Abstract
The expression of endothelins (ETs) and ET-receptors are often upregulated in brain pathology. ET-1, a potent vasoconstrictor, also inhibits the expression of astrocyte glutamate transporters and is mitogenic for astrocytes, glioma cells, neurons and brain capillary endothelia. We have previously shown that mechanical stress stimulates ET-1 production by adult rat astrocytes. We now show in adult astrocytes that ET-1 production is driven by calcium influx through stretch-activated ion channels (SACs) and the ET-1 production correlates with cell proliferation. Mechanical stimulation using biaxial stretch (<20%) of a rubber substrate increased ET-1 secretion, and 4µM GsMTx-4 (a specific inhibitor of SACs) inhibited secretion by 30%. GsMTx-4 did not alter basal ET-1 levels in the absence of stretch. Decreasing the calcium influx by lowering extracellular calcium also inhibited stretch-induced ET-1 secretion without effecting ET-1 secretion in unstretched controls. Furthermore, inhibiting SACs with the less specific inhibitor streptomycin also inhibited stretch-induced ET-1 secretion. The data can be explained with a simple model in which ET-1 secretion depends on an internal Ca2+ threshold. This coupling of mechanical stress to the astrocyte endothelin system through SACs has treatment implications, since all pathology deforms the surrounding parenchyma.
Keywords: stress, GsMtx-4, channel, mechanical, peptide, proliferation
INTRODUCTION
The ability of astrocytes to respond to virtually any CNS disturbance with both stereotyped changes (such as GFAP upregulation) and an adaptable repertoire of other components suggest that astrocytes may possess certain all-purpose sensors to monitor changes in their local environment [19]. Numerous studies have demonstrated that astrocytes possess mechanosensors and their anatomy, an interconnected meshwork of stellate cells, can integrate the effects of mechanical stress from distant sites. The responses to mechanical stimuli include dynamic cytoskeletal components such as GFAP and vimentin, stretch-activated ion channel (SAC) activation, and second-messenger signaling with Ca2+ and IP3 (reviewed in [26]).
ET-1 expression is minimal in quiescent adult astrocytes but robustly upregulated in reactive astrocytes (reviewed in [26]). The expression of ETB receptors is also upregulated in many pathologies [22;28]. ET-1 exerts potent autocrine effects on astrocytes and ET-1 stimulation has been used as an in vitro model for reactive astrocytes in culture [7]. The levels of ET-1 following cerebral hypoxia/ischemia and trauma correlate with the degree of astrocyte reactivity in vivo [30;34]. Since ET-1 is a strong inhibitor of astrocyte glutamate transporter expression [18], the mechanical stress associated with trauma or disease could potentiate neurotoxicity by downregulating clearance of excess glutamate.
Most in vitro studies of the astrocyte ET-system have used cultures from fetal or neonatal animals that may persist in a state of partial reactivity or immaturity [15;37]. Astrocytes from adult rats more closely approximate in vivo conditions - upon subculture they recapitulate the cell cycle kinetics, produce neurotrophic factors, and transiently upregulate GFAP and vimentin as seen in reactive gliosis in vivo. They then reach a state of proliferative quiescence that can be maintained for months in culture [17;29].
We previously demonstrated that mechanical deformation of adult rat astrocytes by stretching flexible-bottomed culture dishes causes an increase in cytoplasmic Ca2+ and inositol triphosphate (IP3), and a substantial increase in ET-1 production and secretion [25]. The trigger for this stretch-induced effect may be the presence of stretch-activated ion channels (SACs), characterized in many cells including neonatal [4;13] and adult rat astrocytes [31]. These channels can be specifically inhibited by a small peptide called GsMTx-4 [5;32].
Here, we demonstrate that the expression of ET-1 by adult rat astrocytes correlates with cell proliferation, becoming negligible in confluent quiescent cultures, akin to the situation in the intact brain. The ET-1 production appears driven by internal Ca2+ that in turn is coupled to an influx through Ca2+ permeable SACs. Production could be reduced by inhibitors of SACs or reduction in the Ca2+ influx by lowered extracellular Ca2+.
MATERIALS AND METHODS
Cell culture
Adult astrocyte cultures derived from stereotactic striatal gelatin implants [17] were obtained from Dr. Robert Plunkett, Department of Neurosurgery, SUNY at Buffalo. The cells were grown on 6-well, flexible-bottomed culture plates coated with collagen I (Bioflex® Plates, Flexcell International Corp., Hillsborough, NC, U.S.A.), maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. After being confluent for one week, the cells were incubated in Starvation Medium containing 0.1% FBS (instead of 10%) for 24-hours. This was then aspirated and replaced with fresh Starvation Medium immediately before testing. Experiments performed under “serum starvation” conditions had less variability that those cultured in normal media, possibly due to a smaller percentage of cells that had not reached a quiescent state or possibly bioactive serum components. The qualitative characteristics of the stretch-induced ET-1 responses and the inhibition of this phenomenon were the same in both media [24].
