Abstract
Increased insulin resistance is frequently associated with chronic liver disease and is a pathophysiological feature of hepatogenous diabetes. Distinctive factors including hepatic parenchymal cell damage, portal-systemic shunting and hepatitis C virus are responsible for the development of hepatogenous insulin resistance/diabetes. Although it remains unclear whether insulin secretion from pancreatic beta cells is impaired as it is in type 2 diabetes, retinopathic and cardiovascular risk is low and major causes of death in cirrhotic patients with diabetes are liver failure, hepatocellular carcinoma and gastrointestinal hemorrhage. Hemoglobin A1c is an inaccurate marker for the assessment and management of hepatogenous diabetes. Moreover, exogenous insulin or sulfonylureas may be harmful because these agents may promote hepatocarcinogenesis. Thus, pathogenesis, cause of death, assessment and therapeutic strategy for hepatogenous insulin resistance/diabetes differ from those for lifestyle-related type 2 diabetes. In this article, we review features of insulin resistance in relationship to chronic liver disease. We also discuss the impact of anti-diabetic agents on interferon treatment and hepatocarcinogenesis.
Keywords: Viral hepatitis, Hyperinsulinemia, Hypoglycemic drug, Hepatoma
INTRODUCTION
An association between diabetes mellitus (DM) and liver cirrhosis was first described by Bohan[1] and named as hepatogenous diabetes by Megyesi et al, in which 57% of cirrhotic patients showed increased insulin resistance[2]. Various pathogenetic factors are involved in development of the insulin resistance[3-7]. Serum insulin levels are higher in diabetic patients with chronic liver disease than those in patients with lifestyle-related DM[8], suggesting that besides over-eating, obesity and physical inactivity, distinctive factors may underlie the pathophysiology of hyperinsulinemia in patients with chronic liver disease.
Since blood glucose is delivered to the liver through the portal vein, hyperinsulinemia in patients with liver cirrhosis may be secondary to either hepatic parenchymal cell damage or to portal-systemic shunting[9-12]. The rate at which insulin is degraded in the liver is reduced in patients with liver cirrhosis[11,12]. Moreover, despite peripheral hyperinsulinemia, insulin levels in the portal and hepatic veins are decreased in cirrhotic patients with portal systemic shunting[9,10]. However, hyperinsulinemia is also seen in patients with chronic hepatitis C virus (HCV) infection who do not show both severe hepatic parenchymal cell damage and portal-systemic shunting[6,8,13-16], indicating that increased hepatic insulin resistance is another factor related to hyperinsulinemia in patients with liver disease, particularly in HCV-related chronic liver disease[8,13,17-21].
PATHOGENESIS OF INSULIN RESISTANCE IN PATIENTS WITH CHRONIC HEPATITIS C
Insulin resistance parallels the liver fibrosis stage[22-26] and is associated with a reduced level of sustained virological response (SVR) to pegylated interferon and ribavirin[27-30]. Thus, insulin resistance is involved in the disease progression and success of treatment and it is important to understand the pathogenesis of insulin resistance in patients with chronic hepatitis C.
Changes in serum levels of leptin, adiponectin, tumor necrosis factor-alpha and interleukin-6 are known to be associated with the development of insulin resistance[31-36]. However, in patients with chronic hepatitis C, changes in these cytokines are not always correlated with insulin resistance[37-39]. On the other hand, insulin resistance is increased in the HCV core cDNA-transfected hepatoma cell lines and mice[8,40] and serum levels of HCV core protein are associated with the development of insulin resistance in patients with chronic hepatitis C[14,41]. Furthermore, insulin resistance is correlated with HCV viral kinetics[42,43] and is improved by clearance of HCV by interferon therapy[44-47]. These findings suggest that HCV per se is an important factor for the development of insulin resistance.
Recently, the relationship between HCV genotype and insulin resistance has been revealed. HCV genotypes 1, 3 and 4 associated with more severe insulin resistance[24,42,48]. In human hepatoma cell lines, HCV genotype 1 up-regulates suppressor of cytokine signaling (SOCS) 3 and causes ubiquitination of insulin receptor substrate (IRS)1/2, which subsequently suppresses insulin-induced phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase and Akt and reduces glucose uptake (Figure 1)[8]. These changes are not seen in hepatoma cell lines infected with HCV genotype 2, suggesting that IRS1/2 degradation through up-regulation of SOCS3 is a genotype-specific mechanism[49]. In agreement with these results of basic research, hepatic expression of SOCS3 is higher in patients with HCV genotype 1 than in those with genotype 2 and increased hepatic expression of SOCS3 is correlated with poor response to antiviral treatment[50,51]. Two further mechanisms are reported in HCV genotype 1: activation of the mammalian target of rapamycin[52] and up-regulation of serine phosphorylation of IRS1(Figure 1)[43]. In addition, amino acid substitutions in the core region of HCV genotype 1b [Gln70 (His70) and/or Met91] have recently been reported as significant predictors of severe insulin resistance[53,54]. Although the underlying molecular mechanisms remain unclear, these findings indicate a unique molecular pathogenesis for insulin resistance in HCV genotype 1.
Figure 1.
