the aminoacylation of tRNA[Ser]Sec with serine by SerRS [12,13]. The G73→A73 mutant tRNA[Ser]Sec transcript was microinjected into Xenopus oocytes, then isolated after overnight incubation and the base modification status of the resulting products analyzed. The data show that the mcm5Um isoform must be aminoacylated prior to Um34 synthesis. To confirm this observation, a potent inhibitor of SerRS, SB-217452 [14], was co-microinjected with the wild type tRNA[Ser]Sec transcript into oocytes. The mcm5U isoform was synthesized without SerRS activity but not the mcm5Um isoform, providing further evidence that an aminoacylated tRNA[Ser]Secmcm5U is the substrate for Um34 methylase.
2. Materials and methods
2.1. Materials
[α-32P]ATP and [α-32P]UTP (specific activity, 3000 Ci/mmol) and [3H]serine (specific activity, 29.5 Ci/mmol) were purchased from Perkin Elmer. All other commercial products were purchased and used as given below.
2.2. Preparation of tRNA[Ser]Sec mutant and tRNA[Ser]Sec and tRNASer wild type transcripts
The wild type tRNA[Ser]Sec and tRNASer vectors were prepared as described [2]. The templates for producing mutant tRNA[Ser]Sec transcripts were generated by PCR using forward primer T7 (5’-TAATACGACTCACTATAGGG-3’) and reverse primers containing the desired mutation(s) at the 3’-end of tRNA[Ser]Sec. For in vitro aminoacylation studies, transcription of tRNA[Ser]Sec was performed using the T7 RiboMAX Express Large Scale RNA Production System as described [2]. 32P-Labeled transcripts were generated using 1 µg of template, 50 µCi of [α-32P]ATP or [α-32P]UTP, 100 units of T7 RNA polymerase (Stratagene), 40 units of RNase inhibitor (Promega), the other components and this mixture incubated, subsequently treated with Dnase I (Alboin), the resulting transcripts isolated and stored until used exactly as described [2,4].
2.3. Isolation of the naturally-occurring tRNA[Ser]Sec and serine (Ser) tRNA1Ser isoforms and tRNA aminoacylation
The naturally-occurring tRNA[Ser]Secmcm5U [3] and Ser tRNA1 isoforms [15] were isolated from bovine liver and purified as described in these studies. Aminoacylation of these isoforms and the corresponding tRNA[Ser]Sec and tRNASer transcripts were aminoacylated with [3H]-serine in the presence of rabbit reticulocyte synthetases as described [16].
2.4. Xenopus oocyte microinjections and RPC-5 chromatography
Preparation of Xenopus oocytes and microinjection of tRNA[Ser]Sec transcripts into oocytes were performed as described [17,18]. After overnight incubation, tRNAs were extracted and chromatographed on a RPC-5 column (19) as described [4,7,20].
2.5. Minor base analysis
tRNAs (approximately 1×105 cpm) within pooled samples of each peak from the RPC-5 column were digested with nuclease P1 in 50 mM ammonium acetate (pH 5.3). Half of the digests were subjected to two-dimensional chromatography on cellulose TLC plates using solvents A and C ([21] and see also [4,18]), and the radioactivity was detected by autoradiography.
3. Results
3.1 Aminoacylation status of wild type and mutant tRNA[Ser]Sec
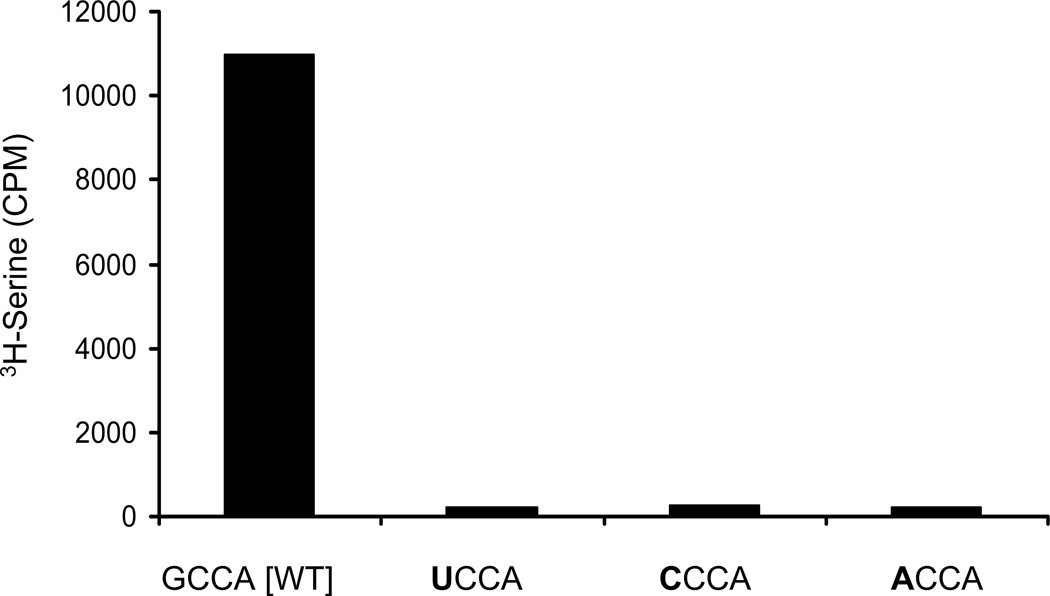
Three mutant tRNA[Ser]Sec isoforms were prepared as described in Materials and Methods to investigate the aminoacylation status of the mcm5U isoform prior to Um34 synthesis. All mutations were made at position 73, the discriminator base and were UCCA, CCCA and ACCA (mutations are shown in bold)
The ability of the UCCA, CCCA and ACCA mutant isoforms to be aminoacylated by SerRS was compared to wild type tRNA[Ser]Sec, GCCA, is shown in Fig. 1. The mutant isoforms were poorly aminoacylated.
