Abstract
Poly(D,L-lactic-co-glycolic acid) (PLGA) is a biodegradable polymer that is widely used for drug delivery. However, the degradation of PLGA alters the local microenvironment and may influence tissue structure and/or function. Here, we studied whether PLGA degradation affects the structure of the arteriolar microcirculation through arteriogenic expansion of maximum lumenal diameters and/or the formation of new smooth muscle-coated vessels. Single microspheres comprised of 50:50 PLGA (521 ± 52.7 μm diameter), 50:50 PLGA with bovine serum albumin (BSA) (547 ± 62.2 μm), 85:15 PLGA (474 ± 52.6 μm), or 85:15 PLGA with BSA (469 ± 57.2 μm) were implanted into mouse dorsal skinfold window chambers, and longitudinal arteriolar diameter measurements were made in the presence of a vasodilator (10−4M adenosine) over 7 days. At the end of the 7-day period, the length density of all smooth muscle-coated microvessels was also determined. Implantation of the window chamber alone elicited a 22% increase in maximum arteriolar diameter. However, the addition of 85:15 and 50:50 PLGA microspheres, bearing either BSA or no protein, elicited a significant enhancement of this arteriogenic response, with final maximum arteriolar diameters ranging from 36 to 46% more than their original size. Interestingly, the influence of PLGA degradation on microvascular structure was limited to lumenal arteriolar expansion, as we observed no significant differences in length density of smooth muscle-coated microvessels. We conclude that the degradation of PLGA microspheres may elicit an arteriogenic response in subcutanteous tissue in the dorsal skinfold window chamber; however, it has no apparent effect on the total length of smooth muscle-coated microvasculature.
Keywords: microcirculation; arteriogenesis; angiogenesis; poly(D,L-lactic-co-glycolic acid); biomaterials
INTRODUCTION
The therapeutic stimulation of arteriogenesis, which is defined as the structural enlargement of arterial diameter, holds promise as a treatment for patients suffering from cardiovascular disease who are not amenable to surgical revascularization.1,2 Numerous investigations have been conducted using gene, protein, or cell therapies to generate therapeutic arteriogenesis in animals. However, to date, the translation of these results to clinical trials has only met with limited success.3–5 With respect to the strategies in which recombinant pro-arteriogenic growth factor proteins are used to stimulate arteriogenesis, achieving sustained delivery and maintaining growth factor bioactivity are significant challenges.
It is possible, however, that these challenges may be overcome by incorporating recombinant growth factors into controlled-release biodegradable polymers that permit the sustained and targeted delivery of biologically stable therapeutic agents, allowing for both temporal and spatial control. For example, poly (D,L-lactic-co-glycolic acid) (PLGA) is a degradable polymer that is both biocompatible and approved by the FDA for therapeutic use. The degradation rate of PLGA, which determines the release rate of an encapsulated protein, may be tuned by the ratio of lactic acid to glycolic acid. Importantly, PLGA has already been used in several animal studies to deliver growth factors and generate neovascularization in different applications.6–10
However, some key questions about the use of PLGA for vascular growth factor delivery have not been explored in detail. For example, it is known that the degradation of PLGA generates an acidic microenvironment that can influence the structure and function of surrounding tissue7,11–14; however, the influence of PLGA on the structural remodeling of existing arterioles is not well-understood and is perhaps best addressed using an animal model that permits the longitudinal observation of individual arterioles over time. The murine dorsal skinfold window chamber model is ideally suited for this purpose because it allows for noninvasive and repeated imaging of the same blood vessels over time.15,16 Of particular relevance to the current study, this model has been used to study the influence of slow degrading 80:20 PLGA scaffolds on angiogenesis, defined as the growth of new microvessels.15 However, the influence of PLGA degradation on the arteriogenic remodeling of individual pre-existing arterioles has yet to be examined.
The goal of this study was, therefore, to determine how the degradation of PLGA microspheres affects the structural lumenal enlargement of existing arterioles in the dorsal skinfold window chamber model. In addition, using whole-mount immunochemistry for SM α-actin, we also studied how PLGA affects the formation and/or lengthening of smooth muscle-coated vessels.
