Highlights
▸ Williams syndrome (WS) is characterized by hypersociability contrasted with non-social fears. ▸ Amygdala response to non-social fear images is increased in WS versus inhibited temperament. ▸ Amygdala response to social fear images is not decreased in WS versus uninhibited temperament. ▸ Comparison groups with similar behavioral traits can elucidate neural bases of disorders.
Keywords: Williams syndrome, Inhibited temperament, Amygdala, Sociability, Anxiety, fMRI
Abstract
Williams syndrome is a neurodevelopmental disorder with an intriguing behavioral phenotype—hypersociability combined with significant non-social fears. Previous studies have demonstrated abnormalities in amygdala function in individuals with Williams syndrome compared to typically developing controls. However, it remains unclear whether the findings are related to the atypical neurodevelopment in Williams syndrome, or are also associated with behavioral traits at the extreme end of a normal continuum. We used functional magnetic resonance imaging (fMRI) to compare amygdala blood-oxygenation-level-dependent (BOLD) responses to non-social and social images in individuals with Williams syndrome compared to either individuals with inhibited temperament (high non-social fear) or individuals with uninhibited temperament (high sociability). Individuals with Williams syndrome had larger amygdala BOLD responses when viewing the non-social fear images than the inhibited temperament control group. In contrast, when viewing both fear and neutral social images, individuals with Williams syndrome did not show smaller amygdala BOLD responses relative to the uninhibited temperament control group, but instead had amygdala responses proportionate to their sociability. These results suggest heightened amygdala response to non-social fear images is characteristic of WS, whereas variability in amygdala response to social fear images is proportionate to, and might be explained by, levels of trait sociability.
1. Introduction
Williams syndrome (OMIM#194050) is a rare neurodevelopmental disorder caused by a known genetic microdeletion on chromosome 7 (7q11.23). Williams syndrome (WS) is generally characterized by: visuospatial construction and memory deficits (Atkinson et al., 2003, Meyer-Lindenberg et al., 2004); mild to moderate intellectual disability (Howlin et al., 1998); and relative strengths in expressive language (Karmiloff-Smith et al., 1997, Setter et al., 2007, Udwin and Yule, 1990) and face processing (Karmiloff-Smith et al., 2004, Riby et al., 2008, Riby et al., 2010). An intriguing component of the WS behavioral phenotype is hypersociability (Doyle et al., 2004) contrasted with significant non-social fears (Dykens, 2003). Individuals with WS are highly sociable (Doyle et al., 2004, Gosch and Pankau, 1994), even with strangers, and highly empathic (Klein-Tasman and Mervis, 2003, Tager-Flusberg and Plesa-Skwerer, 2010, Udwin and Yule, 1991). In contrast, most individuals with WS also have high rates of non-social anxiety, fears and phobias. These non-social fears increase with age and can impede everyday functioning (Davies et al., 1998, Dykens, 2003).
Given that both the non-social fear and hypersociability traits are related to fears (or lack of), the amygdala is likely to be a key brain region mediating these behaviors. The amygdala is involved with multiple aspects of emotion processing including: fear detection (Calder et al., 1996), fear conditioning (LaBar et al., 1998), emotion processing (Yang et al., 2002), and anxiety (for reviews see Davis and Whalen, 2001, Zald, 2003). In individuals with Williams syndrome, amygdala dysfunction – associated with either non-social fear or hypersociability – has been reported in several neuroimaging studies.
When viewing fear-related non-social images, individuals with WS demonstrate larger amygdala BOLD responses than typically developing controls (Meyer-Lindenberg et al., 2005, Munoz et al., 2010). When viewing fear-related social images (i.e., faces) or matching face identities, individuals with WS demonstrate smaller amygdala BOLD responses (Haas et al., 2009, Meyer-Lindenberg et al., 2005, Mimura et al., 2010, Paul et al., 2009) relative to typically developing controls. Smaller amygdala BOLD responses to fear faces correlate with parent reports of increased likelihood to approach a stranger in individuals with WS (Haas et al., 2010). When viewing happy faces, individuals with WS have larger amygdala BOLD responses (Haas et al., 2009). Together, the findings of amygdala responses to social images suggest that the characteristic hypersociability might be the result of a combination of reduced fear responses to negative social stimuli and increased appetitive responses to positive social stimuli.
All of these prior studies used control subjects who were typically developing (Meyer-Lindenberg et al., 2005, Mimura et al., 2010, Paul et al., 2009) or had another neurodevelopmental disorder (Haas et al., 2009). Although the typically developing controls were not behaviorally characterized, we might expect that, at least as a group, they had an average amount of sociability and average amount of non-social fears. As such, the typically developing controls were unlikely to provide a match for either the non-social fear or hypersociability traits. Thus, it is unclear whether differences in amygdala response seen in WS are unique to WS or whether they are also part of the neural basis for the traits of hypersociability and non-social fears in typical development.
To address the issue of matching on traits or phenotypes, the use of carefully selected controls has been recommended (Hodapp and Dykens, 2001). For the study of WS, control groups for the traits of both non-social fear and hypersociability can be provided by the two extreme ends of a single temperament dimension, inhibited temperament. Inhibited temperament (IT) is a trait characterized by a predisposition to respond to novel people, places or events, with avoidance behavior (Kagan et al., 1988a). Individuals with IT chronically show wary, avoidant, or fear responses to novelty. In addition to their characteristic shyness and social anxiety (Schwartz et al., 1999), individuals with IT also typically have significant non-social fears (Goodwin et al., 2004). In contrast, individuals with an uninhibited temperament (UT) typically respond to novelty with positive approach behaviors, are highly social, and have few social or non-social fears (Kagan et al., 1988b). The nonsocial fears and anxieties seen in individuals with IT are similar to those reported in WS. The high sociability associated with UT is similar to the hypersociability characteristic of individuals with WS. Thus, these two extreme temperament groups were targeted to provide trait-based control groups for each of the two aspects of the WS phenotype we are investigating. To our knowledge, control groups targeted for comparison on behavioral traits have not previously been proposed in the study of WS.
