Abstract
Protein kinase C-delta (PKCδ) is a key regulator of human involucrin (hINV) gene expression and is regulated by tyrosine phosphorylation. However, a comprehensive analysis of the requirement for individual PKCδ tyrosine residues is lacking. We show that multiple tyrosine residues influence the ability of PKCδ to increase hINV gene expression. Mutation of individual PKCδ tyrosine residues 52, 64, 155, 187, or 565 does not reduce the ability of PKCδ to increase hINV promoter activity; however, simultaneous mutation of these five tyrosines markedly reduces activity. Moreover, restoration of any one of these residues results in nearly full activity restoration. It is significant that phosphorylation of PKCδ-Y311 is reduced in the five-tyrosine mutant and that mutation of Y311 results in reduced PKCδ activity comparable to that observed in the five-tyrosine mutant. Restoration of any one of the tyrosine residues in the five-tyrosine mutant restores Y311 phosphorylation and biological activity. In addition, reduced phosphorylation of endogenous PKCδ-Y311 is associated with reduced biological activity. These findings indicate that PKCδ activity requires Y311 and a second tyrosine residue; however, any one of the several tyrosine residues can serve in the secondary role.
INTRODUCTION
The protein kinase C (PKC) family comprises at least 11 isoforms that play an important role in the regulation of cell growth and differentiation (Dlugosz and Yuspa, 1994; Newton, 1997). PKC isoforms are classified into three groups on the basis of structural differences in the regulatory domains and differences in cofactor regulation. The classical PKCs (α, βI, βII, and γ) are calcium-, phospholipid-, and diacyl-glycerol-dependent; the novel PKCs (δ, ε, η, θ, and µ) are calcium-independent; and the atypical PKCs (ζ and λ) are calcium- and diacylglycerol-independent (Nishizuka, 1992). All PKC isoforms consist of an N-terminal regulatory domain and a C-terminal catalytic domain (Nishizuka, 1992). The regulatory domain is responsible for the subcellular distribution and contains domains that determine the ligand response of the isoform, whereas the catalytic domain contains the site required for catalytic activity (Steinberg, 2004).
PKC isoforms are important regulators of keratinocyte function. Epidermal keratinocytes express α, δ, ε, η, and ζ isofoms (Osada et al., 1990; Dlugosz et al., 1992; Gherzi et al., 1992; Matsui et al., 1992; Fisher et al., 1993). Human epidermal keratinocytes undergo a tightly controlled differentiation process that results in the formation of terminal dead cells called corneocytes. Corneocytes consist of a stabilized keratin network bounded by an envelope of covalently crosslinked protein called the cornified envelope (Nemes and Steinert, 1999). The cornified envelope is deposited via the action of type I transglutaminase during the final stages of keratinocyte differentiation. The 68 kDa human involucrin (hINV) protein is a highly reactive transglutaminase substrate and component of the scaffold of the cornified envelope (Nemes and Steinert, 1999). The hINV gene is expressed in a differentiation-dependent manner—it is selectively expressed in differentiated suprabasal keratino-cytes (Rice and Green, 1979; Murphy et al., 1984; Eckert et al., 1997). We have previously demonstrated that the novel PKC isoforms, including PKCδ, activate keratinocyte differentiation and increase endogenous involucrin gene expression and hINV promoter activity. This increase is mediated via a novel PKC, Ras, MEKK1, MEK3, p38δ–ERK1/2 signaling cascade, which results in increased levels of AP1, C/EBP, and Sp1 transcription factors, increased binding of these factors to the hINV promoter, and increased gene transcription (Efimova and Eckert, 2000; Efimova et al., 2002; Eckert et al., 2004b). The novel PKCs, including PKCδ, also function with calcium, an agent that increases keratinocyte differentiation, to increase calcium-dependent hINV promoter activity (Deucher et al., 2002). The regulation by PKCδ can be specifically inhibited by a PKCδ inhibitor, rottlerin, and the increase in promoter activity is associated with increased levels of hINV mRNA and protein (Efimova and Eckert, 2000; Efimova et al., 2004). Thus, a physiologic role for PKCδ as a regulator of hINV gene expression is well established.
The effects of tyrosine phosphorylation on the activity of PKCδ are complex and depend on the cell environment and the presence of specific agonists (Denning et al., 1993, 1996; Li et al., 1994, 1996). The PKCδ amino-acid sequence includes 19 tyrosine residues, 9 of which have been identified as sites of phosphorylation (Brodie and Blumberg, 2003). For example, activation of IgE receptors in RBL-2H3 cells leads to phosphorylation of PKCδ-Y52 (Szallasi et al., 1995), and in NIH 3T3 fibroblasts, PKCδ-Y187 is phosphory-lated in response to 12-O-tetradecanoylphorbol-13-acetate (TPA) or platelet-derived growth factor stimulation (Li et al., 1996). These changes in phosphorylation state are frequently associated with altered activity. Phosphorylation of PKCδ on tyrosine residues in v-ras-transformed keratinocytes leads to reduced catalytic activity (Denning et al., 1993). In contrast, H2O2 treatment induces phosphorylation on tyrosine residues 311, 332, and 512 of PKCδ in COS-7 cells, which is associated with increased PKCδ activity (Konishi et al., 2001).
Our previous studies demonstrate that PKCδ expression enhances calcium-dependent hINV promoter activity; calcium treatment does not alter the expression level or induce PKCδ translocation to membranes, but does cause a marked increase in PKCδ tyrosine phosphorylation (Deucher et al., 2002). In spite of the importance of tyrosine phosphorylation on PKCδ function, a comprehensive analysis of the role of specific PKCδ tyrosine residues has not been performed. In this study, we examine the function of individual PKCδ tyrosine residues. Mutation of a single tyrosine residue at position 52, 64, 155, 187, or 565 does not reduce the ability of PKCδ to drive hINV promoter activity. In contrast, simultaneous mutation of tyrosines 52, 64, 155, 187, and 565 markedly reduces the response. Interestingly, restoration of any one of these tyrosine residues restores full activity. In each case, mutants with high activity are highly phosphory-lated on Y311, and mutation of Y311 reduces PKCδ activity comparable to the five-tyrosine mutant. In addition, diet-derived agents (curcumin, apigenin) suppress hINV promoter activity, and this is associated with reduced phosphorylation of PKCδ-Y311. These novel findings indicate that Y311, and at least one additional companion tyrosine residue (tyrosine 52, 64, 155, 187 or 565), must be present for PKCδ activity in keratinocytes.
RESULTS
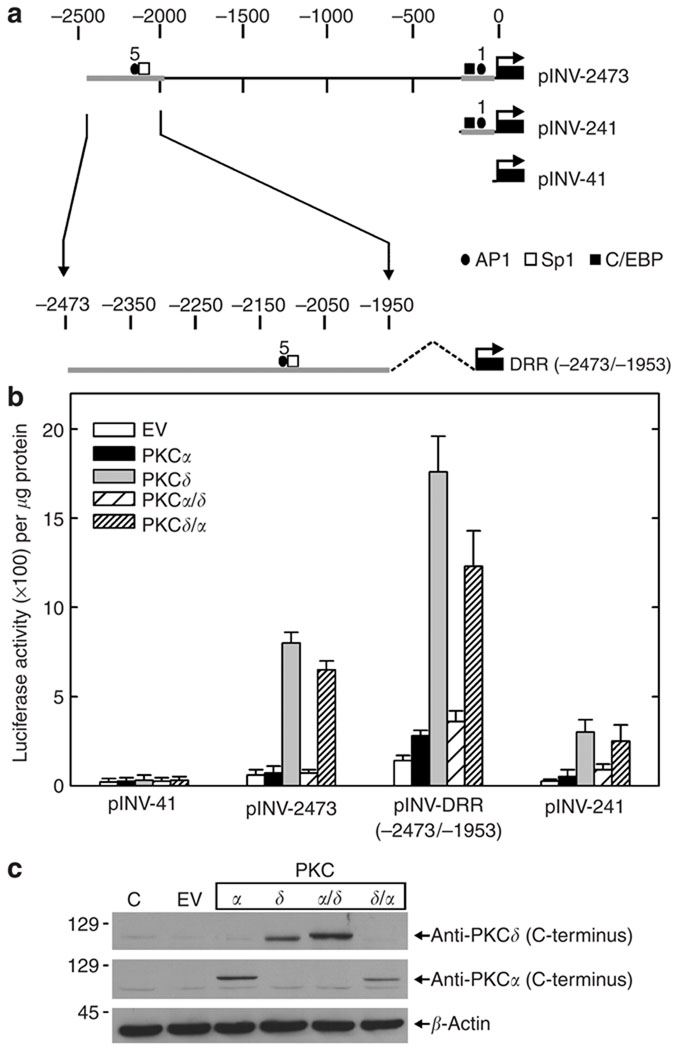
The PKCδ protein encodes two major functional domains—the catalytic domain at the C-terminus and the regulatory domain at the N-terminus (Steinberg, 2004). To understand the role of these domains in regulating involucrin gene expression, we utilized domain-swap mutants. PKCδ/α encodes the PKCδ regulatory domain fused to the PKCα catalytic domain; PKCα/δ encodes the PKCα regulatory domain fused to the PKCδ catalytic domain (Blass et al., 2002). To monitor the impact of the PKC domain-swap mutants on involucrin gene expression, keratinocytes were transfected with the involucrin promoter reporter plasmids indicated in Figure 1a. pINV-2473 encodes the full-length promoter, whereas pINV-DRR(−2473/−1953) and pINV-241 encode the distal and proximal regulatory region, respectively (Welter et al., 1995). These constructs were selected because they encode regulatory domains that are known to respond in a positive manner when keratinocytes are treated with agents that enhance keratinocyte differentiation (Eckert et al., 2004b). pINV-41 encodes the minimal promoter and is not regulated by keratinocyte differentiation agents (Welter et al., 1995). Keratinocytes were transfected with these plasmids in the absence or presence of plasmids encoding PKCδ, PKCα, PKCδ/α, and PKCα/δ, and luciferase activity was monitored at 48 hours post-transfection. As shown in Figure 1b, expression of PKCδ and PKCδ/α increase the activity of all three reporter constructs. In contrast, PKCα and PKCα/δ do not increase activity. None of the PKC expression constructs increases the activity of pINV-41, the hINV minimal promoter plasmid. Figure 1c shows that the key mutants are expressed at a level that permits direct comparison. PKCα and PKCδ/α are present at equal levels, but only PKCδ/α is active—indicating that the PKCδ regulatory domain confers activity on the PKCα backbone. Likewise, the PKCδ/PKCα/δ comparison is also well controlled regarding expression level—in this case, PKCα/δ is not active. These findings are consistent with our previous report indicating that PKCδ increases hINV gene expression and that PKCα is not active (Deucher et al., 2002) and suggest that the PKCδ regulatory domain is required for the PKCδ regulation.