MATERIALS AND METHODS
PLGA microsphere fabrication
Using a single emulsion solvent extraction technique, 250 mg of 85:15 or 50:50 PLGA was dissolved in 1 mL methlyene chloride by vortexing at a medium level for 15 min. For BSA-loaded microspheres, 1mg of bovine serum albumin (BSA) was added to the polymer solution and vortexed at a medium level for an additional 5 min. The polymer solution was slowly added to vigorously stirred (250 rpm) 1% poly-vinyl alcohol and left for 5 h. The resultant microspheres were washed with deionized water, dessicated for 48 h and stored at −80°C.
Profile of protein release from PLGA microspheres
BSA was tagged with rhodamine using an EZ-Label Rhodamine Protein Labeling Kit (Pierce Biotechnology). Rhodamine-labeled BSA microspheres were made following the microsphere fabrication protocol described earlier. Loading efficiency was determined by dissolving 10 mg of the microspheres in 1 mL of methylene chloride, using a spectrophotometer to determine light absorption, and comparing this against standards with a known BSA concentration. Rhodamine-labeled BSA microspheres were then used to generate a release curve for both 85:15 and 50:50 PLGA microspheres. Three milligrams of microspheres were suspended in 1mL of simulated body fluid (SBF)17 and incubated at 37°C. Over a 25 day time period, 100 μL was removed periodically for analysis and a spectrophotometer was used to read fluorescence. The remaining solution was removed and fresh SBF was added to the microspheres. Cumulative fluorescence for each timepoint was calculated and converted to a concentration. On the basis of the data from the loading efficiency study, the concentration at each timepoint was divided by total concentration to generate a percent cumulative release curve.
Dorsal skinfold window chamber
All animal studies were approved by the Animal Research Committee at the University of Virginia and conformed to the American Heart Association Guidelines for the Use of Animals in Research. Thirty-one C57/BL6 male mice weighing between 25 and 30 g were randomly divided into five groups [Untreated (n = 6), 85:15 PLGA (n = 6), 85:15 PLGA-BSA (n = 6), 50:50 PLGA (n = 7), and 50:50 PLGA-BSA (n = 6)] and anesthetized with an intraperitoneal injection of ketamine (0.03 mL), xylazine (0.015 mL), and atropine (0.005 mL) diluted in 0.2 mL of saline. Under sterile conditions, a circular section 12 mm in diameter was surgically excised from the dorsal side of the mouse exposing the microcirculation deep in the dermis. A dorsal skin-fold window chamber was then surgically implanted around the dissected circle of tissue as illustrated in Figure 2(A). After the window chamber had been sutured to the skin, the exposed microcirculation was carefully superfused with sterile Ringer’s solution and then covered with a round coverglass. Mice were allowed 6 days to recover from surgery before PLGA microsphere implantation.
Figure 2.