In summary, prior studies have suggested that amygdala responses to social and non-social stimuli in WS are different from amygdala responses of typically developing controls. While these differences in amygdala response may be the result of WS, an alternative explanation is that amygdala function in WS is the same as in typically developing controls and that the magnitude of amygdala BOLD response is correlated with the behavioral trait. To test this question, we compared individuals with WS to two control groups—typically developing individuals with either extreme IT or extreme UT. If amygdala BOLD response is associated with the traits of non-social fear or hypersociability, we would predict BOLD responses to be increased (or decreased) at the extreme ends of the trait continuum, relative to individuals in the middle of the continuum. For example, a similar amygdala BOLD response to non-social fear images in the WS and IT groups would suggest that amygdala BOLD responses are a function of the non-social fear trait. On the other hand, different amygdala BOLD responses between the WS and IT groups would suggest that amygdala dysfunction is unique to WS, even controlling for the trait. To test whether the non-social fear and hypersociability traits in WS are specific to fear or generalize to other emotions, we use a fully factorial design to examine amygdala responses to non-social and social stimuli across three emotions: fear, neutral, and happy.
2. Methods
2.1. Participants
Thirty individuals with either Williams syndrome (n = 10, 6 females), extreme inhibited temperament (n = 10, 6 females) or extreme uninhibited temperament (n = 10, 6 females) participated in this study. Participants were 18–40 years of age (mean = 23.5, SD = 5.3), predominantly Caucasian, and predominantly right handed. The groups did not differ on age, race or handedness (see Table 1).
Table 1.
Participant characteristics by group.
| Inhibited temperament (IT) |
Williams syndrome (WS) |
Uninhibited temperament (UT) |
IT > WS | UT > WS | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p value | p value | |
| Age | 24.3 | 1.93 | 21.8 | 1.00 | 24.5 | 1.98 | .26 | .24 |
| IQ | 117.30 | 13.49 | 79.20 | 17.84 | 108.33 | 9.03 | <.001 | .002 |
| Temperament | ||||||||
| Retrospective (RSRI) | ||||||||
| Social | 3.91 | .10 | 2.02 | .21 | 1.45 | .07 | <.001 | .02 |
| Nonsocial | 3.54 | .24 | 2.13 | .22 | 1.16 | .08 | <.001 | .002 |
| Current (CSRI) | ||||||||
| Social | 3.00 | .16 | 1.77 | .12 | 1.31 | .08 | <.001 | .006 |
| Nonsocial | 2.76 | .16 | 2.56 | .16 | 1.25 | .05 | .39 | <.001 |
| Personality | ||||||||
| Novelty Seeking | 14.40 | 5.82 | 16.90 | 7.69 | 19.22 | 4.41 | .42 | .44 |
| Harm Avoidance | 14.00 | 6.79 | 12.20 | 4.80 | 3.22 | 1.92 | .50 | <.001 |
| Psychopathology | ||||||||
| Anxiety | 37.50 | 3.83 | 29.55 | 1.97 | 26.22 | 1.27 | .09 | .17 |
| Social anxiety | 83.07 | 23.27 | 28.26 | 19.74 | 23.59 | 14.48 | <.001 | .57 |
| Agoraphobia | 19.40 | 12.75 | 21.11 | 9.38 | 8.44 | 8.33 | .73 | .008 |
| Depression | 5.00 | 4.85 | 2.30 | 2.63 | 2.33 | 3.12 | .14 | .98 |
| Handedness (% right) | 70% | 80% | 80% | .61 | 1.00 | |||
Note: Significant results (p < .05) are in bold.
We recruited participants with WS through an existing database of persons who had either attended or expressed interest in the Vanderbilt Kennedy Center WS Music Camp (http://kc.vanderbilt.edu/site/services/). We recruited the two control groups through advertisement and research participant databases seeking people who were “especially shy or outgoing as a child”. Potential participants completed two questionnaires: the Retrospective Self-Report of Inhibition (RSRI) and the Current Self-Report of Inhibition (CSRI). The RSRI is a 30 item instrument which asks about behaviors during childhood (1–5 Likert scale, 1 = uninhibited, 5 = inhibited). The CSRI, a 31 item instrument, asks about current behaviors (1–5 Likert scale, 1 = uninhibited, 5 = inhibited). Both the RSRI and CSRI are comprised of social and non-social subscales, which provide information on inhibition in the social and non-social realms. Examples of social items are: did you enjoy meeting new children your age? (RSRI); and do you feel comfortable speaking in front of a large group of people? (CSRI). Examples of non-social items are: were you scared of the dark? (RSRI); and do open-air high places bother you? (CSRI). Both the RSRI and CSRI have excellent reliability and construct validity in typically developing groups (Reznick et al., 1992, Rohrbacher et al., 2008). We selected cutoff scores (inhibited ≥2.6, uninhibited ≤1.9) at the top and bottom 15% based on normative data from the general population. We selected only participants whose scores were beyond the cutoff for both the social and non-social subscales on the RSRI and the CSRI. Participants in each temperament group were matched on gender to participants in the WS group.
Participants were excluded on the basis of: failure to pass MRI safety screen; substance abuse or dependence in past 6 months; presence of severe psychiatric illness (such as schizophrenia or bipolar disorder); prior head injury; significant medical illness; and claustrophobia or pregnancy. Presence of anxiety or depression was not an exclusion criterion because both disorders are common in IT and WS. Similarly, we did not exclude participants for use of psychoactive medications because individuals with WS often take medication to control their anxiety.
The Vanderbilt University Institutional Review Board approved the study and we obtained written informed consent after providing participants with a complete description of the study. For participants with WS, the parent or guardian also provided written informed consent.
2.2. Behavioral measures
2.2.1. Temperament and personality
Childhood and current temperament were assessed using the RSRI and CSRI, as described above. Personality was measured with the Novelty Seeking and Harm Avoidance subscales of the Tridimensional Personality Questionnaire (Cloninger, 1987).
2.2.2. Psychopathology
We assessed psychopathology using both a standard clinical interview and continuous measures of anxiety and depression. The Structured Clinical Interview for DSM (SCID; Spitzer et al., 1992) was administered by a trained clinical interviewer. Based on the SCID, one participant with WS had panic disorder and one participant with IT had social phobia.
Current anxiety was measured with the State scale of the State-Trait Anxiety Inventory (Spielberger et al., 1983) and the Social Phobia and Agoraphobia subscales of the Social Phobia and Anxiety Inventory (Turner et al., 1996). Depression was assessed using the Beck Depression Inventory II (Beck et al., 1996).