Figure 1. PKCδ regulatory domain is required for PKCδ activation of hINV promoter activity.
(a) hINV promoter-luciferase reporter gene constructs. The narrow line represents the hINV promoter sequence and the dark box represents the luciferase gene. The arrow indicates the start site and the direction of transcription. The plasmids are named (pINV-2473, pINV-241, etc.) on the basis of the length of the segment, as measured backward from the hINV transcription start site (Eckert and Green, 1986). The scale at the top is in nucleotides. (b) Twenty-five percent confluent keratinocytes were transfected with 1 µg of the indicated involucrin promoter reporter plasmid + 1 µg of empty expression vector (EV) or plasmids encoding PKCα, PKCδ, PKCα/δ, or PKCδ/α. At 48 hours post-transfection, the cells were harvested and lysed for luciferase activity assay. The results depict the mean±SEM, n = 4. (c) Detection of expressed proteins. Keratinocytes were transfected with 1 µg of empty vector (EV) or plasmid encoding the indicated protein. After 48 hours, the cells were harvested and total cell extracts were prepared for electrophoresis and immunodetection of the C-terminus of PKCδ, the C-terminus of PKCα, and β-actin.
We next monitored the impact of selective mutation of PKCδ tyrosine residues on the ability to drive hINV gene expression. Keratinocytes were transfected with each of the involucrin promoter reporter constructs shown in Figure 1a in the presence of the wild-type or mutated PKCδ. As shown in Figure 2a, changing PKCδ tyrosine 52, 64, 155, 187, or 565 to phenylalanine does not attenuate PKCδ-dependent hINV promoter activity. However, reduced activity is observed when all five tyrosine residues are simultaneously mutated, suggesting that a global reduction in tyrosine residue density is required to attenuate biological response. As all three of the hINV promoter constructs respond in a similar fashion, we subsequently focused on only one of the constructs, pINV-241.
Figure 2. Impact of tyrosine mutation on the ability of PKCδ to drive hINV promoter activity.
(a) Twenty-five percent confluent keratinocytes were transfected with 1 µg of indicated involucrin promoter reporter plasmid + 1 µg of empty expression vector (EV) or plasmids encoding PKCδ, PKCδ-Y52F, PKCδ-Y64F, PKCδ-Y155F, PKCδ-Y187F, PKCδ-Y565F, or PKCδ-Δ5(Y52,64,155,187,565F). At 48 hours post-transfection, the cells were harvested and lysed for luciferase activity assay. The results show the mean±SEM, n=6. (b) Detection of expressed PKCδ proteins. Keratinocytes were transfected with 1 µg of empty expression plasmid (EV) or plasmid encoding the indicated PKCδ protein. After 48 hours, the cells were harvested and total cell extracts were prepared for electrophoresis and immunodetection of vector-delivered PKCδ using anti-PKCδ.
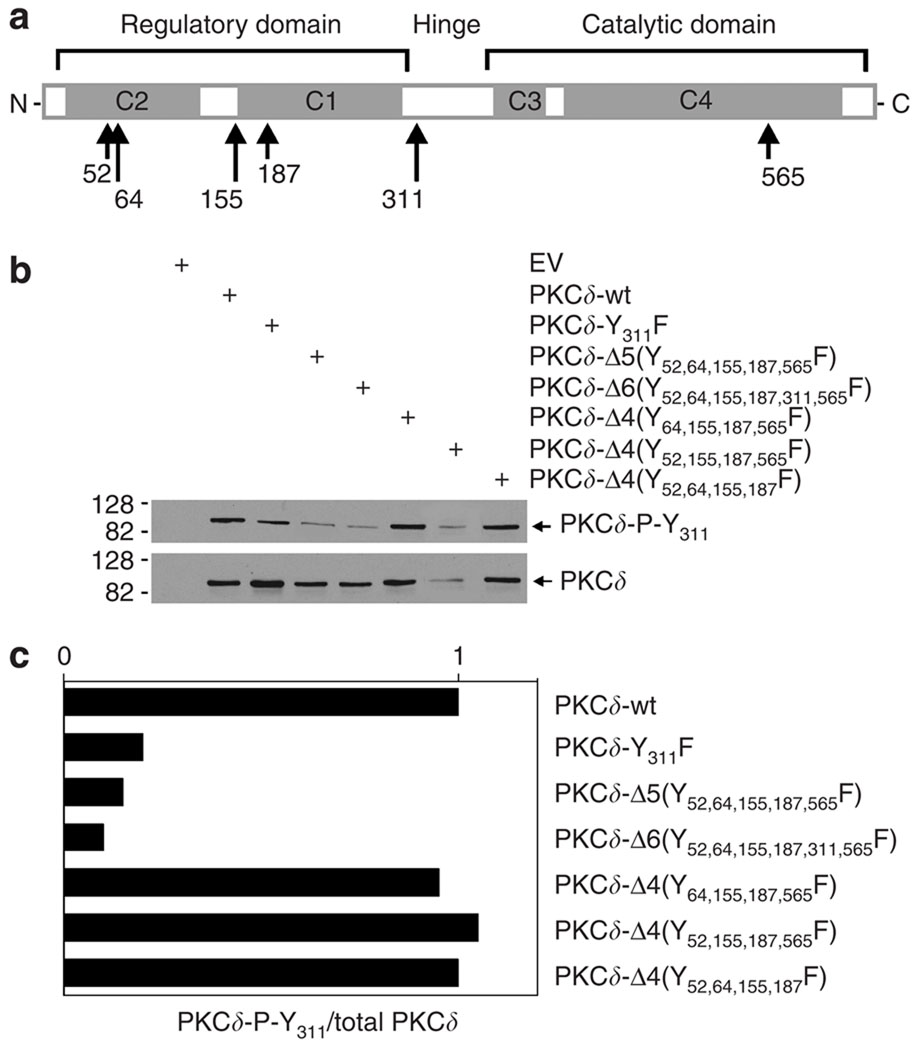
The fact that eliminating five tyrosine residues results in a substantial loss of activity suggests that one or more of these tyrosines may be essential for function. To identify essential PKCδ tyrosine residue(s), we systematically restored individual tyrosines in the context of the PKCδ-Δ5 mutant. To our surprise, as shown in Figure 3b, restoration of any single tyrosine residue (tyrosine 52, 64, 155, 187, or 565) or simultaneous restoration of two residues results in a near-complete restoration of activity, suggesting that no particular tyrosine residue is essential for biological activity. Figure 3a shows that each PKCδ mutant is expressed at a comparable level.
Figure 3. Restoration of one or two tyrosine residues in the PKCδ-Δ5 mutant restores hINV promoter activity.
(a) Expression of vector-delivered proteins. Keratinocytes were transfected with 1µg of empty plasmid vector (EV) or plasmid encoding the indicated protein. After 48 hours, the cells were harvested and total cell extracts were prepared for electrophoresis and immunodetection of vector-delivered PKCδ. (b) Twenty-five percent confluent keratinocytes were transfected with 1µg of pINV-241 promoter reporter plasmid + 1 µg of empty expression vector (EV) or the indicated PKCδ-encoding plasmid. At 48 hours post-transfection, the cells were harvested and lysed for luciferase activity assay. The results show the mean±SEM, n = 4.