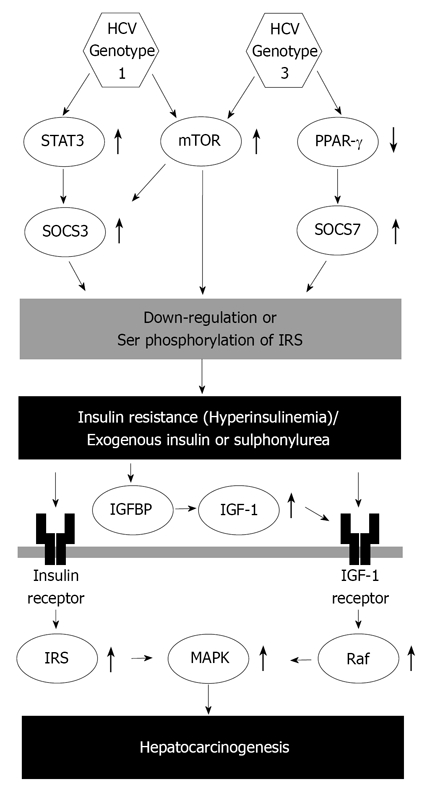
Scheme for HCV genotype difference in the molecular pathogenesis of insulin resistance and hepatocarcinogenesis. HCV: Hepatitis C virus; STAT: Signal transducer and activator of transcription; SOCS: Suppressor of cytokine signaling; mTOR: Mammalian target of rapamycin; PPAR: Peroxisome proliferator-activated receptor; IGFBP: Insulin-like growth factor binding protein; IGF: Insulin-like growth factor; IRS: Insulin receptor substrate; MAPK: Mitogen-activated protein kinase.
HCV genotype 3 also causes down-regulation of IRS1; however, the molecular pathogenesis differs from that of HCV genotype 1. HCV genotype 3 promotes down-regulation of IRS1 by up-regulating SOCS7 but not SOCS3 (Figure 1)[52]. SOCS-7 mRNA expression is independent of signal transducer and activator of transcription 3 and is modulated by peroxisome proliferator-activated receptor gamma activity (Figure 1)[52,55]. HCV genotype 4 is the most common variant in the Middle East and Africa and is increasing in prevalence in Western countries[56]. Infection with HCV genotype 4 is associated with a high prevalence of hepatic steatosis and obesity; however, the impact of adiponectin on insulin resistance remains controversial[57,58] and specific mechanisms of insulin resistance in HCV genotype 4 infection also remain unclear.
Besides direct association of HCV with intracellular insulin signaling, hepatic steatosis is associated with increased BMI and insulin resistance and HOMA index is reported to be a predictor of SVR in patients with HCV non 3 genotypes[27,59-62]. In patients with HCV genotype 3, hepatic steatosis directly correlates with circulating and hepatic viral load, which is mediated by an impaired very-low-density lipoprotein assembly and secretion and by an up-regulation of the sterol depending protein signaling pathway, which regulates de novo lipogenesis and inhibits mitochondrial fatty acid b-oxidation[63,64].
CHANGES IN PANCREATIC BETA CELLS IN PATIENTS WITH LIVER DISEASE
A decrease in islet mass and/or beta-cell dysfunction is a pathogenesis for type 2 DM[65,66]. In patients with chronic liver disease, impairment of insulin secretion is also reported[11,67]; however, insulin resistance/hyperinsulinemia is also characteristic in such patients[8,13,17-21] and it therefore remains unclear whether the pathogenesis of hepatogenous DM is same as that of type 2 DM.
Pancreatic islet hypertrophy is reported in surgical biopsy tissue of patients with liver cirrhosis[68]. Islet hypertrophy and hyperplasia are also reported in thioacetamide-treated rats[69] and in HCV-core transgenic mice[40]. Moreover, Takei et al reported that islets in patients with cirrhosis show higher proliferation and lower apoptosis compare to those in patients with no chronic liver disease[70]. These findings suggest that hyperinsulinemia in cirrhotic patients may be caused by an adaptive response of the pancreatic beta cells to increased insulin resistance.
Although cross-talk between the pancreas and liver is an important issue in the development of insulin resistance, little is known about this relationship. Further studies regarding morphological and pathological changes of pancreatic alpha-or beta cells are required to characterize the pathogenesis of insulin resistance in patients with liver disease.
CAUSES OF DEATH IN DIABETIC PATIENTS WITH LIVER DISEASE
The prevalence of DM in patients with chronic liver disease is reportedly 18%-71%[18,20,71-73]. DM leads to several complications including cardiovascular disease. Generally, the therapeutic strategy in DM is to reduce the incidence of cardiovascular disease and to prevent a subsequent decrease in quality of life and improve prognosis. However, hepatogenous DM is less often associated with a positive family history, retinopathy and cardiovascular diseases[18,74-76]. In fact, major causes of death in cirrhotic patients with DM relate to liver disease or its complications, such as chronic liver failure, hepatocellular carcinoma (HCC) and gastrointestinal hemorrhage[18,19,77-79]. Therefore, the management of DM in patients with liver cirrhosis should aim to reduce such hepatic complications and to improve prognosis. Because the incidence of HCC has been well demonstrated to relate to DM[80], a major target in the management of DM should be to reduce the incidence of HCC in patients with liver cirrhosis.
ASSESSMENT OF DM IN PATIENTS WITH LIVER DISEASE
Plasma glucose and hemoglobin A1c (HbA1c) are generally used for routine assessment and management of patients with type 2 DM, whereas there is less information regarding the association between these markers and HCC incidence or prognosis in patients with liver cirrhosis. HbA1c level in patients with HCC is higher than in patients with liver cirrhosis or in control subjects[81]. In patients with liver cirrhosis, however, HbA1c does not properly represent glycemic control status in cirrhotic patients because of the short lifespan of erythrocytes caused by hypersplenism[82-86]. These data indicate that assessment and management of hepatogenous DM using HbA1c is inaccurate, although poor glucose control is associated with HCC incidence.
Strict control of blood glucose levels may improve survival in HCV patients. In patients with HCV-related liver cirrhosis, the prognosis for patients with hyperglycemia (fasting plasma glucose ≥ 7.0 mmol/L; 126 mg/dL) was worse than for those with normoglycemia[19]. Therefore, fasting plasma glucose < 7.0 mmol/L (126 mg/dL) appears to be meaningful in hepatogenous DM.
