Abstract
The contribution of nitric oxide (NO) to the pathophysiology of asthma remains incompletely defined despite its established pro- and anti-inflammatory effects. Induction of the inducible nitric oxide synthase (iNOS), arginase and superoxide pathways is correlated with increased airway hyperresponsiveness (AHR) in asthmatic subjects. To determine the contributions of these pathways in proximal and distal airways, we compared bronchial wash (BW) to traditional bronchoalveolar lavage (BAL) for measurements of reactive nitrogen/oxygen species, arginase activation, and cytokine/chemokine levels in asthmatic and normal subjects. Levels of NO were preferentially elevated in the BAL, demonstrating higher-level NOS activation in the distal airway compartment of asthmatic subjects. In contrast, DHE+ cells which have the potential to generate reactive oxygen species were found to be increased in both proximal and distal airway compartments of asthmatics compared to controls. Different patterns of cytokines and chemokines were observed, with a predominance of epithelial cell-associated mediators in the BW as compared to macrophage/monocyte-derived mediators in the BAL of asthmatic subjects. Our study demonstrates differential production of reactive species and soluble mediators within the distal airways as compared to the proximal airways in asthma. These results indicate that cellular mechanisms are activated in the distal airways of asthmatics and must be considered in the development of therapeutic strategies for this chronic inflammatory disorder.
Keywords: Nitric oxide, superoxide, arginase, bronchoalveolar lavage, bronchial wash, distal airway
INTRODUCTION
Asthma is a heterogeneous clinical syndrome characterized by intermittent reversible airflow obstruction, airway hyperresponsiveness (AHR), and infiltration of inflammatory cells [1, 2]. Historically, asthma has been conceptualized as a disease of the larger conducting airways with bronchospasm and overproduction of mucus as the primary contributors to clinical symptoms [3]. Recent studies, however, have demonstrated that innate and adaptive immune cells that are central to asthma pathogenesis are increased in both the larger proximal and smaller distal airways, suggesting that inflammation is present throughout the airway in asthmatics [1, 4]. The enrichment of Th2 cytokine mRNA in distal airways further supports a significant role for chronic inflammation of the distal respiratory tract in this disease [1, 4].
Increased levels of reactive nitrogen species (RNS), including nitric oxide (NO), have been observed in exhaled air and bronchoalveolar lavage (BAL) fluid from asthmatics [5, 6]. Reactive oxygen species (ROS), including superoxide (O2•−), are also elevated in BAL cells of asthmatics [7–9]. These reactive species have also been observed in subjects with other inflammatory disorders of the lung [10, 11]. Although both the constitutive nitric oxide synthases (eNOS and nNOS) and the inducible form of the enzyme (iNOS) are expressed in the airway, NO produced by iNOS is at higher concentrations and correlates with asthmatic inflammation, epithelial injury and clinical exacerbations [8, 12–14].
Several studies have also supported a protective function for NO based, at least in part, on its bronchodilatory activity [12–14]. Both pro-inflammatory and anti-inflammatory actions have been seen after allergen challenge and correlate with the up-regulation of iNOS activity [12–14]. It has been postulated that the pro-inflammatory effects of NO are mediated by peroxynitrite, a product of the high reactivity of NO with O2•− [15, 16]. Therefore, it is currently unclear whether NO is primarily a positive effector of inflammation, whether it is primarily generated to attenuate the inflammatory response in asthma, or whether its actions are determined by the balance of its pro- and anti-inflammatory effects.
Superoxide produced via NADPH oxidase [17] has also been shown to contribute to inflammatory responses in the lung with the formation of hydrogen peroxide and/or peroxynitrite in association with the activation of lymphocytes [7, 17, 18] and increased AHR [7]. Additionally, induction of the activity of arginase, a key enzyme in the hepatic urea cycle that is also expressed in leukocytes at sites of inflammation, correlates with asthma exacerbations and AHR; furthermore, down-stream products of arginase, in particular polyamines and L–proline, are associated with enhanced airway remodeling [19].
Studies to date have not demonstrated whether these inflammatory mediators exert their effects in the proximal or distal airway compartments. Most studies have sampled the proximal and distal airways together via a large-volume BAL (instillation of 200 ml of saline) [20, 21] or sampled the proximal airway wall and parenchyma by bronchial biopsies (18); in contrast, a smaller volume bronchial wash (BW; 20–60 ml instillation) preferentially samples the contents of the proximal airway [2, 20, 22].
In this study, we compared cellular constituents, levels of reactive nitrogen and oxygen species and levels of cytokines/chemokines in proximal vs. distal airway samples from asthmatic and normal subjects. We report here that proximal and distal airway compartments of the asthmatic airways demonstrate distinct cytokine/chemokine profiles; additionally, we observed epithelial cell enrichment in proximal airway sampling. We further provide evidence that elevated concentrations of the metabolites of iNOS are localized to the smaller distal airway while cells with the potential to produce ROS are present in both the proximal and the distal airway compartments.
METHODS
Patient Recruitment
Asthmatics (n=8) and normal control subjects (n=8) age 25–65 were recruited through the University of Alabama at Birmingham (UAB) Lung Health Center (Table I). Control subjects had no history of lung disease or respiratory symptoms and had normal lung function as assessed by spirometry. Asthmatics carried a clinical diagnosis and had mild disease as defined by GINA guidelines [23]. They did not require the use of oral or inhaled corticosteroids to maintain symptom control. Subjects were not eligible if they were current smokers or had a greater than 10 pack-year smoking history. This study was approved by the Institutional Review Board at UAB (Protocol # F090116008). Written informed consent was obtained from all participants in this study.
Table 1.
| Normal | Asthmatic | ||
|---|---|---|---|
| n=8 | n=8 | ||
| Mean Age | 51±10 | 50±11 | p=0.85 |
| Male:Female | 3:5 | 1:7 | |
| Caucasian:African American | 1:4 | 1:4 | |
| Former Smoker | 0% | 37.5% | |
| Never Smoker | 100% | 62.5% | |
| Post-Bronchodilator FEV1% | 92% | 89% | |
| BUN | 13.1±3.2 | 10.8±3.7 | p=0.18 |
| BW Return | 3.9±1.4 ml | 4.1±1.5 ml | p=0.79 |
| 1st returned BAL | 11±3.0 ml | 10.9±3.7 ml | p=0.94 |
Collection of airway samples
Airway contents were sampled via fiberoptic bronchoscopy using topical anesthesia to the upper and lower airways (1% lidocaine 200–300 mg) under conscious sedation (intravenous midazolam 1–3 mg and meperidine 50–100 mg). The bronchoscope was wedged in a segmental bronchus of the right middle lobe in the 4th or 5th generation airway as is typical for traditional Bronchoalveolar Lavage (BAL). A bronchial wash (BW) was performed first by instillation of 10 ml of sterile saline warmed to 37°C and then by immediately aspirating fluid to an average return of 3–5 ml by syringe suction followed by storage on ice. This BW procedure is similar to the procedures reported by Grootendorst and colleagues [2] and Keatings and colleagues [22], except that we used a smaller volume of wash fluid in order to limit the sampling to the more proximal portions of the airway and to minimize dilution of the bronchial airway contents. Without moving the bronchoscope, BAL was then performed using 6 aliquots of warmed sterile saline (33 ml each; average recovery 90–120 ml total) and the collected fluid was immediately stored on ice (20, 21). Airway cells and lavage fluids were separated by centrifugation (450 × g, 10 min, 4°C). To minimize dilution of reactive species and other inflammatory mediators from the distal airways, we used the returned fluid from the first 33 ml lavage for the quantitation of the metabolites of NO, urea (as a measure of arginase activity) and cytokines/chemokines. The chelating agent diethylenetriamene pentaacetate (DTPA at 100 μM) was added to the cell-free fluid from the BW and the returned fluid from the first aliquot of the BAL and these samples were stored at −80°C until further analysis was performed. In order to maximize the return of cells from the distal airways of each subject, cells from all of the aliquots of returned BAL fluid were pooled and analyzed together. Both cells and fluids were assayed for metabolites of the free radical pathways by colorimetric assays and by using flow cytometry with fluorescent indicator dyes.