Role of PKCδ-Y311
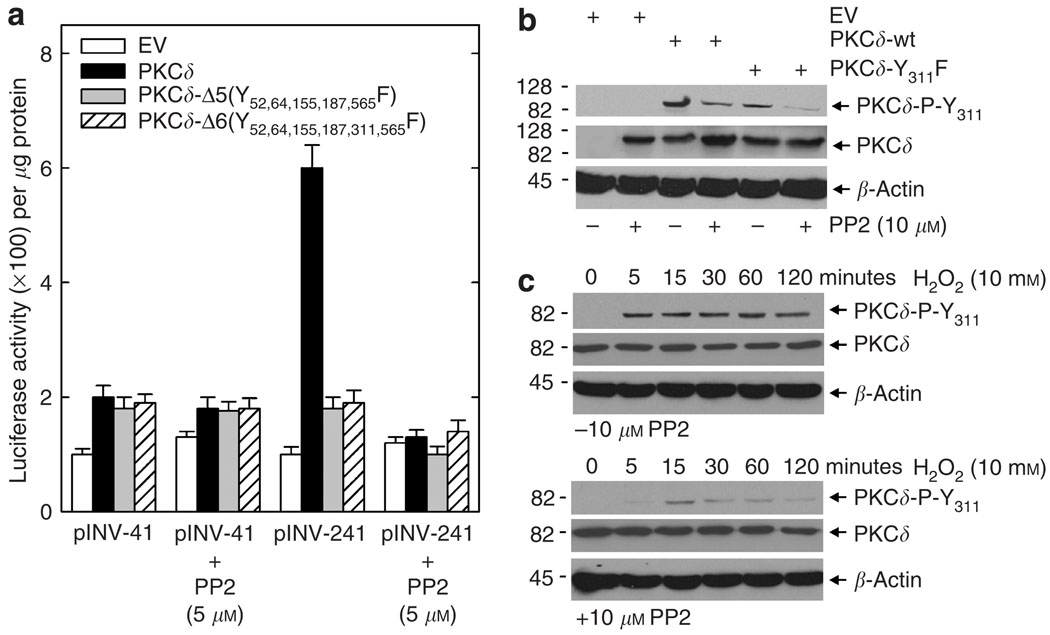
PKCδ-Y311 has been implicated as being required for PKCδ biological activity in human epidermal keratinocytes (Denning et al., 1995, 1998; Deucher et al., 2002; Efimova et al., 2002). We next inquired whether the effects of mutating the various tyrosine residues may be mediated via an impact on Y311 phosphorylation. To test this hypothesis, we transfected keratinocytes with pINV-241 in the presence or absence of PKCδ, PKCδ-Y311F, PKCδ-Δ5, or PKCδ-Δ6 (PKCδ-Δ6 is identical to PKCδ-Δ5, except that Y311 is also mutated). As shown in Figure 4a, the PKCδ-wt-dependent increase in promoter activity is substantially reduced for the PKCδ-Y311F, PKCδ-Δ5, and PKCδ-Δ6 mutants. As shown in Figure 4b, this decrease in activity is associated with reduced phosphorylation of Y311 for all three mutants. It is important to note that the anti-P-Y311 antibody detects residual phosphorylation, presumably because it is not entirely specific for Y311 (Rybin and Steinberg, 2006). These findings demonstrate that mutation of Y311 creates a protein with reduced biological activity. Moreover, these findings indicate that the reduced activity of PKCδ-Δ5 is associated with reduced Y311 phosphorylation.
Figure 4. Phosphorylation level of PKCδ and PKCδ tyrosine mutants at Y311.
(a) Keratinocytes were transfected with 1 µg of pINV-241 plus 1 µg of EV or plasmid encoding the indicated protein. After 48 hours, the cells were harvested and total cell extracts were prepared for luciferase activity assay. Results show the mean±SEM, n = 3. (b) Keratinocytes were transfected with 1 µg of the indicated construct. After 48 hours, extracts were prepared and the level of phosphorylated PKCδ-Y311 and total PKCδ was monitored by immunoblot. β-Actin level was monitored as a control to normalize gel loading.
The above findings show that elimination of five tyrosine residues in PKCδ results in a marked reduction in the functional activity (Figure 3), which is associated with reduced phosphorylation at Y311 (Figure 4). This suggests that the status of tyrosines 52, 64, 155, 187 and 565 may influence phosphorylation at Y311. To assess this, keratino-cytes were transfected with the PKCδ mutants shown in Figure 5a in which individual tyrosine residues were restored and the level of phosphorylation at PKCδ-Y311 was monitored by immunoblot, as was the total expression level of each PKCδ form (Figure 5b). To assess the relative level of PKCδ-Y311 phosphorylation, the blots were scanned and the relative level of Y311 phosphorylation was expressed relative to the total level of protein present (Figure 5c). This analysis reveals that reduced Y311 phosphorylation is observed for PKCδ-Δ5(Y52,64,155,187,565F), PKCδ-Δ6(Y52,64,155,187,311,565F), and PKCδ-Y311. In addition, it is interesting that restoration of tyrosine 52, 64, or 565 restores phosphorylation at Y311. A similar normalization of Y311 phosphorylation is observed following restoration of tyrosine 187 or 565 or when paired sets of tyrosine residues are restored (not shown).
Figure 5. Impact of restoration of tyrosine residues on Y311 phosphorylation.
(a) Structure of PKCδ tyrosine mutants. The overall structure of PKCδ is indicated, including the regulatory and catalytic domains and the hinge region. The locations of the C1, C2, C3, and C4 domains are indicated, as are the positions of the tyrosine residues targeted (tyrosines 52, 64, 155, 187, 311, and 565) in this study. (b) Impact of tyrosine mutations on Y311 phosphorylation. Keratinocytes were transfected with EV or plasmids encoding the indicated PKCδ proteins. After 48 hours, the cells were harvested and the total level of each protein and the extent of phosphorylation at Y311 were assessed by immunoblot. (c) Ratio of Y311 phosphorylation to total PKCδ. The blots shown in (b) were scanned and the results expressed as a ratio of PKCδ-P-Y311 to total PKCδ. Similar phosphorylation differences were observed in each of the four independent experiments.
Reduced PKCδ-Y311 phosphorylation is associated with reduced activity
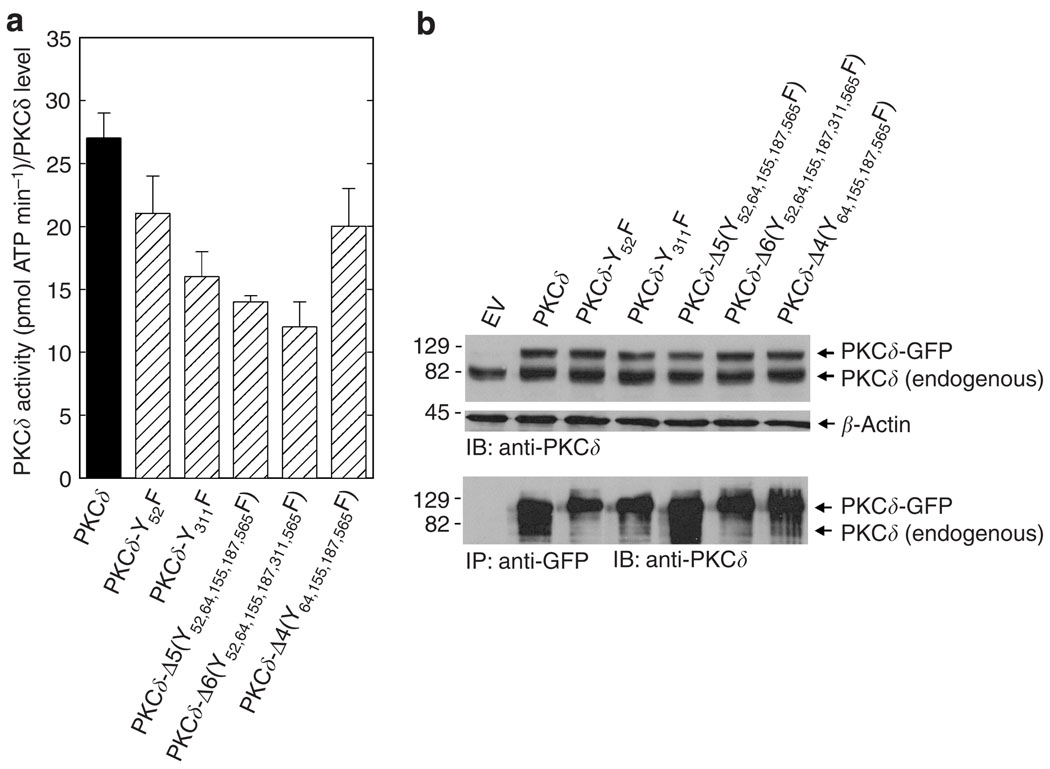
To determine the impact of reduced PKCδ-Y311 phosphorylation on PKCδ activity, keratinocytes were transfected with plasmids encoding wild-type PKCδ and mutant PKCδ proteins as green fluorescent protein (GFP) fusions. After 48 hours, total extracts were prepared and individual PKCδ-GFP fusion proteins were precipitated using anti-GFP. The precipitated PKCδ forms were then monitored for kinase activity as measured by the ability of each to catalyze the incorporation of radioactive phosphate into serine on a PKCδ substrate peptide. As shown in Figure 6a, the PKCδ-Y311F, PKCδ-Δ5, and PKCδ-Δ6 mutants display reduced activity relative to wild-type PKCδ and the PKCδ-Y52F and PKCδ-Δ4 mutants.
Figure 6. PKCδ activity assay.