Fasting serum insulin and homeostasis model assessment of insulin resistance (HOMA-IR) are also used as markers of glucose tolerance. In patients with HCV infection, HCC development is associated with increased fasting serum insulin level and by HOMA-IR[87]. Moreover, HCC recurrence has also been demonstrated to be related to HOMA-IR[88,89]. In addition, prognosis is worse in HCC patients with increased fasting serum insulin level or HOMA-IR[90]. These data suggest that the assessment of insulin is also meaningful in patients with liver cirrhosis. Taken together, fasting plasma glucose and either serum insulin or HOMA-IR are candidate markers for the assessment of hepatogenous insulin resistance/DM. However, further studies are required to clarify the utility of these markers and their target values in terms of complications induced by liver cirrhosis including HCC or prognosis.
IMPACT OF ANTI-DIABETIC AGENTS IN PATIENTS WITH LIVER DISEASE
Exogenous insulin and sulphonylureas
Despite the recognition of this potential link between insulin resistance and life-threatening complications including HCC, there is no common therapeutic strategy for insulin resistance in patients with chronic liver disease. Since insulin is a growth-promoting hormone with mitogenic effects[91], exogenous insulin and sulphonylureas, which increase serum insulin levels, are considered to enhance carcinogenesis. In fact, a large-scale cohort study has reported that exogenous insulin increases the risk of malignancies in patients with DM[92,93]. Exogenous insulin and sulphonylureas are known to promote breast cancer[94], colorectal cancer[95,96] and pancreatic cancer[95,97] in patients with DM. Recently, a possible link between anti-diabetic agents and the risk of cancer is noted in the consensus statement from the American Diabetes Association and the American Cancer Society[98].
An association between anti-diabetic agents and hepatocellular carcinoma (HCC) was first described in 1986 by Lawson et al[99]. In addition, we, along with others, have recently shown that use of exogenous insulin or sulphonylurea increases the development and recurrence of HCC in patients with chronic hepatitis C (Table 1)[80,100-102]. Exogenous insulin or second-generation sulphonylurea increases serum insulin levels. Since insulin has mitogenic and cell proliferative effects, these anti-diabetic agents could be a carcinogenic factor. Insulin binds to insulin receptors and activates the mitogenactivated protein kinase pathway[91,103]. Insulin also cross-reacts with insulin like growth factor (IGF)-1 receptor and activates the Raf cascade, leading to mitosis and cell proliferation[104]. Moreover, excess insulin binds to IGF-binding proteins, resulting in increased levels
Table 1.
Effects of anti-diabetic agents in patients with chronic liver disease
| Anti-diabetic agent | Subjects | Outcome | Reference |
| Exogenous insulin or sulphonylurea | Patients with liver cirrhosis or HCC | Increased HCC risk | [100] |
| Exogenous insulin or sulphonylurea | Patients with chronic hepatitis C | Increased HCC risk | [101] |
| Exogenous insulin | Chronic viral hepatitis patients who had undergone curative resection for HCC | Increased risk of HCC recurrence | [102] |
| Metformin | Treatment-naïve female patients with HCV genotype 1-related chronic hepatitis and insulin resistance | Increased SVR rate | [16] |
| Metformin | Patients diabetes mellitus and liver cirrhosis or HCC | Decreased HCC risk | [101] |
| Metformin | Patients with liver cirrhosis or HCC | Decreased HCC risk | [112] |
| Pioglitazone | Chronic hepatitis C patients who had previously failed to respond to antiviral therapy | No increase in EVR rate | [115] |
| Pioglitazone | Treatment-naïve chronic hepatitis C patients with insulin resistance | Increased SVR rate | [116] |
HCC; hepatocellular carcinoma, EVR; early virological response, SVR; sustained virological response.
of free serum IGF-1 (Figure 1)[87,105-107]. Thus, hyperinsulinemia induced by use of exogenous insulin or sulphonylurea may enhance hepatocarcinogenesis through multiple pathways.
The association of exogenous insulin or second-generation sulphonylurea with HCC was more evident in females than in males[101]. Sex affects the development of HCC and females are less prone to HCC than males[108,109]; therefore, we assume that use of exogenous insulin or a 2nd-generation sulfonylurea may accelerate development of HCC mainly in patients who have negative factor for the development of HCC.
Metformin
Metformin is an oral biguanide with insulin-sensitizing effects. However, biguanides are reported to predispose patients with liver cirrhosis to lactic acidosis and are considered as a contraindication in this situation[110]. Recently, Romero-Gomez et al first reported that adding metformin to peginterferon and ribavirin is safe and improved insulin sensitivity in treatment-naïve patients with HCV genotype 1 infection and DM[16]. In an intent-to-treat analysis, no beneficial effects of metformin on SVR were seen; however, in female patients with insulin resistance, adding metformin to antiviral treatment doubled the SVR rate (58% vs 29%)[16]. Although the reason for this sex difference is still unclear, elevated estradiol-to-testosterone ratio is known to be associated with better response to metformin treatment[111], suggesting a possible association between sex hormones and metformin-induced high SVR rate. Donadon et al and our research group have reported that metformin reduced risk of HCC in patients with DM and chronic liver disease[101,112]. Metformin is also known to attenuate the response of cancer cells to insulin in vitro[113,114]. Thus, metformin has potential benefits as an insulin sensitizer for patients receiving antiviral treatment or those with liver cirrhosis(Table 1).