Fig. 1.
Aminoacylation of wild type and mutant tRNA[Ser]Sec isoforms with serine. Two µgs of wild type and mutant (UCCA, CCCA or ACCA) tRNA[Ser]Sec transcripts were aminoacylated with 3H-serine in the presence of SerRS as described in Materials and Methods. Results shown are counts per minute (CPM) of 3H-serine.
3.2 Microinjection of mutant and wild type tRNA[Ser]Sec isoforms into Xenopus oocytes and identification of base modifications
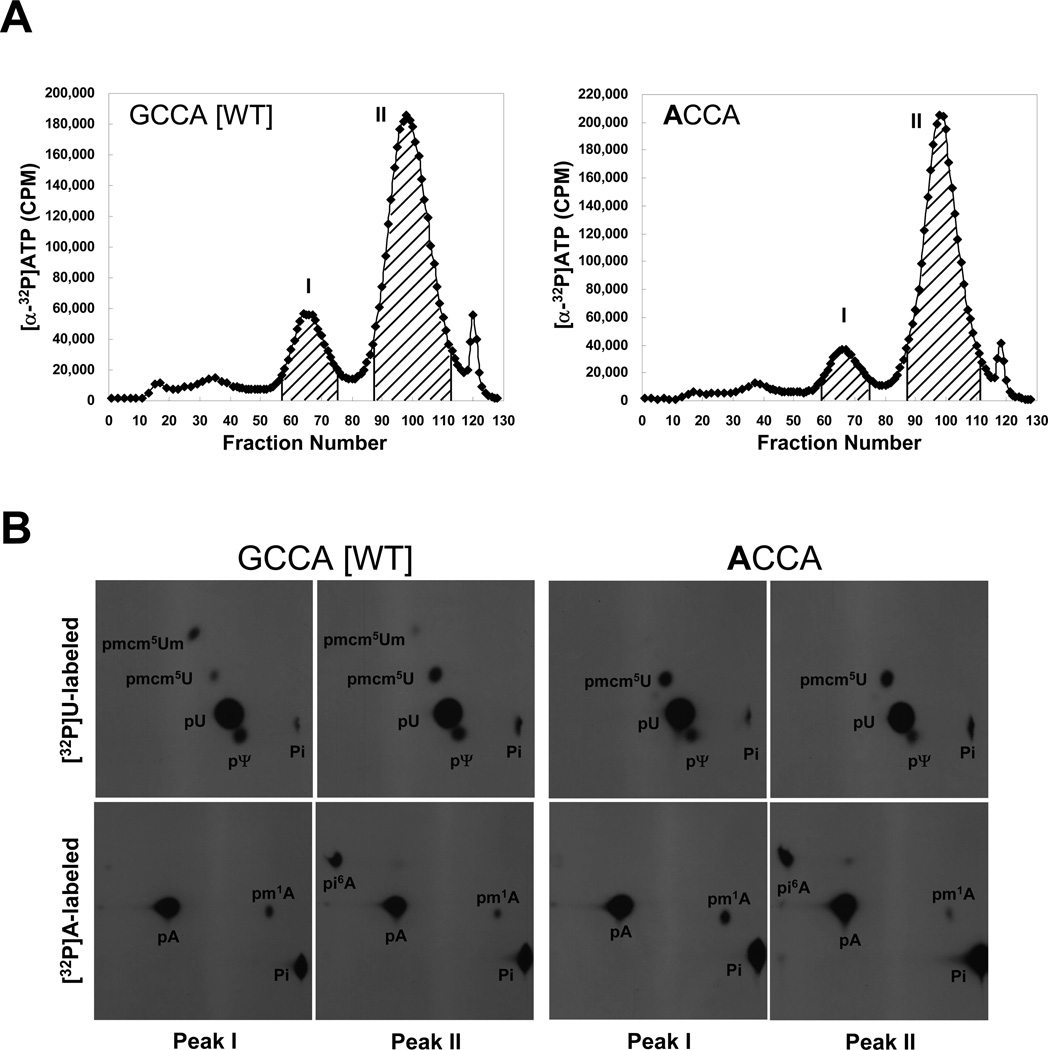
Transcripts of wild type and each mutant tRNA were prepared with either [α-32P]UTP or [α-32P]ATP, microinjected individually into Xenopus oocytes, isolated after overnight incubation and chromatographed on a RPC-5 column. The reason for the use of only [α-32P]UTP and [α-32P]ATP as labels is that the base modifications only occur on U or A nucleosides. The elution profiles of the wild type, GCCA, and discriminator base mutant, ACCA, isoforms labeled with [α-32P]ATP are shown in Fig. 2A. GCCA and ACCA had similar elution profiles that eluted primarily as two peaks, a smaller, front-running peak and a larger, late-running peak, designated as Peaks I and II, respectively. The corresponding [α-32P]UTP-labeled isoforms had similar elution profiles from the RPC-5 column as the [α-32P]ATP-labeled isoforms (compare Supplementary Fig. 1 to Fig. 2A).