(a) Mutants with reduced phosphorylation of PKCδ-Y311 have reduced activity. Keratinocytes in 3 × 60 mm dishes were transfected with 3 µg per dish of the indicated plasmid. After 48 hours, the PKCδ-GFP fusion protein was precipitated from 400 µg of total protein with anti-GFP (ab1218), and the precipitated PKCδ-GFP proteins were assayed for kinase activity. The precipitate was resuspended in 20 µl of kinase buffer, and 5 µl was used in the kinase reaction. Details of this method are provided in the text. Background kinase activity, in the EV control group, was negligible (0.3 pmol ATP minute −1). Similar results were observed in each of the three experiments. The results are presented as PKCδ activity normalized to PKCδ level (pmol ATP per minute per PKCδ level). (b) PKCδ-GFP immunoprecipitation. Keratinocytes were transfected with wild-type and mutant PKCδ forms as indicated. After 48 hours, total cell lysate (upper panel) and anti-GFP immunoprecipitates (lower panel) were electrophoresed and immunoblotted with anti-PKCδ. The expected migration position of endogenous PKCδ is shown. It is important to note that it is not precipitated (see EV lane) by anti-GFP and therefore does not contribute to the PKC activity assay. Similar results were observed in three separate experiments.
Figure 6b shows the level of endogenous and expressed PKCδ and PKCδ-GFP present in the transfected cells. The immunoblot, in the upper panel, indicates that each PKCδ-GFP fusion protein is expressed at a comparable level. The lower panel indicates the amount of PKCδ-GFP precipitated in each group. The level of precipitation of each respective mutant varied by twofold among experiments. However, the activity normalized to the amount of PKCδ present yielded results similar to Figure 6a in each of three separate experiments. Figure 6b also indicates that the precipitates are not contaminated with endogenous PKCδ. These findings indicate that PKCδ-Y311F, PKCδ-Δ5, and PKCδ-Δ6 have partially reduced activity as compared to wild-type PKCδ; however, none of the mutations completely inactivate the kinase.
Subcellular localization
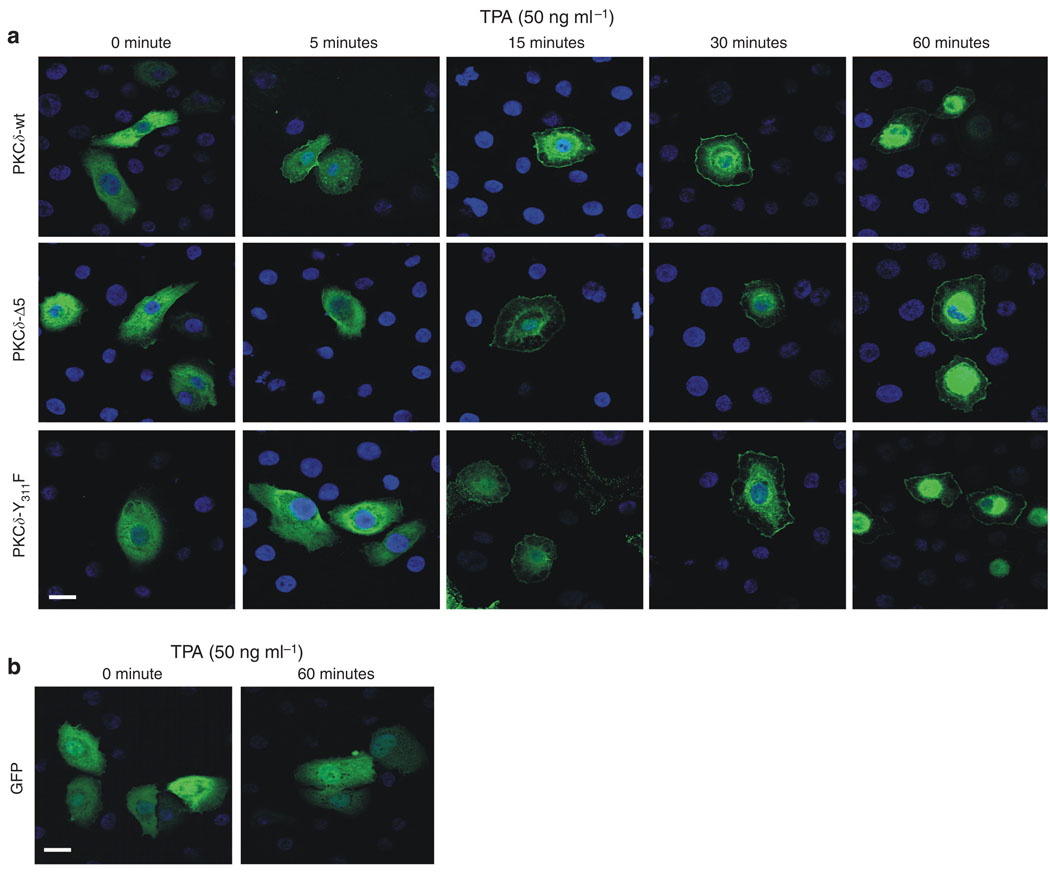
As PKCδ, PKCδ-Δ5, and PKCδ-Y311F have GFP fused at the PKC C-terminus, we were able to directly monitor subcellular distribution by confocal microscopy. Keratinocytes were transfected with plasmids encoding PKCδ-GFP, PKCδ-Δ5-GFP, PKCδ-Y311F-GFP, or GFP. After 48 hours, the cells were treated for 0–60 minutes with 50 ng ml−1 TPA and then fixed for confocal visualization. As shown in Figure 7a, wild-type PKCδ is distributed in the cytoplasm of untreated cells and appears at the plasma membrane beginning at 5 minutes following TPA treatment, and by 15 and 30 minutes it also appears at a perinuclear location. By 60 minutes, most of the PKCδ is present either at the plasma membrane or at the perinuclear location. A similar pattern of redistribution is observed for PKCδ-Δ5 and PKCδ-Y311F. Figure 7b is a control, which shows that GFP does not change compartments following treatment with TPA.
Figure 7. Subcellular distribution of PKCδ mutants in keratinocytes.
(a) Distribution of PKCδ-GFP fusion proteins in keratinocytes. Keratinocytes, growing on glass coverslips, were transfected with plasmids encoding the indicated PKCδ forms, each having GFP fused to the C-terminus. After 48 hours, the cells were treated with 50 ng ml−1 TPA for the indicated time and then harvested and fixed. The distribution of EGFP fluorescence was then examined by confocal microscopy. (b) The distribution pattern of EGFP. The lower panel shows the distribution pattern of GFP in cells transfected with pEGFP-N1. No change in distribution was observed in response to treatment with TPA.
In each case, the shift in subcellular localization was confirmed by biochemical analysis. In this experiment, keratinocytes were transfected with PKCδ, PKCδ-Y311F, and PKCδ-Δ5. After 48 hours, the cells were treated with 50 ng ml−1 TPA for 60 minutes. Figure 8a shows the subcellular distribution of transfected PKCδ forms (present as GFP fusion proteins) and endogenous PKCδ. In cells maintained in the absence of TPA, all of the PKCδ forms distribute equally between the soluble and particular fractions. In contrast, in cells treated with TPA, the ratio of each PKCδ form is threefold enriched in the particulate fraction. Thus, the distribution in resting cells and the pattern of redistribution following TPA treatment is identical for PKCδ and the PKCδ-Δ5 and PKCδ-Y311F mutants, indicating that a change in subcellular distribution of the mutants does not explain their reduced activity. As a control for the cell fractionation protocol, Figure 8b shows that involucrin is, as expected, present in the soluble fraction (Rice and Green, 1979; Eckert et al., 1993). The involucrin distribution does not change in TPA-treated cells (not shown).
Figure 8. Redistribution of PKCδ and PKCδ mutants in TPA-treated keratinocytes.
(a) Twenty-five percent confluent cultures of keratinocytes were transfected with 3 µg of EV (pEGFP-N1) or plasmids encoding PKCδ, PKCδ-Y311F, or PKCδ-Δ5. Each of the transfected PKCδ forms included GFP fused at the PKCδ C-terminus. After 48 hours, the cells were treated for 60 minutes in the presence or absence of 50 ng ml−1 TPA. Soluble and particulate (membrane) fractions were prepared and the distribution of transfected and endogenous PKCδ was monitored by immunoblot using anti-PKCδ. (b) Involucrin distributes in the soluble fraction. Keratinocytes were treated as above and then hINV level was monitored using anti-hINV. It should be noted that these samples were loaded on the basis of total protein concentration and that, if the samples were loaded on the basis of cell equivalents, the amount of membrane-associated PKCδ and hINV would be decreased threefold.
PKCδ-P-Y311 is reduced by treatment with biological agents
We next examined the impact of agents that suppress involucrin gene expression on the level of PKCδ-P-Y311. We recently identified curcumin and apigenin as agents that suppress hINV promoter activity (Balasubramanian et al., 2006). As shown in Figure 9a, treatment with these agents reduces the PKCδ-dependent increase in hINV promoter activity to near control levels. Figure 9b shows that this reduction is associated with reduced phosphorylation of the expressed PKCδ at Y311. We next monitored the impact of treatment with these agents on endogenous PKCδ-P-Y311 level. Figure 9c shows that Y311 phosphorylation of endogenous PKCδ is also reduced following treatment with curcumin or apigenin.
Figure 9. Increased phosphorylation of PKCδ-Y311 is associated with increased ability to PKCδ to increase hINV promoter activity.