Evaluation of Proliferation by BrdU ELISA
Astrocytes were subcultured in 6-well Nunclon™ Surface coated culture plates (VWR, West Chester, PA, U.S.A.) leaving one well free as a control. Proliferation was evaluated using BrdU Cell Proliferation ELISAs (Roche Molecular Biochemicals, Indianapolis, IN, U.S.A.). At each time point the wells were incubated with the BrdU labelling solution for two hours. As a control for non-specific binding of the anti-BrdU antibody, one well (containing cells) of each plate was processed at each time without being loaded with BrdU.
ET-1 measurements
In order to correlate ET-1 levels with the proliferative status of the cells, we made duplicate culture plates for the ET-1 assay and the culture medium was changed weekly. Media samples were taken from the wells at specific times and frozen at −80°C for later analysis.
Cell lysates were prepared at the same time points as follows:
Cells were rinsed twice with a Protease Inhibitor Cocktail (PIC): 5 µg/ml Leupeptin, 2 µg/ml Aprotinin, and 0.7 µg/ml Pepstatin (all from Boehringer Mannheim, Indianapolis, IN, U.S.A.) in Phosphate Buffered Saline (GibcoBRL, Grand Island, NY, U.S.A.), pH 7.4.
1 ml of 0.3% Triton X-100 (Sigma, St. Louis, MO, U.S.A.) in PIC was added to each well and incubated on ice for 30 minutes.
Cell scrapers were used to dissociate the monolayers, after which each lysate was passed several times through a 26-gauge needle to disperse any large aggregates, then frozen at −80°C.
ET-1 was quantified with a Human ET-1 ELISA (Assay Designs, Ann Arbor, MI, U.S.A.), using a HTS-7000™ BioAssay Reader (Applied Biosystems, Foster City, CA, U.S.A.).
Mechanical stretching
A Flexcell FX-3000® Strain Unit (Flexcell International, McKeesport, PA, U.S.A.) was programmed to produce sinusoidal stretch (0.1 Hz, 0–20% elongation). Culture media was sampled from the individual wells after 24-hours of stretching. The sampling occurred during the stretching regimen as previous experiments demonstrated that cessation of stress can serve as an additional stimulus [25].
Calcium Buffering
To buffer the Ca2+ concentration in the culture medium, we used nitrilotriacetic acid (NTA, Kd for Ca2+ = 99.3 µM at 37.0 °C, pH 7.4, 0.15N). Since NTA also binds Mg2+ (Kd =0 .575 mM), the concentration of Mg2+ was augmented to maintain constant free Mg2+ as predicted using the software WinMaxC v.2.05 [1].
GsMtx-4 Preparation
GsMtx-4 from three separate purifications of raw Grammastola spatulata venom was pooled [31]. The pooled sample was tested on outside-out patches of astrocytes to estimate the concentration of the active peptide: assuming a Kd of 500nM and single site binding [31], the percent inhibition of the mean SAC current provided the approximate concentration.
Outside-Out Patch Clamp Recording
The main goal of the patch clamp recordings was to demonstrate the Ca2+ permeability of SACs (Suchyna et al. [31]). The pipette solution contained 140 mM KCl and the bath solution contained 100 mM CaCl2. The patch voltage was hyperpolarized −50mV to reduce the contribution of voltage-activated Ca2+ channels. Currents were sampled at 10 kHz and low-pass filtered at 2 kHz through a four-pole Bessel filter. Electrodes were pulled on a PC-84; (Sutter Instrument Co., Novato, CA, U.S.A), painted with Sylgard-184 (Dow Corning, Midland, MI, U.S.A.), and fire polished. Pressure and suction were applied to the pipette by a pressure clamp [2]. Perfusion was handled by a pressurized bath perfusion system (BPS-8; ALA Scientific Instruments, Westbury, NY, U.S.A.).