2.2.3. Intelligence Quotient (IQ)
We assessed IQ for all participants using the Kaufmann Brief Intelligence Test, Second Edition (Kaufman and Kaufman, 1990).
2.3. fMRI task
To study group differences in amygdala BOLD response to social and non-social stimuli, participants were presented with blocks of social and non-social images across three emotions (happy, fear, and neutral). We used a block-design to enhance our power to detect activation (Birn et al., 2002). We used a passive viewing task because: we were interested in implicit emotional processing, amygdala BOLD response is higher in passive viewing tasks (Costafreda et al., 2008), and passive viewing has relatively low cognitive requirements, which is important given that individuals with WS often have mild to moderate intellectual deficits.
The fMRI procedure consisted of four separate runs, each consisting of six 24-s image blocks interleaved with six 10-s fixation blocks (204-s total per run). Within each run, each combination of stimulus type (social/non-social) by valence (happy/fear/neutral) was presented once, with stimulus type and valence counterbalanced across runs. Within each block, 12 stimuli were presented for 2-s each with no interstimulus interval. Each image was presented only once, and presentation order was randomized within each block.
The social stimuli were happy, fear and neutral facial expressions from the Karolinska Directed Emotional Faces database (Goeleven et al., 2008, Lundqvist et al., 1998). Equal numbers of male and female faces were selected for each emotion. The non-social stimuli were images from the International Affective Picture Set (Lang et al., 1999) selected based on valence and/or arousal ratings (positive, valence ≥6; negative, valence ≤4; neutral, valence 4.01–5.99 and arousal <5). To ensure the images were non-social, the following classes of images were not included: human face(s), erotic images, social interactions, or food. Also, images judged to be risky, threatening, or a known fear for persons with WS were removed for the positive and neutral groups (e.g., fireworks, jaguar, skiing, sky diving). Finally, we added 11 images of stimuli related to fears common in persons with WS (e.g., heights, storms, and fire) to ensure that there would be an adequate representation of specific fear triggers for both the WS and IT groups; the IAPS set already included typical phobic images for the IT group, such as animals bearing teeth, spiders and needles. E-prime software (Version 1.1, Psychology Software Tools, Pittsburgh, PA) was used to present the images.
2.4. MRI data acquisition
Structural (T1) and echo planar imaging (EPI) images were collected on a 3 T Phillips Achieva MRI scanner (Philips Healthcare, Inc., Best, The Netherlands). High resolution T1-weighted anatomical images were collected (256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap). EPI images were acquired using a sequence optimized for the amygdala: 2 s TR, 25 ms TE; 90° flip angle; 1.8 SENSE, 240 mm FOV; 3 mm × 3 mm in plane resolution using an 80 × 80 matrix (reconstructed to 128 × 128), and higher-order shimming to limit susceptibility artifacts. Each volume contained 36 2.5 mm (.25 mm gap) axial oblique slices (tilted 15° anterior higher than posterior relative to the intercommissural plane), which provided complete anterior–posterior coverage and inferior–superior coverage from the bottom of the temporal lobe to the top of the cingulate gyrus.
2.5. Valence ratings
Following the scanner session, study participants rated a subset of images on valence. For the social images, 10 examples of each emotion (fear, neutral, happy) were rated (with social images balanced for gender). The non-social images were selected to represent each of the categories of images seen in the scanner, because responses might be specific to a subcategory of image—for example, fear of spiders but not heights. For the non-social image ratings there were 15 fear, 13 neutral, and 10 happy images. Given the potential group differences in intellectual functioning, we used a visual scale that is appropriate even for use with pre-verbal individuals. Specifically, we modified a standard pediatric pain scale (Wong and Baker, 1988), to include five cartoon faces ranging from very positive to very negative (coded as +2 = very positive, 0 = neutral, −2 = very negative). Participants were asked to rate how each picture “made them feel” by selecting one of five faces. The social images were presented first, followed by the non-social images, with images within each set randomly ordered for each participant.
2.6. Data analysis
2.6.1. Behavioral data
To test for differences in temperament, IQ, personality, and psychopathology, we performed t-tests for the IT versus WS and UT versus WS comparisons. An alpha of .05 was used for all analyses and data were analyzed using SAS (Version 9.1, SAS Institute Inc., Cary, NC).
2.6.2. fMRI data processing
We preprocessed the fMRI data using SPM5 (http://www.fil.ion.ucl.ac.uk/) and Matlab (Version 7.1, The MathWorks, Inc., Natick, MA). The pre-processing steps included: motion correction (aligned to the first slice), coregistration of functional and structural images, normalization into standard stereotactic space (MNI EPI template), resampling to 3 mm × 3 mm × 3 mm voxels, high pass filtering (128 s), and smoothing (6 mm).
We visually inspected EPI images for artifacts, signal dropout, and coverage of the amygdala. For all of the participants, data quality was good with no artifacts and good coverage of the amygdala. While average motion was low (<.5 mm, <1°), four of the participants had motion above our threshold (>3 mm translation or 3° rotation). For these participants, motion was 3.2 mm (WS), 4.3 mm (UT), 4.5° (IT), and 4.8° (WS), although each in only one run. Motion can affect estimation of BOLD signal, especially in small regions such as the amygdala. Although a common strategy for handling motion is to remove participants or runs, we wanted to keep as much data as possible, given the difficulty of recruiting our participant groups. Therefore, we used robust weighted least squares (rWLS) regression (Diedrichsen and Shadmehr, 2005), a modeling method that reduces the contribution of volumes with high motion to the overall general linear model.
2.6.3. fMRI data analysis
The first-level (participant) general linear model (Friston et al., 1994) was estimated using a robust weighted least squares (rWLS) method. Next, contrast images were created for each stimulus type (social/non-social) by emotion (happy/fear/neutral) conditions minus baseline (fixation cross) for each participant. Comparison of each emotion condition to the baseline provided an opportunity to detect differences in brain response to each of the emotions separately. These contrast images were used in the second-level (group) analyses. The second-level analysis included group (WS/IT/UT) as a between-subjects factor and stimulus type (social/non-social) and emotion (fear/neutral/happy) as within-subjects factors.