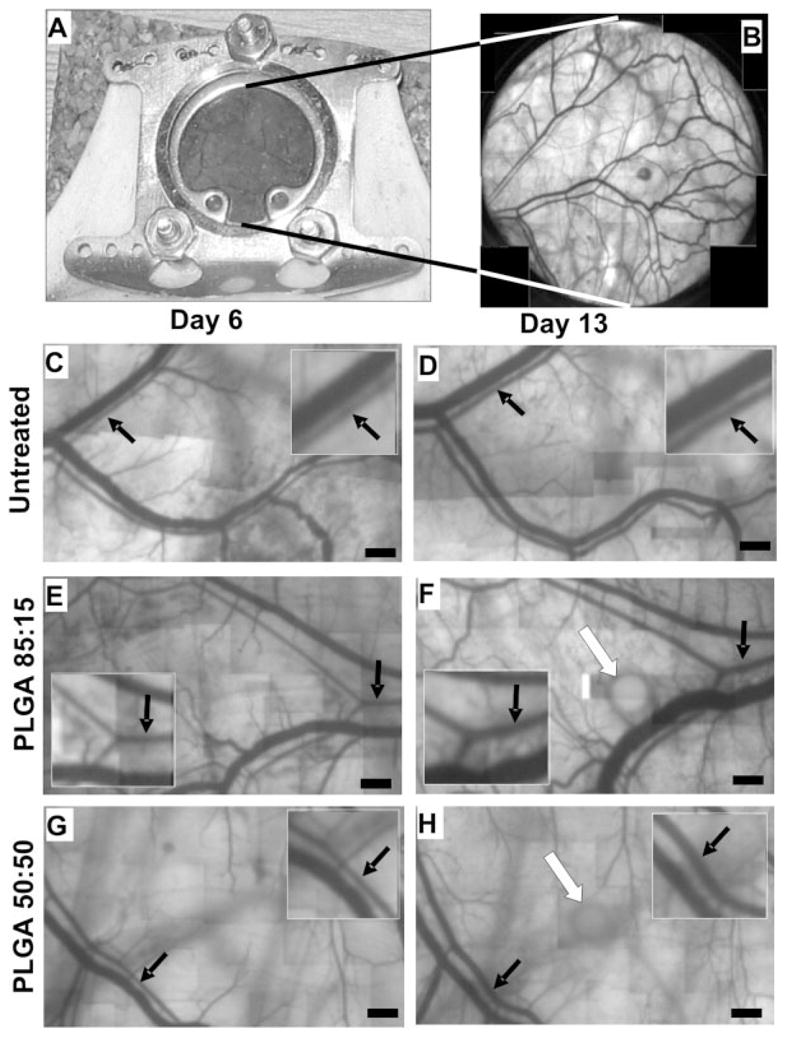
Intravital microscopy images from mouse dorsal skinfold window chambers illustrating the influence of PLGA degradation on arteriolar remodeling. A: Implanted dorsal skinfold window chamber. B: Low magnification (×2.5) outset showing subcutaneous microcirculation. C–H: Representative images of microvessels in dorsal skinfold window chambers of untreated (C,D), 85:15 microsphere (E,F) and 50:50 microsphere (G,H) treated mice at days 6 (C,E,G) and 13 (D,F,H). Black arrows indicate selected arterioles undergoing diameter enlargement. Insets in panels (C-H) are higher magnification images showing the arterioles highlighted with black arrows in the corresponding larger panel. Note that the arterioles in the PLGA microsphere-treated windows undergo a greater increase in diameter when compared with untreated controls. White arrows (F and H) depict PLGA microspheres. PLGA microspheres were occasionally visible during tissue imaging, even though they were situated above the plane of focus. Bars = 400 μm.
Microsphere implantation and noninvasive intravital image acquisition
At each time point, mice were anesthetized using 2.5% isoflurane, and the coverglass was removed from the window chamber to allow access to the exposed subcutaneous microcirculation. On day 6, a location for the microsphere in the immediate vicinity of at least one arteriole/venule pair was selected. The window was topically superfused with adenosine (10−4M) to maximally vasodilate the microcirculation. A Zeiss Axioscope and an Olympus Digital Camera were used to image the entire window (×2.5), and the region of tissue within 5 mm of where the microsphere was about to be implanted (×10). After imaging was completed, the adenosine was suctioned off and the window was twice flushed with Ringer’s solution. Using sterile forceps, a single microsphere with a composition corresponding to the group in which the mouse had been assigned (i.e., 85:15 PLGA, 85:15 PLGA-BSA, 50:50 PLGA, or 50:50 PLGA-BSA) was inserted at the previously selected location and allowed to adhere to the tissue for approximately 10 min. No microsphere was implanted for the untreated control group. At the end of this time, the window was saturated with Ringer’s solution and the coverglass was reattached. On days 9 and 13, the window and region of interest were imaged as described earlier.
