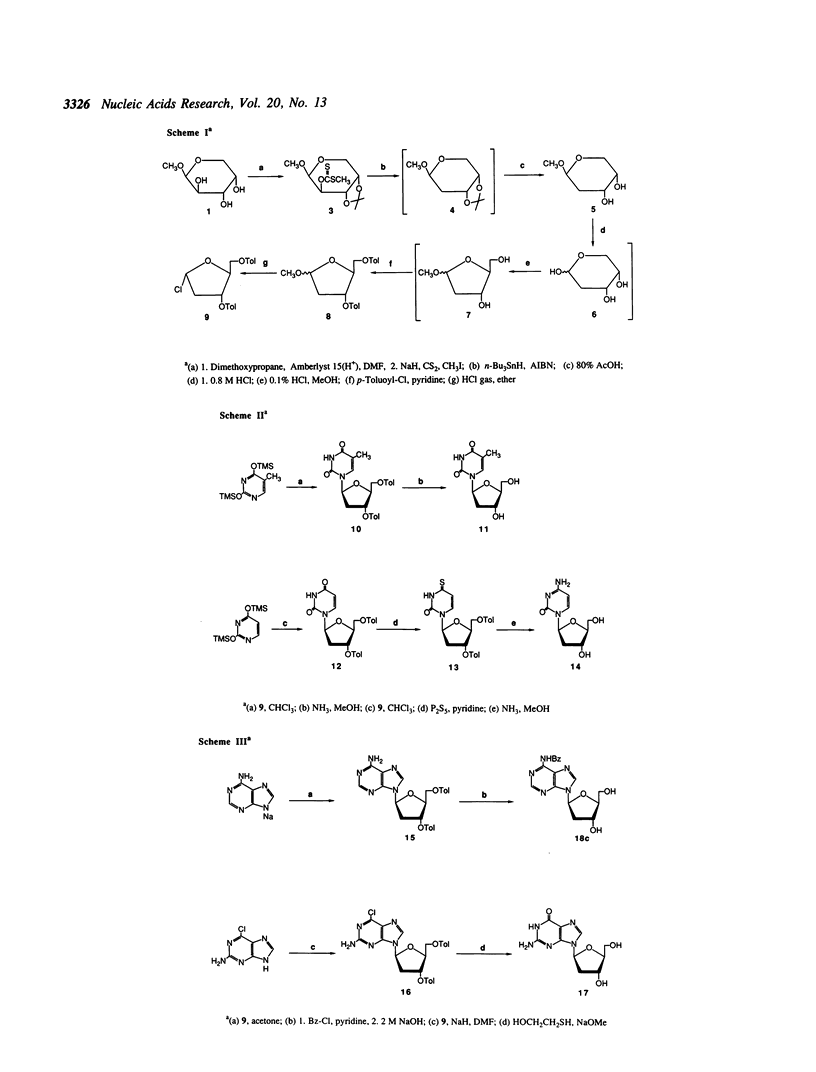
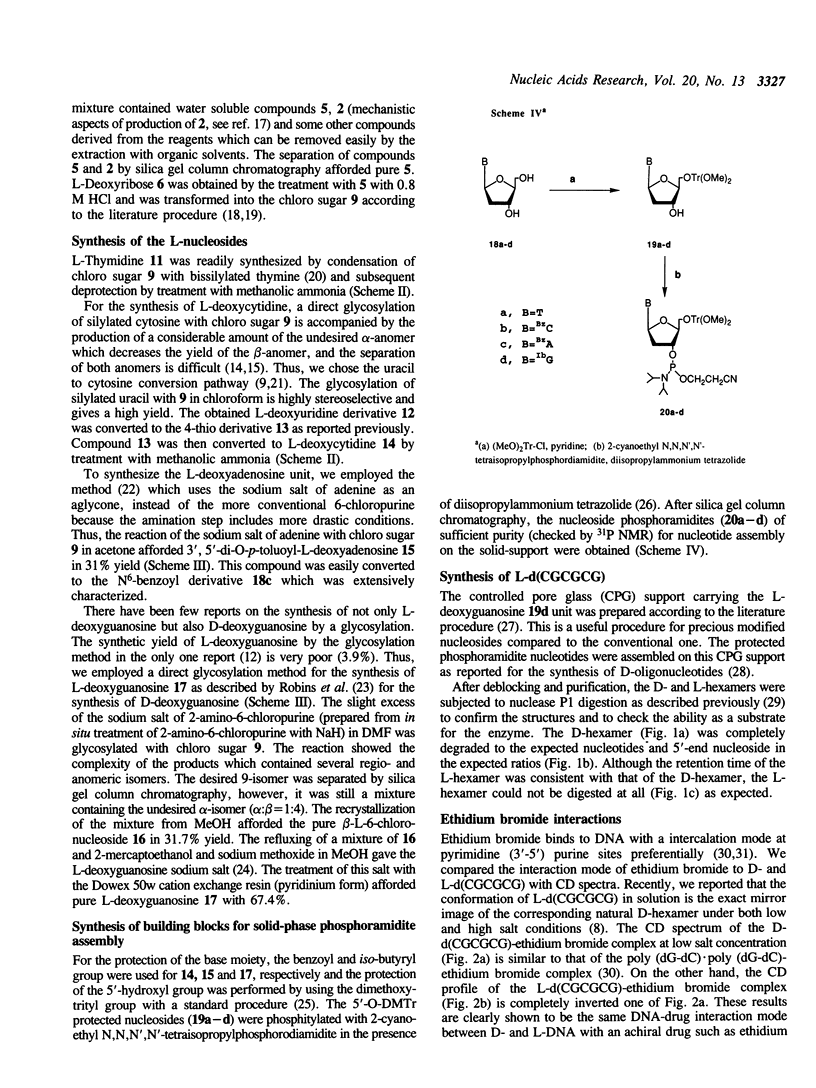
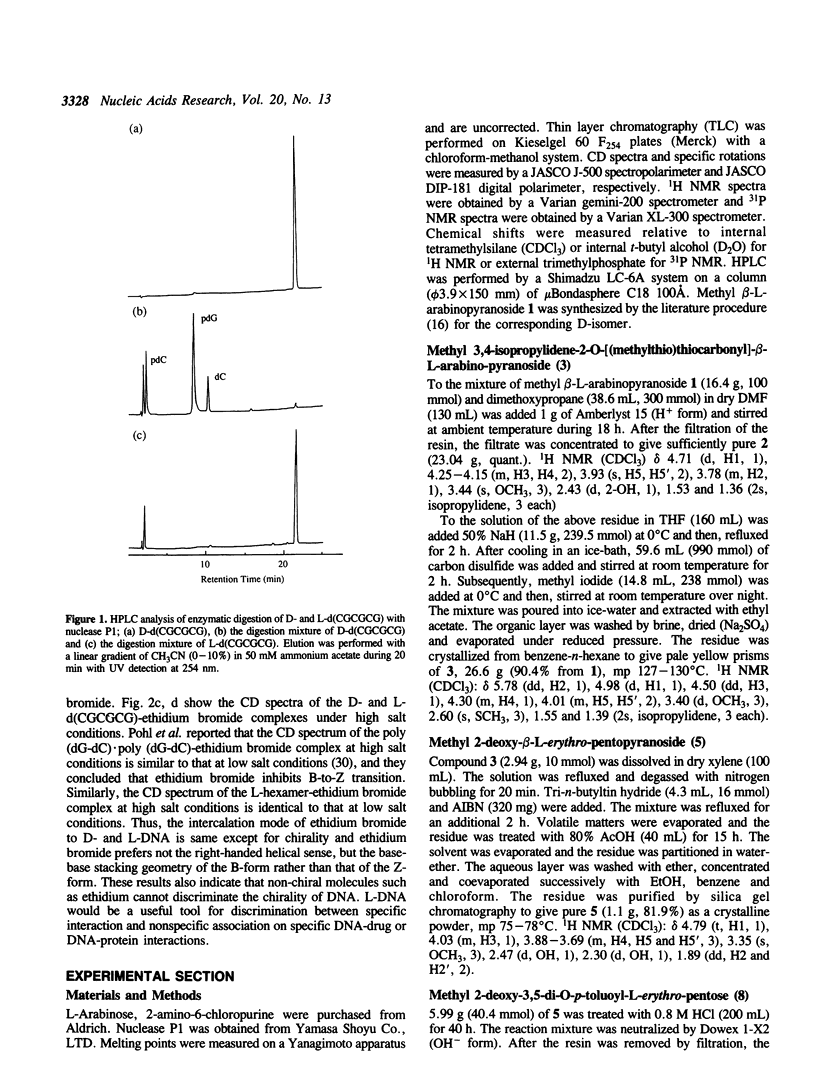
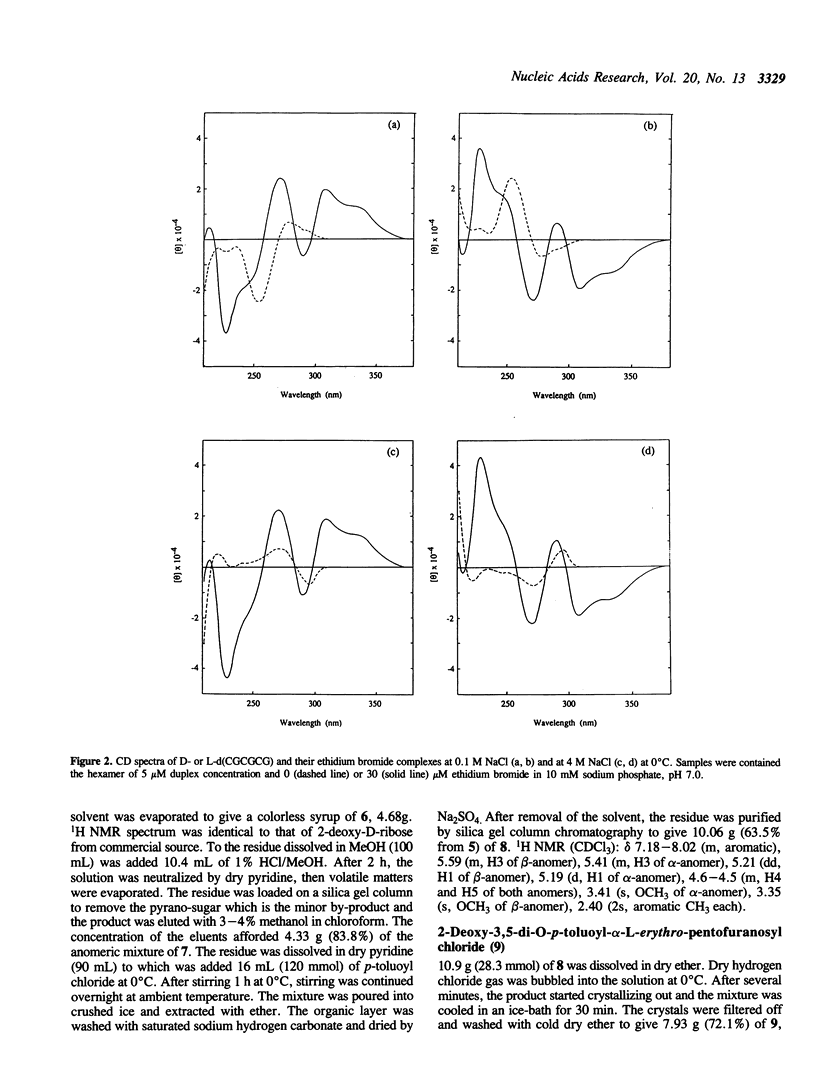
Abstract
We have investigated the conformations of the hexadeoxyribonucleotide, L-d(CGCGCG) composed of L-deoxyribose, the mirror image molecule of natural D-deoxyribose. In this paper, we report the synthesis of four L-deoxynucleosides and the L-oligonucleotide-ethidium bromide interactions. The L-deoxyribose synthon 9 was synthesized from L-arabinose with an over all yield of 28.5% via the Barton-McCombie reaction. The L-deoxynucleosides were obtained by a glycosylation of appropriate nucleobase derivatives with the 1-chloro sugar 9. After derivatization to nucleoside phosphoramidites, L-deoxycytidine and L-deoxyguanosine were incorporated into a hexadeoxynucleotide, L-d(CGCGCG) by a solid-phase beta-cyanoethylphosphoramidite method. This L-hexanucleotide was resistant to digestion