Abstract
Introduction
Despite epilepsy being one of the most prevalent neurological disorders, one third of all patients with epilepsy cannot adequately be treated with available antiepileptic drugs. One of the significant causes for the failure of conventional pharmacotherapeutic treatment is the development of pharmacoresistance in many forms of epilepsy. The problem of pharmacoresistance has called for the development of new conceptual strategies that improve future drug development efforts.
Areas covered
A thorough review of the recent literature on pharmacoresistance in epilepsy was completed and select examples were chosen to highlight the mechanisms of pharmacoresistance in epilepsy and to demonstrate how those mechanistic findings might lead to improved treatment of pharmacoresistant epilepsy. The reader will gain a thorough understanding of pharmacoresistance in epilepsy and an appreciation of the limitations of conventional drug development strategies.
Expert opinion
Conventional drug development efforts aim to achieve specificity of symptom control by enhancing the selectivity of drugs acting on specific downstream targets; this conceptual strategy bears the undue risk of development of pharmacoresistance. Modulation of homeostatic bioenergetic network regulation is a novel conceptual strategy to affect whole neuronal networks synergistically by mobilizing multiple endogenous biochemical and receptor-dependent molecular pathways. In our expert opinion we conclude that homeostatic bioenergetic network regulation might thus be used as an innovative strategy for the control of pharmacoresistant seizures. Recent focal adenosine augmentation strategies support the feasibility of this strategy.
1. Introduction
Epilepsy is one of the most prevalent neurological disorders affecting around 1% of the world population. Historically, epilepsy has been described as a condition of neuronal dysfunction, in which inadequate neuronal inhibition or excessive neuronal excitation causes sudden, brief episodes of altered consciousness or perception, involuntary movements, or convulsions. Consequently, drug development was guided by the principle that excessive neuronal discharges are the cause of the disease. However, despite the development of a whole armamentarium of novel antiepileptic drugs (AEDs) with new mechanisms of action, around one third of all patients with epilepsy experience seizures uncontrolled by conventional pharmacotherapy [1, 2]. The occurrence of severe side effects prevents effective doses of AEDs from being used and in at least some pharmacoresistant individuals effective concentrations of AEDs are not obtained despite increased dosages. The incidence of pharmacoresistance in epilepsy correlates with high seizure frequency and the occurrence of febrile seizures prior to treatment; in addition, certain types of epilepsy such as found in patients with partial seizures are more prone to the development of pharmacoresistance.
Drug development efforts to date have focused on a neurocentric concept of seizure generation with the aim to generate new compounds that are highly selective for downstream targets of neuronal signaling. In addition, model systems used to screen for novel AEDs have been designed almost exclusively on the premise to assess a drug’s effects on neuronal function. The focus on highly selective neuronal targets has however several caveats:
Epilepsy is a disorder of complex network dysfunction [3–5], involving not only an imbalance of neuronal excitation and inhibition, but also disruption of immunomodulatory functions [6, 7], dysfunction of the blood brain barrier [8], altered function of glia – in particular of astrocytes and microglia [6, 9, 10], as well as more complex epigenetic changes [3, 11]. The focus on individual highly selective neuronal downstream targets is therefore unlikely to provide effective treatment for a network disorder, such as epilepsy.
While targeting a downstream component of an epileptogenic network, symptom suppression might go hand in hand with the development of major side-effects.
The focus on a selective downstream target is highly vulnerable to the development of compensatory reactions that might contribute to the development of pharmacoresistance.
Here we urge that future drug development efforts need to focus on “upstream regulators” of network function. Only the synergistic modulation of an entire network will enable us to circumvent drug resistance, which is still a major hurdle in effective seizure treatment of epilepsy. In the following we will discuss mechanisms of pharmacoresistance and strategies to circumvent pharmacoresistance in epilepsy.
2. Mechanisms of drug resistance in epilepsy
Three hypotheses were proposed in recent years in an attempt to explain the phenomenon of pharmacoresistance. As will be outlined in more detail below, those hypotheses are largely based on pharmacodynamic and pharmacokinetic parameters.
2.1. Target hypothesis of pharmacoresistance
This hypothesis is based on pharmacodynamic changes of drug targets. In general, a reduced sensitivity of a given drug target (ion channel, receptor) to its AED is postulated. Pharmacoresistance to carbamazepine due to reduced sensitivity of voltage-gated sodium channels to this drug has most intensively been studied; it remains unclear to date whether pharmacoresistance due to changes in drug targets are a general phenomenon or specific to carbamazepine. In the latter case, electrophysiological recordings performed on hippocampal slices derived from epileptogenic tissue resected from patients with therapy resistant seizures demonstrated loss of use-dependent block of Na+ channels by carbamazepine [12]. Apart from voltage-gated sodium channels, pharmacodynamics changes of GABAA receptors have been evaluated in a rat model of pharmacoresistant TLE. In those studies resistance to phenobarbital was associated with both changes in GABAA receptor subunit composition as well as with a shift to diazepam-insensitive ligand binding properties [13, 14]. In line with those findings changes in composition and properties of GABAA receptors have been linked to status epilepticus resistant to benzodiazepines [15]. To provide a more comprehensive assessment of target changes, a recent patch-clamp study examined the effects of carbamazepine and phenytoin on voltage-gated sodium currents in hippocampal CA1 neurons of sham-control and chronically epileptic rats [16]. Interestingly, in this study significant changes were found in the effects of phenytoin, but not of carbamazepineon the voltage-dependence of current inactivation in chronically epileptic animals. In contrast, carbamazepine had less pronounced effects on the time course of recovery from current inactivation in epileptic compared to control animals, whereas the effects of phenytoin remained unaltered [16]. These findings suggest that the reduction of AED sensitivity in chronic epilepsy is due to different biophysical mechanisms for carbamazepine and phenytoin. Together, currently available data indicate that target mechanisms of drug resistance are AED-specific rather than being based on common general mechanisms.