(a) Twenty-five percent confluent keratinocytes were transfected with 1 µg of hINV-241 + 1 µg of empty expression vector (EV) or 1 µg of the indicated PKCδ-encoding plasmid. At 24 hours post-transfection, the cells were treated with apigenin or curcumin for an additional 24 hours and then harvested and lysed for luciferase activity assay. The results show the mean±SEM, n = 4. (b) Keratinocytes were transfected with 1 µg of EV or the indicated PKCδ-encoding plasmid. After 24 hours, the cells were treated with apigenin or curcumin for an additional 24 hours. Total cell extracts were prepared for immunodetection of vector-expressed total PKCδ and phosphorylated PKCδ-Y311. (c) Apigenin and curcumin suppress Y311 phosphorylation of endogenous PKCδ. Keratinocytes were incubated with 20 µm curcumin or apigenin for 24 hours. Total extracts were prepared and electrophoresed, and total PKCδ and tyrosine-phosphorylated PKCδ were detected by immunoblot.
PKCδ-Y311 phosphorylation is inhibited by src kinase inhibitor
Src is a tyrosine kinase that has been reported to regulate PKCδ tyrosine phosphorylation (Kajimoto et al., 2001, 2004; Rybin et al., 2004; Kaul et al., 2005; Murugappan et al., 2005). To determine whether PKCδ-Y311 phosphorylation is regulated by src family kinases in human keratinocytes, we monitored the impact of treatment with the general src family inhibitor, PP2, on PKCδ-Y311 phosphorylation and promoter activity. As shown in Figure 10a, treatment with PP2 inhibits the PKCδ-dependent increase in hINV promoter activity. Moreover, as shown in Figure 10b, this PP2-dependent reduction in activity is associated with reduced phosphorylation of PKCδ at Y311 and also reduced residual phosphorylation of the PKCδ-P-Y311F mutant. β-Actin levels are not changed by PP2 treatment. This finding indicates that reduced phosphorylation of PKCδ-Y311 is associated with reduced hINV promoter activity.
Figure 10. Src family kinase inhibitor reduces hINV promoter activity and PKCδ-Y311 phosphorylation.
(a) Twenty-five percent confluent keratinocyte cultures were transfected with 1 µg of pINV-41 or pINV-241 plus 1 µg of empty expression vector (EV) or plasmids encoding PKCδ-wt, PKCδ-Δ5(Y52,64,155,187,565F), or PKCδ-Δ6(Y52,64,155,187,311,565F). After 12 hours, the cells were treated with 0 or 5 µm PP2. After an additional 36 hours, the cells were harvested for assay of luciferase activity. The results show the mean±SEM, n = 3. (b) PP2 treatment suppresses phosphorylation of transfected PKCδ-Y311. Keratinocytes were treated with 10 µm PP2 for 60minutes followed by transfection with 2 µg of PKCδ-wt or PKCδ-Y311F. After 24 hours, the cells were harvested for assay of PKCδ-P-Y311 and total PKCδ level by immunoblot. (c) PP2 treatment suppresses phosphorylation of endogenous PKCδ at Y311. Keratinocytes were pretreated for 60 minutes with 10 µm PP2 and then treated for 0–120 minutes with 10mM H2O2. At the indicated times, the cells were harvested and assayed for β-actin, total PKCδ, and PKCδ-P-Y311 levels by immunoblot.
If this regulation is physiologically relevant, we would anticipate that Y311 phosphorylation of endogenous PKCδ should also be controlled by src family kinases. To test this, we monitored the impact of PP2 treatment on hydrogen peroxide-dependent phosphorylation of PKCδ-Y311. H2O2 treatment is known to promote phosphorylation of PKCδ on Y311 (Deucher et al., 2002; Efimova et al., 2004; Kraft et al., 2006). As shown in Figure 10c, pretreatment with PP2 inhibits the H2O2-dependent phosphorylation, suggesting that the phosphorylation of Y311 of endogenous PKCδ is also src family kinase-dependent.
Regulation of endogenous involucrin levels
We previously showed that expression of PKCδ or other novel PKC isoforms in keratinocytes results in an increase in involucrin protein level (Efimova and Eckert, 2000). To assess the impact of individual PKC mutants on endogenous involucrin, keratinocytes were transfected with each of the indicated PKC forms, and hINV protein levels were monitored at 48 hours post-transfection by immunoblot. Figure 11a shows that wild-type PKCδ increases hINV level as compared to empty vector (EV)-transfected cells, but that the PKCδ phosphorylation site mutants (PKCδ-Y311F, PKCδ-Δ5, and PKCδ-Δ6) produced a minimal change in expression. We also compared the PKC domain-swap mutants for their ability to regulate involucrin level. These studies indicate that PKCδ and PKCδ/α increase hINV level, but that PKCα and PKCα/δ do not (Figure 11b).
Figure 11. Impact of PKCδ mutants on the levels of endogenous involucrin protein.
(a and b) Subconfluent cultures of normal human keratinocytes were transfected with 2 µg of EV or vector encoding the indicated PKCδ form per 10 cm2 dish. At 48 hours post-transfection, the cells were harvested and extracts separated by electrophoresis and immunoblotted with anti-involucrin.
DISCUSSION
PKC family members are important regulators of cell differentiation and survival (Denning et al., 1995; Deucher et al., 2002; Cataisson et al., 2003; Efimova et al., 1998, 2004). Activity of these kinases is influenced by subcellular localization, tyrosine phosphorylation, enzymatic processing, and the presence of other PKC isoforms (Denning et al., 1998; Mandil et al., 2001; Deucher et al., 2002; Brodie and Blumberg, 2003; Cataisson et al., 2003; Efimova et al., 2002, 2004; Steinberg, 2004; D’Costa and Denning, 2005; D’Costa et al., 2005; Li et al., 2006). In recent years, it has been appreciated that phosphorylation is an important mechanism that regulates PKCδ function (Brodie and Blumberg, 2003; Steinberg, 2004). PKCδ is sequentially phosphorylated on serine residues as part of the protein maturation process (Brodie and Blumberg, 2003; Steinberg, 2004). However, tyrosine phosphorylation is responsible for regulating activity (Brodie and Blumberg, 2003; Steinberg, 2004). Previous studies in a number of cell types indicate that PKCδ is tyrosine phosphorylated by src family kinases (Kajimoto et al., 2001, 2004; Rybin et al., 2004; Kaul et al., 2005; Murugappan et al., 2005) and by c-abl (Lu et al., 2006). The target tyrosine residues on PKCδ vary in different cell types and in response to different stimuli (Li et al., 1996; Acs et al., 2000; Dundr et al., 2000; Kronfeld et al., 2000; Blass et al., 2002; Zrachia et al., 2002; Okhrimenko et al., 2005; Lu et al., 2006).
PKCδ tyrosine phosphorylation in keratinocytes
PKCδ tyrosine phosphorylation status alters PKCδ activity and downstream responses in keratinocytes. Increased PKCδ tyrosine phosphorylation is associated with reduced PKCδ activity in ras-transformed murine keratinocytes (Yuspa et al., 1994; Denning et al., 1993, 1996). This reduction is associated with reduced cell differentiation (Joseloff et al., 2002) and is reversed by general tyrosine kinase inhibitors (Reynolds et al., 1993) and src kinase inhibitors (Joseloff et al., 2002). In this same cell type, differentiation is stimulated when PKCδ-Y64F or PKCδ-Y565F mutants are expressed, suggesting that phosphorylation of these residues may result in reduced PKCδ activity (Joseloff et al., 2002). In contrast, PKCδ-Y52F, PKCδ-Y155F, and PKCδ-Y187F do not induce differentiation. Thus, in v-ras-transformed murine keratinocytes, increased PKCδ tyrosine phosphorylation, possibly at tyrosines 64 and 565, is associated with decreased kinase activity and reduced cell differentiation (Denning et al., 1993).
In contrast, in normal murine keratinocytes, treatment with the differentiation agent calcium results in increased PKCδ tyrosine phosphorylation, and this increase is associated with enhanced differentiation (Denning et al., 2000). Treatment with EGF or TGFα, which activate src family kinases, also leads to enhanced PKCδ phosphorylation and enhanced differentiation (Denning et al., 1996). Thus, in contrast to the situation in ras-transformed murine keratinocytes, in normal murine keratinocytes, increased PKCδ tyrosine phosphorylation is associated with enhanced keratinocyte differentiation. PKCδ phosphorylation has also been studied in normal human keratinocytes. Calcium treatment of normal human keratinocytes results in increased tyrosine phosphorylation of PKCδ, and PKCδ and calcium act synergistically to increase differentiation as measured by increased involucrin gene expression (Deucher et al., 2002). PKCα antagonizes this action (Deucher et al., 2002). PKCδ is known to be phosphorylated on Y311 in these cells (Efimova et al., 2004), and this phosphorylation is increased following treatment with the apoptosis-inducing agent, hydrogen peroxide (Kraft et al., 2006). In contrast, the differentiating agent okadaic acid does not increase PKCδ phosphorylation at Y311 (Kraft et al., 2006). Thus, information on the role of specific PKCδ tyrosine residues in keratinocytes is limited.