Fig. 2.
Elution profiles of 32P-labeled wild type and mutant tRNA[Ser]Sec isoforms and modified base and nucleoside analyses. In (A), [α-32P]UTP- or [α-32P]ATP-labeled wild type (GCCA [WT]) and the discriminator base (ACCA) tRNA[Ser]Sec transcripts, were prepared, microinjected into Xenopus oocytes, incubated overnight, extracted and chromatographed on a RPC-5 column as described in Materials and Methods. The graphs show the elution profiles of [α-32P]ATP labeled tRNAs (and the [α-32P]UTP elution profiles are shown in Supplementary Fig. 1). X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, and in (B), the digests of the two peaks of [α-32P]UTP- or [α-32P]ATP-labeled were resolved by two-dimensional TLC as described in Materials and Methods and the modified bases and Um34 detected in the digests by autoradiography as described (see text and [4]). The films were exposed for 12–16 h.
The fractions within each peak were pooled as shown in Figures 2A and Supplementary Fig. 1, tRNA within each peak precipitated, collected, digested with nuclease and the resulting 32P-labeled nucleotides separated by 2-D chromatography as given in Materials and Methods. The 32P-labeled U and A modified bases and 32P-labeled Um34 nucleotide from GCCA and ACCA isoforms are shown in Fig. 2B. As expected, the greater hydrophobicity of Peak II was due to the highly modified base at position 37, i6A [3], that was found in this peak of the [α-32P]ATP-labeled isoforms. m1A was found in Peaks I and II in each of the isoforms examined. ψU and mcm5U were also found in each of the [α-32P]UTP-labeled isoforms, while mcm5Um was observed in the wild type, GCCA, isoform but not in the mutant, ACCA, isoform.
The other two 32P-labeled discriminator base mutant tRNA[Ser]Sec isoforms, UCCA and CCCA, were microinjected into Xenopus oocytes, incubated overnight, isolated and chromatographed over the RPC-5 column (Supplementary Fig. 2A). The resulting two major peaks were then isolated and analyzed for their minor base and Um34 compositions (Supplementary Fig. 2B) as described above for the other tRNA[Ser]Sec isoforms. Both these discriminator base mutant isoforms yielded virtually identical chromatographic and base analysis results as observed above for the ACCA isoform (compare Supplementary Fig. 1A,B to Fig. 3A,B, respectively, and see Table 1).
Table 1.
Relative amounts of modified nucleotides in wild type tRNA[Ser]Sec and mutants and the SB-217452 treated tRNA[Ser]Seca
| U-labeled |
A-labeled |
|||||
|---|---|---|---|---|---|---|
| pmcm5Ub | pmcm5Umb | pψc | pm1Ad | pi6Ae | ||
| GCCA[WT] | I | ± | + | + | + | − |
| GCCA[WT] | II | ++ | ± | + | ± | + |
| ACCA | I | ++ | − | + | + | − |
| ACCA | II | ++ | − | + | ± | + |
| CCCA | I | ++ | − | + | + | − |
| CCCA | II | ++ | − | + | ± | + |
| UCCA | I | ++ | − | + | + | − |
| UCCA | II | ++ | − | + | ± | + |
| GCCA[WT] | I | ± | + | + | + | − |
| GCCA[WT] | II | ++ | ± | + | + | + |
| GCCA[WT] +SB−217452 |
I | ++ | − | + | + | − |
| GCCA[WT] +SB−217452 |
II | ++ | − | + | + | + |
The amount of each modified nucleotide was calculated using a PhosphorImager and is relative to the amount of pU or pA.
++: 15–25 %; +: 5–15 %; ±: 1–5 %; −: < 1 % of pU.
+: 15–25 % of pU.
+: 10–20 %; ±: 1–10 % of pA.