2.2. Transporter hypothesis of pharmacoresistance
This hypothesis is largely based on pharmacokinetic changes that determine drug availability, and a large body of literature supports a strong correlation between the overexpression of drug efflux transporters in the blood brain barrier (BBB) and pharmacoresistance to AEDs [8, 17–20]. Evidence for the transporter hypothesis is based on findings that a variety of multidrug transporters are expressed at increased levels in endothelial cells of the BBB from tissue resected from patients with pharmacoresistant epilepsy [8, 21, 22]. Those findings could be replicated in several animal models of epilepsy. Thus, members of the ATP-binding cassette (ABC) transporter family, such as P-glycoprotein and breast cancer resistance protein were found to be upregulated in rodent models as a consequence of seizure activity [23–26] and were shown to be associated with lower brain levels of AEDs [27]. Moreover, AED-resistant rats expressed higher levels of P-glycoprotein than non-resistant rats [26]. Mechanistically, in transport assays it was further demonstrated that several AEDs are substrates of P-glycoprotein and that several AEDs are indeed transported by ABCtransporters [28–30]. The strongest support for the transporter hypothesis is based on therapeutic studies, in which the selective P-glycoprotein inhibitor tariquidar was able to reverse AED resistance in rats [31, 32]. More recently, it was demonstrated that targeting the glutamate/cyclooxygenase-2 mediated signaling pathway that leads to upregulation of P-glycoprotein in epilepsy with a cyclooxygenase-2 inhibitor controlled P-glycoprotein expression and promoted the delivery of phenytoin to the brain of chronic epileptic rats [33]. While those rodent-based in vivo and in vitro studies support the transporter hypothesis it is important to validate the functional and mechanistic aspects of the hypothesis in human patients with pharmacoresistant epilepsy. In a recent study a humanized dynamic in vitro BBB model was developed that is based on co-cultures of human microvascular endothelial cells and astrocytes from epilepsy patients or controls [34]. Using this model system, it was demonstrated that the permeability of this reconstructed BBB for phenytoin was reduced by one order of magnitude when cells from pharmacoresistant patients were used, an effect that was reversible after the addition of the P-glycoprotein inhibitor tariquidar [34]. A similar conclusion was derived in an independent approach using a directional transport assay that was independent of permeability components, in which transport of phenytoin and several other AEDs by human P-glycoprotein was demonstrated [35]. Together, those data suggest that AEDs are indeed substrates for drug efflux transporters in human patients with pharmacoresistant epilepsy.
2.3. The intrinsic severity hypothesis of pharmacoresistance
This hypothesis is based on epidemiological data suggesting that long-term treatment outcome in epilepsy and responsiveness to AEDs depends on severity and frequency of seizures in the early phase of epilepsy [36]. In those epidemiological studies a higher number of seizures prior to treatment onset and seizure clustering were associated with a higher incidence for the development of pharmacoresistance [37–39]. Although supported by epidemiological data the neurobiological basis of this hypothesis remains understudied. It would be interesting to see whether there is a genetic basis linking intrinsic seizure severity with pharmacoresistance. Clinically, patients with higher seizure frequency at onset of treatment might eventually become treatable with AEDs at higher serum concentration of the drugs. Importantly, the intrinsic severity hypothesis was questioned in a prospective study, in which 77 children with new onset TLE were followed with formal reviews performed at 7 and 14 years following seizure onset. This prospective study demonstrated that neither initial frequency nor early seizure remission were predictive of final outcome [40]. Together, while a wide range of epidemiological data support the intrinsic severity hypothesis, there is little molecular evidence in support of the hypothesis and some clinical studies are not in agreement with the hypothesis.
2.4. Genetic basis of pharmacoresistance
If pharmacoresistance can be linked to increased expression of drug efflux transporters, then overexpression of the genes or polymorphisms within genes coding for those transporters might causally be linked to pharmacoresistance. Pharmacogenetic studies that link polymorphisms in drug transporter genes appear to be more frequent in non-responders to AEDs and have critically been reviewed elsewhere [41]. Those studies need to be treated with caution, however, because data are derived from patients that have been treated with AEDs for many years [41]. Attention has focused on polymorphisms in the gene encoding P-glycoprotein, MDR1, and a C/T polymorphism at base 3435 has been associated with resistance to phenytoin and pentobarbital, both drugs transported by human P-glycoprotein [42, 43]. Importantly, a CC genotype was associated with lower phenobarbital levels in CSF than the CT or TT genotype at position 3435 [43]. Although some pharmacogenomics data appear to indicate a genetic association between polymorphisms in drug transporter genes and pharmacoresistance, causal relationships have not yet been established.
3. Strategies to circumvent drug resistance and the role of the blood brain barrier (BBB)
Circumvention of drug resistance is problematic because of difficulties defining what is meant by drug resistance, what factors and mechanisms are responsible for drug resistance, and what biomedical strategies are available to counter drug resistance. Clearly, adjunctive treatment of epilepsies with AEDs is disappointingly ineffective [44] and thus investigators are searching for new drugs targeting mechanisms other than those affected by current AEDs, ways to increase BBB permeability of new AEDs, new targets that regulate drug resistance, and ways to decrease drug efflux away from sites of action in brain. The BBB is a complicated and dynamic process that includes tight junctions between adjacent endothelial cells, closely apposed astrocytes and podocytes, and to a lesser extent a basement membrane. The BBB constitutes a physical and function barrier, important for the supply of nutrients and drugs into the brain and as a protection against the entry of unwanted and potentially harmful cells and substances. Although there is very strong evidence that the BBB may be at least transiently opened when and where epileptic foci occur, it is not clear yet whether BBB disruption occurs prior to or as a consequence of seizures [45]. Furthermore, most AEDs are lipophilic and as such can enter the brain by diffusing through endothelial membranes. Nevertheless, disruption of the BBB can increase serum protein extravasation and because lipophilic AEDs such as phenytoin are highly bound to serum protein the levels of free phenytoin in brain are decreased especially in brain regions where there is BBB disruption and this could predispose patients to pharmacoresistance [46]. But the BBB is not the only possible explanation for drug resistance (see also above) because pharmacoresistance has been observed in brain slices where the BBB is not functional especially under conditions where there is overexpression of drug efflux pumps [47].
As outlined above, AEDs are substrates for P glycoprotein and other carrier proteins that efflux drugs across the BBB [45]. As such the BBB (see above) plays a prominent role in the transporter hypothesis of drug resistance in epilepsy. P-glycoprotein also known as the multidrug resistance protein (MRP) is a member of the ABC family of transporters and these active transporters help defend the brain in part by regulating the efflux of lipophilic substances and drugs from the brain. Overexpression of these transporters in patients with pharmacoresistant epilepsy and in patients receiving AEDs has been shown by many investigators, and this likely participates in preventing effective drug levels of AEDs from being reached in brain of pharmacoresistant epileptic patients [8]. Indeed, inhibitors of these transporters have been shown to increase drug levels in brain of pharmacoresistant patients. However, it is still uncertain the extent to which increased drug transporter expression levels are directly linked to pharmacoresistant epilepsy [45] and a better strategy might be to control the over-expression of these transporters by targeting transcriptional activation thus preserving basal transporter functions [48]. Alternatively, AEDs and other anti-epileptic approaches that are less specific and that do not rely on multidrug transporters might be advantageous to patients living with pharmacoresistant epilepsy.