For the group-level analysis, we compared the WS group to each of the control groups in separate analyses. That is, the WS group was compared to the IT control group (targeted for comparison on high non-social fear) for the non-social stimuli contrasts: non-social fear > fixation; non-social neutral > fixation; and non-social happy > fixation. The WS group was compared to the UT control group (targeted for comparison on low social fear/hypersociability) for the social stimuli contrasts: social fear > fixation; social neutral > fixation; and social happy > fixation.
To provide a comparison with two prior studies (Meyer-Lindenberg et al., 2005, Munoz et al., 2010), we also directly tested for a dissociation in response to the non-social and social images. Amygdala BOLD response for non-social > social and social > non-social images were compared separately within each group (WS/IT/UT) and emotion (fear/neutral/happy).
Given the a priori interest in the amygdala, statistical analyses were restricted to amygdala search regions. The left and right amygdala regions of interest (ROI) were defined using the AAL templates (Tzourio-Mazoyer, 2002) implemented in WFU Pick Atlas (Maldjian, 2003). SPM5 was used to test for contrasts within each amygdala. To control for Type I error, we used cluster-based thresholding methods. The AlphaSim Monte Carlo simulation program (5000 iterations; http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) was used to provide the cluster-size threshold given the dimensions of the amygdala clusters, voxel-wise uncorrected p-value of .05 and smoothing kernel of 6 mm FWHM. A contiguous cluster size >11 voxels (297 mL) controlled for family-wise error for each amygdala at α = .05. Results in the tables, text and figure legends are presented with the uncorrected p-values and the cluster size. For the amygdala, p-values <.05 with cluster sizes (k) > 11 voxels are significant at a family-wise corrected α = .05. Exploratory whole brain analyses were performed to identify other potential neural substrates of WS. As with the region of interest analysis, we compared the WS group to each of the two control groups separately. To reduce the number of exploratory analyses, we focused on the fear stimuli since fear responses were key for the traits of interest. For the whole brain analysis a voxel-wise p-value of .005 and a contiguous cluster size >25 controlled for family-wise error at α = .05.
2.6.4. Valence ratings
To test for differences in the valence ratings, we tested for group differences for the non-social (WS versus IT) and social (WS versus UT) images for each of the three emotions (happy/fear/neutral). Data were analyzed using SAS (Version 9.2, SAS Institute Inc., Cary, NC).
3. Results
3.1. Behavioral data
We compared individuals with WS to the two temperament control groups (IT/UT) on measures related to the traits of interest including temperament, personality, and anxiety and depression (see Table 1).
3.1.1. Williams syndrome versus inhibited temperament
The WS and IT control groups were similarly high on measures of current non-social inhibition (although different during childhood) and also similar on the personality measures of Harm Avoidance and Novelty Seeking, and the psychopathology measures of trait anxiety, agoraphobia, and depression.
As expected, the major difference between the WS and IT control group was on the measures of social fear. The IT control group had more social inhibition and social anxiety than the WS group. IQs in the IT control group were significantly higher than the WS group.
3.1.2. Williams syndrome versus uninhibited temperament
The WS and UT control groups had similarly low reports of social anxiety, trait anxiety and depression. The groups were also similar on the personality measure of Novelty Seeking. Although the UT control group rated themselves as significantly more uninhibited that the WS group, both groups were on the uninhibited extreme of the continuum (top 15% on social subscale of CSRI).
Validating expected differences in non-social fears, the WS group had significantly higher (more inhibited) scores on the retrospective and current non-social inhibition subscales and on the personality measure of Harm Avoidance, compared to the UT control group. The WS group also reported higher scores on the agoraphobia subscale of the SPAI. IQs in the UT control group were significantly higher than the WS group.
3.2. fMRI data
3.2.1. Nonsocial images: Williams syndrome versus inhibited temperament
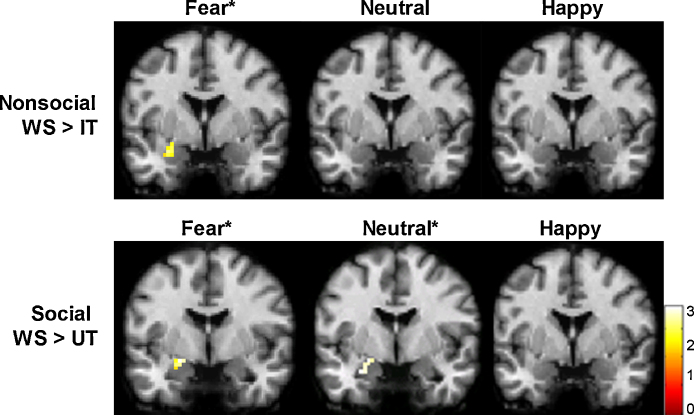
To examine the amygdala BOLD response of individuals with WS when viewing non-social images, we compared individuals with WS to individuals with IT, the control group targeted for comparison on the high non-social fear trait. When viewing fear non-social images, individuals with WS had significantly larger BOLD responses in the left amygdala relative to the IT control group (see Fig. 1; WS: M = 2.06, SD = 1.41; IT: M = .92, SD = .66). When viewing neutral and happy non-social images, amygdala BOLD responses were similar between the two groups.
Fig. 1.
Amygdala BOLD signal varies by group, stimulus type, and emotion. Top panel: Amygdala BOLD response to fear non-social images (versus baseline) is larger for individuals with Williams syndrome compared to those with inhibited temperament, the control group targeted for comparison on non-social fear (peak voxel: x = −27, y = −3, z = −21; z = 2.84, p = .002; cluster size = 17). Bottom panel: Amygdala BOLD response to fear and neutral social images (versus baseline) is larger for individuals with Williams syndrome compared to those with uninhibited temperament, the control group targeted for comparison on sociability (fear: peak voxel: x = −21, y = −3, z = −12; z = 2.81, p = .002, cluster size = 11; neutral: peak voxel = −27, y = −3, z = −21; z = 2.16, p = .015, cluster size = 14). Statistical parametric t-maps are displayed on a single-subject MNI template brain and thresholded at uncorrected p < .05 and cluster size >11 voxels. Color bar shows t-values.