+: 35–55 %; −: < 1 % of pA.
3.3 Inhibition of SerRS in vitro and in Xenopus oocytes
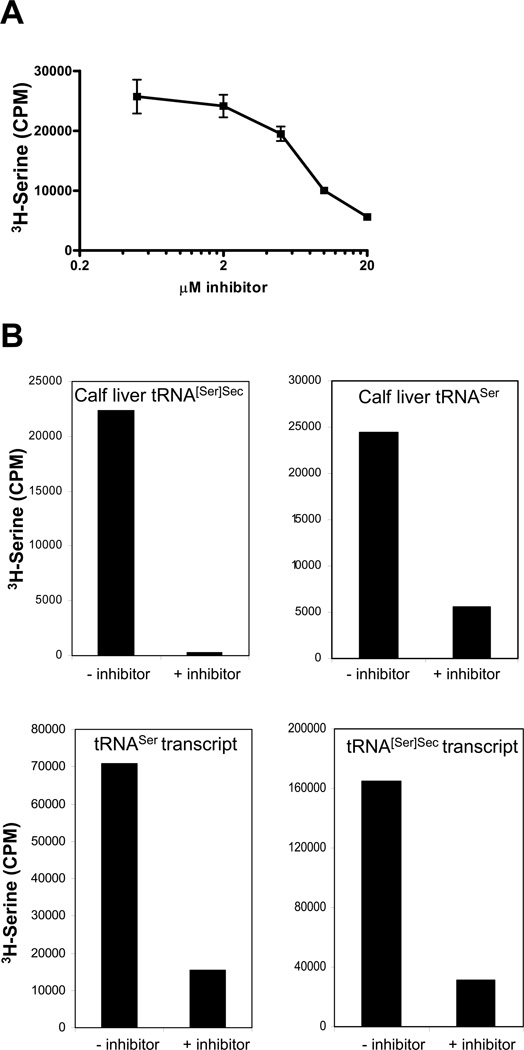
We examined the effect of the reported inhibitor of SerRS in yeast, SB-217452 [14], on the aminoacylation of Ser tRNA using total calf liver tRNA and mammalian SerRS at varying concentrations of inhibitor (Fig. 3A). Since the maximal level of SerRS inhibition occurred at 20 µM SB-217452 with total calf liver tRNA, the inhibition of SerRS using the naturally-occurring isoforms, tRNA[Ser]Secmcm5U and tRNA1Ser, and on the wild type tRNA[Ser]Sec and tRNASer transcripts, was then analyzed (Fig. 3B). The levels of aminoacylation of each of these isoforms were dramatically reduced demonstrating that this inhibitor effectively inhibited mammalian SerRS.
Fig. 3.
In vitro inhibition of SerRS. In (A), total calf liver tRNA (3 µg) was aminoacylated with 3H-serine as described in Materials and Methods with various concentrations of SB-217452. In (B), naturally-occurring, purified tRNA[Ser]Sec, purified calf liver tRNASer and tRNA[Ser]Sec, and tRNASer transcripts (0.075 A260 unit) were aminoacylated with 3H-serine in the presence or absence of 20 µM SB-217452. Results shown are counts per minute (CPM) of 3H-serine.
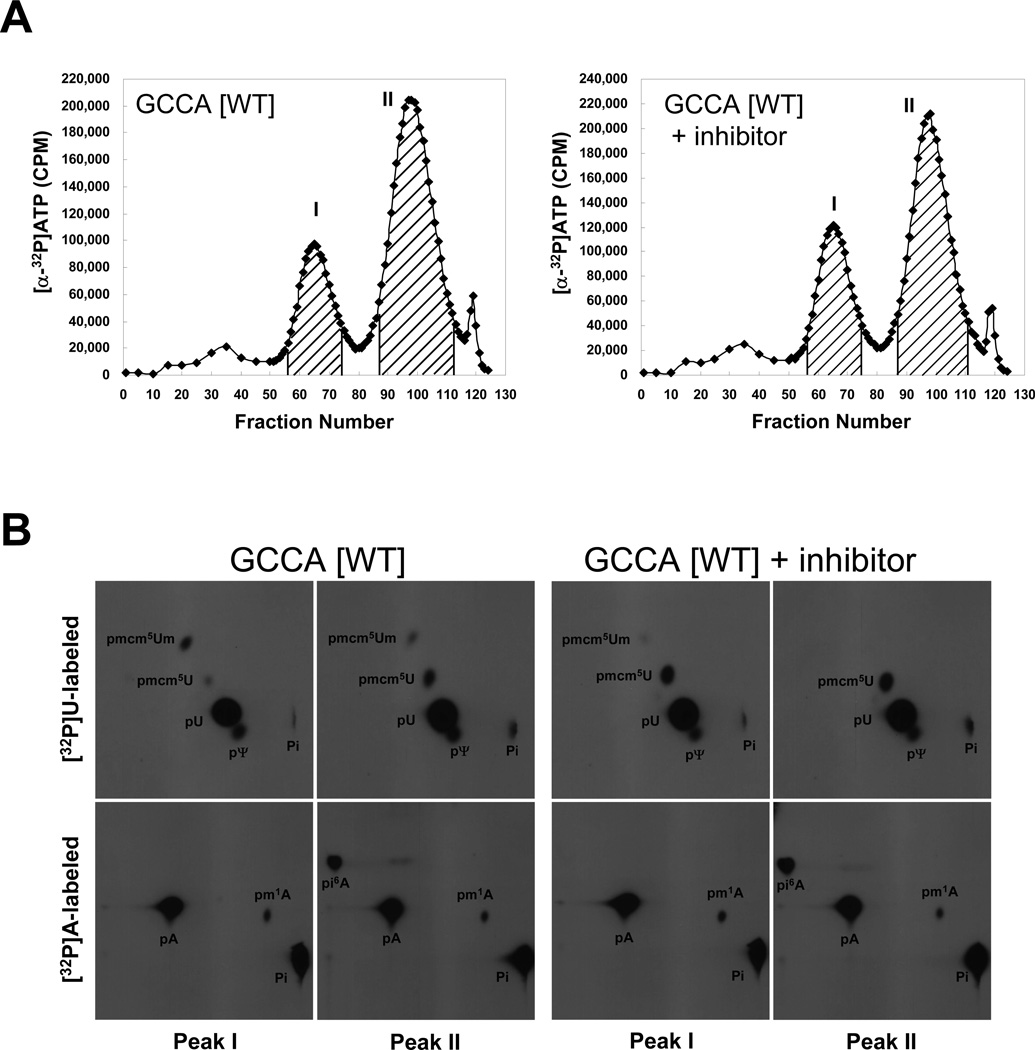
The wild type tRNA[Ser]Sec transcript, labeled with [α-32P]ATP or [α-32P]UTP was microinjected into Xenopus oocytes with or without SB-217452, incubated overnight, isolated, chromatographed on the RPC-5 column and the resulting peaks isolated (Fig. 4A and Supplementary Fig. 3, respectively) and minor base and Um34 analyses determined (Fig. 4B). As shown in the figure, all modifications occurred in tRNA[Ser]Sec that was not exposed to the inhibitor but the Um34 modification was more than 90% reduced in the presence of the inhibitor (see Table 1). These data are further considered in the Discussion.
Fig. 4.