RESULTS
Production and secretion of ET-1 following subculture
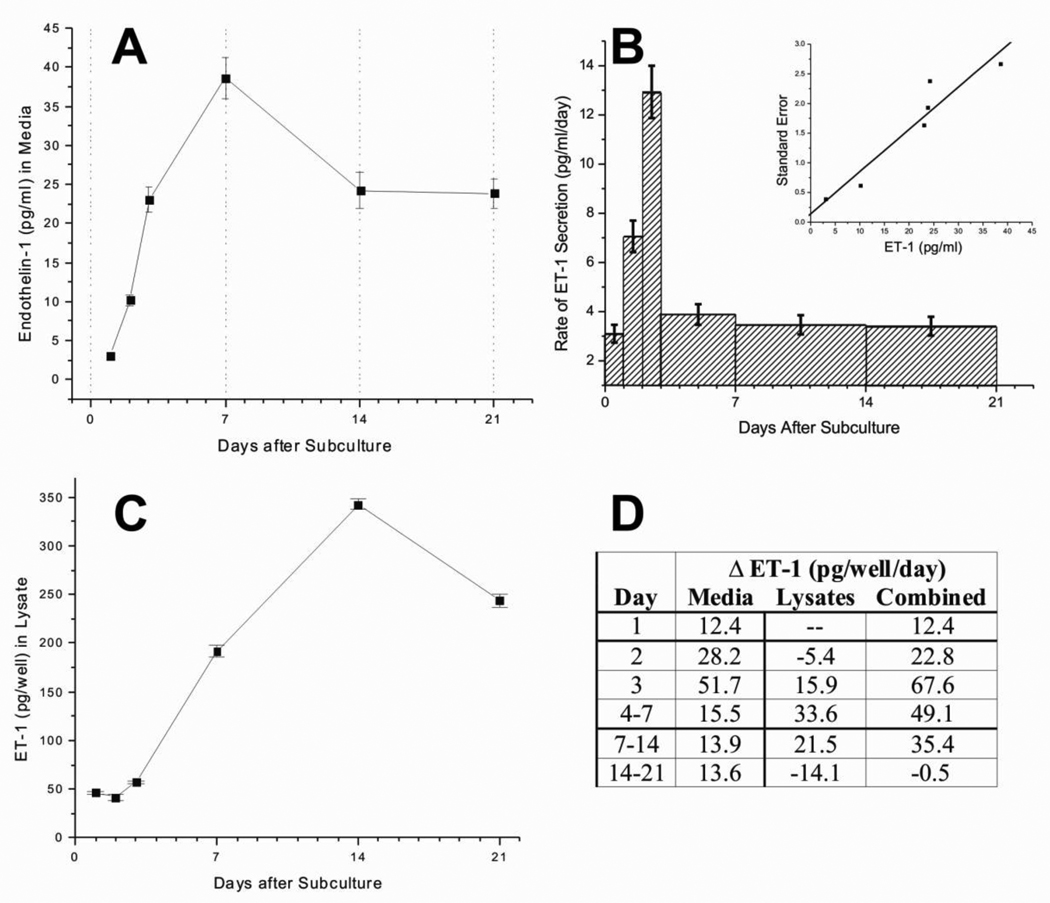
Figure 1A shows the concentration of ET-1 in media at specific times after subculture. Separate replicate wells were used for each time point to maintain equal volumes. The concentration of ET-1 accumulates over time in the media so we normalized the data to rate of ET-1 secretion per day as shown in Figure 1B. We found a linear relationship between the standard error and the ET-1 concentration measured by ELISA, so the standard error values for Figure 1B were extrapolated from this fit (see figure inset). The rate of ET-1 secretion into the medium was highest during the third day after subculture. It then rapidly declined and plateaued for the remainder of the three-week time course.
Figure 1. ET-1 production and secretion by adult rat astrocyte cultures.
Panel A shows ET-1 measured in media. Media was changed every 7 days (dotted lines) so that measurements on days 1,2,3, and 7 represent ET-1 accumulated since subculturing (time 0), whereas measurements on days 14 and 21 represent ET-1 accumulated over the previous week (since the last media change). Separate replicate wells were used for each time-point so media volumes remained constant. Panel B shows the average change in ET-1 concentration per day, over a length of time indicated by the width of the bars. Standard errors were extrapolated from a linear fit of SEs from panel A (see inset). Panel C shows ET-1 measured in cell lysates. Panel D shows the combined rate of change of ET-1 levels in the media and lysates per well over the 21-day time course. Each well had 4 ml of media, so multiplying the values in panels A and B by 4 provides pg/well. Error bars = ±SE.
Intracellular ET-1, as inferred from the lysates, remained steady until day 3 and then increased, reaching a peak at day 14 and then started to decline (Figure 1C). Figure 1D shows the combined rate of ET-1 production (i.e. change in lysates plus media) per culture well. Since the freshly subcultured cells presumably contain some amount of ET-1 even before attaching to the dishes, we called the "Day 1" amounts zero. The lysates showed a slight decrease on Day 2 (Figure 1C) suggesting that production did not begin until after the first 2 days. The increased ET-1 in the media from days 14–21 is nearly equal to the amount lost from the lysates (Figure 1D). Apparently pre-formed ET-1 was secreted from the cells during this time, i.e. synthesis of ET-1 had ceased by day 14.