ELISA for detection of SP-D and RAGE
Cell-free BW and BAL fluid from both study groups were prepared and measurements were made using ELISA kits for surfactant protein–D (SP-D; Biovendor, Candler, NC) and receptor for advanced glycation end products (RAGE; R & D systems, Minneapolis, MN) following the manufacturers’ recommendations.
Measurement of nitrite
For detection of metabolites of NO (NOx), majorly nitrite and nitrate as described in the result section, by tri-iodide dependent chemiluminiscence, cell-free BW and the return of the first BAL fluid were incubated for 20 min at room temperature (RT) in the dark with or without 0.5% w/v sulfanilamide (stock in 2.5 M HCl) and with or without 0.2% w/v HgCl2. Samples were then injected into a reaction chamber containing tri-iodide and the metabolites of NO were detected by chemiluminiscence (CLD 88sp, ECO MEDICS or Sievers NOA) [24]. The nitrite data were first normalized to standard curves (made from known concentrations of nitrite) followed by subtraction of the levels of nitrite that were present in the saline used for the lavage procedure. Results were then reported as the final nitrite concentrations.
Measurement of nitrate
For quantitation of levels of nitrate in cell-free BW and the return of the first BAL fluid, these samples were methanol extracted at a 1:1 ratio to remove proteins. Concentrations of nitrate were measured in methanol extracts on a EiCOM NOx Analyzer (EiCOM, San Diego, CA) and by comparison to respective standard curves. Final nitrate concentrations were derived following subtraction of the levels of nitrate present in the saline used for the lavage procedure.
Measurement of arginase activity
The concentration of urea, an end product of the arginase pathway, was used as an estimate of the arginase activity in the supernatants of the first collection of the returned BAL and the BW fluids. Detection of urea was carried out with use of (a) Quantichrom Urea Assay kit (DIUR-500) (BioAssay Systems, Hayward, CA) which measured existing concentrations of urea and (b) Quantichrom arginase assay kit (DARG-200) (BioAssay Systems) which measured levels of urea produced from L-arginine in vitro from arginase activity that was present in the samples. 50 μl of urea depleted BAL and the BW fluid were used for the latter analyses and the concentrations of urea generated from L-arginine were determined based on comparisons to a urea standard reference curve, with absorbances at 520 nm measured by spectrophotometry following the manufacturer’s recommendations.
Synthesis of ornithine-lactam

Ornithine-lactam was prepared according to the procedures described by Lobenhoffer [25]. A solution of (L)-Ornithine hydrochloride (1) (0.2 g, 1.19 mmol) in toluene (30 mL) and 3N NaOH (0.4 mL, 1.2 mmol) was stirred at room temperature for 10 min. Al2O3 (0.8 g, 4 wt. eq.) was added and the reaction mixture was refluxed using a Dean-Stark trap for 6h. The byproduct water produced during the reaction was removed periodically by the Dean-Stark trap. TLC examination (MeOH/CHCl3, 1:4) revealed that the reaction was complete. The reaction mixture was then allowed to attain room temperature. Al2O3 was filtered off and washed with MeOH/CH2Cl2 (1:9, 30mL). The filtrate and washings were combined and evaporated under high vacuum in a rotary evaporator to yield the crude product, which was crystallized from hexanes to afford ornithine-lactam (2) (0.11 g, 81 %); mp 35 – 38 °C; 1H NMR (CDCl3) δ 1.43 – 2.27 (m, 6H), 3.17 – 3.38 (m, 3H), 6.52 (bs, 1H); 13C NMR (CDCl3) δ 21.6, 29.9, 42.6, 51.6, 175.2; MS (ES+) m/z 115 (M+H).
Chromatography and mass spectrometric detection of ornithine and ornithine-lactam
Chromatography: The gradient was generated with a Shimadzu Prominence UFLC system consisting of a vacuum degasser, binary pump, and a temperature controlled autosampler. The injection volume was 10 μL and the samples were maintained in the autosampler at 4° C. The column was a Phenomenex Jupiter C5 2.00 mm i.d. × 250 mm (5μ) with a guard column. Mobile phase A was 0.1% TFA, 0.1% HCOOH in water; mobile phase B was methanol + 0.1% HCOOH. The gradient was as follows: 0 – 2 min = 0%B, 5 – 6 min = 100%B, 6.5 – 10 min = 0%B and the flow rate was 0.4 mL/min.
Tandem Mass Spectrometry (MS/MS): Data were acquired with an Applied Biosystems API-4000 triple-quadrupole mass spectrometer equipped with APCI (atmospheric pressure chemical ionization) operating in positive ion mode. The mass spectrometer and HPLC were controlled with Analyst 1.4.2 in multiple reaction monitoring mode. The mass transition for ornithine was m/z 133.1 (M + H)+ to the m/z 70.1 fragment. The mass transition for ornithine lactam was m/z 115.2 (M + H)+ to the m/z 70.1 fragment. The dwell time was 40 ms for all mass transitions. The APCI optimized conditions include the following: curtain gas = 10, collision gas = 3, nebulizer gas = 35, nebulizer current = 5, source temperature = 450 and all gases used were nitrogen. Concentrations of ornithine and ornithinelactam in ng/ml were derived from standard curves.
Measurement of DHE+ cells
For the flow cytometric detection of DHE+ cells which have the potential to produce ROS, cells from BW or pooled BAL were incubated for 20 min at RT with dihydroxyethidium (DHE; 5mM stabilized stock solution in DMSO), 10 μM in PBS; Molecular Probes, Eugene, OR) following the manufacturer’s recommendations [26, 27]. Cells were then washed twice in PBS and the percent positive cells were determined by flow cytometry. The total number of DHE+ cells/ml were derived as follows: (total cell numbers from BW or BAL X percentage of DHE+ cells in BW or BAL) / total volume of BW or BAL fluid.
Cell Count and Differential
The total numbers of viable lavage cells were determined using trypan blue and a hemacytometer. Cytospin preparations of BW and BAL cells were prepared and stained with Diff-Quik (Baxter, McGaw Park, IL). Slides were subjected to blinded manual differential cell counts in which 300 cells were counted per slide, and the percents of total cells and different cell populations were determined. Cellularity in the BW and BAL fluid were reported as epithelial cells, monocytes and macrophages combined, and all other white blood cells (Other WBC) combined. Where indicated, the fraction of eosinophils within the Other WBC pool was reported.
Cytokine analysis
For the detection of cytokines in the BW and first collection of the returned BAL fluid, samples were centrifuged to remove the cellular debris and duplicate measurements were made from both samples using the 42 plex MILLIPLEX MAP Human Cytokine/Chemokine Panel (Millipore, Billerica, MA) and cytokine concentrations in pg/ml were measured as described before [28, 29]. Fluorescent signals were measured using a Luminex 100 laser (Biorad, Hercules, CA) and compared to the internal standard curves, established following the manufacturer’s recommendations.