To provide a comparison with other studies that use neutral images as a baseline, we also compared the WS and IT groups when viewing the fear/happy images relative to the neutral images. Even when using neutral images as the baseline, the individuals with WS still showed a significantly increased left amygdala BOLD response when viewing the nonsocial fear images (peak voxel: x = −27, y = −3, z = −21; z = 2.55, p = .005; cluster size = 12). When viewing the happy images relative to the neutral images, amygdala BOLD responses were similar between the groups.
3.2.2. Social images: Williams syndrome versus uninhibited temperament
To examine the amygdala response of individuals with WS when viewing social images (faces), we compared the WS group with the UT control group, targeted for comparison on the hypersociability/low social fear trait. When viewing fear faces, the WS group had larger amygdala BOLD response relative to the UT control group, an unexpected finding (Fig. 1; WS: M = 1.37, SD = 1.39; UT: M = .44, SD = .40). Similarly, left amygdala BOLD responses for the WS group were larger when viewing neutral social images, compared to the UT control group (Fig. 1; WS: M = 1.31, SD = 1.23; UT: M = .01, SD = 1.30). When viewing happy social images, BOLD responses were similar in both groups.
To provide a comparison with previous studies that have used neutral faces as a baseline, we also compared the WS and UT groups when viewing the fear/happy faces relative to the neutral faces. For the fear faces, the two groups did not differ significantly in amygdala BOLD response, as expected given the group differences (in the same direction) for both the fear and neutral faces. However, when comparing the happy faces to the neutral faces, the UT group had significantly greater left amygdala BOLD response than the WS group (peak voxel: x = −27, y = 3, z = −21; z = 3.38, p < .001; cluster size = 28). Thus, using neutral faces as a baseline produced different results, driven by the fact that individuals with WS show larger amygdala BOLD responses to neutral faces compared to the UT control group.
3.2.3. Non-social/social dissociation
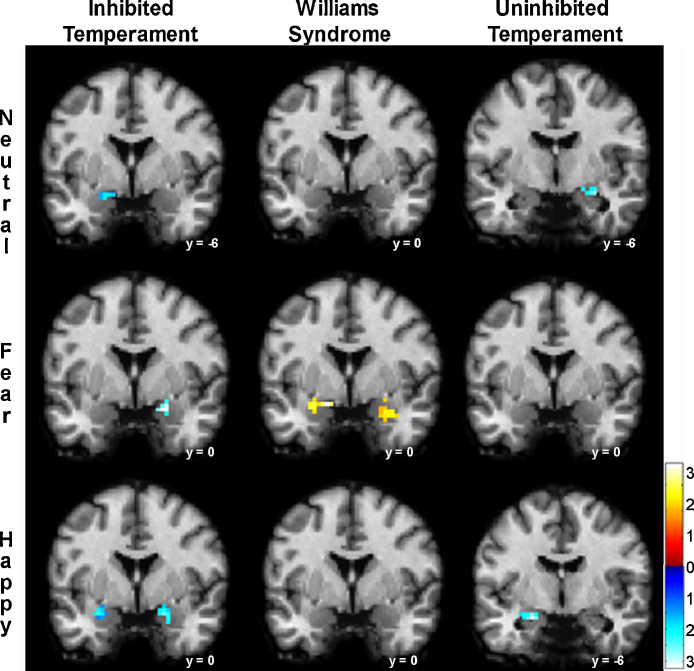
To specifically test for a dissociation in amygdala response to non-social and social images, we compared the amygdala BOLD response to non-social versus social images within each group and each emotion (see Fig. 2). In the WS group, there was a dissociation in the responses to fear non-social and social images in both the left and right amygdala, with BOLD responses larger for non-social fear images, but no differences for the neutral or happy images. In the IT group, amygdala BOLD responses were larger when viewing social images across all three emotions (fear/neutral/happy). Among those in the UT group, amygdala BOLD responses were larger for the social neutral and happy images, but were not significantly different for the fear images.
Fig. 2.
Nonsocial–social dissociation in amygdala BOLD response differs by group. Left column: For the inhibited temperament group, amygdala BOLD response was smaller when viewing non-social relative to social images across all three emotional conditions: neutral (peak voxel: x = −15, y = −6, z = −18; z = 3.71, p < .001, cluster size = 17); fear (peak voxel: x = 21, y = 0, z = −18; z = 2.82, p = .002, cluster size = 20); and Happy (left peak voxel: x = −15, y = −6, z = −18; z = 4.18, p < .001, cluster size = 31; right peak voxel: x = 21, y = −3, z = −18; z = 3.35, p < .001, cluster size = 33). Middle column: For the Williams syndrome group, the amygdala BOLD response was larger when viewing fear non-social images relative to fear social images (left peak voxel: x = −15, y = 0, z = −15, z = 3.16, p = .001, cluster size = 23; right peak voxel: x = 33, y = 0, z = −21, z = 2.20, p = .02, cluster size = 23). Right column: For the uninhibited temperament group, amygdala BOLD responses were smaller when viewing non-social relative to social images in the Neutral (peak voxel: x = 30, y = −3, z = −21; z = 3.04, p = .001, cluster size = 18) and Happy (peak voxel: x = −21, y = −6, z = −18; z = 3.09, p = .001, cluster size = 15) conditions. Statistical parametric t-maps are displayed on a single-subject MNI template brain and thresholded at uncorrected p < .05 and cluster size > 11 voxels. Color bar shows t-values.
3.2.4. Whole brain analysis
To determine whether between-group differences in BOLD response in other brain regions might be related to the WS traits, we performed exploratory whole brain analyses. As with the amygdala region of interest analysis, we compared the WS group to the IT control group for the non-social images and to the UT control group for the social images. When viewing the fear non-social images, individuals with WS had larger BOLD responses in bilateral visual cortex (BA 18, BA 19), right medial prefrontal cortex, and right dorsolateral prefrontal cortex, compared to the IT control group (see Table 2). Compared to the WS group, the IT control group had larger BOLD responses in a bilateral area of visual cortex (middle occipital gyrus) inferior to the region activated in the WS group.
Table 2.