Correlation between ET-1 production and astrocyte proliferation
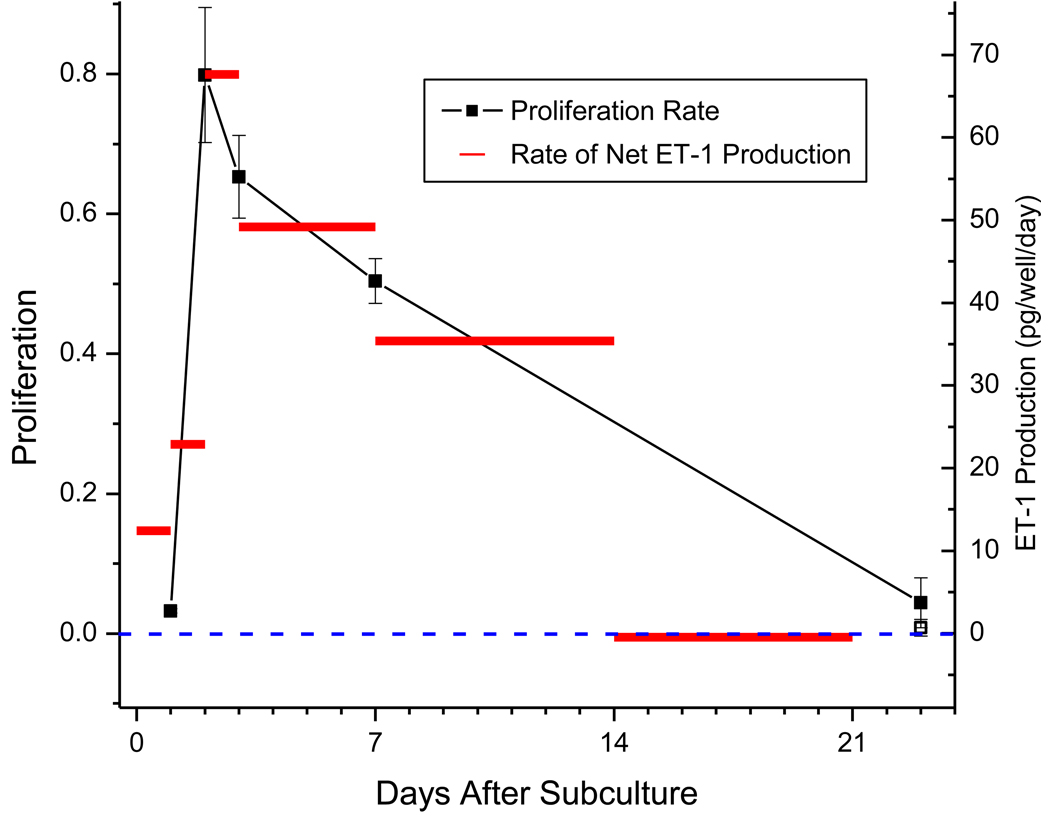
The squares in Figure 2 show the average rate of DNA synthesis per culture well as a function of time. Each data point represents the amount of BrdU incorporated into DNA per well during a two hour load, and thus reflects both the percentage of cells that are actively proliferating and the total number of cells per well. The proliferation measurement on day 1 was very low - probably because although a high percentage of the cells were in S phase, there were a small number of cells in each well. The proliferation rate peaked two days after subculture. At day 23 the rate of proliferation was comparable to that on Day 1. The open-box data point at day 23 is nearly zero and represents the proliferative activity following a two-day serum starvation (0.1% serum in medium instead of 10%). The slight discrepancy between this point and the normal media wells suggests that a small percentage of cells were still proliferating in the normal media.
Figure 2. ET-1 production rate correlates with proliferation (DNA synthesis).
The open-box data point at day 23 represents DNA synthesis following 2-days of serum starvation (0.1% serum). The left Y-axis has been normalized from zero to one. The horizontal red bars are the values from the “Combined” column in panel D of Figure 1, the average change in ET-1 concentration per day averaged over the length of time indicated by the width of the bars. Error bars = ±SE.
The red bars in Figure 2 show the average change in ET-1 concentration per day over the length of time indicated by the width of the bars (data from Figure 1D). There is a clear correlation between ET-1 production and cell proliferation. Rates were highest during the first week and then subsided during the next two weeks. By the end of the three-week time course, both proliferation and ET-1 production had essentially ceased. The correlation does not imply causality, but the dependence of ET-1 secretion on Ca2+ influx (below) suggests that ET-1 is driving proliferation rather than the opposite.
Lowering extracellular calcium attenuates stretch-induced ET-1 secretion
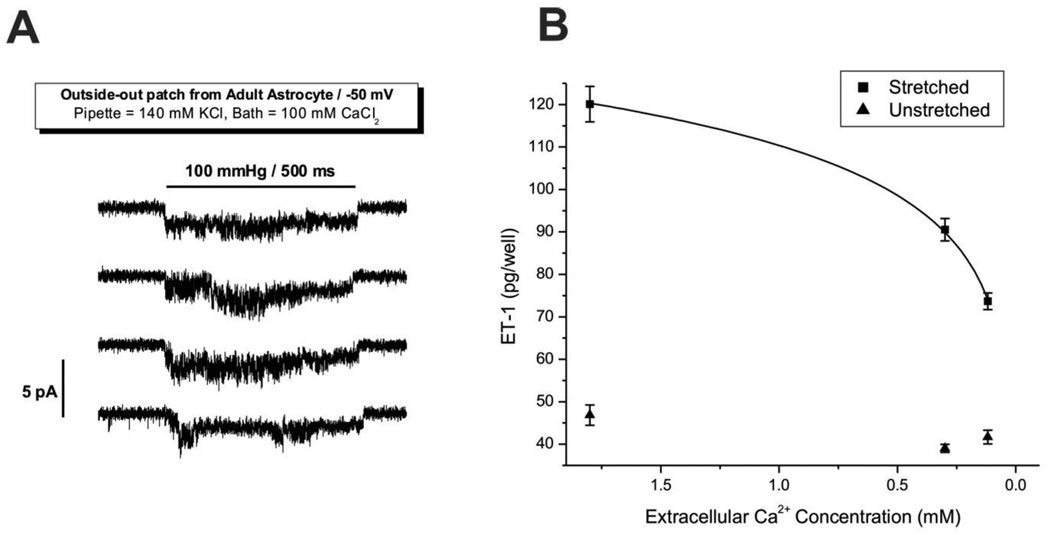
If ET-1 secretion is driven by intracellular Ca2+ changes from stretch-induced Ca2+ influx, it should be inhibited by lowering extracellular Ca2+. We used the 24-hour sinusoidal deformation regimen and varied extracellular Ca2+. As shown in Figure 3B, lowering the extracellular Ca2+ concentration decreased the stretch-induced secretion of ET-1 (squares) in a nonlinear manner, but it had no effect on ET-1 secretion in the absence of stretch (triangles). Therefore, the decrease in ET-1 secretion in the stretched wells is not due to other stretch-independent secondary effects of lowering external Ca2+. This also indicates that the Ca2+-chelator NTA did not alter ET-1 secretion through nonspecific mechanisms. There was a slight, but statistically insignificant decrease of ET-1 in the unstretched wells, but a background effect is expected since the probability of an ion channel being closed is always greater than zero.