Pioglitazone
Pioglitazone is a thiazolidinedione with insulin-sensitizing effects. Recently, Overbeck et al reported that adding pioglitazone to pegylated interferon-alpha and ribavirin improves insulin resistance; however, none of the patients achieved a satisfactory virological response after 12 wk of treatment (Table 1)[115]. On the other hand, Khattab et al reported that pioglitazone improves sustained virological response to antiviral therapy in hepatitis C patients with insulin resistance (Table 1)[116]. The effect of pioglitazone on SVR therefore remains controversial; however, a difference in enrolled subjects may account for this discrepancy. The study by Overbeck et al enrolled patients with chronic hepatitis C who previously failed to respond to peginterferon plus ribavirin therapy[115], whereas the study by Khattab et al enrolled naïve chronic hepatitis C patients with insulin resistance[116]. Thus, pioglitazone may not enhance the effect of antiviral therapy in intractable chronic hepatitis C. However, insulin resistance is reduced in both studies and pioglitazone may therefore be able to improve insulin resistance-related complications in patients with HCV infection. Further study will need to focus on the effects of pioglitazone, not only on antiviral treatment but also on the development of hepatic fibrosis, hepatocarcinogenesis and patient prognosis.
Dipeptidyl peptidase-4 inhibitors
Dipeptidyl peptidase (DPP)-4 inactivates incretin hormones including glucagon-like peptide-1 (GLP-1)[117,118], which enhances insulin secretion and reduces body weight[119,120]. DPP-4 inhibitors are therefore used as anti-diabetic agents[117,118]. DPP-4 is also known as CD26, an immune-regulation molecule expressed on T-cells[121], and transfection of a HCV non-structural genome region is reported to increase DPP-4 expression in a hepatoma cell line[122]. Treatment of HCV-infected patients with interferon decreases serum DPP-4 activity, which is related to interferon-induced immune activation[123]. Although changes in DPP-4 activity after interferon treatment may just represent indirect evidence, one would think that changes in DPP-4 activity could be involved in the pathogenesis of HCV-related insulin resistance.
Although changes in GLP-1 and DPP-4 remain unclear in hepatogenous insulin resistance, we previously investigated changes of these molecules in patients with HCV infection[124]. The serum level of the active GLP-1 in HCV-infected patients is significantly lower than that in hepatitis B virus-infected patients and healthy subjects. On the other hand, DPP-4 is up-regulated in the serum, ileum and liver of HCV-infected patients more than that of hepatitis B-infected patients and healthy subjects. Taken together, it seems that inactivation of GLP-1 through up-regulation of DPP-4 is a possible pathogenetic mechanism for HCV-related insulin resistance.
DPP-4 inhibitors are now available in the clinical setting and decrease plasma glucose levels as well as HbA1c levels with a low incidence of hypoglycemia in patients with type 2 diabetes mellitus[125,126]. Unlike other anti-diabetic agents, DPP-4 inhibitors are metabolized in the kidney and rarely cause hepatic dysfunction[127,128]. Moreover, GLP-1 analogs improve insulin sensitivity in insulin-resistant obese fa/fa Zucker rats[129] and DPP-4 inhibitors increase hepatic glucose uptake[130]. Thus, further study will be focus on the effects of DPP-4 inhibitors on HCV-related insulin resistance.
COFFEE CONSUMPTION
In various studies including a large prospective study, patients with HCV-related liver disease with a regular coffee consumption show a lower rate of disease progression such as hepatic fibrosis[131-133] and HCC[134-138]. Recently, it was also reported that more than 3 cups per day coffee drinkers are three times more likely to have a virological response to peginterferon plus ribavirin treatment than non-drinkers[139]. Since coffee consumption increases insulin sensitivity[140] and inhibits the development of non-alcoholic fatty liver disease in healthy subjects[141], coffee intake may be protective by mechanisms modulating insulin sensitivity and resulting in a reduced extent of liver steatosis in patients with HCV infection.
CONCLUSION
In this paper, we summarize the features of insulin resistance in relationship to chronic liver disease. Pathogenesis, assessment and cause of death in insulin resistance related to liver disease differ from those of lifestyle-related insulin resistance. Furthermore, exogenous insulin or sulfonylureas may be harmful because these agents may promote hepatocarcinogenesis. There is, therefore, a need for a unique therapeutic strategy for hepatogenous insulin resistance.
Footnotes
Supported by the Grant-in-Aid for Young Scientists (B)(No. 22790874 to T.K.) and a Grant-in-Aid for Scientific Research (C)(No. 21590865 to M.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Health and Labour Sciences Research Grants for Research on Hepatitis from the Ministry of Health, Labour and Welfare of Japan
Peer reviewers: Papandreou Dimitrios, PhD, MD, RD, Assi-
stant Professor of Nutrition, Department of Health Science, University of Nicosia, Cyprus; Head of Pediatric Obesity Unit, Aristotle University of Thessaloniki, School of Medicine, Ahe-
pa General Hospital, P. Mela 22 GR 54622, Greece; Ignazio Grattagliano, MD, General & Internal Medicine, University of Bari, P.zza G. Cesare, 111, 70043 Bari, Italy
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
References
- 1.Bohan EM. Diabetes mellitus and cirrhosis of the liver; a case report. Del Med J. 1947;19:212–215. [PubMed] [Google Scholar]
- 2.Megyesi C, Samols E, Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967;2:1051–1056. doi: 10.1016/s0140-6736(67)90334-0. [DOI] [PubMed] [Google Scholar]