Elution profiles and modified base analysis of wild type tRNA[Ser]Sec isoforms following inhibition of SerRS. In (A), [α-32P]UTP- or [α-32P]ATP-labeled wild type (GCCA [WT]) tRNA[Ser]Sec transcript was microinjected into Xenopus oocytes with or without 20 µM SB-217452, incubated overnight, extracted, and chromatographed on a RPC-5 column as described in Materials and Methods. The graphs show the elution profiles of [α-32P]ATP labeled tRNAs. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, and in (B), the digests resolved by two-dimensional TLC as described in Materials and Methods and the modified bases and Um34 detected by autoradiography (see text and [4]). The films were exposed for 12–16 h.
4. Discussion
An important question regarding the function and regulation of tRNA[Ser]Sec is whether this tRNA is aminoacylated prior to its methylation on the ribosyl moiety at position 34 or does the methylation occur independently of aminoacylation. If the Um34 methylase requires Sec-tRNA[Ser]Sec as a substrate, this would provide further insight into why selenium is required for Um34 synthesis [3,5,6], and in turn, for the requirement of the element for stress-related selenoprotein expression (e.g., GPx1) [2,7–9]. We used two different approaches to elucidate the aminoacylation status of tRNA[Ser]Sec. A G73→A73 mutation was prepared wherein the discriminator base of tRNA[Ser]Sec was changed resulting in poor aminoacylation. Interestingly, all the known base modifications, mcm5U, i6A, ψU and m1A at positions 34, 37, 55 and 58, respectively, occurred in tRNA[Ser]Sec following microinjection and incubation of the mutant transcript into Xenopus oocytes. However, the nucleoside Um34 modification did not occur. The other two discriminator base mutants, UCCA and CCCA, also yielded the same results.
We also used a reported inhibitor of SerRS, SB-217452, which is an enzymatically processed product of albomycin δ2 [14]. We examined the ability of this compound to inhibit the aminoacylation of the wild type tRNA[Ser]Sec and tRNASer transcripts and the naturally-occurring tRNA[Ser]Secmcm5U and tRNA1Ser isoforms in vitro. The data showed that SB-217452 was a relatively potent inhibitor of mammalian SerRS in vitro and likely in Xenopus oocytes since all base modifications in tRNA[Ser]Sec occurred in oocytes with the exception of the Um34 modification that supported our findings with the discriminator base mutations.