A PKCδ five-tyrosine mutant has reduced ability to regulate involucrin gene expression
In this study, we examined the impact of PKCδ tyrosine phosphorylation on PKCδ-dependent regulation of involucrin gene expression in normal human keratinocytes. Involucrin is a marker of keratinocyte differentiation that is expressed in the suprabasal epidermal layers (Robinson et al., 1996; Eckert et al., 1993, 1997). Our previous studies show that a PKCδ, Ras, MEKK1, MEK3 pathway that acts via an ERK1/2–p38d complex regulates differentiation in keratinocytes (Efimova and Eckert, 2000; Efimova et al., 1998, 2002, 2003). Activation of this pathway ultimately results in increased AP1, Sp1, and C/EBP transcription factor binding to the hINV promoter upstream regulatory region and increased gene expression (Efimova and Eckert, 2000; Efimova et al., 1998, 2002, 2003).
Our studies show that mutation of individual tyrosine residues at position 52, 64, 155, 187, or 565 does not reduce the ability of vector-expressed PKCδ to increase hINV promoter activity. However, simultaneous mutation of all five residues results in a substantial loss of the ability to increase hINV promoter activity and endogenous hINV protein level. It is also informative that restoration of any one of these residues results in the complete restoration of PKCδ function. This is surprising, as we had anticipated that only selected tyrosines would be important. In C6 glioma cells, etoposide treatment causes apoptosis, a response that is inhibited when the PKCδ-Δ5 mutant is expressed (Blass et al., 2002). Mutation of individual PKCδ tyrosine residues in this cell type indicates that tyrosines 64 and 187 are required for etoposide-dependent apoptosis (Blass et al., 2002). This is clearly a different situation from this study, where restoration of any of the five PKCδ tyrosine residues restores biological activity.
PKCδ-Y311 has a key role
To extend these studies, we examined the role of PKCδ-Y311. Phosphorylation at PKCδ-Y311 is known to be regulated in keratinocytes. Hydrogen peroxide treatment increases PKCδ-Y311 phosphorylation and causes apoptosis/differentiation (Efimova et al., 2004), whereas treatment with the dietary chemopreventive agent, apigenin, decreases PKCδ-Y311 phosphorylation and suppresses differentiation (Balasubramanian et al., 2006). Therefore, we decided to assess whether the reduction in the activity of the five-tyrosine mutant could be due to a change in Y311 phosphorylation. This analysis revealed a surprising role for Y311. First, the results showed that reduced ability of PKCδ-Δ5(Y52,64,155,187,565F) to drive involucrin gene expression is associated with reduced phosphorylation of Y311. Second, restoration of any one of the five-tyrosine residues permits not only phosphorylation at Y311 but also PKCδ-dependent activation of involucrin gene expression. Finally, the reduction in functional activity of the PKCδ-Δ5 mutant is comparable to that observed upon mutation of Y311. As an additional confirmation, we monitored the impact of these mutations on PKCδ activity. This analysis revealed that each PKCδ mutant that displayed reduced ability to increase hINV promoter activity also displayed reduced PKCδ-Y311 phosphorylation and reduced functional kinase activity. Thus, Y311 is required for PKCδ function.
These findings indicate that any one of the five tyrosine residues, from among tyrosines 52, 64, 155, 187, and 565, can partner with Y311. The mechanistic basis for this observation is not known. It is possible that one of these tyrosines must be present to provide a docking site for the tyrosine kinase that phosphorylates PKCδ at Y311. Alternatively, phosphorylation of Y311 plus phosphorylation of one of these other tyrosine may be required to activate PKCδ. We presently favor the latter hypothesis.
Phosphorylation of PKCδ-Y311 is functionally important in other systems and this residue is a target of src family kinases. Src family kinase-dependent phosphorylation of PKCδ-Y311 is associated with increased thromboxane A2 production in platelets (Murugappan et al., 2005). Src family kinase-dependent PKCδ-Y311 phosphorylation is also observed in cardiomyocytes, following treatment with TPA or hydrogen peroxide (Rybin et al., 2004), and in hydrogen peroxide-treated dopaminergic neuronal cells (Kaul et al., 2005). Treatment of HeLa cells with ceramide results in apoptosis, which is associated with PKCδ activation and phosphorylation at Y311 and Y322 (Kajimoto et al., 2004). Hydrogen peroxide treatment results in PKCδ phosphorylation on tyrosine residues 311, 332, and 512 in COS-7 cells (Konishi et al., 2001). Our present studies indicate that src kinase is important for phosphorylation of PKCδ-Y311 in human keratinocytes, as treatment with src kinase inhibitor reduces the phosphorylation level. This is consistent with the role of src kinases in phosphorylating PKCδ in murine keratinocytes (Joseloff et al., 2002).
Subcellular distribution does not have an impact
One possible explanation for the reduced biological activity of the PKCδ-Y311F, PKCδ-Δ5, and PKCδ-Δ6 mutants is an impact of the mutation on the subcellular distribution of the PKCδ form. This is an important issue, as PKC isoforms are known to redistribute in response to treatment with physiological agents, and this is thought to be important for biological activity. For example, PKCδ has been reported to move to the particulate insoluble fraction in murine keratinocytes (Denning et al., 1995). We therefore examined whether the PKCδ-Y311F, PKCδ-Δ5, or PKCδ-Δ6 mutants display an altered distribution compared to wild-type PKCδ. In resting cells, all mutants displayed a similar cytoplasmic distribution, with some also present in the membrane fraction. Following treatment with TPA, each of the mutants moves to the plasma membrane and perinuclear region on a similar time course, a change that is confirmed by biochemical fractionation studies. Thus, there appears to be no difference in the behavior of these mutants with respect to intracellular distribution and stimulus-dependent movement, suggesting that an altered intracellular distribution is not likely to explain the altered biological activity of the mutants.
An essential role for the PKCδ regulatory domain
An additional point of importance is the observation that the PKCδ regulatory domain is required for PKCδ activity in keratinocytes. This is supported by data obtained from experiments using domain-swap mutants, which show that PKCδ/δ and PKCδ/α are active whereas PKCα/α and PKCα/δ are not active. This suggests that tyrosine 565 in the PKCδ catalytic domain (see Figure 6) can be substituted by tyrosines in the PKCα catalytic domain, but that features of the PKCδ regulatory domain are uniquely required for activity. Additional studies will be required to identify these feature(s). These findings are in contrast to those reported in C6 glioma cells, where both the PKCδ regulatory and catalytic domains are required for function (Blass et al., 2002).
Y311 is a physiologically important residue
The above studies indicate that mutation of Y311 to phenylalanine results in reduced ability of vector-delivered PKCδ to drive involucrin promoter activity and increase endogenous involucrin protein level. However, an important aspect is the response of the system following treatment with physiological or pharmacologic agents. To assess this, we treated keratinocytes with several agents that suppress involucrin gene expression. An example is the treatment of keratinocytes with dietary chemopreventive agents. These agents suppress keratinocyte differentiation and reduce involucrin gene expression (Balasubramanian et al., 2006; Eckert et al., 2004a, 2006). The studies described in this report indicate that these agents also suppress phosphorylation of PKCδ at Y311. Thus, following treatment with exogenous dietary agents, PKCδ-Y311 phosphorylation is suppressed, and this is associated with reduced involucrin gene expression. A particularly good example of this response is the ability of the dietary-constituent, apigenin, to reduce PKCδ-Y311 phosphorylation. Additional evidence of a physiological role for this tyrosine is the loss of PKCδ-dependent activation of involucrin gene expression in PKCδ-Y311 mutants (Balasubramanian et al., 2006). These results are consistent with a general hypothesis that PKCδ-dependent activation of keratinocyte differentiation requires phosphorylation of PKCδ-Y311 and the presence of a second tyrosine residue. Further studies will be necessary to identify the precise role of the secondary tyrosine.
MATERIALS AND METHODS
Antibodies and reagents
Keratinocyte serum-free medium, gentamicin, and trypsin were purchased from Life Technologies Inc (Carlsbad, CA). Antibodies for detection of PKCδ (sc-937) and PKCα (sc-208) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin (A5441) was purchased from Sigma (St Louis, MO). Rabbit polyclonal anti-PKCδ-P-Y311 (murine) (44–950) was obtained from BioSource International Inc (Camarillo, CA). Horseradish peroxidase-conjugated sheep anti-mouse IgG (NA931) and donkey anti-rabbit IgG (NA934) antibodies were purchased from Amersham Biosciences (Cleveland, OH). Apigenin (no. A3145) and curcumin (no. C1386) were obtained from Sigma. FuGENE 6 transfection reagent (no. 1814443) was purchased from Roche Diagnostics Corporation (Indianapolis, IN). PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4,d]pyrimidine) is an src family kinase inhibitor. All experiments were approved by the appropriate institutional board and the study plan was conducted according to the Declaration of Helsinki Principles.
Plasmids
The hINV reporter plasmids, encoding various lengths of hINV promoter upstream regulatory region fused to the luciferase reporter gene in pGL2-basic, have been described (Welter and Eckert, 1995; Efimova et al., 1998). The PKCδ tyrosine mutants are identified by indicating the position of the mutant tyrosine (e.g., PKCδ-Y52F, PKCδ-Δ5(Y52,64,155,187,565F)). Some of the mutants used in this study were previously described (Blass et al., 2002) and others were constructed as part of this study. PKCα/δ refers to the chimera of the PKCα regulatory and the PKCδ catalytic domain; PKCδ/α refers to the reciprocal chimera. PKC chimeras have been used to assign domain function of PKC isoforms (Acs et al., 1997). All PKCδ constructs have GFP fused to the PKCδ C-terminus. Empty expression vector, pEGFP-N1, was purchased from Clontech (Palo Alto, CA).