Immunochemical labeling for smooth muscle α-actin
After imaging on day 13, animals were euthanized and perfusion fixed by cardiac puncture with 4% paraformaldehyde in PBS. The window chamber tissue was then harvested, treated with 3 mg/mL collagenase for 30 min to facilitate antibody access, and incubated overnight with 1:200 IA4 monoclonal anti-α-smooth muscle actin (SMA) Cy3 conjugate (Sigma).
Microvascular remodeling measurements
Digital images were assembled into a montage. Image J software (NIH) was used to analyze all images. Arteriolar structural diameter measurements were made along vessels present in the region of interest at both day 6 and 13. All visible vessels stained positive for SMA were imaged and traced. The length density metric was generated by normalizing the total length of SMA+ microvessel in the region of interest to the total en-face surface area of the region of interest.
Statistics
All data are presented as means ± SE. All data were first tested for normality. Arteriolar diameter changes [Fig. 3(A, B)] and the length-density of smooth muscle-coated vessels [Fig. 4(D)] were analyzed by One-Way Analysis of Variance (ANOVA) followed by pairwise comparisons with Tukey’s t-tests. All statistical analyses were performed using SigmaStat software. Significance was assessed at p < 0.05.
Figure 3.
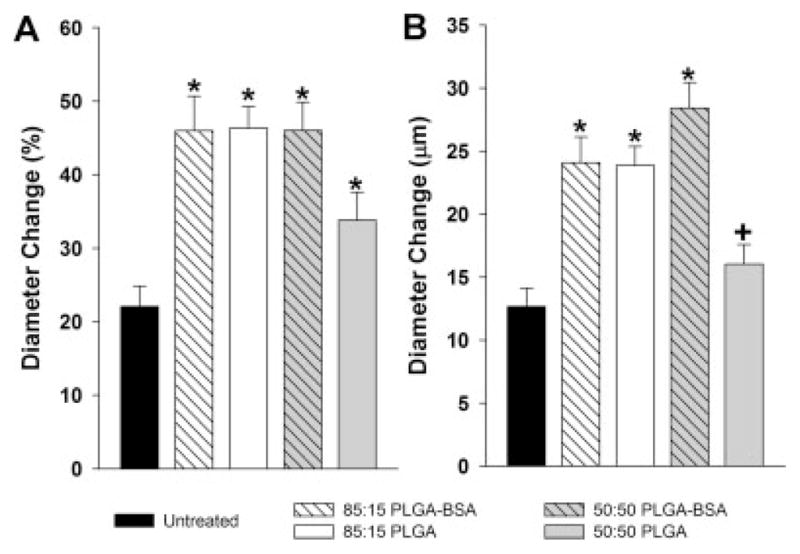
Bar graphs showing arteriolar diameter changes in untreated control, BSA-loaded 85:15 PLGA, 85:15 PLGA, BSA-loaded 50:50 PLGA, and 50:50 PLGA-treated mice. A: Increase in arteriolar diameter from day 6 to 13 as a percentage, where day 6 is the day of microsphere implantation. B: Absolute increases in diameter from day 6 to 13. *indicates significantly different (p < 0.05) than untreated control. +indicates significantly different (p < 0.05) than 50:50 PLGA+BSA.
Figure 4.
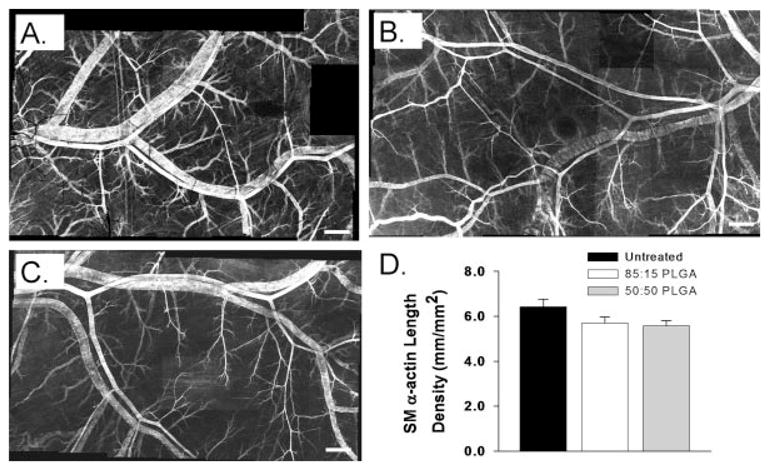
A–C: Confocal microscopic images of dorsal skinfold window chamber tissues from untreated (A), 85:15 PLGA (B), and 50:50 PLGA (C) mice that have been immunostained as whole-mounts for SM α-actin at day 13. Bars indicate 500 μm. D: Length densities of smooth muscle-coated microvessels in the region of interest at day 13 in untreated, 85:15 PLGA, and 50:50 PLGA-treated microvascular networks. No significant differences were observed between groups.