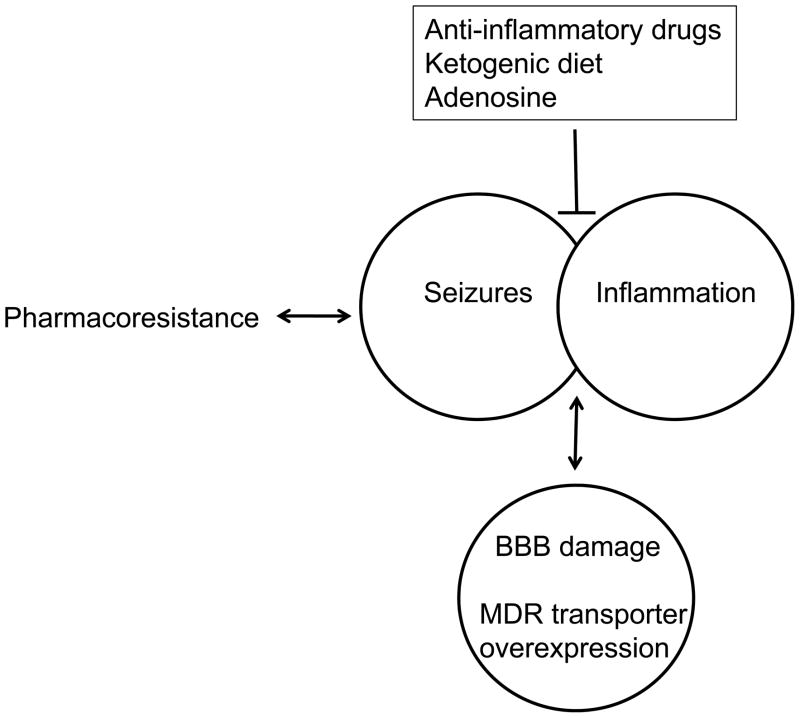
It appears clear that inflammation, disruption of the BBB, and the presence of epilepsy are intimately linked(Fig. 1) [45, 49]. A variety of inflammatory mediators including cytokines, matrix metalloproteinases, arachidonic acid, and derivatives of arachidonic acid have all been shown to negatively affect BBB integrity. Conversely, anti-inflammatory strategies including the use of non-selective COX inhibitors, selective COX-2 inhibitors, and corticosteroids have been used to protect the BBB, block P glycoprotein overexpression, and treat medically refractory epilepsy [48, 50]. Ketogenic diets (see below) continue to be used effectively for the treatment of medically refractory epilepsies and ketogenic strategies appear able to control seizures, epilepsy and inflammation [49]. Indeed, a ketogenic diet has been shown to decrease preexisting neuroinflammation in patients with obesity-associated fatty liver disease [51], affect a variety of inflammatory pathways and decrease local inflammation induced in rat hindpaws [52]. Consistent with the hypothesis that increased adenosine production is a mechanism by which a ketogenic diet acts (see below), adenosine is well known to have anti-seizure and anti-inflammatory properties, and drugs such as methylxanthines that affect adenosine receptor function can protect against BBB disruption [53–55]. While age- and model-specific anticonvulsant effects of the methylxanthine caffeine have been reported [56] it is generally accepted that higher doses of methylxanthines have proconvulsant effects [57]. Thus, a ketogenic diet and adenosine with its documented anticonvulsant properties may find use as alternate strategies for the treatment of pharmacoresistant epilepsies.
Figure 1. Role of the blood brain barrier in pharmacoresistance.
Approximately 30% of people living with epilepsies are pharmacoresistant to anti-epileptic drugs (AEDs). The resulting seizures can result from and lead to inflammation. The seizures, inflammation and pharmacoresistance are at least associated with and possibly result from damage to the blood brain barrier (BBB) and increased expression levels of multiple drug resistant (MDR) transporters. Drug targets alternate to AEDs are being sought to control pharmacoresistant seizures and the development of epilepsy. Although not yet investigated in human patients, animal models suggest therapeutic potential for anti-inflammatory drugs(e.g. cyclooxygenase 2 inhibitors); Likewise, ketogenic diets and the purine nucleoside adenosine show therapeutic promise based on mechanisms that are not currently targeted by conventional AEDs.
4. Homeostatic bioenergetic network regulation – a novel concept to avoid drug resistance
Mechanisms of pharmacoresistance and strategies to circumvent pharmacoresistance as discussed above are inherently linked to current concepts and dogmas of pharmaceutical drug development efforts. The “holy grail” of drug development is specificity, thought to be achievable by selecting drug targets that act as far downstream as possible in specific signal transduction pathways. Thus, highly selective AEDs are ideally specific for only one target/pathway (e.g. a particular type of ion channel in neurons), which bears the inherent risk of compensation. Compensation of blockade of a single target (or a few targets) can most easily be achieved by pharmacodynamics changes affecting interactions between target and drug, or by pharmacokinetic changes affecting the effective concentration of an AED. Therefore, it is not surprising that current AED treatment regimens are prone to the development of pharmacoresistance and that drugs with a broader spectrum of action, such as valproate are still the mainstay for epilepsy therapy.
Epilepsy is a complex disorder of network dysfunction not only involving an imbalance between excitation and inhibition (targeted by current AEDs). Apart from changes in neuronal physiology – the measurable outcome of current AED efficacy, epilepsy involves dysfunction of glial control of brain homeostasis [9, 58–60], inflammatory processes [6, 7], biochemical alterations [61], bioenergetic changes [55, 62], and epigenetic changes [3, 63, 64]. Control of homeostatic bioenergetic network regulation might provide an alternative strategy to treat epilepsy(Fig. 2) by affecting several pathogenic mechanisms involved in epilepsy synergistically. It is obvious that this goal cannot be achieved by focusing on downstream targets; in contrast, if it were possible to modify master regulators that act upstream of several biochemical and receptor-dependent signaling pathways it might be possible to rationally treat a complex disorder such as epilepsy on the network level. Modification of a whole network is unlikely to lead to compensatory changes and drug resistance as encountered with current AEDs that act on highly specific downstream targets. In the following we will focus on the purine ribonucleoside adenosine, which – based on its biochemical properties – is prototype of ahomeostatic bioenergetic network regulator.
Figure 2. Regulation of bioenergetic network homeostasis.
Conventional antiepileptic drugs (AED) act with high specificity on selected pathways and are therefore prone to compensatory reactions that contribute to pharmacoresistance. In contrast, a homeostatic bioenergetic network regulator, such as adenosine, is uniquely able to effect entire networks on multiple receptor-dependent, biochemical, bioenergetic, and epigenetic pathways.