- 3.Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356–4364. doi: 10.3748/wjg.15.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943–1952. doi: 10.3748/wjg.v16.i16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009;16:4843–4857. doi: 10.2174/092986709789909620. [DOI] [PubMed] [Google Scholar]
- 6.Khattab MA. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol. 2009;15:3472–3479. doi: 10.3748/wjg.15.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–1547. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch J, Gomis R, Kravetz D, Casamitjana R, Terés J, Rivera F, Rodés J. Role of spontaneous portal-systemic shunting in hyperinsulinism of cirrhosis. Am J Physiol. 1984;247:G206–G212. doi: 10.1152/ajpgi.1984.247.3.G206. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto N, Ashida H, Kotoura Y, Nishioka A, Nishiwaki M, Utsunomiya J. Analysis of hepatic encephalopathy after distal splenorenal shunt--PTP image and pancreatic hormone kinetics. Hepatogastroenterology. 1993;40:360–364. [PubMed] [Google Scholar]
- 11.Iwasaki Y, Ohkubo A, Kajinuma H, Akanuma Y, Kosaka K. Degradation and secretion of insulin in hepatic cirrhosis. J Clin Endocrinol Metab. 1978;47:774–779. doi: 10.1210/jcem-47-4-774. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DG, Alberti KG, Faber OK, Binder C. Hyperinsulinism of hepatic cirrhosis: Diminished degradation or hypersecretion? Lancet. 1977;1:10–13. doi: 10.1016/s0140-6736(77)91652-x. [DOI] [PubMed] [Google Scholar]
- 13.Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–362. doi: 10.1111/j.1478-3231.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi T, Nagao Y, Tanaka K, Ide T, Harada M, Kumashiro R, Sata M. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109–114. [PubMed] [Google Scholar]
- 15.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M, et al. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702–1708. doi: 10.1002/hep.23206. [DOI] [PubMed] [Google Scholar]
- 17.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 18.Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677–681. doi: 10.1046/j.1440-1746.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwon SY, Kim SS, Kwon OS, Kwon KA, Chung MG, Park DK, Kim YS, Koo YS, Kim YK, Choi DJ, et al. Prognostic significance of glycaemic control in patients with HBV and HCV-related cirrhosis and diabetes mellitus. Diabet Med. 2005;22:1530–1535. doi: 10.1111/j.1464-5491.2005.01687.x. [DOI] [PubMed] [Google Scholar]
- 20.Pazhanivel M, Jayanthi V. Diabetes mellitus and cirrhosis liver. Minerva Gastroenterol Dietol. 2010;56:7–11. [PubMed] [Google Scholar]
- 21.Thuluvath PJ, John PR. Association between hepatitis C, diabetes mellitus, and race. a case-control study. Am J Gastroenterol. 2003;98:438–441. doi: 10.1111/j.1572-0241.2003.07256.x. [DOI] [PubMed] [Google Scholar]
- 22.Petta S, Cammà C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, Cabibi D, Di Stefano R, Ferraro D, et al. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31:507–515. doi: 10.1111/j.1478-3231.2011.02453.x. [DOI] [PubMed] [Google Scholar]
- 23.Chu CJ, Hung TH, Hwang SJ, Wang YJ, Yang CF, Lin HC, Lee FY, Lu RH, Chang CY, Chang FY, et al. Association of insulin resistance with hepatic steatosis and progression of fibrosis in Chinese patients with chronic hepatitis C. Hepatogastroenterology. 2008;55:2157–2161. [PubMed] [Google Scholar]
- 24.Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48:723–731. doi: 10.1002/hep.22392. [DOI] [PubMed] [Google Scholar]
- 25.Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136–1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 27.Khattab M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol. 2010;105:1970–1977. doi: 10.1038/ajg.2010.110. [DOI] [PubMed] [Google Scholar]
- 28.Grasso A, Malfatti F, De Leo P, Martines H, Fabris P, Toscanini F, Anselmo M, Menardo G. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984–990. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 32.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 33.Ruge T, Lockton JA, Renstrom F, Lystig T, Sukonina V, Svensson MK, Eriksson JW. Acute hyperinsulinemia raises plasma interleukin-6 in both nondiabetic and type 2 diabetes mellitus subjects, and this effect is inversely associated with body mass index. Metabolism. 2009;58:860–866. doi: 10.1016/j.metabol.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 34.de Vinuesa SG, Goicoechea M, Kanter J, Puerta M, Cachofeiro V, Lahera V, Gómez-Campderá F, Luño J. Insulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: effects of angiotensin II blockade. J Am Soc Nephrol. 2006;17:S206–S212. doi: 10.1681/ASN.2006080916. [DOI] [PubMed] [Google Scholar]
- 35.Manco M, Fernandez-Real JM, Equitani F, Vendrell J, Valera Mora ME, Nanni G, Tondolo V, Calvani M, Ricart W, Castagneto M, et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metab. 2007;92:483–490. doi: 10.1210/jc.2006-0960. [DOI] [PubMed] [Google Scholar]
- 36.Borst SE, Conover CF, Bagby GJ. Association of resistin with visceral fat and muscle insulin resistance. Cytokine. 2005;32:39–44. doi: 10.1016/j.cyto.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne-Carrie N, Grando-Lemaire V, Roulot D, Capeau J, Trinchet JC, et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol. 2010;53:827–833. doi: 10.1016/j.jhep.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66–73. doi: 10.1002/hep.21703. [DOI] [PubMed] [Google Scholar]
- 39.Liu CJ, Chen PJ, Jeng YM, Huang WL, Yang WS, Lai MY, Kao JH, Chen DS. Serum adiponectin correlates with viral characteristics but not histologic features in patients with chronic hepatitis C. J Hepatol. 2005;43:235–242. doi: 10.1016/j.jhep.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 40.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu H, Takatsuka K, Yoshida A, Yoshimatsu E, Matsui K, Iwabuchi S. Partial splenic embolization reverses insulin resistance in patients with liver cirrhosis. Intern Med. 2009;48:747–751. doi: 10.2169/internalmedicine.48.1649. [DOI] [PubMed] [Google Scholar]