Whole brain findings for non-social fear images: Williams syndrome versus inhibited temperament.
| Brain region (hemisphere) | Cluster size | p value | z score | x | y | z |
|---|---|---|---|---|---|---|
| Williams syndrome > inhibited temperament | ||||||
| Cuneus/BA19 (L) | 31 | <.001 | 4.37 | −18 | −96 | 24 |
| Cuneus/BA18 (R) | 55 | <.001 | 4.97 | 18 | −87 | 24 |
| Lingual gyrus (L) | 35 | <.001 | 4.45 | −12 | −87 | −12 |
| Dorsolateral prefrontal cortex/BA46 (R) | 40 | <.001 | 3.83 | 39 | 33 | 21 |
| Medial prefrontal cortex/BA10 (R) | 82 | <.001 | 3.79 | 30 | 63 | 0 |
| Amygdala (L)a | 18 | .002 | 2.84 | −27 | −3 | −21 |
| Inhibited temperament > Williams syndrome | ||||||
| Middle occipital gyrus (L) | 68 | <.001 | 4.79 | −18 | −96 | −3 |
| Middle occipital gyrus (R) | 75 | <.001 | 4.57 | 33 | −90 | 12 |
Small volume corrected.
When viewing the fear social images, individuals with WS had larger BOLD responses in visual cortex (cuneus, BA18), compared to the UT control group. The UT control group had larger BOLD responses in visual cortex (middle occipital gyrus) slightly inferior to those in the WS group (see Table 3).
Table 3.
Whole brain findings for social fear images: Williams syndrome versus uninhibited temperament.
| Brain region (hemisphere) | Cluster size | p value | z score | x | y | z |
|---|---|---|---|---|---|---|
| Williams syndrome > uninhibited temperament | ||||||
| Cuneus/BA18 (L) | 52 | <.001 | 4.37 | −15 | −96 | 24 |
| Uninhibited temperament > Williams syndrome | ||||||
| Middle occipital gyrus (L) | 136 | <.001 | 5.52 | −12 | −99 | −6 |
3.3. Valence ratings
3.3.1. Nonsocial images: Williams syndrome versus inhibited temperament
In order to examine group differences in subjective ratings of the non-social images, we compared the WS and IT control groups on ratings of the fear, neutral, and happy non-social images. Both groups rated the fear images as most unpleasant and the happy images as most pleasant (see Table 4). For the fear non-social images, both the WS and IT control groups had similar ratings of unpleasantness. However, for the neutral and happy images, individuals with WS rated the images as significantly more pleasant than those in the IT control group (p < .01 for both).
Table 4.
Stimulus valence ratings by group.
| Inhibited temperament (IT) |
Williams Syndrome (WS) |
IT > WS | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |
| Non-social stimuli | |||||
| Fear | −0.70 | .54 | −0.61 | .97 | .81 |
| Neutral | 0.17 | .22 | 1.25 | .74 | <.001 |
| Happy | 0.90 | .48 | 1.77 | .35 | <.001 |
| Uninhibited temperament (UT) |
Williams syndrome (WS) |
UT > WS | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |
| Social stimuli | |||||
| Fear | −0.47 | .88 | −0.30 | 1.12 | .71 |
| Neutral | 0.07 | .35 | 0.00 | .27 | .64 |
| Happy | 0.96 | .64 | 1.49 | .34 | .04 |
Note: Significant results (p < .05) are in bold.
3.3.2. Social images: Williams syndrome versus uninhibited temperament
Next, we compared the WS and UT control groups on ratings of the social images across the three emotions of fear, neutral, and happy. Similar to the results from the non-social images, both the WS and UT control groups rated the fear images as most unpleasant and the happy images as most pleasant (see Table 4). For the fear and neutral social images, the WS and UT control groups had similar ratings. However, individuals with WS rated the happy faces as significantly more pleasant than did those in the UT control group (p = .04).
4. Discussion
We compared individuals with Williams syndrome (WS) to two novel control groups to determine whether amygdala BOLD responses to social and non-social images are characteristic of WS per se, or are associated with traits at the extreme end of a normal continuum. When viewing non-social images, individuals with WS showed larger amygdala BOLD response to fear images, even relative to the targeted comparison group with inhibited temperament (IT), suggesting that this brain response to fear non-social images is characteristic of WS and cannot be explained by the behavioral trait alone. The finding of increased BOLD response to non-social fear images replicates two prior studies (Meyer-Lindenberg et al., 2005, Munoz et al., 2010). The increased amygdala BOLD response to non-social images was specific to the fear stimuli, as there were no between group differences for the neutral or happy non-social images. When viewing fear non-social images, individuals with WS also showed increased BOLD signal in the medial prefrontal and dorsolateral cortices in regions very close to those reported by Meyer-Lindenberg et al. (2005). Thus, increased amygdala and prefrontal cortex BOLD responses to fear non-social images appear to be a unique characteristic of WS, and not merely attributable to the trait of high non-social fear.
In contrast, when viewing social images, individuals with WS did not have the expected smaller amygdala BOLD responses relative to the uninhibited temperament (UT) control group, but instead had larger amygdala BOLD responses to the fear faces. However, when using neutral faces as a baseline, these two high sociability groups had similar amygdala responses to the fear faces. These findings conflict with prior reports of smaller amygdala BOLD responses to fear faces relative to a typically developing group (Haas et al., 2009, Meyer-Lindenberg et al., 2005, Mimura et al., 2010). The discrepancy might be due to study design differences; for example, the present study used a block design with passive viewing and the other studies used event-related designs with a required motor response. However, our findings for the non-social images did replicate findings from one of these studies (Meyer-Lindenberg et al., 2005) suggesting that the discrepancy is not due solely to study differences. Alternatively, the findings from the present study may indicate that the smaller amygdala response to fear faces in those with WS reported in the other studies was a function of the high sociability associated with WS, and the fact that the control subjects were not selected to have high sociability. Providing some initial support for this view, a previous study by Haas et al. (2010) reported that amygdala activation to fear (versus neutral) faces was correlated with a measure of social fearlessness. Thus, reduced amygdala BOLD responses to fear faces may be associated with a trait related to sociability in both individuals with and without Williams syndrome.
We found that the abnormal amygdala response reported in previous studies of WS was present in a non-WS control group with high sociability (the UT control group), which suggests that amygdala response to fear is associated with sociability in both groups. However, individuals with WS also display indiscriminate sociability, a trait that is not shared in typically developing individuals with UT. This difference between groups suggests that indiscriminate sociability might not be explained by smaller amygdala responses to social fear stimuli, but instead might be explained by functional differences in other brain regions or circuits. For example, studies of neuropsychological profiles (Porter et al., 2007) and of brain BOLD response (Mobbs et al., 2007) suggest that prefrontal cortex deficits produce difficulties in response inhibition. Future studies should attempt to dissect the various components of the sociability phenotype in Williams syndrome in order to identify neural response differences that are specific to the disorder.