RESULTS
Profile of BSA release from PLGA microspheres
To determine the loading efficiency and release rate, rhodamine-labeled BSA was loaded into both 85:15 (slow degrading) and 50:50 (fast degrading) PLGA microspheres. Loading efficiency was determined to be 45% for 85:15 PLGA microspheres and 63% for 50:50 PLGA microspheres (data not shown). The cumulative release rate curve showed that after seven days, 1.5% of total protein was released from 85:15 PLGA microspheres, and 11% of total protein was released from 50:50 PLGA microspheres (Fig. 1).
Figure 1.
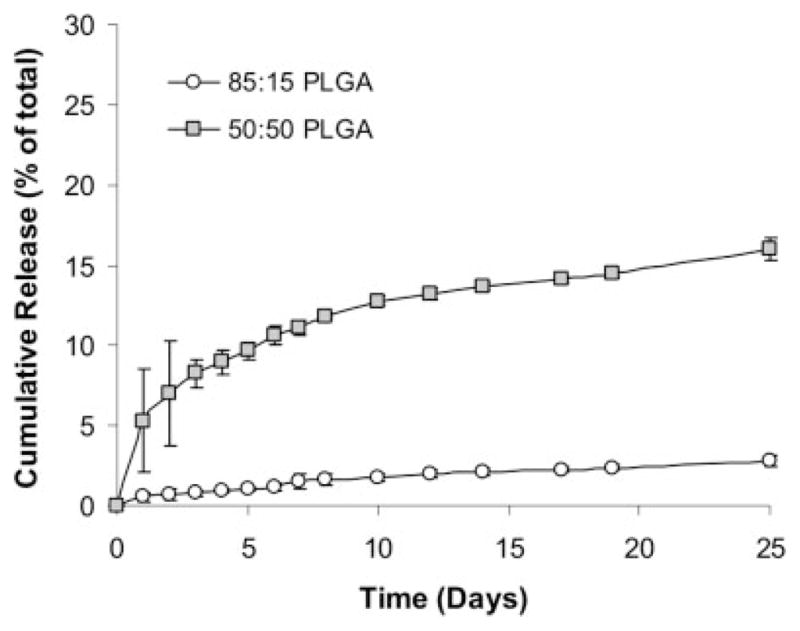
Line graph illustrating the cumulative release of BSA from 85:15 and 50:50 PLGA microspheres over a period of 25 days. Note that, as expected, the 50:50 formulation degrades more rapidly than the 85:15 formulation.
Arterioles in untreated control microvascular networks exhibit a moderate arteriogenesis response
Implantation of a window chamber on the mouse dorsal skinfold [Fig. 2(A)] permitted intravital observation of the subcutaneous microcirculation [Fig. 2(B)]. Microvascular networks in the untreated control group were imaged on days 6, 9, and 13 and longitudinal diameter measurements were made on selected arterioles within a ~5 mm diameter region of interest [Fig. 2(C, D)]. Implantation of the window chamber alone caused arteriolar diameter to increase by 9% (4 μm) from day 6 to 9 and by 13% (9 μm) from day 9 to 13 (data not shown). Over the entire seven day time course, the average arteriolar diameter increased by 22% (13 μm) [Fig. 3(A,B)].