4.1. Network regulation through adenosine
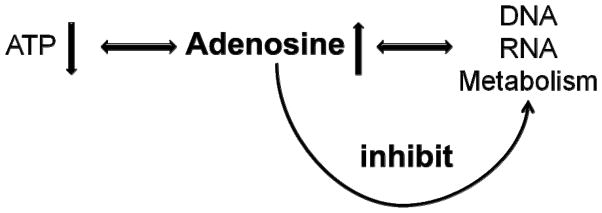
In the 1950s Miller and Urey performed experiments on chemical evolution in an attempt to reconstruct primordial components of our Earth’s atmosphere. Importantly, given the right conditions, the purine base adenine can form spontaneously from hydrogen cyanide. Therefore, adenine likely played already a role in prebiotic evolution. Biochemically, adenine, and its ribosylated form adenosine, are part of 5′-adenosine triphosphate (ATP, reflecting the energy state of a system) as well as of the nucleic acids RNA and DNA (reflecting the metabolic capacity of a cellular system)(Fig. 3). Therefore, it is tempting to speculate, that early on in evolution adenosine assumed the role of a bioenergetic feedback regulator. Thus, if ATP levels (i.e. energy supplies in the environment) go down, adenosine-levels (the natural degradation product of ATP) increase and this rise in adenosine is a prime candidate to provide metabolic feedback inhibition on all processes that consume energy. Due to those – evolutionary ancient – properties, adenosine has been termed a retaliatory metabolite. The earliest forms of life depended on very basic biochemical regulatory networks long before more sophisticated regulatory systems such as receptors, signal transduction pathways, protein kinases, etc., evolved. Due to its early evolutionary origin, adenosine has most likely retained its primordial regulatory functions and thus not only exerts its “modern” receptor-mediated functions, but also exerts receptor-independent functions as a key regulator of basic biochemistry. Adenosine is an obligatory end product of transmethylation reactions [65]. If adenosine is not constantly removed by its key regulating enzyme adenosine kinase (ADK) the S-adenosylhomocysteine hydrolase reaction is driven towards the synthesis of S-adenosylhomocysteine, which is a potent inhibitor of transmethylation reactions [66]. This biochemical property of adenosine poises the molecule to induce epigenetic changes. Interestingly, a nuclear isoform of ADK undergoes tightly controlled developmental expression switches during early postnatal brain development [67].
Figure 3. Adenosine is bioenergetic feedback regulator.
The biochemistry of adenosine, being a building block of both 5′-adenosinetriphosphate (ATP) as well as of nucleic acids allows it to act as bioenergetic feedback regulator. When energy levels drop (e.g. due to excessive energy consumption during a seizure) ATP levels drop and adenosine – a degradation product of ATP – rises. Increased levels of adenosine provide inhibition to a wide range of metabolic activities in a biochemical strategy to conserve energy.
On the receptor level, adenosine is known to activate four types of G-protein coupled adenosine receptors termed A1, A2A, A2B, and A3. In brain the high affinity receptors for adenosine, A1 and A2A, have been characterized most extensively. The A1R is expressed at high levels in the limbic system and is associated with inhibitory G-proteins. Activation of presynaptic A1Rs blocks calcium channels and thereby reduces the release of excitatory neurotransmitters, such as glutamate, whereas activation of postsynaptic A1Rs increases potassium flux across the membrane and thereby stabilizes the postsynaptic membrane potential. Due to those properties, activation of A1Rs is associated with potent antiepileptic and neuroprotective properties [68–70]. Conversely, loss of the A1R is associated with spontaneous electrographic seizures and with seizure- and trauma-induced mortality [71, 72]. In contrast, the A2AR is associated with stimulatory G-proteins and due to its heterologous association with other receptor subtypes (e.g. dopamine D2 receptors, TrkB receptors) this receptor is uniquely suited to modulate neuronal networks on different levels [73, 74]. Whereas activation of the A1R can contribute to the generation of a globally inhibited network with potent anticonvulsant downstream effects [75, 76], activation of the A2AR can potentiate specific functions within the inhibited network and thereby improve the signal to noise ratio of signal transduction in neuronal networks [77]. [78]Although A2AR activation can have pro-convulsive effects [76, 79] and may modify the efficacy of AEDs [80], it appears that the net-effects of combined A1R and A2AR activation are anticonvulsant [81]. An additional layer of complexity in the net-effects of adenosine is afforded by a possible anticonvulsant role of the A3R [82] and its interaction with AEDs [83]. Given the multiple activities of adenosine as an upstream regulator, controlling not only basic biochemistry (through adenosine receptor independent mechanisms) but also specific receptor-mediated functions of relevance for epilepsy, adenosine is a prime candidate to modulate network activity in epilepsy in the broader sense of “homeostatic bioenergetic network regulation”.
4.2. Suppression of pharmacoresistant seizures by adenosine
Based on the mechanisms discussed above, adenosine is considered to be an endogenous anticonvulsant of the brain. Indeed, adenosine is released during seizures and likely is an endogenous agent contributing to seizure arrest [84]. Conversely, adenosine deficiency – due to overexpression of ADK – has been associated with spontaneous seizures [71] and increased susceptibility to neuronal injury [85]. Therefore, adenosine augmentation therapies should constitute a rational approach to suppress seizures in epilepsy. But would this strategy be effective in pharmacoresistant epilepsy? Using a mouse model of pharmacoresistant temporal lobe epilepsy that is based on the intrahippocampal injection of the excitotoxin kainic acid (KA) it was demonstrated that spontaneous seizures that were resistant to carbamazepine, valproate, and phenytoin, could completely be suppressed by systemic treatment with the adenosine A1R agonist 2-chloro-N6-cyclopentyladenosine (CCPA). Likewise, seizures were completely suppressed by systemic application of the ADK inhibitor 5-iodotubercidin, an effect that was reversed after injecting the animals with the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine(DPCPX), indicating that seizure suppression by 5-iodotubercidin was based on increasing the tone of the endogenous anticonvulsant adenosine [86]. These studies suggest that adenosine – being an endogenous metabolite – is uniquely suited to suppress seizures that are resistant to conventional AEDs. However, the systemic augmentation of adenosine receptor signaling as achieved in these studies was associated with cardiovascular and sedative side effects [75, 83]. Likewise, the potentiation of anticonvulsant activity of classical AEDs by adenosine receptor agonists was associated with neurotoxic and peripheral side effects in at least some studies [80, 83], while peripheral side effects have not routinely been investigated in other studies [87, 88]. Thus, in order to avoid peripheral side effects and to utilize adenosine signaling for antiepileptic therapy, different approaches are needed.
4.3. Ketogenic diet – a dietary tool to circumvent pharmacoresistance
Manipulating an endogenous molecule such as adenosine offers ideal opportunities to avoid pharmacoresistance and limit side effects, and recent evidence suggests there may be global metabolic strategies which regulate adenosine. A well-established dietary therapy – the ketogenic diet – is a metabolic treatment used most often to treat pediatric and pharmacoresistant epilepsy [89], and recent evidence suggests it may be acting by increasing A1R activity. Briefly, it had been observed historically that when persons with epilepsy stopped eating they would experience relief from their seizures [89]. Because fasting cannot be continued indefinitely, and seizures returned upon eating, the ketogenic diet was developed in the early 1900’s as an epilepsy therapy which would mimic metabolic changes occurring during fasting but still allow the patient to ingest calories [89]. Like fasting, the ketogenic diet reduced seizures. This dietary therapy is highly effective, yet it fell out of favor with the advent of AEDs [89]. Nevertheless, it remains as effective as AEDs (more than 50% seizure control in more than 50% of patients) and it can resolve medically refractory epilepsy – suggesting that it targets mechanisms other than those targeted by traditional AEDs [89]. Recent retrospective and prospective evidence [90], combined with a continued need for new epilepsy therapies, have re-energized interest in this metabolic strategy.