- 42.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606–2612. doi: 10.1128/JVI.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado-Borrego A, Jordan SH, Negre B, Healey D, Lin W, Kamegaya Y, Christofi M, Ludwig DA, Lok AS, Chung RT. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:458–462. doi: 10.1016/j.cgh.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawaguchi Y, Mizuta T, Oza N, Takahashi H, Ario K, Yoshimura T, Eguchi Y, Ozaki I, Hisatomi A, Fujimoto K. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int. 2009;29:871–877. doi: 10.1111/j.1478-3231.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Tapias JM, Diago M, Escartín P, Enríquez J, Romero-Gómez M, Bárcena R, Crespo J, Andrade R, Martínez-Bauer E, Pérez R, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451–460. doi: 10.1053/j.gastro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Duseja A, Dhiman RK, Chawla Y, Thumburu KK, Kumar A, Das A, Bhadada S, Bhansali A. Insulin resistance is common in patients with predominantly genotype 3 chronic hepatitis C. Dig Dis Sci. 2009;54:1778–1782. doi: 10.1007/s10620-009-0844-y. [DOI] [PubMed] [Google Scholar]
- 49.Persico M, Russo R, Persico E, Svelto M, Spano D, Andolfo I, La Mura V, Capasso M, Tiribelli C, Torella R, et al. SOCS3 and IRS-1 gene expression differs between genotype 1 and genotype 2 hepatitis C virus-infected HepG2 cells. Clin Chem Lab Med. 2009;47:1217–1225. doi: 10.1515/CCLM.2009.280. [DOI] [PubMed] [Google Scholar]
- 50.Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F, et al. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009–1015. doi: 10.1002/hep.21782. [DOI] [PubMed] [Google Scholar]
- 51.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 53.Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, et al. Amino acid substitutions in the hepatitis C virus core region of genotype 1b are the important predictor of severe insulin resistance in patients without cirrhosis and diabetes mellitus. J Med Virol. 2009;81:1032–1039. doi: 10.1002/jmv.21473. [DOI] [PubMed] [Google Scholar]
- 54.Sumida Y, Kanemasa K, Hara T, Inada Y, Sakai K, Imai S, Yoshida N, Yasui K, Itoh Y, Okanoue T, et al. Impact of amino acid substitutions in hepatitis C virus genotype 1b core region on liver steatosis and glucose tolerance in non-cirrhotic patients without overt diabetes. J Gastroenterol Hepatol. 2011;26:836–842. doi: 10.1111/j.1440-1746.2010.06568.x. [DOI] [PubMed] [Google Scholar]
- 55.Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010;91:1678–1686. doi: 10.1099/vir.0.020644-0. [DOI] [PubMed] [Google Scholar]
- 56.Moucari R, Ripault MP, Martinot-Peignoux M, Voitot H, Cardoso AC, Stern C, Boyer N, Maylin S, Nicolas-Chanoine MH, Vidaud M, et al. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut. 2009;58:1662–1669. doi: 10.1136/gut.2009.185074. [DOI] [PubMed] [Google Scholar]
- 57.Ashour E, Samy N, Sayed M, Imam A. The relationship between serum adiponectin and steatosis in patients with chronic hepatitis C genotype-4. Clin Lab. 2010;56:103–110. [PubMed] [Google Scholar]
- 58.Derbala M, Rizk N, Al-Kaabi S, Amer A, Shebl F, Al Marri A, Aigha I, Alyaesi D, Mohamed H, Aman H, et al. Adiponectin changes in HCV-Genotype 4: relation to liver histology and response to treatment. J Viral Hepat. 2009;16:689–696. doi: 10.1111/j.1365-2893.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira AC, Parise ER, Catarino RM, Lanzoni V, Leite-Mor MM, Simon KA, Junqueira VB. Insulin resistance and not steatosis is associated with modifications in oxidative stress markers in chronic hepatitis C, non-3 genotype. Free Radic Res. 2009;43:1187–1194. doi: 10.3109/10715760903247249. [DOI] [PubMed] [Google Scholar]
- 60.Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:26–37. doi: 10.1111/j.1478-3231.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 61.Anty R, Gelsi E, Giudicelli J, Mariné-Barjoan E, Gual P, Benzaken S, Saint-Paul MC, Sadoul JL, Huet PM, Tran A. Glucose intolerance and hypoadiponectinemia are already present in lean patients with chronic hepatitis C infected with genotype non-3 viruses. Eur J Gastroenterol Hepatol. 2007;19:671–677. doi: 10.1097/MEG.0b013e3281532b9a. [DOI] [PubMed] [Google Scholar]
- 62.Hui JM, Kench J, Farrell GC, Lin R, Samarasinghe D, Liddle C, Byth K, George J. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J Gastroenterol Hepatol. 2002;17:873–881. doi: 10.1046/j.1440-1746.2002.02813.x. [DOI] [PubMed] [Google Scholar]
- 63.McPherson S, Jonsson JR, Barrie HD, O’Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;49:1046–1054. doi: 10.1016/j.jhep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 64.van der Poorten D, Shahidi M, Tay E, Sesha J, Tran K, McLeod D, Milliken JS, Ho V, Hebbard LW, Douglas MW, et al. Hepatitis C virus induces the cannabinoid receptor 1. PLoS One. 2010;5:e12841 pii. doi: 10.1371/journal.pone.0012841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stock MJ. An automatic, closed-circuit oxygen consumption apparatus for small animals. J Appl Physiol. 1975;39:849–850. doi: 10.1152/jappl.1975.39.5.849. [DOI] [PubMed] [Google Scholar]
- 66.Abdel-Halim SM, Guenifi A, Khan A, Larsson O, Berggren PO, Ostenson CG, Efendić S. Impaired coupling of glucose signal to the exocytotic machinery in diabetic GK rats: a defect ameliorated by cAMP. Diabetes. 1996;45:934–940. doi: 10.2337/diab.45.7.934. [DOI] [PubMed] [Google Scholar]
- 67.Narita R, Abe S, Kihara Y, Akiyama T, Tabaru A, Otsuki M. Insulin resistance and insulin secretion in chronic hepatitis C virus infection. J Hepatol. 2004;41:132–138. doi: 10.1016/j.jhep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Saitoh M. [Studies on histopathology of pancreas in portal hypertension] Nippon Shokakibyo Gakkai Zasshi. 1984;81:1444–1452. [PubMed] [Google Scholar]