with nuclease P1. The conformations of L-d(CGCGCG) were an exact mirror image of that of the corresponding natural one as described previously, and the conformations of the L-d(CGCGCG)-ethidium bromide complex were also the mirror images of those of the D-d(CGCGCG)-ethidium bromide complex under both low and high salt conditions. These results suggest that ethidium bromide prefers not a right-handed helical sense, but the base-base stacking geometry of the B-form rather than that of the Z-form. Thus, L-DNA would be a useful tool for studying DNA-drug interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Hau J. F., Czernecki S., Le Diguarher T., Perlat M. C., Valery J. M., Thuong N. T. Synthesis and physicochemical properties of oligonucleotides built with either alpha-L or beta-L nucleotides units and covalently linked to an acridine derivative. Nucleic Acids Res. 1991 Aug 11;19(15):4067–4074. doi: 10.1093/nar/19.15.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damha M. J., Giannaris P. A., Zabarylo S. V. An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res. 1990 Jul 11;18(13):3813–3821. doi: 10.1093/nar/18.13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. W., Martinez A. P., Goodman L., Henry D. W. Guanine, thioguanine, and related nucleosides by the mercuric cyanide--silyl method. An improved synthesis of -2'-deoxythioguanosine. J Org Chem. 1972 Sep 22;37(19):2923–2927. doi: 10.1021/jo00984a001. [DOI] [PubMed] [Google Scholar]
- Morvan F., Génu C., Rayner B., Gosselin G., Imbach J. L. Sugar modified oligonucleotides. III (1). Synthesis, nuclease resistance and base pairing properties of alpha- and beta-L-octathymidylates. Biochem Biophys Res Commun. 1990 Oct 30;172(2):537–543. doi: 10.1016/0006-291x(90)90706-s. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Iwai I. Studies on synthetic nucleosides. I. Trimethylsilyl derivatives of pyrimidines and purines. Chem Pharm Bull (Tokyo) 1964 Mar;12(3):352–356. doi: 10.1248/cpb.12.352. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Ethidium bromide-(dC-dG-dC-dG)2 complex in solution: intercalation and sequence specificity of drug binding at the tetranucleotide duplex level. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3343–3347. doi: 10.1073/pnas.73.10.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins M. J., Khwaja T. A., Robins R. K. Purine nucleosides. XXIX. The synthesis of 2'-deoxy-L-adenosine and 2'-deoxy-L-guanosine and their alpha anomers. J Org Chem. 1970 Mar;35(3):636–639. doi: 10.1021/jo00828a019. [DOI] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata H., Ueno M., Yamasaki F., Akagi M. Interstrand cross-linking of the hexadeoxynucleotide d(TACGTA) upon reaction with trans-diamminedichloroplatinum(II). Biochem Biophys Res Commun. 1991 Mar 15;175(2):537–542. doi: 10.1016/0006-291x(91)91598-7. [DOI] [PubMed] [Google Scholar]