Figure 3. Stretch-induced ET-1 secretion is dependent on extracellular calcium.
Panel A shows that adult astrocyte SACs can conduct Ca2+ in response to mechanical stress. An outside-out patch was exposed to 500 millisecond pressure pulses (100 mmHg as noted with the horizontal bar) with 1.5 seconds between pulses. The holding potential was −50 mV and a downward deflection denotes Ca2+ influx. Panel B shows ET-1 concentration measured in media following a 24-hour sinusoidal stretching regimen (squares, 0.1 Hz, 20% linear strain) and in unstretched control cultures (triangles). Extracellular Ca2+ levels in the media were lowered by buffering with NTA. The curve is a fit of the data to a 2-parameter function (see Supplementary Material for derivation): Y= P1*ln[Ca2+]out + P2 − 42.26, P1=16.06 ± 0.3, P2 = 152.7 ± 0.5.
To demonstrate that the SACs conduct Ca2+ in response to stretch, we perfused outside-out patches with saline where Ca2+ was the only cation and demonstrated inward single currents with stretch (Figure 3A). The kinetics were similar to those previously published where the channel conducted monovalent ions [31].
Inhibition of stretch-induced ET-1 secretion by GsMtx-4 and streptomycin
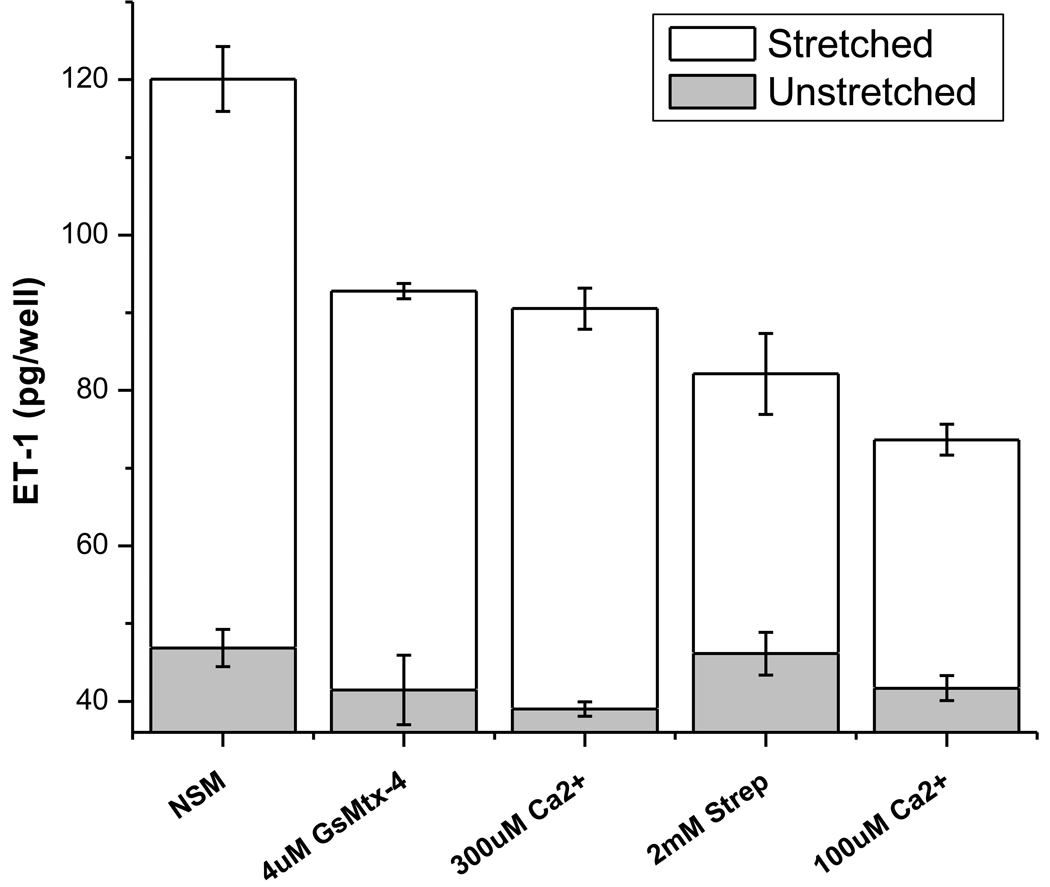
In adult rat astrocytes, GsMtx-4 blocks SACs with a Kd of ~500nM while exhibiting minimal effects on voltage-sensitive currents [31]. The mechanism of action is as a gating modifier that involves both the channel and its lipid environment [32]. Adding 4µM GsMtx-4 to the culture media caused a 23% reduction in the amount of ET-1 detected in the media following a 24-hour cyclic deformation regimen (P<0.003). It had no significant effect on secretion of unstretched controls (Figure 4). This corresponds to a ~30% inhibition of the stretch-induced ET-1 secretion (i.e. after subtracting the unstretched baseline controls). Streptomycin, a nonspecific inhibitor of cationic SACs (2mM), caused somewhat larger inhibition of stretch-induced secretion of 32% (P<0.0001), while again having no effect on unstretched controls. This corresponds to ~50% inhibition of stretch-induced ET-1 secretion.
Figure 4. ET-1 in the media increases with stretch and Ca2+ influx.
NSM = “normal starvation media” that has 1.8 mM Ca2+. The grey bars are from unstretched controls. Error bars = ±SE. The data show stimulation by the mechanical input and inhibition by changes that reduce Ca2+ influx. (24h of cyclic stretch, 0.1 Hz, 0–20% strain).
Assuming a 1:1 stoichiometry of GsMTx-4 binding to SACs, and a Kd of ~500nM [31], 4µM of GsMtx-4 should block ~90% of SACs, but it resulted in only a ~30% inhibition of stretch-induced ET-1 secretion, implying a non-linear relationship between the Ca2+ influx through SACs and ET-1 secretion. This is not surprising, as astrocytes express several other ion channels capable of conducting Ca2+, including voltage- and ligand-gated channels [36] that are known to be stretch-sensitive (Ca2+ [6], Na+ [3] and K+ channels [10]). Voltage dependent channels may be indirectly activated by stretch when SACs cause depolarization.