- 69.Nagy I, Hajnal F, Mohácsi G, Németh J, Lászik Z, Pap A. Pancreatic trophism in experimental liver cirrhosis. Int J Pancreatol. 1993;14:157–166. doi: 10.1007/BF02786122. [DOI] [PubMed] [Google Scholar]
- 70.Takei K, Suda K. [Study of mechanisms of pancreatic fibrosis and structural changes in liver cirrhotic patients] Nippon Shokakibyo Gakkai Zasshi. 1997;94:92–100. [PubMed] [Google Scholar]
- 71.Buzzelli G, Chiarantini E, Cotrozzi G, Relli P, Matassi L, Romanelli RG, Gentilini P. Estimate of prevalence of glucose intolerance in chronic liver disease. Degree of agreement among some diagnostic criteria. Liver. 1988;8:354–359. doi: 10.1111/j.1600-0676.1988.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 72.Del Vecchio Blanco C, Gentile S, Marmo R, Carbone L, Coltorti M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res Clin Pract. 1990;8:29–36. doi: 10.1016/0168-8227(90)90093-9. [DOI] [PubMed] [Google Scholar]
- 73.Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 74.Marchesini G, Ronchi M, Forlani G, Bugianesi E, Bianchi G, Fabbri A, Zoli M, Melchionda N. Cardiovascular disease in cirrhosis--a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94:655–662. doi: 10.1111/j.1572-0241.1999.00931.x. [DOI] [PubMed] [Google Scholar]
- 75.Fujiwara F, Ishii M, Taneichi H, Miura M, Toshihiro M, Takebe N, Ishida W, Kaneko Y, Kato A, Suzuki K, et al. Low incidence of vascular complications in patients with diabetes mellitus associated with liver cirrhosis as compared with type 2 diabetes mellitus. Tohoku J Exp Med. 2005;205:327–334. doi: 10.1620/tjem.205.327. [DOI] [PubMed] [Google Scholar]
- 76.Kuriyama S, Miwa Y, Fukushima H, Nakamura H, Toda K, Shiraki M, Nagaki M, Yamamoto M, Tomita E, Moriwaki H. Prevalence of diabetes and incidence of angiopathy in patients with chronic viral liver disease. J Clin Biochem Nutr. 2007;40:116–122. doi: 10.3164/jcbn.40.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119–125. doi: 10.1016/0270-9139(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 78.El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122:1822–1828. doi: 10.1053/gast.2002.33650. [DOI] [PubMed] [Google Scholar]
- 79.Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, Suzuki M, Kanda T, Kawano S, Hiramatsu N, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70–75. doi: 10.1111/j.1572-0241.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 80.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025–3032. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bando Y, Kanehara H, Toya D, Tanaka N, Kasayama S, Koga M. Association of serum glycated albumin to haemoglobin A1C ratio with hepatic function tests in patients with chronic liver disease. Ann Clin Biochem. 2009;46:368–372. doi: 10.1258/acb.2009.008231. [DOI] [PubMed] [Google Scholar]
- 83.Cacciatore L, Cozzolino G, Giardina MG, De Marco F, Sacca L, Esposito P, Francica G, Lonardo A, Matarazzo M, Varriale A. Abnormalities of glucose metabolism induced by liver cirrhosis and glycosylated hemoglobin levels in chronic liver disease. Diabetes Res. 1988;7:185–188. [PubMed] [Google Scholar]
- 84.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. doi: 10.1507/endocrj.k10e-138. [DOI] [PubMed] [Google Scholar]
- 85.Koga M, Kasayama S, Kanehara H, Bando Y. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81:258–262. doi: 10.1016/j.diabres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Nomura Y, Nanjo K, Miyano M, Kikuoka H, Kuriyama S, Maeda M, Miyamura K. Hemoglobin A1 in cirrhosis of the liver. Diabetes Res. 1989;11:177–180. [PubMed] [Google Scholar]
- 87.Hung CH, Wang JH, Hu TH, Chen CH, Chang KC, Yen YH, Kuo YH, Tsai MC, Lu SN, Lee CM. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–2271. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Imai K, Takai K, Nishigaki Y, Shimizu S, Naiki T, Hayashi H, Uematsu T, Sugihara J, Tomita E, Shimizu M, et al. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol Res. 2010;40:376–382. doi: 10.1111/j.1872-034X.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 89.Miuma S, Ichikawa T, Taura N, Shibata H, Takeshita S, Akiyama M, Motoyoshi Y, Ozawa E, Fujimoto M, Kawashimo H, et al. The level of fasting serum insulin, but not adiponectin, is associated with the prognosis of early stage hepatocellular carcinoma. Oncol Rep. 2009;22:1415–1424. doi: 10.3892/or_00000583. [DOI] [PubMed] [Google Scholar]
- 90.Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N, et al. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–552. [PubMed] [Google Scholar]
- 91.Barker BE, Fanger H, Farnes P. Human Mammary Slices in Organ Culture. I. Method of Culture and Preliminary Observations on the Effect Of Insulin. Exp Cell Res. 1964;35:437–448. doi: 10.1016/0014-4827(64)90134-x. [DOI] [PubMed] [Google Scholar]
- 92.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 95.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 96.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 97.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lawson DH, Gray JM, McKillop C, Clarke J, Lee FD, Patrick RS. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med. 1986;61:945–955. [PubMed] [Google Scholar]
- 100.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–486. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 102.Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T, Ohta T, Shimizu K, Nakamoto Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939–1946. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 103.Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol. 2000;20:6323–6333. doi: 10.1128/mcb.20.17.6323-6333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 105.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 106.Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001;54:138–144. doi: 10.1136/mp.54.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003–1015. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 108.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 109.Wands J. Hepatocellular carcinoma and sex. N Engl J Med. 2007;357:1974–1976. doi: 10.1056/NEJMcibr075652. [DOI] [PubMed] [Google Scholar]