The above studies demonstrate that tRNA[Ser]Sec is aminoacylated for Um34 synthesis to occur. However, these studies in themselves do not demonstrate whether any one specific amino acid or all of the known amino acid attachments to tRNA[Ser]Sec, serine, phosphoserine, Sec and Cys [22], could serve as substrates for the Um34 methylase. The following observations suggest that Sec-tRNA[Ser]Sec, and only Sec attached to tRNA[Ser]Sec, serves as a substrate for the Um34 methylase: 1) the Um34 methylation step requires selenium [3,5–7] suggesting that the methylase either requires this element as a cofactor or it requires selenium in the form of Sec as its substrate. Since eukaryotes are not known to utilize selenium as a cofactor (whereas its use as Sec explains biological functions of dietary selenium), the more likely possibility is that the Um34 methylase requires Sec as its substrate; 2) the synthesis of GPx1 is dependent on the mcm5Um isoform [8,9], and interestingly, thiophosphate can replace selenophosphate in the Sec biosynthetic pathway yielding Cys-tRNA[Ser]Sec and the resulting Cys is inserted into TR1 and TR3 but apparently not into GPx1 ([22] and see also [3]) the Sec tRNA transcript serves as a substrate for seryl-tRNA synthetase, phosphoseryl-tRNA[Ser]Sec kinase, Sec synthase and selenophosphate synthetase [1,23], but the Um34 methylase has a requirement for the fully modified tRNA[Ser]Sec [4] that must be aminoacylated (this study); although this observation in itself does not demonstrate that Sec must be attached to its tRNA, in light of the above considerations, the most logical conclusion is that indeed Sec must be attached for Um34 methylation to occur.
In the event Sec-tRNA[Ser]Sec is the only substrate for the Um34 methylase, this final step in the maturation of Sec-tRNA[Ser]Sec would provide an important autoregulatory step in the synthesis of the stress-related selenoprotein subclass and may account, at least in part, for the hierarchy of selenoprotein expression. It has been known for many years that the expression of some selenoproteins in mammalian cells and tissues, such as TR1 (designated as housekeeping selenoproteins), is fairly insensitive to selenium status while others, such as GPx1 (designated as stress-related selenoproteins), are highly sensitive and are poorly expressed under conditions of selenium deficiency, which is a phenomenon known as selenoprotein hierarchy [24,25]. Stress-related selenoproteins are not essential to the animal’s survival and are synthesized by the Um34 containing isoform [8,9]. However, housekeeping selenoproteins are essential to the animal’s survival and are synthesized by the precursor, non-Um34 isoform. Whether housekeeping selenoproteins can also be synthesized by the mcm5Um isoform has not be resolved.
Interestingly, mice maintained on a selenium deficient diet contain about a 1:1 ratio of Sec/Cys in liver TR1 and about a 9:1 ratio in mice fed a selenium adequate diet [22]. Although further studies are required to show unequivocally that Cys cannot be inserted into GPx1 by this de novo synthetic pathway for synthesizing Cys, the available data suggest that this is the case as noted above. Clearly, additional work has to be done to fully elucidate how selenium status regulates the two selenoprotein subclasses and hierarchy of selenoprotein expression, but the observation that the tRNA[Ser]Sec isoform likely has Sec attached provides an important step in the complete understanding of these processes.
Highlights.
-
▸
Two isoforms of selenocysteine (Sec) tRNA[Ser]Sec differ by a single methyl group (Um34) and synthesize different subclasses of selenoproteins.
-
▸
The relationship between tRNA[Ser]Sec aminoacylation and Um34 synthesis was investigated.
-
▸
The data show that tRNA[Ser]Sec must be aminoacylated, most likely with Sec, for Um34 synthesis to occur.
-
▸
The data provide an explanation why selenium is essential for the function of Um34 methylase.
-
▸
The data provide further insights into the hierarchy of selenoprotein expression.
Supplementary Material
Elution profiles of 32P-labeled wild type and mutant Sec tRNA[Ser]Sec isoforms. [α-32P]UTP-labeled wild type (GCCA [WT]) and discriminator base (ACCA) mutant tRNA[Ser]Sec isoforms were prepared, microinjected into Xenopus oocytes, incubated overnight, extracted, and chromatographed on a RPC-5 column as described in Materials and Methods of the manuscript. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, the digests resolved by two dimensional TLC (see Materials and Methods of the manuscript for details) and the occurrence of the modified bases and Um34 assessed as shown in Figure 2B in the manuscript.
Elution profiles of 32P-labeled wild type and mutant Sec tRNA[Ser]Sec isoforms and modified base and nucleoside analyses. In (A), [α-32P]UTP- or [α-32P]ATP-labeled discriminator base mutant (UCCA, CCCA) tRNA[Ser]Sec isoforms, were prepared, microinjected into Xenopus oocytes, incubated overnight, extracted and chromatographed on a RPC-5 column as described in Materials and Methods of the manuscript. The graphs shown are [α-32P]ATP-labeled (upper panel) and [α-32P]UTP (lower panel) tRNAs. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, and (B) the digests resolved by two-dimensional TLC as described in Materials and Methods of the manuscript and the modified bases and Um34 detected by autoradiography as described (see text of the manuscript and [4]). The films were exposed for 12–16 h.