Arterioles in microvascular networks exposed to PLGA microspheres exhibit a significantly enhanced arteriogenesis response
Following the same protocol as for the untreated mice, we waited six days after surgery to implant either a single PLGA microsphere or a single BSA-loaded PLGA microsphere in the region of interest (Fig. 2). Mean implanted microsphere diameters (±SD) were 521 ± 52.7 μm (50:50 PLGA), 547 ± 62.2 μm (50:50 PLGA-BSA), 474 ± 52.6 μm (85:15 PLGA), or 469 ± 57.2 μm (85:15 PLGA-BSA). All four PLGA microsphere treatment groups exhibited statistically significant increases in maximum arteriolar diameter over the untreated control group [Fig. 3(A)]. Specifically, for the 85:15 PLGA, 85:15 PLGA-BSA, and 50:50 PLGA-BSA groups, changes in maximum arteriolar diameter were more than twofold greater than in untreated controls. Maximum arteriolar diameter changes were not as pronounced for the 50:50 PLGA group; however, they were still 64% greater than untreated controls. Similar results were also observed when diameter changes were considered as absolute differences [Fig. 3(B)]. Here, of the four treatment groups, only the 50:50 PLGA microsphere group did not exhibit a statistically significant increase over untreated controls. On average, the three groups that did show significance exhibited diameter changes that were ~ 10 to 15 μm greater than untreated controls.
PLGA microspheres do not elicit statistically significant changes in smooth muscle-coated microvessel length density
To determine the length density of vessels positive for SM α-actin (i.e. smooth muscle-coated microvesels), windows were harvested on day 13, immunostained for SM α-actin, and all visible vessels in the region of interest were traced. The total length of SM α-actin+ microvessels was then normalized to the area of the region of interest. This metric was generated for the untreated control, 85:15 PLGA, and 50:50 PLGA groups. No significant differences in the length density of smooth muscle-coated microvessels were observed (Fig. 4) when considering statistical significance at p < 0.05. However, the 50:50 PLGA group was significantly less than the untreated control group at p < 0.07, whereas the 85:15 PLGA was significantly less than the untreated controls at p < 0.15.
DISCUSSION
The purpose of this study was to determine whether the degradation of PLGA, a biodegradable polymer that is finding utility as a fundamental component of vascular growth factor delivery systems, affects arteriogenic remodeling in the murine dorsal skinfold window chamber model. Our central finding is that, indeed, the degradation of both fast (50:50) and slow (85:15) degrading PLGA microspheres can elicit the structural lumenal enlargement of arterioles in surrounding dermal tissue. These increases in arteriolar diameter are not, however, accompanied by statistically significant changes in the total length of smooth muscle-coated microvessels. Nonetheless, these length density results should be interpreted carefully because, when considered at p < 0.07, the 50:50 PLGA group actually exhibited a surprising decrease in length density when compared with the untreated control group.
Dorsal skinfold window chamber model
The dorsal skinfold window chamber was chosen for these studies because it permits the longitudinal intravital observation of individual microvessels through time. Therefore, the data presented in Figure 3 are particularly robust and sensitive because the differences were generated from repeated diameter measurements at specific sites in the arteriolar network. In addition, all measurements were taken after the microcirculation had been maximally vasodilated via superfusion of the tissue with Ringer’s solution containing 10−4M adenosine. Because this step was taken, we are confident that the increase in arteriolar diameters observed here was caused by changes in arteriolar wall structure and not by differences in arteriolar vasoactivity elicited by PLGA degradation.
In the context of the current study, another interesting observation of the dorsal skinfold window chamber model was that implantation of the chamber itself caused a mean structural arteriolar diameter increase of 22%. Surgical implantation of the window chamber causes inflammation in the tissue and likely alters local microvascular hemodynamics. Both of these factors are known to affect arteriogenesis,18–20 therefore it was perhaps not surprising that arteriogenic remodeling was observed. Indeed, we have recently shown that the deletion of C-Chemokine Receptor-2 (CCR2), which is the receptor for the inflammatory cytokine Monocyte Chemotactic Protein-1 (MCP1), from bone marrow-derived cells abolished this baseline arteriogenic response.21 However, it is still unclear as to whether the requirement of CCR2 expression by bone marrow-derived cells was linked to hemodynamic changes and/or to inflammation directly; therefore, there is some uncertainty as to the source of the baseline arteriogenic remodeling in this model. Nonetheless, in this application, the baseline arteriogenesis also conferred some advantage because it mimicked the response that would be created by a growth factor encapsulated within the PLGA microspheres; therefore, the influence of PLGA degradation on arteriogenesis was tested under microvessel remodeling conditions that appropriately simulated the intended therapeutic application.