Statistical Analysis
Nitrite, urea values, volumes of BW and BAL, BUN and age of the study subjects were analyzed using t tests. Paired t tests were used for comparisons of methods (e.g. BAL vs. mini-BAL) within a group and group t tests for comparisons between normals and asthmatics. Cytokine values were not normally distributed, so non-parametric tests were used. The Wilcoxon Signed Rank test was used for comparisons of methods between BAL and BW within the same individuals and the Mann Whitney U test for comparisons between normal and asthmatic subjects. Kruskal-Wallis non-parametric ANOVA was used for comparisons of the two groups. ANOVA was used for comparisons of DHE+ cells. A p value <0.05 was considered significant for all analyses reported here.
RESULTS
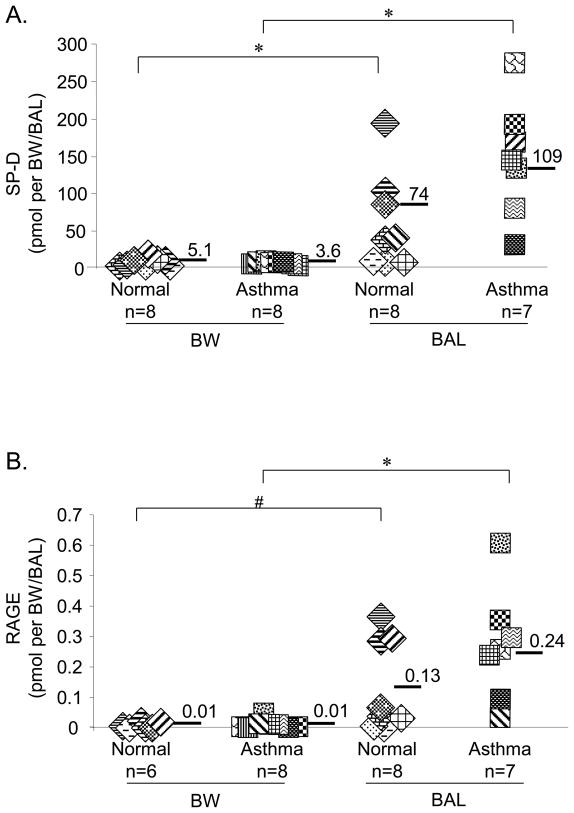
Levels of SP-D and RAGE were significantly higher in BAL fluid compared to BW
To investigate the contributions of the proximal compared to the distal airway for the production of mediators of asthmatic inflammation, we first tested whether BW and BAL were representative of these two distinct compartments of the airway. We measured levels of SP-D and RAGE, which are generally associated with the alveolar space of the lung [30, 31]. The total amounts of both SP-D (p=0.01) and RAGE (p=0.01) were significantly higher in the BAL compared to the BW (Figures 1A and 1B). The levels of SP-D (comparing BW: p=0.83, comparing BAL: p=0.52) and RAGE (comparing BW: p=0.88, comparing BAL: p=0.47) were not significantly different between asthmatic and normal control samples. Altogether, these data demonstrate that BW and BAL sample two different regions of the airway and indicate enrichment of these alveolar space-associated proteins in the BAL samples.
Figure 1. Bronchial wash (BW) and Bronchoalveolar lavage (BAL) fluids have significantly different levels of SP-D and RAGE.
(A) Total number of picomoles of SP-D (measured by ELISA) comparing BW and BAL samples collected from asthmatic and control subjects, *p=0.01 BW vs. BAL from normals and BW vs. BAL from asthmatics; (B) Comparison of RAGE levels in picomoles (measured by ELISA) between BW and BAL samples from normal and asthmatic subjects. Data are means ± S.D. #p=0.02 BW vs. BAL fluid from normals and *p=0.01 BW vs. BAL from asthmatics.
Elevated nitrite and nitrate concentrations were associated with asthmatic BAL and not BW fluid
To determine whether performing BW with a low volume would provide an enrichment of reactive nitrogen species (RNS), we compared the concentrations of NO and its metabolites in cell-free fluid collected by BW and BAL collected from normal subjects and subjects with asthma. The total NOx levels (nitrite + nitrate) were significantly higher in BAL fluid from asthmatic subjects (9.75±3.98 μM, p<0.001) compared to BAL fluid from normals (0.65±0.73 μM; Fig. 2). Strikingly, only very low levels of NOx were recovered in the BW from asthmatic subjects (0.69±0.76 μM, p=0.012 compared to BAL from asthmatics).
Figure 2. Total NOx levels are increased in the BAL fluid from asthmatics and not in the BW fluid compared to controls.
Total levels of nitrite and nitrate in BAL and BW fluid of normal and asthmatic subjects were measured by methods of chemiluminiscence and liquid chromatography. **p<0.001 comparing total nitrite and nitrate levels sampled by BAL from normal vs. asthmatics; *p=0.01 comparing total nitrite and nitrate levels in BAL vs BW fluid from asthmatics; #p=0.05 comparing total nitrite and nitrate levels in normal BW vs. BW fluid from asthmatics. Both nitrite and nitrate levels are reported as saline subtracted values.
Quantitation of the levels of nitrite within the total NOx in BW and BAL fluid collected from the airways of asthmatic and normal subjects was carried out using methods of chemiluminiscence which can detect several different metabolites of NO including nitrite, S-nitrosothiols (RS-NO) and N-nitrosamine species (Rx-NO) [24]. The mean nitrite concentration in BAL fluid from asthmatic subjects was 7.29 ± 2.65 μM, which was significantly higher compared to the BAL samples from normal subjects (0.002 ± 0.02μM, p<0.001) (Supplementary Fig. 1). On the other hand, dramatically lower concentrations of nitrite were detected in the BW from these same subjects (0.01 ± 0.02 μM) (p=0.01). Nitrite levels were not statistically different in BW fluid from normal subjects (0.01 ± 0.01 μM) when compared to asthmatic subjects (p=0.28). Furthermore, nitrite levels in BAL and BW fluid were not significantly different within the normal subjects. (p=0.48). The fact that nitrite detected in BAL fluid of asthmatics was at micromolar concentrations suggested that it is highly likely that the source of this nitrite was the inducible NOS rather than either of the constitutive NOS enzymes. Nitrite was the major NO metabolite detected by the chemiluminescence technique, representing 99% of the total metabolites.
Significant levels of nitrate have been reported in the airway fluid of asthmatic subjects [5]. Consequently, we measured the concentrations of nitrate in the BW and BAL fluid. Within the total nitrite + nitrate shown in Fig. 2, we detected enhanced levels of nitrate in the BAL fluid of asthmatic subjects (2.45 ± 2.53 μM), but not significantly different as compared to normal controls (0.64 ± 0.73 μM) (p=0.083) (Supplementary Fig. 2).
Levels of RSNO of ≤1 pmol were detected as mercury sensitive peaks which were present in only a limited number of samples. While the levels of RSNO to be anticipated in BW and BAL samples such as those analyzed here are not fully established, our failure to detect consistent levels of RSNO species may underscore the need for specific techniques for preserving the specimens if this type of analysis is desired. Our procedures for collection and storage of BW and BAL fluid did not include protection from visible light, and thus were not optimized for detection of nitrosothiol species.