DISCUSSION
Following subculture, both ET-1 production and cell proliferation peaked during the first week and then declined over the next two, becoming negligible by ~3 weeks. This time course is similar to the expression of GFAP and vimentin described by Langan et al [17] for the adult rat astrocytes – the expression of these markers of the reactive phenotype was upregulated during the first two weeks following subculture and declined to baseline during the third week, and this paralleled the reactive glial reaction in vivo adjacent to an explant. The cessation of proliferation and ET-1 production after the cells became confluent suggests that astrocyte cultures derived from adult rats may enter a quiescent phenotype comparable to their in vivo behavior, as opposed to neonatal/fetal cells that may persist in a state of partial reactivity or immaturity.
As in our prior experiments [25], a two day mechanical stretching regimen produced significant ET-1 compared to unstretched controls. The response to mechanical stress seems to be the result of SAC activation. Inhibition of the channels by GsMtx-4 and streptomycin inhibited stretch-induced ET-1 secretion but did not alter levels in unstretched controls. Coupling between channel activation and ET-1 production seems to be via Ca2+ influx through the channels since lowering extracellular Ca2+ decreased the stretch-induced ET-1 secretion without affecting levels in unstretched controls. GsMTx-4 is a gating modifier whereas streptomycin is an open channel blocker [11;16] so while they both inhibit the channels they should have different side effects and the Kds are quite different. The lack of specificity of streptomycin may account for the greater inhibition compared to GsMtx-4, as it inhibits voltage-gated Ca2+ channels [21;27] which may be indirectly activated by depolarization caused by stretch-induced Ca2+ influx.
The coupling of Ca2+ influx to ET-1 expression may follow that seen for Ca2+ waves and IP3 [26]. As intracellular Ca2+ stores are released, the intracellular Ca2+ concentration remains elevated for some period of time. If the amount of ET-1 secreted into the media ([ET-1]media) depends on the probability of reaching a threshold for release of Ca2+ stores, then ET-1 secretion should be linearly related to total Ca2+ influx (i.e., through all influx pathways assuming no changes in Ca+2 efflux). If the flux of Ca2+ varies logarithmically with the ion concentration (the driving force of the Nernst potential), the relationship between [ET-1]media and [Ca2+]out can be modeled with a two-parameter function:
where P1 and P2 are constants that can be obtained by curve fitting (see Supplementary Material for detailed derivation).