It is well known that metabolic dysfunction is associated with many neurological disorders, including epilepsy but often it is unclear if the metabolic abnormalities contribute to, result from, or simply evolve in parallel with the disorder [55]. In sharp contrast to this cause-and-effect conundrum, the ketogenic diet is a clear case where the metabolic change occurs first: eating a very high fat, low carbohydrate diet – and thus forcing the body to generate ATP from ketones rather than glucose – dramatically reduces seizures [55]. Therefore, the metabolic change precipitated by adhering to this diet precedes the change in seizure threshold. The diet is associated with some side effects – initial lethargy, constipation, and increased risk of kidney stones – but in many patients these effects are mild [89]. A bigger issue is that the diet requires strict adherence, which is particularly difficult for adults.
Recent evidence suggests that ketogenic diet may be acting via increasing A1R activity, thus opening up a new strategy for manipulating this endogenous anticonvulsant [91]. With adenosine as a key link between metabolism and neuronal activity it is a likely candidate to play this role [55], and a global adenosine-increasing metabolic treatment this could be an effective strategy for generalized refractory seizures (where surgery is not an option) and for patients who experience side effects from AEDs – particularly children. In addition, while most children are on the diet for 6 months to 2 years, there are reports of adults who have been on a ketogenic diet for epilepsy for nearly 30 years without adverse effects [92].
Increasingly, the ketogenic diet is gaining acceptance as the treatment of choice or at least a second-line therapy for some seizure disorders as outlined in a recent International consensus statement [93–95], and it is used increasingly around the world and in many different cultures [89]. In addition, the ketogenic diet may control two interacting pathologies of epilepsy – seizures and inflammation [49]. Taken together, the potential to increase an endogenous modulator – such as adenosine – by altered endogenous metabolism – such as ketogenic diet therapy, or an analogous pharmaceutical strategy -offer the potential for few side effects and limited pharmacoresistance. In addition, adenosine acts to promote sleep and reduce anxiety, thus potentially resolving known co-morbidities of epilepsy simultaneously with improved seizure control.
4.4. Novel strategies for seizure suppression in epilepsy
To make use of adenosine augmentation as a therapy of epilepsy, focal treatment approaches have been developed in order to avoid peripheral and central side effects associated with systemic adenosine augmentation.
In an approach aimed at providing a focal source for adenosine, biopolymer silk was engineered to release adenosine at predefined doses and release kinetics [96]. Silk is a natural occurring protein that can be manufactured in all-aqueous solutions and that provides a biodegradable source for focal drug delivery, with the degradation products being amino acids [97]. Since both silk, as well as adenosine are already FDA-approved for different conditions, silk-based adenosine delivery appears to be a promising strategy for future clinical applications. In a recent study, silk-based implants engineered to release a constant dose of adenosine for a limited time span of 10 days were implanted into the infrahippocampal fissure of fully kindled (epileptic) rats [98], a widely used animal model with predictive value for the efficacy of potential AEDs in human epilepsy. Before implantation, all animals reproducibly displayed generalized stage 4 to 5 seizures prior to polymer implantation. While this seizure activity was maintained in rats receiving control implants or a sham procedure, seizure activity was almost completely suppressed in recipients of adenosine-releasing implants for a duration of 10 days [98]. As the release of adenosine from the polymers began to wear off beyond day 10, seizures gradually recurred indicating that seizure suppression was due to the paracrine activity of adenosine released from the polymer [98]. Adenosine-releasing silk-based polymers implanted prior to the onset of kindling significantly suppressed the progressive course of seizure expression. This effect was dose dependent [96] and was maintained even after expiration of adenosine-release from the polymer suggesting longer-lasting disease-modifying effects of transient adenosine delivery [98].
In an attempt to provide sustained delivery of adenosine to the epileptic brain, murine embryonic stem cells were engineered to release adenosine based on biallelic genetic disruption of the Adk gene. After in vitro differentiation into adenosine-releasing neural progenitor cells, the cells were grafted into the infrahippocampal fissure of rats one week prior to the onset of kindling [99] or into the infrahippocampal fissure of mice 24h after triggering epileptogenesis with intraamygdaloid injection of KA[71]. While recipients of control graft or those subjected to a corresponding sham procedure were without lasting therapeutic benefits, recipients of adenosine releasing embryonic stem cell-derived neural progenitor cells were characterized by robust suppression of kindling epileptogenesis [99], and KA-injected mice with the implants failed to develop any spontaneous seizure activity three weeks after KA injection, a time point when control animals typically displayed about 4 seizures per hour [71]. These studies demonstrate the therapeutic potential of adenosine released from engineered stem cells.
Finally, adeno associated virus-based gene therapy vectors were constructed to express an antisense construct directed against adenosine kinase [100]. Spontaneously epileptic Adk-tg mice that exhibit seizures due to their inborn adenosine deficiency received a unilateral intrahippocampal injection of the ADK antisense virus or of an empty control virus. Only recipients of the ADK knockdown virus displayed almost complete suppression of seizures in the injected hemisphere, while seizures in the contralateral hippocampus were not affected [100].
5. Conclusion
The examples outlined above suggest that focal adenosine augmentation by various strategies is very effective in suppressing seizures in a variety of epilepsy models. In contrast to systemic adenosine augmentation no overt side effects were noted in these studies. In conclusion, utilization of the adenosine system, either by metabolic manipulation or by focal augmentation strategies, appears to be a strategy to circumvent pharmacoresistance (i) by making use of an endogenous agent, and (ii) by providing upstream control of complex network regulation.