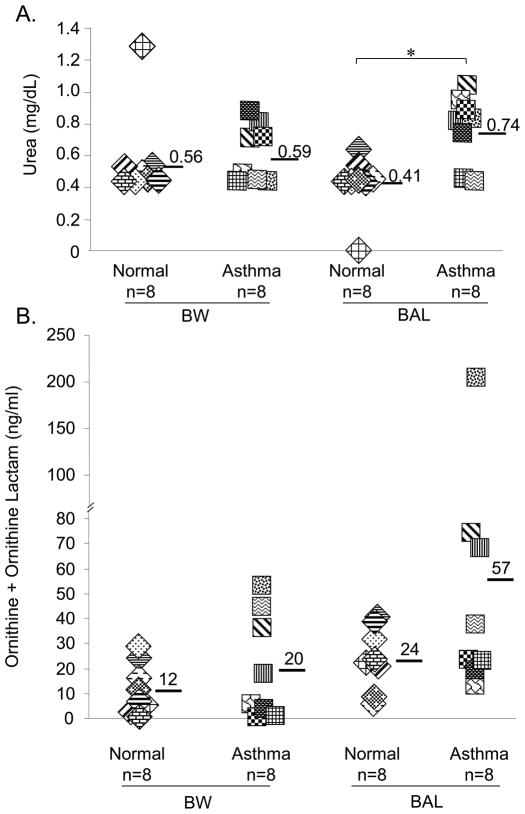
Increased levels of metabolites of the arginase pathway were detected in the asthmatic BAL and not the BW fluid
High arginase activity and elevated production of L-ornithine and urea, both metabolites of the arginase pathway, have been correlated with airway inflammation in mice as well as in humans [15, 19]. We compared the concentration of urea, as an indicator of arginase activity, using BAL and BW fluid from asthmatic and normal patients. As shown in Fig. 3a, the recovery of urea in the BAL fluid from asthmatic subjects (0.74 ± 0.21 mg/dL) was significantly higher than in the BAL fluid from normals (0.41 ± 0.18 mg/dL, p=0.01), with a similar trend to that observed for nitrite. The average concentrations of urea in the BAL samples from asthmatic subjects appeared to be different from those in BW samples from both normal and asthmatic subjects, but these differences did not reach statistical significance (0.56 ± 0.28 mg/dL in BW from normal subjects; 0.59 ± 0.17 mg/dL in BW from asthmatic subjects; for both comparisons, p=0.06).
Figure 3. Urea, a metabolite of the arginase pathway, is increased, whereas, ornithine is not enhanced significantly in the BAL fluid of asthmatics.
(A) Total urea levels were measured in BAL and BW fluid of normal and asthmatic subjects as an indicator for arginase activity. *p=0.01 comparing BAL fluid from normal vs. asthmatic subjects. The arginase activity in BAL fluid compared to BW fluid of asthmatics was p=0.06. (B) Total levels of ornithine and ornithine-lactam detected in BW and BAL fluids from asthmatic and normal subjects by LC-MS/MS.
In order to assess de novo synthesis of urea from arginase activity, we next depleted existing urea from the BW and BAL fluids and assayed for arginase activity in the residual samples. As shown in Supplementary Fig. 3, in samples from asthmatic subjects, arginase activity was significantly higher in the BAL when compared to BW fluid (p=0.02). In contrast, the differences in arginase activity comparing BAL samples of asthmatics and normals (p=0.69) as well as similar comparisons for BW fluid (p=0.44) did not reach statistical significance.
We then determined whether ornithine, another metabolite of the arginase pathway was enhanced in the airways of asthmatics compared to control subjects. As shown in Fig. 3b, although both ornithine (p=0.15) and ornithine-lactam (p=0.31) (the atypical metabolite of ornithine) were enriched in the BAL fluid of asthmatics compared to normals, the differences in levels of these arginase metabolites were not statistically different for all comparisons (total ornithine+ornithine-lactam (p=0.19) as well as individual concentrations of ornithine and ornithine-lactam) (Supplementary Fig. 4 & 5).
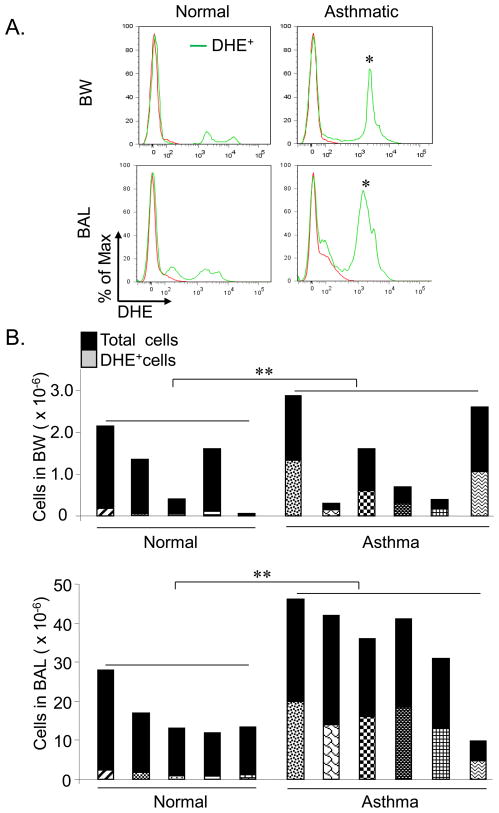
DHE+ cells which have the potential to produce ROS are increased in both BW and BAL fluid from asthmatic subjects
Although elevated levels of NO were localized primarily to the smaller, distal airways that were sampled by BAL, the cells capable of producing ROS were found throughout the asthmatic airway, both in the large, proximal as well as the small, distal airways. As shown in Fig. 4A, 43 ± 8% of the cells recovered in the BW fluid from asthmatic subjects stained positively with DHE, the indicator for ROS, compared to 7 ± 4% of the cells in BW fluid from normal controls (p<0.05). The percentages of DHE+ cells in BAL samples of asthmatics were also significantly different (42.8 ± 3.1%) as compared to the normal subjects (8.3 ± 2.2%, p<0.05). The total numbers of DHE+ cells were significantly higher in the pooled BAL (p=0.004) and BW (p=0.004) of asthmatics compared to normal controls (Fig. 4B). The total numbers of DHE+ cells in pooled BAL was significantly higher compared to BW of asthmatics (p=0.031) (Fig. 4B); however given the larger volume of the BAL compared to the BW, the concentrations of DHE+ cells/ml were not significantly different between pooled BAL and BW in asthmatics (p=0.917) (Supplementary Fig. 6A). The numbers of DHE+ cells/ml were also significantly higher in asthmatics when compared to normal controls for both BW (p=0.004) and BAL (p=0.004) samples (Supplementary Fig. 6A). The total BAL cell numbers were significantly higher in asthmatics compared to controls (p=0.041), while the total BW cell numbers were not significantly different between the study groups (Fig. 4B & Supplementary Fig. 6B). The number of cells recovered following instillation of the first aliquot of saline was variable and constituted 13.98±12 and 13.13±9.77 percent of total number of cells recovered in pooled BAL of asthmatics and normal controls respectively (Fig. 6B). These results indicate that DHE+ cells which have the potential to produce ROS are present in both proximal and distal airway compartments, and that the numbers of these cells are increased in the airways of asthmatic subjects as compared to normals.
Figure 4. DHE+ cells with the potential to produce ROS are present in increased numbers in BAL and BW of asthmatic subjects compared to normal controls.