Elution profiles of 32P-labeled wild type tRNA[Ser]Sec isoforms following inhibition of SerRS. [α-32P]UTP-labeled wild type (GCCA [WT]) tRNA[Ser]Sec was microinjected into Xenopus oocytes with or without 20µM SB-217452, incubated overnight, extracted, and chromatographed on a RPC-5 column as described in Materials and Methods. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, the digests resolved by 2-D TLC and the occurrence of the modified bases and Um34 assessed as shown in Figure 4B in the manuscript.
Acknowledgements
This work was supported by the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health (to DLH); the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (program number 2009-0094020 to BJL and JYK); National Institutes of Health (grant numbers GM061603, GM065204 and CA080946 to VNG), and American Heart Association (grant number 09BGIA2070029 to SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Xu XM, Carlson BA, Zhang Y, Mix H, Kryukov GV, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. New developments in selenium biochemistry: selenocysteine biosynthesis in eukaryotes and archaea. Biol. Trace Elem. Res. 2007;119:234–241. doi: 10.1007/s12011-007-8003-9. [DOI] [PubMed] [Google Scholar]
- 2.Carlson BA, Xu XM, Kryukov GV, Rao M, Berry MJ, Gladyshev VN, Hatfield DL. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl. Acad. Sci. U S A. 2004;101:12848–12853. doi: 10.1073/pnas.0402636101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond AM, Choi IS, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE, Burk RF, McCloskey JA, Hatfield DL. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec) J. Biol. Chem. 1993;268:14215–14223. [PubMed] [Google Scholar]
- 4.Kim LK, Matsufuji T, Matsufuji S, Carlson BA, Kim SS, Hatfield DL, Lee BJ. Methylation of the ribosyl moiety at position 34 of selenocysteine tRNA[Ser]Sec is governed by both primary and tertiary structure. RNA. 2000;6:1306–1315. doi: 10.1017/s1355838200000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatfield D, Lee BJ, Hampton L, Diamond AM. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991;19:939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chittum HS, Baek HJ, Diamond AM, Fernandez-Salguero P, Gonzalez F, Ohama T, Hatfield DL, Kuehn M, Lee BJ. Selenocysteine tRNA[Ser]Sec levels and selenium-dependent glutathione peroxidase activity in mouse embryonic stem 14 cells heterozygous for a targeted mutation in the tRNA[Ser]Sec gene. Biochemistry. 1997;36:8634–8639. doi: 10.1021/bi970608t. [DOI] [PubMed] [Google Scholar]
- 7.Moustafa ME, Carlson BA, El-Saadani MA, Kryukov GV, Sun QA, Harney JW, Hill KE, Burk RF, Combs GF, Feigenbaum L, Mansur DB, Berry MJ, Diamond AM, Gladyshev VN, Lee BJ, Hatfield DL. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing an isopentenyladenosine mutant selenocysteine tRNA[Ser]Sec transgene. Mol. Cell. Biol. 2001;21:3840–3852.. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson BA, Xu XM, Gladyshev VN, Hatfield DL. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J. Biol. Chem. 2005;280:5542–5548. doi: 10.1074/jbc.M411725200. [DOI] [PubMed] [Google Scholar]
- 9.Carlson BA, Xu XM, Gladyshev VN, Hatfield DL. Um34 in selenocysteine tRNA is required for the expression of stress-related selenoproteins in mammals. In: Grosjean H, editor. Fine-Tuning of RNA Functions by Modification and Editing. Topics in Current Genetics. Vol 12. Berlin/Heidelberg/New York: Springer-Verlag; 2005. pp. 431–438. [Google Scholar]
- 10.Carlson BA, Moustafa ME, Sengupta A, Schweizer U, Shrimali R, Rao M, Zhong N, Wang S, Feigenbaum L, Lee BJ, Gladyshev VN, Hatfield DL. Selective restoration of the selenoprotein population in a mouse hepatocyte selenoproteinless background with different mutant selenocysteine tRNAs lacking Um34. J. Biol. Chem. 2007;282:32591–32602. doi: 10.1074/jbc.M707036200. [DOI] [PubMed] [Google Scholar]
- 11.Crothers DM, Seno T, Söll DG. Is there a discriminator site in transfer RNA? Proc. Natl. Acad. Sci. USA. 1972;69:3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohama T, Yang DC, Hatfield DL. Selenocysteine tRNA and serine tRNA are aminoacylated by the same synthetase, but may manifest different identities with respect to the long extra arm. Arch. Biochem. Biophys. 1994;315:293–301. doi: 10.1006/abbi.1994.1503. [DOI] [PubMed] [Google Scholar]