Comparisons with other studies
Although the direct influence of PLGA degradation on structural lumenal expansion of existing arterioles has not, to our knowledge, been directly investigated before this study, the influence of PLGA scaffold degradation on perfusion restoration in the ischemic hindlimb has been reported by Sun et al.9 Because perfusion restoration in this model has been largely attributed to lumenal arteriogenic remodeling,22 their data can be, at least indirectly, compared with Figure 3. In apparent contrast with our results, Sun et al.9 found no changes in perfusion with PLGA implantation alone, which would suggest that arteriogenesis did not occur. There are, however, many differences between our study and that of Sun et al.,9 therefore it is not difficult to explain this discrepancy. Indeed, significant differences in tissue type, animal model, PLGA mass, and perhaps distance between the implant and the measurement site exist between the two studies. Furthermore, Sun et al.9 did not measure perfusion in the presence of a vasodilator, therefore potential differences in lumenal structure may have been masked by different levels of vascular tone.
In addition, the influence of PLGA degradation on the number of smooth muscle-coated vessels per unit area (i.e. smooth muscle-coated vessel density) in surrounding tissue has been studied.6,9 In agreement with our results reported in Figure 4, the implantation of a scaffold comprised of 85:15 PLGA exerted no change in the density of smooth muscle-coated vessels. Unfortunately, Sun et al.9 did not assess the diameters of these vessels, therefore it is not possible to directly compare their results with Figure 3. Richardson et al.6 reported smooth muscle-coated vessel density in muscle tissue surrounding a blank PLGA-based scaffold; however, no values were given for completely uninstrumented tissue, therefore the influence of the scaffold itself cannot be assessed.
Our study was designed to reveal if differences in PLGA degradation rate affect arteriogenesis, with the results indicating that, in the absence of an encapsulated protein, slow (85:15) and fast (50:50) degrading PLGA elicit different absolute changes in maximum arteriolar diameter [Fig. 3(B)]. To our knowledge, the effect of PLGA degradation rate on arteriogenesis has not been studied; however, Sung et al.7 have examined the related process of angiogenesis, which refers to the growth of new capillaries from existing vessels, within slow and fast degrading scaffolds in the absence of an encapsulated protein. Although the considerable differences between our study and that conducted by Sung et al.7 make it quite possible that their observations were created by quite different mechanisms, Sung et al.7 did show that a slower degradation rate can lead to an enhanced remodeling response, thereby generally supporting our observations.
Putative mechanisms for the arteriogenesis response
Although our study did not explicitly investigate the mechanisms through which arteriogenic responses are created by PLGA microspheres, we hypothesize that PLGA degradation creates an acidic microenvironment that, in turn, affects morphogenic vascular cell behaviors and stimulates arteriogenesis. Indeed, although limited, there is evidence linking acidic conditions to blood vessel remodeling. For example, the presence of an acidic microenvironment has been shown enhance the expression of both vascular endothelial growth factor and fibroblast growth factor 2 in vitro.23 In addition, more recently, it has been shown that the deletion of a pH-sensing G-Protein coupled receptor (GPR4) during development creates a phenotype that is indicative of poor vessel maturation.24 Importantly, vessel maturation involves the proliferation and recruitment of mural cells (i.e. smooth muscle cells and pericytes), behaviors that are also critical for arteriogenesis. Therefore, it is reasonable to postulate that an acidic microenvironment could enhance GPR4 signaling which, in turn, could lead to the enhanced arteriogenesis via the proliferation and/or recruitment of smooth muscle.