(A) Representative overlaid histogram plots of BW and BAL cells from normal and asthmatic subjects stained with the indicator for ROS, Dihydroxyethidium (DHE). The percentages of DHE+ cells were determined by flow cytometry. *p<0.05 comparing BW cells from asthmatics with normal controls and BAL cells from asthmatics vs. normal controls. (B) Stacked histograms showing total cell numbers and numbers of DHE+ cells in pooled BAL and BW samples from 6 asthmatics compared to 5 normal control subjects.
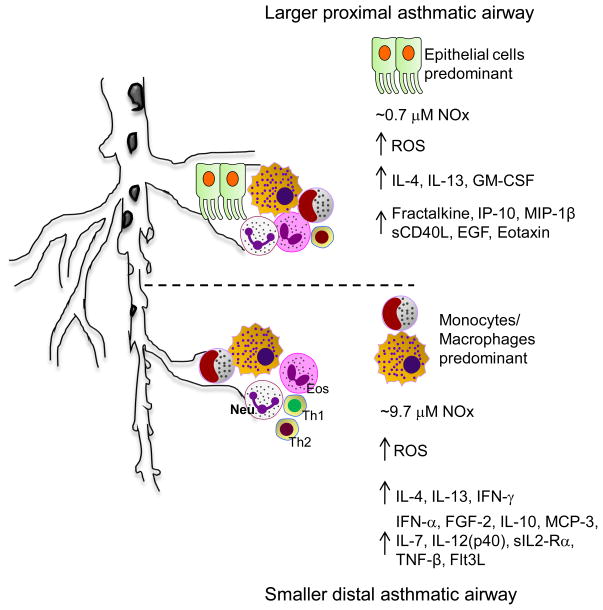
Figure 6.
Schematic representation of the inflammatory cytokine/chemokine signaling and inflammatory cellular contributions in larger, proximal versus smaller, distal airways in asthmatic subjects.
Differential sampling of cell populations by BW and BAL in samples from asthmatic compared to normal subjects
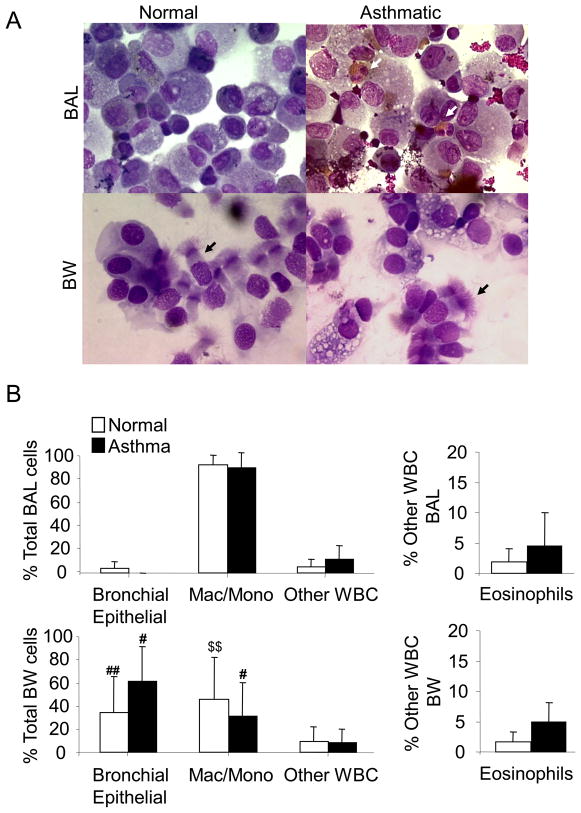
Prior studies have shown that the cells recovered from the BW and the BAL differ, with more epithelial cells recovered using the BW procedure and a greater number of macrophages recovered in the BAL fluid [32]. As shown in the cytospin preparations stained with Diff-Quik in Fig. 5A & B, BW fluid samples from asthmatic and normal subjects had a significantly increased proportion of ciliated bronchial epithelial cells when compared to the BAL samples from both asthmatic (p=0.04) and normal (p=0.008) subjects. Within the asthmatic population, macrophages and monocytes were significantly increased in the BAL preparation (88.33 ± 12.11%) (p=0.04) as compared to the BW (30.73 ± 29.12%). The normal subjects also had significant differences in percentages of monocyte/macrophage cells in the BAL (90.4 ± 7.81%) compared to BW (45.16 ± 35.81%; p=0.005). The percentages of eosinophils in the BW and BAL collected from mild asthmatics recruited for this study appeared elevated, but were not significantly different (BW: p=0.15, BAL: p=0.66) compared to their respective normal controls (Fig. 5B).
Figure 5. BW and BAL sample different cell populations.
(A) Representative images (1000 X) of DIFF Quik stained cytospin preparations of BW and traditional BAL cells from asthmatic and control subjects. White arrows designate eosinophils and black arrows highlight ciliated columnar epithelial cells (B) Blinded analysis of cell differentials (expressed as percents of total) from asthmatic and control subjects sampled using the BW and BAL techniques. #p=0.04 comparing the number of epithelial cells in BAL vs. BW fluid obtained from asthmatic subjects and ##p=0.008 comparing epithelial cells recovered by BAL vs. BW from normal subjects. #p=0.04 comparing the number of macrophage/monocyte cells in BAL vs. BW fluid obtained from asthmatic subjects. $$p=0.005 comparing the number of macrophage/monocyte cells in BAL vs. BW fluid obtained from normal subjects. Eosinophils are represented as % Other WBC excluding monocytes and macrophages. p=0.15 comparing numbers of eosinophils in BW fluid of asthmatics vs. controls and p=0.66 comparing BAL fluid from asthmatics vs. controls.
Differential cytokine profiling in the BW and BAL samples of asthmatic compared to normal subjects
We next determined whether unique profiles of cytokines and chemokines differentiated the proximal and distal airways. We compared BW to BAL fluid using the 42 plex MILLIPLEX MAP Human Cytokine/Chemokine Panel (Millipore, Billerica, MA). BW samples from subjects with asthma were distinctly enriched for epithelial cell-associated inflammatory products, with increased concentrations of Fractalkine [33], IL-8, IP-10, MCP-1, EGF, IL-1RA and Eotaxin as compared to the BW from control subjects. Notably, Fractalkine, IL-8, MCP-1 and IL-1RA were also elevated in the BAL fluid from the asthmatics when compared to normal subjects (Table II & Supplementary Fig. 7A). Generally, reduced levels of epithelial cell-associated cytokines were observed in the BAL. An exception was IL-1RA, which can be derived from both epithelial and inflammatory cells and was increased 3.9-fold in the BAL fluid when compared to the BW fluid of asthmatic subjects.
Table II.