- 13.Amberg R, Mizutani T, Wu XQ, Gross HJ. Selenocysteine synthesis in mammalia: An identity switch from tRNA(Ser) to tRNA(Sec) J. Mol. Biol. 1996;263:8–19. doi: 10.1006/jmbi.1996.0552. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Y, Roy H, Patil PB, Ibba M, Chen S. Characterization of two seryl-tRNA synthetases in albomycin-producing Streptomyces sp. strain ATCC 700974. Antimicrob. Agents Chemother. 2009;53:4619–4627. doi: 10.1128/AAC.00782-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staehelin M, Rogg H, Baguley BC, Ginsberg T, Wehrli W. Structure of a mammalian serine tRNA. Nature. 1968;219:1363–1365. doi: 10.1038/2191363a0. [DOI] [PubMed] [Google Scholar]
- 16.Hatfield D, Matthews CR, Rice M. Aminoacyl-transfer RNA populations in mammalian cells chromatographic profiles and patterns of codon recognition. Biochim. Biophys. Acta. 1979;564:414–423. doi: 10.1016/0005-2787(79)90032-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee BJ, de la Pena P, Tobian J, Zasloff M, Hatfield D. Unique pathway of expression of an opal suppressor phosphoserine tRNA. Proc. Natl. Acad. Sci. U S A. 1987;84:6384–6388. doi: 10.1073/pnas.84.18.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BJ, Kang SG, Hatfield DL. Transcription of Xenopus selenocysteine tRNA Ser (formerly designated opal suppressor phosphoserine tRNA) gene is directed by multiple 5'-extragenic regulatory elements. J. Biol. Chem. 1989;264:9696–9702. [PubMed] [Google Scholar]
- 19.Kelmers AD, Heatherly DE. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal. Biochem. 1971;44:486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- 20.Choi IS, Diamond AM, Crain PF, Kolker JD, McCloskey JA, Hatfield DL. Reconstitution of the biosynthetic pathway of selenocysteine tRNAs in Xenopus oocytes. Biochemistry. 1994;33:601–605. doi: 10.1021/bi00168a027. [DOI] [PubMed] [Google Scholar]
- 21.Silberklang M, Gillum AM, RajBhandary UL. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;1979:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- 22.Xu XM, Turanov AA, Carlson BA, Yoo MH, Everley RA, Nandakumar R, Sorokina I, Gygi SP, Gladyshev VN, Hatfield DL. Targeted insertion of cysteine by decoding UGA codons with mammalian selenocysteine machinery. Proc .Natl. Acad. Sci. U S A. 2010;107:21430–21434. doi: 10.1073/pnas.1009947107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2006;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maquat LE. Nonsense-mediated mRNA decay: Splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell. Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 25.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep. 2009;29:329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elution profiles of 32P-labeled wild type and mutant Sec tRNA[Ser]Sec isoforms. [α-32P]UTP-labeled wild type (GCCA [WT]) and discriminator base (ACCA) mutant tRNA[Ser]Sec isoforms were prepared, microinjected into Xenopus oocytes, incubated overnight, extracted, and chromatographed on a RPC-5 column as described in Materials and Methods of the manuscript. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, the digests resolved by two dimensional TLC (see Materials and Methods of the manuscript for details) and the occurrence of the modified bases and Um34 assessed as shown in Figure 2B in the manuscript.
Elution profiles of 32P-labeled wild type and mutant Sec tRNA[Ser]Sec isoforms and modified base and nucleoside analyses. In (A), [α-32P]UTP- or [α-32P]ATP-labeled discriminator base mutant (UCCA, CCCA) tRNA[Ser]Sec isoforms, were prepared, microinjected into Xenopus oocytes, incubated overnight, extracted and chromatographed on a RPC-5 column as described in Materials and Methods of the manuscript. The graphs shown are [α-32P]ATP-labeled (upper panel) and [α-32P]UTP (lower panel) tRNAs. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, and (B) the digests resolved by two-dimensional TLC as described in Materials and Methods of the manuscript and the modified bases and Um34 detected by autoradiography as described (see text of the manuscript and [4]). The films were exposed for 12–16 h.
Elution profiles of 32P-labeled wild type tRNA[Ser]Sec isoforms following inhibition of SerRS. [α-32P]UTP-labeled wild type (GCCA [WT]) tRNA[Ser]Sec was microinjected into Xenopus oocytes with or without 20µM SB-217452, incubated overnight, extracted, and chromatographed on a RPC-5 column as described in Materials and Methods. X and Y axis represent fraction number and CPM, respectively. Peaks I and II were pooled as shown by the hatched areas in the figures, collected, digested with nuclease, the digests resolved by 2-D TLC and the occurrence of the modified bases and Um34 assessed as shown in Figure 4B in the manuscript.