6. Expert Opinion
During the past decades pharmaceutical drug development efforts were largely based on the premise to develop highly specific drugs by targeting downstream effectors of cellular communication pathways. While specificity might have value to suppress symptoms or select endophenotypes of a condition, this approach bears the inherent risk for the development of pharmacoresistance. Today the pharmacological treatment options for epilepsy have improved only incrementally compared to treatment options 40 years ago, and more than 30% of all patients with epilepsy remain refractory to treatment. Not surprisingly, older drugs with a broader spectrum of action, such as valproate, are still the mainstay for antiepileptic therapy. Targeting downstream pathways with exogenous and highly selective drugs can lead to compensatory responses both on pharmacodynamic, as well as pharmacokinetic levels, a phenomenon commonly referred to as “pharmacoresistance”. In order to improve treatment options for complex network disorders, such as epilepsy, the current dogmas of pharmaceutical drug development efforts, i.e. selectivity and focus on (largely neuronal) downstream targets, need to be brought into revised perspective and new conceptual strategies need to be developed. A possible conceptual improvement for the treatment of epilepsy might therefore be the focus on non-selective upstream regulators and endogenous regulators of brain homeostasis. Adenosine is a homeostatic bioenergetic network regulator, which affects basic biochemical and bioenergetic homeostatic networks, but in addition can exert more specific functions via a variety of molecular pathways that are linked to adenosine receptors. Complex interactions between biochemical, and receptor-dependent, mechanisms, lead to anticonvulsive and neuroprotective net effects that are mediated by an increased tone in adenosine. Since adenosine deficiency is a pathological hallmark of the epileptic brain, adenosine reconstitution would be based on a concise neurochemical rationale. Rather than leading to excessive adenosine signaling, augmentation of adenosine signaling to the epileptic brain could be a unique way to reconstitute normal adenosine signaling. In contrast to conventional drugs, adenosine is an endogenous metabolite, and therefore not a substrate for drug efflux transporters. As an upstream regulator, adenosine is capable to synergistically regulating the homeostasis of complex networks. Therefore, compensatory mechanisms (“pharmacoresistance”) are unlikely to limit the therapeutic effectiveness of adenosine. Adenosine homeostasis can be modified therapeutically by strategies as diverse as the ketogenic diet or stem cell and gene therapies. Importantly, adenosine augmentation is capable of suppressing seizures that are refractory to conventional pharmacotherapy. Adenosine augmentation therapies can be targeted to specific brain regions by focal application modalities that include the regionally restricted implantation of adenosine releasing devices or cells. Several experimental adenosine augmentation approaches have shown that the focal delivery of adenosine to an epileptogenic brain region can potently suppress epileptic seizures and newer studies indicate that even a transient enhancement of adenosine signaling can have long-lasting disease-modifying effects. The future for epilepsy therapy might thus rely less on conventional drug therapies but rather on targeted and localized strategies that can restore homeostasis of complex networks. Eventually, only rationally designed therapeutic strategies that affect whole networks might provide a cure for epilepsy, whereas conventional blockade of select pathways might at the best provide symptom control.
Article highlights box.
Pharmacoresistance can be caused by pharmacodynamics changes of drug targets. It can also be caused by pharmacokinetic changes that determine drug availability; overexpression of drug efflux transporters is a major contributor to pharmacoresistance.
Epidemiological data suggest a correlation between initial seizure severity and subsequent emergence of pharmacoresistance while pharmacogenomic data suggest an association between polymorphisms in drug transporter genes and pharmacoresistance, but causal relationships have not yet been established.
Overexpression of multiple drug resistant proteins (transporters) can result in pharmacoresistance in part by limiting drug concentrations in brain.
The blood brain barrier (BBB) restricts the entry of AEDs into brain and evidence exists that the BBB is disrupted when and where epileptic seizures occur.
A complex network disorder, such as epilepsy, might best be treated by regulating homeostasis and bioenergetics of network function, rather than using drugs that are highly selective for single downstream targets.
Adenosine is a homeostatic bioenergetic network regulator that can provide seizure control by a combination of receptor-mediated, biochemical, and bioenergetic functions.
Adenosine augmentation therapies effectively suppress seizures in a variety of rodent models of epilepsy.
A ketogenic diet can suppress seizures by augmentation of adenosine signaling.
Novel adenosine augmentation strategies to prevent seizures in experimental epilepsy include silk-based adenosine delivery, stem cell transplantation and gene therapy.
Eventually, only rationally designed therapeutic strategies that affect whole networks might provide a cure for epilepsy, whereas conventional blockade of select pathways might at the best provide symptom control.
Footnotes
Declaration of Interest: All of the authors are supported by R01NS061844, and R01NS065957 from the National Institutes of Health (NIH) and by 2P20RR017699 from the NCRR, a division of the NIH. In addition to this, D Boison is supported by the Legacy Foundations while his co-author JD Geiger is supported by the CHDI.
Bibliography
- 1.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010 Jan;9(1):68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 2.Vajda FJE. Pharmacotherapy of epilepsy: New armamentarium, new issues. Journal of Clinical Neuroscience. 2007 Sep;14(9):813–23. doi: 10.1016/j.jocn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010 Jul;39(1):53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cymerblit-Sabba A, Schiller Y. Network dynamics during development of pharmacologically induced epileptic seizures in rats in vivo. J Neurosci. 2010 Feb 3;30(5):1619–30. doi: 10.1523/JNEUROSCI.5078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roopun AK, Simonotto JD, Pierce ML, et al. A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex. Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):338–43. doi: 10.1073/pnas.0912652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezzani A, Ravizza T, Balosso S, et al. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49( Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 7*.Ravizza T, Gagliardi B, Noe F, et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008 Jan;29(1):142–60. doi: 10.1016/j.nbd.2007.08.012. This paper emphasizes the importance of inflammatory pathways for the development of epilepsy. [DOI] [PubMed] [Google Scholar]
- 8.Lazarowski A, Czornyj L, Lubienieki F, et al. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48( Suppl 5):140–9. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 9.Seifert G, Carmignoto G, Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010 May;63(1–2):212–21. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008 Dec 5;322(5907):1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 11.Aronica E, Fluiter K, Iyer A, et al. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010 Feb 26;31:1100–07. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 12.Jandova K, Pasler D, Antonio LL, et al. Carbamazepine-resistance in the epileptic dentate gyrus of human hippocampal slices. Brain. 2006 Dec;129(Pt 12):3290–306. doi: 10.1093/brain/awl218. [DOI] [PubMed] [Google Scholar]