Cytokine and Chemokine profiles
| Bronchial Wash | BAL | Normal | Asthma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine (pg/ml) | p value | Cytokine (pg/ml) | p value | p value | p value | ||||
| Normal (n=5) | Asthma (n=8) | Asthma vs. Normal | Normal (n=6) | Asthma (n=6) | Asthma vs. Normal | BW vs. BAL | BW vs. BAL | ||
| Th2 | IL-4 | N.D. * | 3.1±0.8 | 0.002 | 1.2±0.4 | 6.6±3.1 | 0.002 | 0.06 | 0.02 |
|
| |||||||||
| IL-13 | 0.1±0.1 | 1.4±0.5 | 0.002 | 2.1±0.3 | 8.6±1.0 | 0.002 | 0.06 | 0.02 | |
|
| |||||||||
| GM-CSF | 1.2±0.5 | 5.6±1.9 | 0.003 | N.D. | N.D. | - | 0.06 | 0.02 | |
|
| |||||||||
| Th1 | IFNγ | N.D. | N.D. | - | 1.2±0.1 | 1.3±0.1 | 0.24 | 0.06 | 0.02 |
|
| |||||||||
| IL-12(p40) | N.D. | N.D. | - | N.D. | 4.3±1.1 | 0.002 | - | 0.02 | |
|
| |||||||||
| Epithelial associated | EGF | 1.3±0.5 | 5.0±1.1 | 0.002 | N.D. | N.D. | - | 0.06 | 0.02 |
|
| |||||||||
| Eotaxin | 1.5±0.2 | 4.0±0.7 | 0.002 | N.D. | N.D. | - | 0.06 | 0.02 | |
|
| |||||||||
| Fractalkine | N.D. | 5.9±1.2 | 0.002 | N.D. | 4.5±5.5 | 0.18 | - | 0.75 | |
|
| |||||||||
| IL-8 | 1.8±0.6 | 23.0±8.9 | 0.002 | 7.7±2.1 | 15.5±5.2 | 0.02 | 0.06 | 0.17 | |
|
| |||||||||
| IL-15 | N.D. | N.D. | - | 2.2±0.3 | 2.3±0.4 | 0.69 | 0.06 | 0.02 | |
|
| |||||||||
| IL-1ra | 19.0±3.3 | 73.5±78.6 | 0.002 | 16.9±14.0 | 286.0±149.1 | 0.002 | 0.71 | 0.02 | |
|
| |||||||||
| IP-10 | 33.1±27.7 | 224.3±103.8 | 0.002 | 81.2±4.2 | 83.6±2.2 | 0.48 | 0.06 | 0.02 | |
|
| |||||||||
| MCP-1 | 12.8±5.3 | 156.7±92.9 | 0.002 | 43.1±17.6 | 124.2±36.9 | 0.009 | 0.06 | 0.75 | |
|
| |||||||||
| RANTES | 1.2±0.4 | 5.0±1.2 | 0.002 | N.D. | 56.3±4.3 | 0.002 | 0.06 | 0.02 | |
|
| |||||||||
| Asthma associated | FGF2 | N.D. | N.D. | - | N.D. | 26.1±4.6 | 0.002 | - | 0.02 |
|
| |||||||||
| Fit-3 ligand | 1.1±0.1 | 2.8±0.3 | 0.002 | 0.6±0.4 | 11.2±6.9 | 0.04 | 0.06 | 0.04 | |
|
| |||||||||
| IL-1α | 0.6±0.5 | 6.5±2.0 | 0.002 | N.D. | 14.0±5.9 | 0.002 | 0.06 | 0.04 | |
|
| |||||||||
| IL-1β | N.D. | N.D. | N.D. | 7.0±1.0 | 0.002 | - | 0.02 | ||
|
| |||||||||
| IL-6 | 0.2±0.4 | 3.7±1.0 | 0.002 | 4.4±0.6 | 7.2±0.8 | 0.002 | 0.06 | 0.02 | |
|
| |||||||||
| IL-10 | N.D. | N.D. | - | N.D. | 15.2±5.4 | 0.002 | - | 0.02 | |
|
| |||||||||
| TGFα | N.D. | N.D. | - | N.D. | 2.5±0.7 | 0.002 | - | 0.02 | |
|
| |||||||||
| SCD40-L | 0.9±1.3 | 0.8±1.0 | 0.83 | N.D. | N.D. | - | 0.06 | 0.04 | |
|
| |||||||||
| MIP-1B | 0.1±0.1 | 5.7±1.9 | 0.002 | 12.1±21.5 | 8.8±9.8 | 0.93 | 0.31 | 0.34 | |
|
| |||||||||
| TNFβ | N.D. | N.D. | - | 2.6±0.3 | 6.0±0.7 | 0.002 | 0.02 | 0.02 | |
|
| |||||||||
| IL-7 | N.D. | 1.6±0.2 | 0.002 | 71.6±1.7 | 169.0±9.3 | 0.002 | 0.06 | 0.02 | |
|
| |||||||||
| MDC | 1.5±0.2 | 6.3±3.0 | 0.002 | N.D. | 48.5±27.8 | 0.01 | 0.05 | 0.04 | |
|
| |||||||||
| IFNα2 | N.D. | N.D. | - | 72.2±0.8 | 84.0±4.9 | 0.002 | 0.06 | 0.02 | |
|
| |||||||||
| PDGF-AA | 1.7±0.8 | 10.6±3.9 | 0.002 | 58.5±28.9 | 64.2±15.5 | 0.69 | 0.14 | 0.02 | |
|
| |||||||||
| sIL-2Ra | N.D. | N.D. | - | N.D. | 63.8±2.6 | 0.002 | - | 0.02 | |
N.D.= not detected
Both the BW and the BAL fluids from subjects with asthma manifested an increase in other cytokines and chemokines that have been previously reported to be associated with asthma. The Th2 cell-derived cytokines, IL-4 and IL-13 showed a 4–5 fold increase in the BAL fluid from asthmatic subjects when compared to controls, with a similar trend noted for the BW fluid (Table II & Supplementary Fig. 7B). GM-CSF was detected only in the BW, with a 4.6 fold increase in samples from asthmatics when compared to controls. The BAL fluid from asthmatic patients had a unique cytokine profile when compared to the BW fluid, with significantly elevated concentrations of asthma-associated cytokines and chemokines including FGF-2, IL-10, TGF-α, TNF-β, IL-12(p40), IL-7, sIL2-Rα and IFN-α (Table II & Fig. 7C). Interestingly, IL-5 was not detected in the BW or the BAL of either study group. Flt3 ligand (Flt3L) was increased in both the BW and BAL fluid from asthmatic subjects, but was significantly higher in the BAL fluid when compared to the BW. The concentrations of IL-1α, IL-6, and MDC were significantly higher in the BAL samples as compared to the BW samples in subjects with asthma (Table II & Fig. 7C). Taken together, our studies indicate that the inflammatory cytokine and chemokine profiles are distinct in the airways of asthmatic subjects when compared to the airways of subjects with no known lung disease. Our data also suggests that the two sampling methods identify unique patterns of inflammatory signaling molecules within each airway compartment.
DISCUSSION
This study demonstrates that the more proximal and the more distal airway compartments in humans are characterized by distinct profiles of cellular activation, generation of reactive nitrogen and oxygen species and production of cytokines and chemokines (Fig. 6). We utilized a protocol modified from previously reported techniques to sample airway cells and fluid from the proximal airways (using a small volume BW) and the more distal airways and alveolar spaces (using conventional BAL) of asthmatics and normal subjects [34–36]. Levels of SP-D and RAGE, proteins produced largely by alveolar epithelial cells, were significantly enriched in BAL samples compared to BW fluid, confirming that BW and BAL preferentially sample the proximal and distal airways, respectively. Nitrite, a metabolite of nitric oxide, was elevated in the distal airways of asthmatic subjects compared to healthy controls, consistent with the known association of high exhaled NO and asthmatic inflammation in humans [7, 8, 37]. Nitrate concentrations were also elevated in the BAL of asthmatic subjects, consistent with previous reports [5]. Although apparently enhanced compared to the levels of nitrate in BAL of normal subjects, these differences were not statistically significant. Elevated total NOx levels (nitrite + nitrate) were limited to the distal airways, not the proximal airways, indicating, for the first time, that there are regional differences in the concentrations of NO and presumably also NOS enzymatic activity in the airways of asthmatic subjects. We speculate that these differences in localized concentrations of NO may also reflect differences in regional demands for NO to regulate inflammation in asthmatic airways.