The selection of a baseline condition may critically affect study results. Previous studies of amygdala BOLD response in individuals with WS have used neutral images as the baseline condition, to which the fear and happy conditions were compared. In the present study, we explicitly examined amygdala responses to neutral non-social images and neutral faces. Whereas the WS and IT control groups had similar amygdala responses to the neutral non-social images, individuals with WS had a larger amygdala BOLD response to neutral faces relative to the UT control group. Because increased amygdala BOLD response has been associated with viewing both fear and happy faces in typically developing adults (Sergerie et al., 2008, Zald, 2003), it is difficult to interpret this finding. However, given that the valence findings suggest a possible positivity bias, we propose that the increased amygdala response to neutral faces might indicate that neutral faces are perceived more like happy faces in individuals with WS. Studies of emotion discrimination using morphed facial emotions could contribute to the interpretation of this intriguing finding. Regardless of the interpretation, group differences in amygdala BOLD response to neutral faces changed the results for both the fear and happy face conditions, suggesting that baseline conditions should be considered carefully in future studies.
To our knowledge, previous studies of WS have not examined ratings of emotional stimuli. In this study, individuals with WS did not differ from the two control groups on valence ratings of fear for either the non-social or the social images. However, individuals with WS had more positive ratings of both the social happy and nonsocial happy images, suggesting a possible positivity bias in WS.
This study had several limitations. First, similar to previous studies, the sample size for this study was small, which could limit generalization of our findings. Second, because we used a passive viewing task, it is possible that attention during the task differed by group. While attention can affect BOLD signal, a previous study reported that in individuals with Williams syndrome reaction times were not correlated with BOLD responses to either fear or happy faces, despite generally slower reaction times (Haas et al., 2009). Third, although individuals with WS were as low on social anxiety as the UT control group, the WS group rated themselves as less socially uninhibited than the uninhibited temperament control group. While behaviorally the WS participants in this study were characteristically sociable, it is possible that our questionnaires did not assess a key aspect of sociability in WS, or that individuals with WS are not able to report accurately on their own sociability. Future studies should explore alternative methods for measuring non-social fear and hypersociabilty in individuals with WS. Fourth, while we controlled for Type I error for each of the emotion contrasts, we did not additionally control for possible Type I errors due to testing multiple contrasts. Thus, it will be important for the results to be replicated in subsequent studies. Fifth, the scope of this study was limited to the role of the amygdala in the neural basis of the non-social fear and high sociability in WS. However, as the current findings suggest, the full story is likely to be much more complex, involving other brain regions perhaps related to response inhibition and reward circuitry. Regional differences in response inhibition and reward circuitry were not probed under the current protocol but could be investigated in future studies using other paradigms. Future studies should also aim to identify genes that are associated with these disproportionate amygdala responses to non-social fear. Finding these genetic associations could provide important new clues to genetic risk factors or pathways related to specific fears and phobia in the general population.
This study demonstrates the importance of using novel, targeted control groups for the study of neurodevelopmental disorders such as WS. Our study design allowed for direct comparison of brain responses underlying similar behavioral traits in groups with typical versus atypical neurodevelopment. In this way, we were able to disambiguate the neural processes that co-occur with specific behavioral traits from those that are exclusively seen in the genetic disorder of Williams syndrome.
Acknowledgements
We thank the individuals with Williams syndrome and their families for participating in this study. We thank Elizabeth Roof for research assistance, and Elisabeth Dykens and David Zald for input on study design. This research was supported in part by funding from the National Institute of Mental Health NIMH (K01-MH083052 to JUB), NIH Roadmap for Medical Research Postdoctoral Fellowship – Biobehavioral Intervention Training Program (T32 MH75883, TATW), a Hobbs Discovery Grant from the Vanderbilt Kennedy Center, the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH), and the Vanderbilt University Institute of Imaging Science. Portions of this work were presented at the Society for Neuroscience, Chicago, IL, 2009.
References
- Atkinson J., Braddick O., Anker S., Curran W., Andrew R., Wattam-Bell J., Braddick F. Neurobiological models of visuospatial cognition in children with Williams syndrome: measures of dorsal-stream and frontal function. Dev. Neuropsychol. 2003;23:139–172. doi: 10.1080/87565641.2003.9651890. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychology Corporation; San Antonio, TX: 1996. Manual for Beck Depression Inventory II (BDI-II) [Google Scholar]
- Birn R.M., Cox R.W., Bandettini P.A. Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. Neuroimage. 2002;15:252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Calder A.J., Young A.W., Rowland D., Perrett D.I., Hodges J.R., Etcoff N.L. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn. Neuropsychol. 1996;13:699–745. [Google Scholar]
- Cloninger C.R. A systematic method for clinical description and classification of personality variants—a proposal. Arch. Gen. Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., Brammer M.J., David A.S., Fu C.H.Y. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res. Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Davies M., Udwin O., Howlin P. Adults with Williams syndrome—preliminary study of social, emotional and behavioural difficulties. Br. J. Psychiatry. 1998;172:273–276. doi: 10.1192/bjp.172.3.273. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. Neuroimage. 2005;27:624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T.F., Bellugi U., Korenberg J.R., Graham J. Everybody in the world is my friend” hypersociability in young children with Williams syndrome. Am. J. Med. Genet. A. 2004;124:263–273. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- Dykens E.M. Anxiety, fears, and phobias in persons with Williams syndrome. Dev. Neuropsychol. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Friston K., Holmes A., Worsley K., Poline J., Frith C., Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Goeleven E., De Raedt R., Leyman L., Verschuere B. The Karolinska Directed Emotional Faces: a validation study. Cogn. Emotion. 2008;22:1094–1118. [Google Scholar]