- 13.Bethmann K, Fritschy JM, Brandt C, et al. Antiepileptic drug resistant rats differ from drug responsive rats in GABA A receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis. 2008 Aug;31(2):169–87. doi: 10.1016/j.nbd.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Volk HA, Arabadzisz D, Fritschy JM, et al. Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABA(A) receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol Dis. 2006 Mar;21(3):633–46. doi: 10.1016/j.nbd.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006 Mar;5(3):246–56. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 16.Schaub C, Uebachs M, Beck H. Diminished response of CA1 neurons to antiepileptic drugs in chronic epilepsy. Epilepsia. 2007 Jul;48(7):1339–50. doi: 10.1111/j.1528-1167.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 17.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006 Jan;129(Pt 1):18–35. doi: 10.1093/brain/awh682. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann U, Kann O, Remy S, et al. Novel mechanisms underlying drug resistance in temporal lobe epilepsy. Adv Neurol. 2006;97:85–95. [PubMed] [Google Scholar]
- 19.Loscher W. How to explain multidrug resistance in epilepsy? Epilepsy Curr. 2005 May–Jun;5(3):107–12. doi: 10.1111/j.1535-7511.2005.05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loscher W. Drug transporters in the epileptic brain. Epilepsia. 2007;48( Suppl 1):8–13. doi: 10.1111/j.1528-1167.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 21.Ak H, Ay B, Tanriverdi T, et al. Expression and cellular distribution of multidrug resistance-related proteins in patients with focal cortical dysplasia. Seizure. 2007 Sep;16(6):493–503. doi: 10.1016/j.seizure.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Kubota H, Ishihara H, Langmann T, et al. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006 Mar;68(3):213–28. doi: 10.1016/j.eplepsyres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Yang Z, Yang J, et al. Increased P-glycoprotein expression and decreased phenobarbital distribution in the brain of pentylenetetrazole-kindled rats. Neuropharmacology. 2007 Oct;53(5):657–63. doi: 10.1016/j.neuropharm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann K, Gastens AM, Volk HA, et al. Expression of the multidrug transporter MRP2 in the blood-brain barrier after pilocarpine-induced seizures in rats. Epilepsy Res. 2006 Apr;69(1):1–14. doi: 10.1016/j.eplepsyres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Volk H, Potschka H, Loscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem. 2005 Apr;53(4):517–31. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- 26*.Volk HA, Loscher W. Multidrug resistance in epilepsy: rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain. 2005 Jun;128(Pt 6):1358–68. doi: 10.1093/brain/awh437. This paper highlights the important contribution of overexpressed multi drug transporters for the development of pharmacoresistance in epilepsy. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet EA, van Schaik R, Edelbroek PM, et al. Region-specific overexpression of P-glycoprotein at the blood-brain barrier affects brain uptake of phenytoin in epileptic rats. J Pharmacol Exp Ther. 2007 Jul;322(1):141–7. doi: 10.1124/jpet.107.121178. [DOI] [PubMed] [Google Scholar]
- 28**.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005 Aug;6(8):591–602. doi: 10.1038/nrn1728. Comprehensive review on the role of drug efflux transporters for the development of drug resistance. [DOI] [PubMed] [Google Scholar]
- 29.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005 May;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005 Jan;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt C, Bethmann K, Gastens AM, et al. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol Dis. 2006 Oct;24(1):202–11. doi: 10.1016/j.nbd.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.van Vliet EA, van Schaik R, Edelbroek PM, et al. Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia. 2006 Apr;47(4):672–80. doi: 10.1111/j.1528-1167.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet EA, Zibell G, Pekcec A, et al. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010 Feb;58(2):404–12. doi: 10.1016/j.neuropharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Cucullo L, Hossain M, Rapp E, et al. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007 Mar;48(3):505–16. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 35.Luna-Tortos C, Fedrowitz M, Loscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008 Dec;55(8):1364–75. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 36.Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 2008 Sep–Oct;8(5):127–30. doi: 10.1111/j.1535-7511.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sillanpaa M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood-onset epilepsy. Brain. 2008 Apr;131(Pt 4):938–44. doi: 10.1093/brain/awn037. [DOI] [PubMed] [Google Scholar]
- 38.Sillanpaa M, Schmidt D. Delayed time to first remission identifies poor long-term drug response of childhood-onset epilepsy: a prospective population-based study. Epilepsy Behav. 2009 Nov;16(3):507–11. doi: 10.1016/j.yebeh.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Sillanpaa M, Schmidt D. Early seizure frequency and aetiology predict long-term medical outcome in childhood-onset epilepsy. Brain. 2009 Apr;132(Pt 4):989–98. doi: 10.1093/brain/awn357. [DOI] [PubMed] [Google Scholar]
- 40.Spooner CG, Berkovic SF, Mitchell LA, et al. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006 Dec 26;67(12):2147–53. doi: 10.1212/01.wnl.0000248189.93630.4f. [DOI] [PubMed] [Google Scholar]
- 41.Loscher W, Klotz U, Zimprich F, et al. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009 Jan;50(1):1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 42.Ebid AH, Ahmed MM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit. 2007 Jun;29(3):305–12. doi: 10.1097/FTD.0b013e318067ce90. [DOI] [PubMed] [Google Scholar]
- 43.Basic S, Hajnsek S, Bozina N, et al. The influence of C3435T polymorphism of ABCB1 gene on penetration of phenobarbital across the blood-brain barrier in patients with generalized epilepsy. Seizure. 2008 Sep;17(6):524–30. doi: 10.1016/j.seizure.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: systematic review and meta-analysis. Epilepsia. 2010 Jan;51(1):7–26. doi: 10.1111/j.1528-1167.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- 45.Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006 Nov;47(11):1761–74. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 46.Marchi N, Teng Q, Ghosh C, et al. Blood-brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010 Sep 24;1353:176–86. doi: 10.1016/j.brainres.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wahab A, Albus K, Gabriel S, et al. In search of models of pharmacoresistant epilepsy. Epilepsia. 2010 Jul;51( Suppl 3):154–9. doi: 10.1111/j.1528-1167.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 48.Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010 Aug;51(8):1333–47. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- 49.Nabbout R, Vezzani A, Dulac O, et al. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol. 2011 Jan;10(1):99–108. doi: 10.1016/S1474-4422(10)70214-3. [DOI] [PubMed] [Google Scholar]
- 50.Granata T, Marchi N, Carlton E, et al. Management of the patient with medically refractory epilepsy. Expert Rev Neurother. 2009 Dec;9(12):1791–802. doi: 10.1586/ern.09.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tendler D, Lin S, Yancy WS, Jr, et al. The effect of a low-carbohydrate, ketogenic dieton nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007 Feb;52(2):589–93. doi: 10.1007/s10620-006-9433-5. [DOI] [PubMed] [Google Scholar]
- 52.Ruskin DN, Kawamura M, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4(12):e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Lan X, Roche I, et al. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem. 2008 Nov;107(4):1147–57. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Gawryluk JW, Wagener JF, et al. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends in Neurosciences. 2008 Jun;31(6):273–78. doi: 10.1016/j.tins.2008.02.009. This is the first publication suggesting a molecular link between a ketogenic diet and adenosine augmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tchekalarova J, Kubova H, Mares P. Postnatal caffeine treatment affects differently two pentylenetetrazol seizure models in rats. Seizure. 2009 Sep;18(7):463–9. doi: 10.1016/j.seizure.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Boison D. Methylxanthines, seizures and excitotoxicity. Handb Exp Pharmacol. 2010:200. doi: 10.1007/978-3-642-13443-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberheim NA, Tian GF, Han X, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008 Mar 26;28(13):3264–76. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006 Oct;54(5):358–68. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 60**.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007 Feb;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. The concept of the tripartite synapseis a rationale explanation why astrocytes play an important role in epilepsy. [DOI] [PubMed] [Google Scholar]