Our observations are consistent with the recent reports that the distal airway alveolar contributions of NO, as sampled by exhaled air, are increased in asthmatic patients with poor asthma control or with more severe disease [38]. We also observed a statistically significant increase in arginase activity in BAL fluid of asthmatics compared to BAL fluid from normal subjects. In subjects with asthma, arginase activity appeared increased in BAL fluid compared to BW, although statistical significance was not reached (p=0.06). Levels of ornithine and ornithine-lactam, both resulting from induction of arginase, were increased in the BAL fluid from asthmatics as compared to normal controls, although statistical significance was not reached (p=0.19). Analyses of these metabolites in a larger sample size are warranted to definitively evaluate the contribution of arginase.
Another interesting observation in our study was that ciliated airway epithelial cells were primarily recovered in the BW fluid, and not in the BAL fluid. The induction of iNOS and the constitutive NOS enzymes in human airway epithelial cells has been previously reported and, in turn, epithelial cell-derived NO has been implicated in asthmatic inflammation [39, 40]. Our studies, however, detected dramatically lower concentrations of both nitrite and urea in the epithelial cell-enriched BW samples compared to the BAL samples that contained few or no detectable epithelial cells. This suggests that epithelial cells derived from the proximal airway may not produce significant amounts of reactive nitrogen species. In contrast, the differences in the levels of reactive nitrogen species observed in the distal as opposed to the proximal airways may reflect the different phenotypes of airway epithelial cells in the proximal vs. the distal airways, especially with regard to the expression/activation of the NOS enzymes. This hypothesis is consistent with earlier reports that the levels of NO in the airways may be associated especially with alveolar epithelial cells [41], which are abundant in the distal airway. The contributions of other inflammatory cells, such as eosinophils and neutrophils, and their associated peroxidases have also not been addressed in our study and cannot, therefore, be ruled out as participants in the establishment of the RNS and ROS milieu in asthmatic airways. The cytokine and chemokine profile noted in BW samples that was remarkable for its enrichment of epithelial cell-derived mediators, and the presence of an inflammatory profile in BAL fluid that was enriched with Th2 cytokines further support the differences in cellular composition/activation and their relative contributions to the inflammatory process from the more proximal and the more distal airway compartments.
Interestingly, irrespective of the regional differences of mediators we have observed, there was significant enrichment of inflammatory cytokines and chemokines in both the BW and the BAL samples from subjects with asthma as compared to normal controls, underscoring the presence of significant asthmatic inflammation even in the mild asthmatic subjects recruited for this study. The data reported here are also consistent with both epithelial cell-associated and Th2 cytokines being increased, as already recognized to be associated with asthmatic inflammation [1, 4, 42, 43].
Although there are substantial regional differences in the production of RNS, cytokines and chemokines in the airways of subjects with asthma, it is important to note that DHE+ cells were present in both proximal and distal airway compartments, with significant increases detected in asthmatic subjects compared to controls. Although ROS production per se can only be determined by quantitation of kinetics of cytochrome c reduction in the presence or absence of inhibitors of the ROS pathway, our data suggest that increased numbers of cells which have the potential to produce ROS are present in both the proximal and distal airways of asthmatics. We acknowledge, however, that other lung cell types not recovered using conventional BAL, including airway and alveolar epithelial cells, may contribute to total ROS production. Additionally, precise comparisons between the immune cell mediators, including ROS, RNS, and arginase metabolites cannot be performed here because we measured mediators and metabolites in the returned fluid from the first aliquot of BAL, whereas we analyzed the numbers of DHE+ and other cells in the pooled samples from all 6 aliquots of BAL. Nevertheless, our data are consistent with the reported contribution of ROS and superoxide to the inflammatory process in asthma [7, 17, 18, 44].
The different patterns of localization of high concentrations of NO and ROS during asthmatic inflammation reported here suggest that these reactive species are likely to have fundamentally different functions in airway responses. NO is an important endogenous regulatory molecule with dual roles of both enhancing and suppressing inflammation. The potential protective effects of NO have been linked to its broncho- and vasodilatory properties [45], with inhaled nitric oxide reversing antigen-induced bronchoconstriction in animal models. The anti-inflammatory properties of NO have been evident in the ability of NO to suppress the production of inflammatory cytokines as well as to inhibit the activation of NF-κB [46, 47]. The pro-inflammatory properties of NO and its reaction product peroxynitrite have also been well documented.
Our observations that the major portion of airway NO is recovered from the more distal airway raises additional questions of the pro- and anti-inflammatory actions of this mediator in the alveolar airway compartment, which has received relatively less attention in asthma research. The identification and phenotypic characterization of the cellular sources of reactive species and a systematic analysis of the function of these ROS- and RNS-producing cells will improve our understanding of the inflammatory cascade in different compartments of the asthmatic airway. Furthermore, these studies will aid in the development of new therapeutic strategies to target more efficiently the distal airway component of asthma pathophysiology.
Supplementary Material
Acknowledgments
The authors thank Ann Kelley for coordinating the recruitment of participants for this study through the UAB Lung Health Center. We acknowledge Angela P. Brandon for technical assistance with the chemiluminiscence assay. This work was supported by funding from the UAB Center for Clinical and Translational Science 5UL1RR025777 (DDC), UAB Center for Free Radical Biology (DDC), UAB Comprehensive Arthritis Musculoskeletal and Autoimmunity Center (DDC), UAB Lung Health Center, NIH 1F32HL095341-01A1 (JD), HL073907 (DDC) and CA131653 (JL). The mass spectrometer was purchased with funds from a Shared Instrumentation grant from the National Center for Research Resources (S10 RR19231, S. Barnes, PI).The operation of the UAB Targeted Metabolomics and Proteomics Laboratory is additionally supported by federal grants (U54 CA100949, S. Barnes, PI; P30 AT050948, C. Elmets, PI; and P30 DK079337, A. Agarwal, PI) and the UAB Lung Health Center.