- Goodwin R.D., Fergusson D.M., Horwood L.J. Early anxious/withdrawn behaviours predict later internalising disorders. J. Child Psychol. Psychiatry. 2004;45:874–883. doi: 10.1111/j.1469-7610.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Gosch A., Pankau R. Social–emotional and behavioral-adjustment in children with Williams–Beuren syndrome. Am. J. Med. Genet. 1994;53:335–339. doi: 10.1002/ajmg.1320530406. [DOI] [PubMed] [Google Scholar]
- Haas B.W., Mills D., Yam A., Hoeft F., Bellugi U., Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J. Neurosci. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.W., Hoeft F., Searcy Y.M., Mills D., Bellugi U., Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48:1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodapp R.M., Dykens E.M. Strengthening behavioral research on genetic mental retardation syndromes. Am. J. Ment. Retard. 2001;106:4–15. doi: 10.1352/0895-8017(2001)106<0004:SBROGM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Howlin P., Davies M., Udwin O. Cognitive functioning in adults with Williams syndrome. J. Child Psychol. Psychiatry. 1998;39:183–189. [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Snidman N., Gibbons J., Johnson M.O. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A., Grant J., Berthoud I., Davies M., Howlin P., Udwin O. Language and Williams syndrome: how intact is “intact”? Child Dev. 1997;68:246–262. [PubMed] [Google Scholar]
- Karmiloff-Smith A., Thomas M., Annaz D., Humphreys K., Ewing S., Brace N., Van Duuren M., Pike G., Grice S., Campbell R. Exploring the Williams syndrome face-processing debate: the importance of building developmental trajectories. J. Child Psychol. Psychiatry. 2004;45:1258–1274. doi: 10.1111/j.1469-7610.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. American Guidance Services, Inc.; Circle Pines, NM: 1990. Kaufman Brief Intelligence Test. [Google Scholar]
- Klein-Tasman B.P., Mervis C.B. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev. Neuropsychol. 2003;23:269–290. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. University of Florida, Center for Research in Psychophysiology; Gainesville, FL: 1999. International Affective Picture System (IAPS) [Computer software] [Google Scholar]
- Lundqvist D., Flykt A., Ohman A. Department of Clinical Neuroscience, Psychology Section, Karolinska Institute; 1998. The Karolinska Directed Emotional Faces – KDEF. [Google Scholar]
- Maldjian J.A. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Hariri A.R., Munoz K.E., Mervis C.B., Mattay V.S., Morris C.A., Berman K.F. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat. Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Kohn P., Mervis C.B., Kippenhan J.S., Olsen R.K., Morris C.A., Berman K.F. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Mimura M., Hoeft F., Kato M., Kobayashi N., Sheau K., Piggot J., Mills D., Galaburda A., Korenberg J.R., Bellugi U., Reiss A.L. A preliminary study of orbitofrontal activation and hypersociability in Williams syndrome. J. Neurodev. Disord. 2010;2:93–98. doi: 10.1007/s11689-009-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Eckert M.A., Mills D., Korenberg J., Bellugi U., Galaburda A.M., Reiss A.L. Frontostriatal dysfunction in Williams syndrome. Biol. Psychiatry. 2007;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Munoz K.E., Meyer-Lindenberg A., Hariri A.R., Mervis C.B., Mattay V.S., Morris C.A., Berman K.F. Abnormalities in neural processing of emotional stimuli in Williams syndrome vary according to social vs. non-social content. Neuroimage. 2010;50:340–346. doi: 10.1016/j.neuroimage.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B.M., Snyder A.Z., Haist F., Raichle M.E., Bellugi U., Stiles J. Amygdala response to faces parallels social behavior in Williams syndrome. Soc. Cogn. Affect. Neurosci. 2009;4:278–285. doi: 10.1093/scan/nsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M.A., Coltheart M., Langdon R. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. 2007;45:2839–2849. doi: 10.1016/j.neuropsychologia.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Reznick J.S., Hegeman I.M., Kaufman E.R., Woods S.W., Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Dev. Psychopathol. 1992;4:301–321. [Google Scholar]
- Riby D.M., Doherty-Sneddon G., Bruce V. Exploring face perception in disorders of development: evidence from Williams syndrome and autism. J. Neuropsychol. 2008;2:47–64. doi: 10.1348/174866407x255690. [DOI] [PubMed] [Google Scholar]
- Riby D.M., Doherty-Sneddon G., Bruce V. Atypical unfamiliar face processing in Williams syndrome: what can it tell us about typical familiarity effects? Cognit. Neuropsychiatry. 2010;13:47–58. doi: 10.1080/13546800701779206. [DOI] [PubMed] [Google Scholar]
- Rohrbacher H., Hoyer J., Beesdo K., Hofler M., Bittner A., Lieb R., Wittchen H.U. Psychometric properties of the Retrospective Self Report of Inhibition (RSRI) in a representative German sample. Int. J. Methods Psychiatr. Res. 2008;17:80–88. doi: 10.1002/mpr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Snidman N., Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child Adolesc. Psychiatr. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Setter J., Stojanovik V., Van Ewijk L., Moreland M. Affective prosody in children with Williams syndrome. Clin. Linguist. Phon. 2007;21:659–672. doi: 10.1080/02699200701539056. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Mind Garden; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory (Form Y) [Google Scholar]
- Spitzer R.L., Williams J.B.W., Gibbon M., First M.B. The Structured Clinical Interview for DSM-III-R (SCID). 1. History, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H., Plesa-Skwerer D. Social engagement in Williams syndrome. In: Marshall P.J., Fox N.A., editors. The Development of Social Engagement: Neurobiological Perspectives. Oxford University Press; New York: 2010. pp. 331–334. [Google Scholar]
- Turner S.M., Beidel D.C., Dancu C.V. Multi-Health Systems; North Tonawanda, NY: 1996. SPAI: Social Phobia and Anxiety Inventory. [Google Scholar]
- Tzourio-Mazoyer N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Udwin O., Yule W. Expressive language of children with Williams Syndrome. Am. J. Med. Genet. 1990:108–114. doi: 10.1002/ajmg.1320370620. [DOI] [PubMed] [Google Scholar]
- Udwin O., Yule W. A cognitive and behavioral-phenotype in Williams syndrome. J. Clin. Exp. Neuropsychol. 1991;13:232–244. doi: 10.1080/01688639108401040. [DOI] [PubMed] [Google Scholar]
- Wong D.L., Baker C.M. Pain in children: comparison of assessment scales. Pediatr. Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- Yang T.T., Menon V., Eliez S., Blasey C., White C.D., Reid A.J., Gotlib I.H., Reiss A.L. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]