Limitations of the study
Our results indicate that the degradation of PLGA microspheres can amplify arteriogenesis in the dorsal skinfold window chamber; however, our study has some limitations, therefore these results should be interpreted carefully. First, while the dorsal skinfold window chamber is ideal for making repeated measurements, the stimuli for the baseline arteriogenesis response generated in this model, beyond the requirement of CCR2 expression by bone marrow-derived cells, are not well understood. Furthermore, the remodeling response of the arterioles in subcutaneous tissue may not be representative of skeletal and cardiac muscle, which are the tissues in which therapeutic arteriogenesis is most commonly attempted. Second, although the creation of an acidic microenvironment is a likely explanation for the observed response, the molecular and cellular mechanisms linking PLGA degradation to arteriogenesis were not elucidated. Finally, while our observations indicate that PLGA degradation causes an increase in maximum arteriolar diameter, it is important to note that we did not assess the influence of PLGA degradation on arteriolar wall structure. Therefore, potential changes in wall thickness due to altered smooth muscle proliferation, smooth muscle hypertrophy, and/or extracellular matrix deposition were not assessed.
References
- 1.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 3.Baklanov D, Simons M. Ateriogenesis: Lessons learned from clinical trials. Endothelium. 2003;10:217–223. doi: 10.1080/10623320390246397. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, Stegmann TJ, Udelson JE, Rosengart TK. Clinical trials in coronary angiogenesis: Issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 5.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, Pratt C, Kleiman N, Group AGT. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 6.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 7.Sung H-K, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharmaceutical Research. 2005;22:1110–1116. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide-co-glycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Controlled Release. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 11.Barbensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv Drug Deliver Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 12.Lewandorski KU, Grosser JD, Wise DL, Trantolo DJ, Hasirci V. Tissue responses to molecularly reinforced polylactide-co-glycolide implants. J Biomater Sci Polymer Ed. 2000;11:401–414. doi: 10.1163/156856200743788. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal MC, Anthanasiou KA. Technique to control pH in vicinity of biodegradable PLA-PGA implants. J Biomed Mater Res. 1997;38:105–114. doi: 10.1002/(sici)1097-4636(199722)38:2<105::aid-jbm4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 15.Rücker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mülhaupt R, Gellrich N-C, Menger MD. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27:5027–5038. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, Vujaskovic Z, Dewhirst MW, Arcasoy MO. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS One. 2007;2(6):e549. doi: 10.1371/journal. pone. 0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 18.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 19.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92:929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 20.Price RJ, Less JR, Van Gieson EJ, Skalak TC. Hemodynamic stresses and structural remodeling of anastomosing arteriolar networks: Design principles of collateral arterioles. Microcirculation. 2002;9:11–124. doi: 10.1038/sj/mn/7800127. [DOI] [PubMed] [Google Scholar]
- 21.Nickerson MM, Owens GK, Skalak TC, Price RJ. Arteriogenesis and microvascular capacitance in the mouse dorsal skin-fold window are dependent on CCR2 expression by bone marrow-derived cells. Eighth World Congress of Microcirculation; Milwaukee, WI. 2007. [Google Scholar]
- 22.Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol. 2002;283:H2012–H2020. doi: 10.1152/ajpheart.00257.2002. [DOI] [PubMed] [Google Scholar]
- 23.D’Arcangelo D, Facchiano F, Barlucchi LM, Melillo G, Illi B, Testolin L, Gaetano C, Capogrossi MC. Acidosis inhibits endothelial cell apoptosis and function and induces basic fibroblast growth factor and vascular endothelial growth factor expression. Circ Res. 2000;86:312–318. doi: 10.1161/01.res.86.3.312. [DOI] [PubMed] [Google Scholar]
- 24.Yang LV, Radu CG, Roy M, Lee S, McLaughlin J, Teitell MA, Iruela-Arispe ML, White ON. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol Cell Biol. 2007;27:1334–1347. doi: 10.1128/MCB.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