LIST OF ABBREVIATIONS
- AHR
Airway Hyper-responsiveness
- BAL
Bronchoalveolar lavage
- BW
Bronchial wash
- FEV1
Forced Expiratory Volume in One Second
- iNOS
Inducible nitric oxide synthase
- NO
Nitric oxide
- RAGE
Receptor for Advanced Glycation End products
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SP-D
Surfactant protein-D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gelfand EW, Kraft M. The importance and features of the distal airways in children and adults. J Allergy Clin Immunol. 2009;124:S84–87. doi: 10.1016/j.jaci.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 2.Grootendorst DC, Sont JK, Willems LN, Kluin-Nelemans JC, Van Krieken JH, Veselic-Charvat M, Sterk PJ. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997;27:769–779. [PubMed] [Google Scholar]
- 3.Leff A. Pathogenesis of asthma. Neurophysiology and pharmacology of bronchospasm. Chest. 1982;81:224–229. doi: 10.1378/chest.81.2.224. [DOI] [PubMed] [Google Scholar]
- 4.Hyde DM, Hamid Q, Irvin CG. Anatomy, pathology, and physiology of the tracheobronchial tree: emphasis on the distal airways. J Allergy Clin Immunol. 2009;124:S72–77. doi: 10.1016/j.jaci.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 5.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Israel E, Jarjour N, Moore W, Peters S, Teague G, Gaston B, Erzurum SC. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 181:1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 8.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 9.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 2001;163:166–172. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- 11.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 12.Kharitonov SA, O’Connor BJ, Evans DJ, Barnes PJ. Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med. 1995;151:1894–1899. doi: 10.1164/ajrccm.151.6.7767537. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov SA, Yates D, Springall DR, Buttery L, Polak J, Robbins RA, Barnes PJ. Exhaled nitric oxide is increased in asthma. Chest. 1995;107:156S–157S. doi: 10.1378/chest.107.3_supplement.156s. [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Leick-Maldonado EA, Yano L, Leme AS, Capelozzi VL, Martins MA, Tiberio IF. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2006;35:457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- 15.Maarsingh H, Bossenga BE, Bos IS, Volders HH, Zaagsma J, Meurs H. L-arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur Respir J. 2009;34:191–199. doi: 10.1183/09031936.00105408. [DOI] [PubMed] [Google Scholar]
- 16.Sadeghi-Hashjin G, Folkerts G, Henricks PA, Muijsers RB, Nijkamp FP. Peroxynitrite in airway diseases. Clin Exp Allergy. 1998;28:1464–1473. doi: 10.1046/j.1365-2222.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 17.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 19.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol. 2009;158:652–664. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baughman RP. Technical aspects of bronchoalveolar lavage: recommendations for a standard procedure. Semin Respir Crit Care Med. 2007;28:475–485. doi: 10.1055/s-2007-991520. [DOI] [PubMed] [Google Scholar]
- 21.Elston WJ, Whittaker AJ, Khan LN, Flood-Page P, Ramsay C, Jeffery PK, Barnes NC. Safety of research bronchoscopy, biopsy and bronchoalveolar lavage in asthma. Eur Respir J. 2004;24:375–377. doi: 10.1183/09031936.04.00063003. [DOI] [PubMed] [Google Scholar]
- 22.Keatings VM, Evans DJ, O’Connor BJ, Barnes PJ. Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluid. Thorax. 1997;52:372–374. doi: 10.1136/thx.52.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 24.Moller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Jr, Denicola A. Membrane “lens” effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 25.Martens-Lobenhoffer J, Becker A, Freude H, Bode-Boger SM. Identification and quantification of the atypical metabolite ornithine-lactam in human plasma by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2284–2289. doi: 10.1016/j.jchromb.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Horvathova M, Wsolova L, Jahnova E. Simultaneous flow cytometric evaluation of phagocytosis and oxidative burst in human polymorphonuclear cells. Bratisl Lek Listy. 2005;106:63–66. [PubMed] [Google Scholar]
- 27.Mahfouz R, Sharma R, Lackner J, Aziz N, Agarwal A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil Steril. 2009;92:819–827. doi: 10.1016/j.fertnstert.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 28.Brasier AR, Victor S, Boetticher G, Ju H, Lee C, Bleecker ER, Castro M, Busse WW, Calhoun WJ. Molecular phenotyping of severe asthma using pattern recognition of bronchoalveolar lavage-derived cytokines. J Allergy Clin Immunol. 2008;121:30–37. e36. doi: 10.1016/j.jaci.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brasier AR, Victor S, Ju H, Busse WW, Curran-Everett D, Bleecker E, Castro M, Chung KF, Gaston B, Israel E, Wenzel SE, Erzurum SC, Jarjour NN, Calhoun WJ. Predicting intermediate phenotypes in asthma using bronchoalveolar lavage-derived cytokines. Clin Transl Sci. 3:147–157. doi: 10.1111/j.1752-8062.2010.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jambo K, French N, Zijlstra E, Gordon S. AIDS patients have increased surfactant protein D but normal mannose binding lectin levels in lung fluid. Respiratory Research. 2007;8:42. doi: 10.1186/1465-9921-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential Proteomic Analysis of Bronchoalveolar Lavage Fluid in Asthmatics following Segmental Antigen Challenge. Molecular & Cellular Proteomics. 2005;4:1251–1264. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Grootendorst DC. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997;27:769–779. [PubMed] [Google Scholar]
- 33.Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, Durand-Gasselin I, Varga EM, Simonneau G, Emilie D, Durham SR, Humbert M. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–1146. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 34.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodman L, Sutcliffe A, Kaur D, Berry M, Bradding P, Pavord ID, Brightling CE. Chemokine concentrations and mast cell chemotactic activity in BAL fluid in patients with eosinophilic bronchitis and asthma, and in normal control subjects. Chest. 2006;130:371–378. doi: 10.1378/chest.130.2.371. [DOI] [PubMed] [Google Scholar]
- 36.Comhair SA, Lewis MJ, Bhathena PR, Hammel JP, Erzurum SC. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am J Respir Crit Care Med. 1999;159:1824–1829. doi: 10.1164/ajrccm.159.6.9810044. [DOI] [PubMed] [Google Scholar]
- 37.Verleden GM, Dupont L, Lamont J, Buyse B, Delcroix M, Van Raemdonck D, Lerut T, Vanhaecke J, Demedts MG. Is there a role for measuring exhaled nitric oxide in lung transplant recipients with chronic rejection? J Heart Lung Transplant. 1998;17:231–232. [PubMed] [Google Scholar]
- 38.Puckett J, Taylor R, Leu S-Y, Guijon O, Aledia A, Galant S, George S. Clinical patterns in asthma based on proximal and distal airway nitric oxide categories. Respiratory Research. 11:47. doi: 10.1186/1465-9921-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharitonov SA, Yates D, Robbins RA, Barnes PJ, Logan-Sinclair R, Shinebourne EA. Increased nitric oxide in exhaled air of asthmatic patients. The Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 40.Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radical Biology and Medicine. 2006;41:515–527. doi: 10.1016/j.freeradbiomed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, Calhoun W, Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164:5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 42.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Current Opinion in Immunology. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Current Opinion in Immunology. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Cinci L, Masini E, Bencini A, Valtancoli B, Mastroianni R, Calosi L, Bani D. Suppression of allergen-induced respiratory dysfunction and airway inflammation in sensitized guinea pigs by MnII(Me2DO2A), a novel superoxide scavenger compound. Free Radical Biology and Medicine. 48:1525–1534. doi: 10.1016/j.freeradbiomed.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 45.Laursen BE, Stankevicius E, Pilegaard H, Mulvany M, Simonsen U. Potential Protective Properties of a Stable, Slow-releasing Nitric Oxide Donor, GEA 3175, in the Lung. Cardiovascular Drug Reviews. 2006;24:247–260. doi: 10.1111/j.1527-3466.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 46.Raychaudhuri B, Dweik R, Connors MJ, Buhrow L, Malur A, Drazba J, Arroliga AC, Erzurum SC, Kavuru MS, Thomassen MJ. Nitric oxide blocks nuclear factor-kappaB activation in alveolar macrophages. Am J Respir Cell Mol Biol. 1999;21:311–316. doi: 10.1165/ajrcmb.21.3.3611. [DOI] [PubMed] [Google Scholar]
- 47.Olson N, Kasahara DI, Hristova M, Bernstein R, Janssen-Heininger Y, van der Vliet A. Modulation of NF-κB and HIF-1 by S-Nitrosoglutathione Does Not Alter Allergic Airway Inflammation in Mice. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2010-0035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.