Site-directed mutagenesis of PKCδ
PKCδ-Δ5(Y52,64,155,187,565F) was used as a template for the site-directed mutagenesis to restore mutated tyrosine residues, using the QuikChange Site-Directed Mutagenesis Kit from Stratagene (no. 200518, La Jolla, CA). The following oligonucleotides served as primers for mutagenesis: 5′-GCCCACCATGTATCCCGAGTGGAA GACAACG, converts phenylalanine 52 to tyrosine (bold letters); 5′-CGACGCCCACATCTACGAAGGCCGTGTTATCC, converts pheny-lalanine 64 to tyrosine (bold letters); 5′-GGCCAAGATCCACTATA TAAAGAACCACG, converts phenylalanine 155 to tyrosine (bold letters); 5′-CTCAACAAGCAAGGCTATAAATGCAGGCAATGC, converts phenylalanine 187 to tyrosine (bold letters); 5′-GGACACAC CACACTATCCCCGCGGGATCACC, converts phenylalanine 565 to tyrosine (bold letters). Using PKCδ plasmid as template, 5′-GGA CACACCACACTTTCCCCGTTGGATCACC was used to change tyrosine 565 to phenylalanine (bold letters). Another oligonucleotide, 5′-GTCTGTCGGAATATTCCAGGGATTTGAG, was used to convert Y311 to phenylalanine (bold letters). The mutations were confirmed by DNA sequencing.
Cell culture, transfection, and hINV promoter assay
Normal human foreskin keratinocytes were grown as described previously (Welter and Eckert, 1995). All use of human tissues complies with federal and institutional guidelines. For plasmid DNA transfection, third-passage keratinocytes, cultured in 35 mm diameter dishes, were transfected when 25% confluent. FuGENE 6 transfection reagent (12 µl) was mixed with 200 µl of keratinocyte serum-free medium, and 4 µg of plasmid DNA was added. The mixture was incubated at room temperature for 15 minutes and then added dropwise to the cells in dishes containing 2 ml of keratinocyte serum-free medium. After 48 hours, keratinocytes were rinsed twice with 1 × phosphate-buffered saline, incubated in 100 µl of cell culture lysis reagent (Promega, Madison, WI) for 15 minutes, and harvested by scraping. The cell lysates were assayed immediately using a Berthold luminometer and Promega luciferase assay reagent. Cell lysate (20 µl) and 100 µl of luciferin solution were reacted for 4 seconds and light output was monitored during the next 20 seconds. All assays were performed in duplicate, and each experiment was repeated a minimum of three times. Luciferase activity is normalized per µg of protein.
Immunoblot analysis
Total cell lysates were prepared from cultured human epithelial keratinocytes at 48 hours post-transfection or at 24 hours after apigenin or curcumin treatment. Cells were washed with phosphate-buffered saline and incubated on ice in lysis buffer (20 mm Tris-HCl pH 7.5, 150mm NaCl, 2 mm Na2EDTA, 2 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 µg ml−1 leupeptin, and 1 mm PMSF) for 5 minutes. Lysates were collected, sonicated intermittently for 20 seconds, and then centrifuged at 15,000 × g for 10 minutes at 4°C to pellet cell debris. Protein concentrations were determined by Bio-Rad protein assay. Equivalent amounts of protein were electrophoresed on denaturing polyacrylamide gels and subsequently transferred to nitrocellulose for immunoblot. The membranes were blocked and incubated with appropriate primary and secondary antibodies, and proteins were visualized using chemiluminescence reagents.
Microscopy
Human epidermal keratinocytes were plated onto 22 × 22 mm coverslips and transfected with 1 µg of plasmid encoding wild-type or mutant PKCδ-GFP. After 48 hours, keratinocytes were fixed with 3.7% paraformaldehyde in phosphate-buffered saline at room temperature for 20 minutes. After two washes with 1 × phosphate-buffered saline, the cells were mounted on glass slides with mounting medium and the distribution of the PKCδ-GFP fusion proteins was examined by confocal fluorescence microscopy.
Subcellular fractionation
Keratinocytes were grown in 60 mm diameter dishes and, when 25% confluent, were transfected with 3 µg of plasmid DNA encoding PKCδ-wt, PKCδ-Y311F, PKCδ-Δ5, or EV. At 48 hours post-transfection, cells were exposed to 50 ng TPA per ml for 60 minutes. Keratinocytes were then rinsed with 1 × phosphate-buffered saline and lysed in 150 µl of buffer containing 20 mm HEPES (pH 7.4), 250 mm sucrose, 150mm NaCl, 0.5 mm EGTA, 0.5 mm EDTA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 8 µg ml−1 aprotinin, 20 µm leupeptin, 200 µm NaVO3, and 10mm NaF. Cell lysates were sonicated intermittently for 20 seconds and centrifuged for 1 hour at 4°C. The supernatant was collected as the soluble protein fraction. The membrane pellet was washed with 500 µl of lysis buffer and centrifuged for 1 hour at 4°C. The membrane pellet was resuspended in 150 µl of the lysis buffer containing 1% Triton X-100. Protein concentrations were determined by Bio-Rad protein assay. An equivalent amount of protein was electrophoresed on 10% polyacrylamide denaturing gels, and PKCδ and involucrin levels were assayed by immunoblot.
PKCδ activity assay
Keratinocytes were transfected with 3 µg of EV or plasmid encoding PKCδ, PKCδY52F, PKCδ-Y311F, PKCδ-δ5(Y52,64,155,187,565f), PKCδ-Δ6(Y52,64,155,187,311,565F), or PKCδ-Δ4(Y64,155,187,565F). At 48 hours post-transfection, cells were lysed in 20 mM Tris-HCl (pH 7.5) containing 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm sodium orthovanadate, 1 µg ml−1 leupeptin, 2 mm phenylmethylsulfonyl fluoride, and 0.5 mm dithiothreitol. Protein lysate (400 µg) was precleared by mixing with 25 µl of Protein A/G PLUS-agarose beads (Santa Cruz Biotechnology), and the suspension was rotated for 2 hours at 4°C. The preparation was centrifuged at 4,000 r.p.m. for 1 minute and the supernatant was incubated with 2 µg of mouse monoclonal anti-GFP antibody (ab1218) for 3 hours, followed by incubation with 25 µl of Protein A/G PLUS-agarose beads overnight. The suspension was then centrifuged at 4,000 r.p.m. for 1 minute and the beads were washed three times with lysis buffer and once with kinase assay buffer (50 mm Tris-HCl (pH 7.5), 10mm β-glycerophosphate, 4 mm dithiothreitol, 20 mm MgCl2, and 0.2 mm sodium orthovanadate). The beads were resuspended in 20 µl of kinase buffer. PKCδ activity was assayed using freshly prepared immunoprecipitate. Activity was measured using the SignaTECT Protein Kinase C Assay System (Promega, no. 7470) according to the manufacturer’s instructions. Biotinylated peptide Neurogranin(28–43) (AAKIQASFRGHMARKK) is used as PKC substrate in this assay. The serine indicated by the asterisk is the target serine.
ACKNOWLEDGMENTS
This work utilized the facilities of the Skin Diseases Research Center of Northeast Ohio (NIH, AR39750) and was supported by grants to R.L.E. (RO1AR046494) and C.B. (RO1CA109196) from the National Institutes of Health.