The (limited) data in Figure 3B is well fit by the function (black curve) yielding P1 and P2 (16.06 ± 0.3 and 152.7 ± 0.5 respectively). From this we estimate the intracellular Ca2+ concentration ([Ca2+]in) to be ~ 75 nM (see Supplementary Material). This average intracellular Ca2+ concentration during the 24-hour stretching regimen represents a steady state between the stress-enhanced Ca2+ influx, intracellular storage and efflux. Using Fura-2 (Molecular Probes, Eugene, OR, U.S.A.), Niggel et al. measured the basal [Ca2+]in for the adult rat astrocytes ~30–40nM [23]. Other labs have estimated a basal [Ca2+]in of 50–90 nM in neonatal rat astrocytes [8;35]. Therefore, 75 nM obtained from the model seems reasonable.
There was measureable ET-1 in the media from the unstretched cells under all conditions (Figures 3B and 4). We previously demonstrated that ET-1 in media from unstretched controls remains constant from as early as one hour after the initial media change [28] so that the act of changing media appears to induce (via fluid shear stress) a rapid transient release of ET-1. Macarthur et al. [20] observed a similar transient secretion of ET-1 in both stretched and unstretched endothelial cell cultures, which they attributed to the mechanical disturbance of changing media. Tong et al. showed that HEK-293 cells exhibit an increase in Ca2+ when culture medium is changed with aspiration [33]. However, they also showed that if the medium was changed by an “overflow” method with no shear stress there was no observable calcium change.
These experiments have significant implications for in situ pathology. CNS trauma and diseases deform the surrounding parenchyma (and thus the astrocyte network). The effects may be quite general. Recent studies suggest that ET-1 inhibits astrocyte gap-junctional communication [12], thus potentially providing feedback regulation of mechanically induced intercellular Ca2+ and IP3 signaling [26]. The upregulation of astrocyte ET-1 observed in many pathologies and its potent effects on astrocytes, neurons and the cerebral vasculature suggests that mechanical induction of the astrocytes’ endothelin system may be common to many disorders. GsMtx-4 provides a unique tool to study SAC activity in the CNS and the utility is illustrated by this work and by the stimulatory effect for GsMTx-4 on neurite growth [9;14]. SAC pharmacology may therefore provide a new class of therapeutic agents for nervous system pathology.
HIGHLIGHTS.
Endothelin-1 expression by adult rat astrocytes correlates with cell proliferation.
Stretch-induced ET-1 is inhibited by GsMtx-4, a specific inhibitor of Ca2+ permeant SACs.
The less specific SAC inhibitor streptomycin also inhibits ET-1 secretion.
Stretch-induced ET-1 production depends on a calcium influx.
SAC pharmacology may provide a new class of therapeutic agents for CNS pathology.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Jeffrey Niggel and Sang Chul Lee for the data on resting Ca2+ in the astrocytes, and JST and NIH for support (FS). FS and TMS are part owners of Tonus Therapeutics that is developing drugs for mechanosensitive ion channels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bers D, Patton C, Nuccitelli R. A Practical Guide to the Preparation of Ca Buffers, A Practical Guide to the Study of Ca2+ in Living Cells. San Diego: Academic Press; 1994. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- 2.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Arch. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 3.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. The Journal of Physiology. 2010;588:4969–4985. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman CL, Ding J, Sachs F, Sokabe M. Mechanotransducing ion channels in astrocytes. Brain Research. 1992;584:272–286. doi: 10.1016/0006-8993(92)90906-p. [DOI] [PubMed] [Google Scholar]
- 5.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: History, properties, mechanisms and pharmacology. Toxicon. 2007;49:249–270. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese B, Tabarean IV, Juranka P, Morris CE. Mechanosensitivity of N-type calcium channel currents. Biophys.J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egnaczyk GF, Pomonis JD, Schmidt JA, Rogers SD, Peters C, Ghilardi JR, Mantyh PW, Maggio JE. Proteomic analysis of the reactive phenotype of astrocytes following endothelin-1 exposure. Proteomics. 2003;3:689–698. doi: 10.1002/pmic.200300407. [DOI] [PubMed] [Google Scholar]
- 8.Gebke E, Muller AR, Pehl U, Gerstberger R. Astrocytes in sensory circumventricular organs of the rat brain express functional binding sites for endothelin. Neuroscience. 2000;97:371–381. doi: 10.1016/s0306-4522(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb PA, Barone T, Sachs F, Plunkett R. Neurite outgrowth from PC12 cells is enhanced by an inhibitor of mechanical channels. Neuroscience Letters. 2010;481:115–119. doi: 10.1016/j.neulet.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage-gated K+ channel. Biophys.J. 2001;80:2678–2693. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacological Reviews. 1996;48:231–252. [Review] [PubMed] [Google Scholar]