- 110.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 111.Horng SG, Wang TH, Wang HS. Estradiol-to-testosterone ratio is associated with response to metformin treatment in women with clomiphene citrate-resistant polycystic ovary syndrome (PCOS) Chang Gung Med J. 2008;31:477–483. [PubMed] [Google Scholar]
- 112.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 113.Goodwin PJ. Insulin in the adjuvant breast cancer setting: a novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26:833–834. doi: 10.1200/JCO.2007.14.7132. [DOI] [PubMed] [Google Scholar]
- 114.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 115.Overbeck K, Genné D, Golay A, Negro F. Pioglitazone in chronic hepatitis C not responding to pegylated interferon-alpha and ribavirin. J Hepatol. 2008;49:295–298. doi: 10.1016/j.jhep.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 116.Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30:447–454. doi: 10.1111/j.1478-3231.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 117.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 118.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 119.Brubaker PL. Minireview: update on incretin biology: focus on glucagon-like peptide-1. Endocrinology. 2010;151:1984–1989. doi: 10.1210/en.2010-0115. [DOI] [PubMed] [Google Scholar]
- 120.Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med. 2010;123:S19–S27. doi: 10.1016/j.amjmed.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Ohnuma K, Yamochi T, Hosono O, Morimoto C. CD26 T cells in the pathogenesis of asthma. Clin Exp Immunol. 2005;139:13–16. doi: 10.1111/j.1365-2249.2005.02683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harada T, Kim DW, Sagawa K, Suzuki T, Takahashi K, Saito I, Matsuura Y, Miyamura T. Characterization of an established human hepatoma cell line constitutively expressing non-structural proteins of hepatitis C virus by transfection of viral cDNA. J Gen Virol. 1995;76(Pt 5):1215–1221. doi: 10.1099/0022-1317-76-5-1215. [DOI] [PubMed] [Google Scholar]
- 123.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 124.Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 125.Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2010;30:463–484. doi: 10.1592/phco.30.5.463. [DOI] [PubMed] [Google Scholar]
- 126.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med. 2010;123:S28–S37. doi: 10.1016/j.amjmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 127.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 128.De Block CE, Van Gaal LF. GLP-1 receptor agonists for type 2 diabetes. Lancet. 2009;374:4–6. doi: 10.1016/S0140-6736(09)60942-9. [DOI] [PubMed] [Google Scholar]
- 129.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 130.Perreault L, Man CD, Hunerdosse DM, Cobelli C, Bergman BC. Incretin action maintains insulin secretion, but not hepatic insulin action, in people with impaired fasting glucose. Diabetes Res Clin Pract. 2010;90:87–94. doi: 10.1016/j.diabres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 131.Costentin CE, Roudot-Thoraval F, Zafrani ES, Medkour F, Pawlotsky JM, Mallat A, Hézode C. Association of caffeine intake and histological features of chronic hepatitis C. J Hepatol. 2011;54:1123–1129. doi: 10.1016/j.jhep.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 132.Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201–209. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, Lee WM, Lok AS, Di Bisceglie AM, Bonkovsky HL, et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50:1360–1369. doi: 10.1002/hep.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, Tsugane S; Japan Public Health Center-Based Prospective Study Group. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev. 2009;18:1746–1753. doi: 10.1158/1055-9965.EPI-08-0923. [DOI] [PubMed] [Google Scholar]
- 135.Ohishi W, Fujiwara S, Cologne JB, Suzuki G, Akahoshi M, Nishi N, Takahashi I, Chayama K. Risk factors for hepatocellular carcinoma in a Japanese population: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:846–854. doi: 10.1158/1055-9965.EPI-07-2806. [DOI] [PubMed] [Google Scholar]
- 136.Wakai K, Kurozawa Y, Shibata A, Fujita Y, Kotani K, Ogimoto I, Naito M, Nishio K, Suzuki H, Yoshimura T, et al. Liver cancer risk, coffee, and hepatitis C virus infection: a nested case-control study in Japan. Br J Cancer. 2007;97:426–428. doi: 10.1038/sj.bjc.6603891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohfuji S, Fukushima W, Tanaka T, Habu D, Tamori A, Sakaguchi H, Takeda T, Kawada N, Seki S, Nishiguchi S, et al. Coffee consumption and reduced risk of hepatocellular carcinoma among patients with chronic type C liver disease: A case-control study. Hepatol Res. 2006;36:201–208. doi: 10.1016/j.hepres.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 138.Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528–534. doi: 10.1016/j.jhep.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 139.Freedman ND, Curto TM, Lindsay KL, Wright EC, Sinha R, Everhart JE; HALT-C Trial Group. Coffee Consumption is Associated with Response to Peginterferon and Ribavirin Therapy in Patients with Chronic Hepatitis C. Gastroenterology. 2011. p. Mar 1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arnlöv J, Vessby B, Risérus U. Coffee consumption and insulin sensitivity. JAMA. 2004;291:1199–1201. doi: 10.1001/jama.291.10.1199-b. [DOI] [PubMed] [Google Scholar]
- 141.Catalano D, Martines GF, Tonzuso A, Pirri C, Trovato FM, Trovato GM. Protective role of coffee in non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2010;55:3200–3206. doi: 10.1007/s10620-010-1143-3. [DOI] [PubMed] [Google Scholar]