Abbreviations
- EV
empty vector
- GFP
green fluorescent protein
- hINV
human involucrin
- PKC
protein kinase C
- PKCδ
protein kinase C-delta
- TPA
12-O-tetradecanoylphorbol-13-acetate
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4,d]pyrimidine
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Acs P, Beheshti M, Szallasi Z, Li L, Yuspa SH, Blumberg PM. Effect of a tyrosine 155 to phenylalanine mutation of protein kinase cdelta on the proliferative and tumorigenic properties of NIH 3T3 fibroblasts. Carcinogenesis. 2000;21:887–891. doi: 10.1093/carcin/21.5.887. [DOI] [PubMed] [Google Scholar]
- Acs P, Wang QJ, Bogi K, Marquez AM, Lorenzo PS, Biro T, et al. Both the catalytic and regulatory domains of protein kinase C chimeras modulate the proliferative properties of NIH 3T3 cells. J Biol Chem. 1997;272:28793–28799. doi: 10.1074/jbc.272.45.28793. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Zhu L, Eckert RL. Apigenin inhibition of involucrin gene expression is associated with a specific reduction in phosphorylation of protein kinase Cdelta Tyr311. J Biol Chem. 2006;281:36162–36172. doi: 10.1074/jbc.M605368200. [DOI] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S, et al. Activation of cutaneous protein kinase Calpha induces keratino-cyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol. 2003;171:2703–2713. doi: 10.4049/jimmunol.171.5.2703. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Denning MF. A caspase-resistant mutant of PKC-delta protects keratinocytes from UV-induced apoptosis. Cell Death Differ. 2005;12:224–232. doi: 10.1038/sj.cdd.4401558. [DOI] [PubMed] [Google Scholar]
- D’Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2005;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Cheng C, Dempsey PJ, Coffey RJ, Jr, Threadgill DW, et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol. 2000;9:192–199. doi: 10.1034/j.1600-0625.2000.009003192.x. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Howett MK, Yuspa SH. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Threadgill DW, Magnuson T, Yuspa SH. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Williams EK, Szallasi Z, Blumberg PM, Yuspa SH. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- Denning MF, Wang Y, Nickoloff BJ, Wrone-Smith T. Protein kinase Cdelta is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)alpha and PKCδelta. J Biol Chem. 2002;277:17032–17040. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Mischak H, Mushinski JF, Yuspa SH. Transcripts encoding protein kinase C-alpha, -delta, -epsilon, -zeta, and -eta are expressed in basal and differentiating mouse keratinocytes in vitro and exhibit quantitative changes in neoplastic cells. Mol Carcinog. 1992;5:286–292. doi: 10.1002/mc.2940050409. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Yuspa SH. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J Invest Dermatol. 1994;102:409–414. doi: 10.1111/1523-1747.ep12372171. [DOI] [PubMed] [Google Scholar]
- Dundr M, Misteli T, Olson MO. The dynamics of postmitotic reassembly of the nucleolus. J Cell Biol. 2000;150:433–446. doi: 10.1083/jcb.150.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Balasubramanian S. Antioxidants regulate normal human keratinocyte differentiation. Biochem Pharmacol. 2004a;68:1125–1131. doi: 10.1016/j.bcp.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Balasubramanian S. Opposing action of curcumin and green tea polyphenol in human keratinocytes. Mol Nutr Food Res. 2006;50:123–129. doi: 10.1002/mnfr.200500125. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, et al. Regulation of involucrin gene expression. J Invest Dermatol. 2004b;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Green H. Structure and evolution of the human involucrin gene. Cell. 1986;46:583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Yaffe MB, Crish JF, Murthy S, Rorke EA, Welter JF. Involucrin—structure and role in envelope assembly. J Invest Dermatol. 1993;100:613–617. doi: 10.1111/1523-1747.ep12472288. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol Cell Biol. 2004;24:8167–8183. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Deucher A, Kuroki T, Ohba M, Eckert RL. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–31760. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- Efimova T, LaCelle P, Welter JF, Eckert RL. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Tavakkol A, Leach K, Burns D, Basta P, Loomis C, et al. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-beta II in psoriasis. J Invest Dermatol. 1993;101:553–559. doi: 10.1111/1523-1747.ep12365967. [DOI] [PubMed] [Google Scholar]
- Gherzi R, Sparatore B, Patrone M, Sciutto A, Briata P. Protein kinase C mRNA levels and activity in reconstituted normal human epidermis: relationships to cell differentiation. Biochem Biophys Res Commun. 1992;184:283–291. doi: 10.1016/0006-291x(92)91190-2. [DOI] [PubMed] [Google Scholar]
- Joseloff E, Cataisson C, Aamodt H, Ocheni H, Blumberg P, Kraker AJ, et al. Src family kinases phosphorylate protein kinase C delta on tyrosine residues and modify the neoplastic phenotype of skin keratinocytes. J Biol Chem. 2002;277:12318–12323. doi: 10.1074/jbc.M111618200. [DOI] [PubMed] [Google Scholar]
- Kajimoto T, Ohmori S, Shirai Y, Sakai N, Saito N. Subtype-specific translocation of the delta subtype of protein kinase C and its activation by tyrosine phosphorylation induced by ceramide in HeLa cells. Mol Cell Biol. 2001;21:1769–1783. doi: 10.1128/MCB.21.5.1769-1783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, et al. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem. 2004;279:12668–12676. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J Biol Chem. 2005;280:28721–28730. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, et al. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci USA. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft CA, Efimova T, Eckert RL. Activation of PKCdelta and p38delta MAPK during okadaic acid dependent keratinocyte apoptosis. Arch Vermatol Res. 2006;229:79–83. doi: 10.1007/s00403-006-0727-4. [DOI] [PubMed] [Google Scholar]
- Kronfeld I, Kazimirsky G, Lorenzo PS, Garfield SH, Blumberg PM, Brodie C. Phosphorylation of protein kinase Cdelta on distinct tyrosine residues regulates specific cellular functions. J Biol Chem. 2000;275:35491–35498. doi: 10.1074/jbc.M005991200. [DOI] [PubMed] [Google Scholar]
- Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem. 2006;281:3237–3243. doi: 10.1074/jbc.M512167200. [DOI] [PubMed] [Google Scholar]
- Li W, Chen XH, Kelley CA, Alimandi M, Zhang J, Chen Q, et al. Identification of tyrosine 187 as a protein kinase C-delta phosphorylation site. J Biol Chem. 1996;271:26404–26409. doi: 10.1074/jbc.271.42.26404. [DOI] [PubMed] [Google Scholar]
- Li W, Mischak H, Yu JC, Wang LM, Mushinski JF, Heidaran MA, et al. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- Lu W, Finnis S, Xiang C, Lee HK, Markowitz Y, Okhrimenko H, et al. Tyrosine 311 is phosphorylated by c-Abl and promotes the apoptotic effect of PKCdelta in glioma cells. Biochem Biophys Res Commun. 2006;352:431–436. doi: 10.1016/j.bbrc.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandil R, Ashkenazi E, Blass M, Kronfeld I, Kazimirsky G, Rosenthal G, et al. Protein kinase Calpha and protein kinase Cdelta play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 2001;61:4612–4619. [PubMed] [Google Scholar]
- Matsui MS, Chew SL, DeLeo VA. Protein kinase C in normal human epidermal keratinocytes during proliferation and calcium-induced differentiation. J Invest Dermatol. 1992;99:565–571. doi: 10.1111/1523-1747.ep12667411. [DOI] [PubMed] [Google Scholar]
- Murphy GF, Flynn TC, Rice RH, Pinkus GS. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Invest Dermatol. 1984;82:453–457. doi: 10.1111/1523-1747.ep12260945. [DOI] [PubMed] [Google Scholar]
- Murugappan S, Shankar H, Bhamidipati S, Dorsam RT, Jin J, Kunapuli SP. Molecular mechanism and functional implications of thrombin-mediated tyrosine phosphorylation of PKCdelta in platelets. Blood. 2005;106:550–557. doi: 10.1182/blood-2004-12-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Okhrimenko H, Lu W, Xiang C, Ju D, Blumberg PM, Gomel R, et al. Roles of tyrosine phosphorylation and cleavage of protein kinase Cdelta in its protective effect against tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis. J Biol Chem. 2005;280:23643–23652. doi: 10.1074/jbc.M501374200. [DOI] [PubMed] [Google Scholar]
- Osada S, Mizuno K, Saido TC, Akita Y, Suzuki K, Kuroki T, et al. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990;265:22434–22440. [PubMed] [Google Scholar]
- Reynolds NJ, Talwar HS, Baldassare JJ, Henderson PA, Elder JT, Voorhees JJ, et al. Differential induction of phosphatidylcholine hydrolysis, diacylglycerol formation and protein kinase C activation by epidermal growth factor and transforming growth factor-alpha in normal human skin fibroblasts and keratinocytes. Biochem J. 1993;294(Part 2):535–544. doi: 10.1042/bj2940535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Robinson NA, LaCelle PT, Eckert RL. Involucrin is a covalently crosslinked constituent of highly purified epidermal corneocytes: evidence for a common pattern of involucrin crosslinking in vivo and in vitro. J Invest Dermatol. 1996;107:101–107. doi: 10.1111/1523-1747.ep12298323. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Sabri A, Elouardighi H, Schaefer E, Steinberg SF. Stimulus-specific differences in PKCdelta localization and activation mechanisms in cardiomyocytes. J Biol Chem. 2004;279:19350–19361. doi: 10.1074/jbc.M311096200. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Steinberg SF. Immunoblotting PKC-delta: a cautionary note from the bench. Am J Physiol Cell Physiol. 2006;290:C750–C756. doi: 10.1152/ajpcell.00395.2005. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi Z, Denning MF, Chang EY, Rivera J, Yuspa SH, Lehel C, et al. Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC delta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem Biophys Res Commun. 1995;214:888–894. doi: 10.1006/bbrc.1995.2370. [DOI] [PubMed] [Google Scholar]
- Welter JF, Crish JF, Agarwal C, Eckert RL. Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J Biol Chem. 1995;270:12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- Welter JF, Eckert RL. Differential expression of fos and jun family members c-fos, fosB, Fra-1, Fra-2, c-jun, junB and junD during human epidermal keratinocyte differentiation. Oncogene. 1995;11:2681–2687. [PubMed] [Google Scholar]
- Yuspa SH, Dlugosz AA, Cheng CK, Denning MF, Tennenbaum T, Glick AB, et al. Role of oncogenes and tumor suppressor genes in multistage carcinogenesis. J Invest Dermatol. 1994;103:90S–95S. doi: 10.1111/1523-1747.ep12399255. [DOI] [PubMed] [Google Scholar]
- Zrachia A, Dobroslav M, Blass M, Kazimirsky G, Kronfeld I, Blumberg PM, et al. Infection of glioma cells with Sindbis virus induces selective activation and tyrosine phosphorylation of protein kinase C delta. Implications for Sindbis virus-induced apoptosis. J Biol Chem. 2002;277:23693–23701. doi: 10.1074/jbc.M111658200. [DOI] [PubMed] [Google Scholar]