- 12.Herrero-González S, Valle-Casuso JC, Sánchez-Alvarez R, Giaume C, Medina JM, Tabernero A. Connexin43 is involved in the effect of endothelin-1 on astrocyte proliferation and glucose uptake. Glia. 2009;57:222–233. doi: 10.1002/glia.20748. [DOI] [PubMed] [Google Scholar]
- 13.Islas L, Pasantes-Morales H, Sanchez JA. Characterization of stretch-activated ion channels in cultured astrocytes. Glia. 1993;8:87–96. doi: 10.1002/glia.440080204. [DOI] [PubMed] [Google Scholar]
- 14.Jacques-Fricke BT, Seow YQ, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. Journal of Neuroscience. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimelberg HK. Current methods and approaches to studying astrocytes: A forum position paper. Neurotoxicology. 1999;20:703–712. [PubMed] [Google Scholar]
- 16.Kroese AB, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear.Res. 1989;37:203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 17.Langan TJ, Plunkett RJ, Asada H, Kelly K, Kaseloo P. Long-Term Production of Neurotrophic Factors by Astrocyte Cultures From Hemiparkinsonian Rat Brain. Glia. 1995;14:174–184. doi: 10.1002/glia.440140303. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann C, Eisner F, Engele J. Role of endothelins as mediators of injury-induced alterations of glial glutamate turnover. J.Neurosci.Res. 2008;86:660–667. doi: 10.1002/jnr.21512. [DOI] [PubMed] [Google Scholar]
- 19.Little AR, O'Callagha JP. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–618. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 20.Macarthur H, Warner TD, Wood EG, Corder R, Vane JR. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem.Biophys.Res.Commun. 1994;200:395–400. doi: 10.1006/bbrc.1994.1462. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa T, Kakehata S, Akaike N, Komune S, Takasaka T, Uemura T. Effects of Ca2+ antagonists and aminoglycoside antibiotics on Ca2+ current in isolated outer hair cells of guinea pig cochlea. Brain Res. 1992;580:345–347. doi: 10.1016/0006-8993(92)90966-d. [DOI] [PubMed] [Google Scholar]
- 22.Nakagomi S, Kiryu-Seo S, Kiyama H. Endothelin-converting enzymes and endothelin receptor B messenger RNAs are expressed in different neural cell species and these messenger rnas are coordinately induced in neurons and astrocytes respectively following nerve injury. Neuroscience. 2000;101:441–449. doi: 10.1016/s0306-4522(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 23.Niggel J, Sigurdson W, Sachs F. Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J Membr.Biol. 2000;174:121–134. doi: 10.1007/s002320001037. [DOI] [PubMed] [Google Scholar]
- 24.Ostrow LW. State University of New York at Buffalo, School of Medicine and Biomedical Sciences, Department of Physiology and Biophysics. 2005. Mechanosensation and Endothelin in Astrocytes [Thesis/Dissertation] [Google Scholar]
- 25.Ostrow LW, Langan TJ, Sachs F. Stretch-induced endothelin-1 production by astrocytes. Journal of Cardiovascular Pharmacology. 2000;36:S274–S277. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- 26.Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes--hypothetical roles in CNS pathophysiology. Brain Research Reviews. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Redman RS, Silinsky EM. Decrease in calcium currents induced by aminoglycoside antibiotics in frog motor nerve endings. Br.J Pharmacol. 1994;113:375–378. doi: 10.1111/j.1476-5381.1994.tb16998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SD, Peters CM, Pomonis JD, Hagiwara H, Ghilardi JR, Mantyh PW. Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia. 2003;41:180–190. doi: 10.1002/glia.10173. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JP, Wilson DJ. Preparation and characterization of type 1 astrocytes cultured from adult rat cortex, cerebellum, and striatum. Glia. 1992;5:75–80. doi: 10.1002/glia.440050111. [DOI] [PubMed] [Google Scholar]
- 30.Siren AL, Knerlich F, Schilling L, Kamrowski-Kruck H, Hahn A, Ehrenreich H. Differential glial and vascular expression of endothelins and their receptors in rat brain after neurotrauma. Neurochemical Research. 2000;25:957–969. doi: 10.1023/a:1007552408463. [DOI] [PubMed] [Google Scholar]
- 31.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a Peptide Toxin from Grammostola spatulata Spider Venom that Blocks Cation-selective Stretch-activated Channels. Journal of General Physiology. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 33.Tong JF, Du GG, Chen SRW, MacLennan DH. HEK-293 cells possess a carbachol- and thapsigargin-sensitive intracellular Ca2+ store that is responsive to stop-flow medium changes and insensitive to caffeine and ryanodine. Biochemical Journal. 1999;343:39–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang MCS, Lo ACY, Cheung PT, Chung SSM, Chung SK. Perinatal hypoxia-/ischemia-induced endothelin-I mRNA in astrocyte-like and endothelial cells. Neuroreport. 2001;12:2265–2270. doi: 10.1097/00001756-200107200-00044. [DOI] [PubMed] [Google Scholar]
- 35.Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. Journal of Neuroscience. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res.Brain Res.Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 37.Wu VW, Schwartz JP. Cell culture models for reactive gliosis: new perspectives. J Neurosci.Res. 1998;51:675–681. doi: 10.1002/(SICI)1097-4547(19980315)51:6<675::AID-JNR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.