- 61.Iyer AM, Zurolo E, Boer K, et al. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience. 2010 May 19;167(3):929–45. doi: 10.1016/j.neuroscience.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 62.Williamson A, Patrylo PR, Pan J, et al. Correlations between granule cell physiology and bioenergetics in human temporal lobe epilepsy. Brain. 2005 May;128(Pt 5):1199–208. doi: 10.1093/brain/awh444. [DOI] [PubMed] [Google Scholar]
- 63.Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009 Nov;8(11):1056–72. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- 64.Karagiannis TC, Kn H, El-Osta A. The epigenetic modifier, valproic acid, enhances radiation sensitivity. Epigenetics. 2006 Jul–Sep;1(3):131–7. doi: 10.4161/epi.1.3.2896. [DOI] [PubMed] [Google Scholar]
- 65.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annual Review of Nutrition. 2008;28:273–93. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 66.Boison D, Scheurer L, Zumsteg V, et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99(10):6985–90. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studer FE, Fedele DE, Marowsky A, et al. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006 Sep 29;142(1):125–37. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11(1):25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 69.Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;193:535–87. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- 70.Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signaling. 2005;1:111–34. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T, Ren G, Lusardi T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118(2):571–82. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fedele DE, Li T, Lan JQ, et al. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200(1):184–90. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 73.Sebastiao AM, Ribeiro JA. Tuning and fine-tuning of synapses with adenosine. Curr Neuropharmacol. 2009;7(3):180–94. doi: 10.2174/157015909789152128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen JF, Sonsalla PK, Pedata F, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007 Dec;83(5):310–31. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 75.Malhotra J, Gupta YK. Effect of adenosine receptor modulation on pentylenetetrazole-induced seizures in rats. Br J Pharmacol. 1997 Jan;120(2):282–8. doi: 10.1038/sj.bjp.0700869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeraati M, Mirnajafi-Zadeh J, Fathollahi Y, et al. Adenosine A(1) and A(2A) receptors of hippocampal CA1 region have opposite effects on piriform cortex kindled seizures in rats. Seizure-European Journal of Epilepsy. 2006 Jan;15(1):41–48. doi: 10.1016/j.seizure.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Cunha RA. Different cellular sources and different roles of adenosine: A(1) receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A(2A) receptor-mediated facilitation of plasticity. Neurochemistry International. 2008 Jan;52(1–2):65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 78**.Hasko G, Pacher P, Vizi ES, et al. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005 Oct;26(10):511–6. doi: 10.1016/j.tips.2005.08.004. Outstanding summary of mechanisms by which the adenosine system interacts with immunological functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Yacoubi M, Ledent C, Parmentier M, et al. Adenosine A2A receptor deficient mice are partially resistant to limbic seizures. Naunyn Schmiedebergs Arch Pharmacol. 2009 Sep;380(3):223–32. doi: 10.1007/s00210-009-0426-8. [DOI] [PubMed] [Google Scholar]
- 80.Czuczwar SJ, Szczepanik B, Wamil A, et al. Differential effects of agents enhancing purinergic transmission upon the antielectroshock efficacy of carbamazepine, diphenylhydantoin, diazepam, phenobarbital, and valproate in mice. J Neural Transm Gen Sect. 1990;81(2):153–66. doi: 10.1007/BF01245835. [DOI] [PubMed] [Google Scholar]
- 81.De Sarro G, De Sarro A, Di Paola ED, et al. Effects of adenosine receptor agonists and antagonists on audiogenic seizure-sensible DBA/2 mice. Eur J Pharmacol. 1999;371(2–3):137–45. doi: 10.1016/s0014-2999(99)00132-6. [DOI] [PubMed] [Google Scholar]
- 82.von Lubitz DKJE, Carter MF, Deutsch SI, et al. The effects of adenosine A3 receptor stimulation on seizures in mice. Eur J Pharmacol. 1995;275(1):23–9. doi: 10.1016/0014-2999(94)00734-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borowicz KK, Kleinrok Z, Czuczwar SJ. N6-2-(4-aminophenyl)ethyl-adenosine enhances the anticonvulsive activity of antiepileptic drugs. Eur J Pharmacol. 1997 May 30;327(2–3):125–33. doi: 10.1016/s0014-2999(97)89651-3. [DOI] [PubMed] [Google Scholar]
- 84.Lado FA, Moshe SL. How do seizures stop? Epilepsia. 2008 Oct;49(10):1651–64. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007 Jan;27(1):1–5. doi: 10.1038/sj.jcbfm.9600334. This paper highlights the role of adenosine kinase as upstream regulator of the endogenous neuroprotectant adenosine. [DOI] [PubMed] [Google Scholar]
- 86.Gouder N, Scheurer L, Fritschy J-M, et al. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24(3):692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borowicz KK, Luszczki J, Czuczwar SJ. 2-Chloroadenosine, a preferential agonist of adenosine A1 receptors, enhances the anticonvulsant activity of carbamazepine and clonazepam in mice. Eur Neuropsychopharmacol. 2002 Apr;12(2):173–9. doi: 10.1016/s0924-977x(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 88.Luszczki JJ, Kozicka M, Swiader MJ, et al. 2-Chloro-N-6-cyclopentyladenosine enhances the anticonvulsant action of carbamazepine in the mouse maximal electroshock-induced seizure model(A) Pharmacological Reports. 2005 Nov–Dec;57(6):787–94. [PubMed] [Google Scholar]
- 89.Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic Diets: An Update for Child Neurologists. J Child Neurol. 2009 Jun 17;24(8):979–88. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 90.Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008 Jun;7(6):500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 91*.Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010 Mar 17;30(11):3886–95. doi: 10.1523/JNEUROSCI.0055-10.2010. This study investigates key mechanisms of the ketogenic diet in an in vitro preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kossoff EH, Rowley H, Sinha SR, et al. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008 Feb;49(2):316–9. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 93.Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009 Feb;50(2):304–17. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 94.Kelley SA, Kossoff EH. Doose syndrome (myoclonic-astatic epilepsy): 40 years of progress. Dev Med Child Neurol. 2010 Nov;52(11):988–93. doi: 10.1111/j.1469-8749.2010.03744.x. [DOI] [PubMed] [Google Scholar]
- 95.Kossoff EH, Hedderick EF, Turner Z, et al. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008 Sep;49(9):1504–9. doi: 10.1111/j.1528-1167.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 96**.Wilz A, Pritchard EM, Li T, et al. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008 Sep;29(26):3609–16. doi: 10.1016/j.biomaterials.2008.05.010. This paper demonstrates the therapeutic benefit of seizure control via focal silk-based adenosine-augmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szybala C, Pritchard EM, Wilz A, et al. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–35. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li T, Steinbeck JA, Lusardi T, et al. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007 May;130(Pt 5):1276–88. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 100.Theofilas P, Brar S, Stewart K-A, et al. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52(3):589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]