Abstract
Imatinib (trade name Gleevec) preserves fertility in female mice treated with the common chemotherapeutic agent cisplatin. Imatinib seems to block an apoptotic pathway activated by cisplatin in ovarian germ cells (pages 1179– 1185). The findings could lead to new ways to protect germ cells from the damaging effects of cancer treatment.
For many individuals with cancer, the decision to protect their fertility from the damaging effects of radiation and chemotherapy is complicated by their age, marital status, the time they have to delay treatment and, sometimes, the uncertainty of surviving their disease. In the past two years, a remarkable alignment between oncologists and fertility specialists has increased access to information about the fertility threats of treatment and the options for fertility preservation.[1] Men and pubertal boys, for instance, now routinely have access to sperm banks and home sperm collection kits.[2] But girls and young women have traditionally been left behind in both the discussion about fertility threats and the options available to them. That, too, is quickly changing.
The current treatment options for young women with cancer who can delay the start of cancer treatment is hormonal intervention followed by cryopreservation of oocytes or fertilized eggs.[1]If the patient has no time to wait, she can opt to have one ovary removed and cryopreserved for her later use.[3] An experimental option for use of the tissue is to transplant pieces of the cryopreserved ovarian cortex back onto her nonfunctional remaining ovary. This process has resulted in the resumption of endocrine function and in live human births.[3] The procedure is only possible for individuals with low risk of preexisting contaminating cancer cells in the tissue. For the high-risk group, maturation of eggs within ovarian follicles in vitro has been successful in mice and is being adapted to humans.[4,5]
The most important breakthrough in this field would be the development of chemotherapeutics that do not harm the resting germ cell or, what I call fertoprotective adjuvant therapy, which would protect the eggs from the damaging effects of drugs. In this issue of Nature Medicine, Gonfloni et al.[6] examine a combination therapy in the latter category. They show that an approved drug, imatinib, may be the first drug in this class.
One of the chemotherapeutic agents commonly used to treat cancer is cisplatin, which acts by cross-linking DNA and inducing the cell’s apoptosis machinery; the effect is the elimination of rapidly growing cancer cells as well as normal cells such as oocytes.[7] The ‘off target’ effects of cisplatin cause substantial illness during the drug treatment and infertility long after drug treatment is over.[8]
After birth, the primordial oocyte pool, also known as the ‘ovarian reserve’, lies in a protected dormant state until individual oocytes are called upon to enter the growing population that will result in the release of a single mature oocyte each month.[9] The tumor suppressor protein p63 is expressed in the dormant and growing oocyte, but elimination of the gene does not interrupt normal development of the egg nor does it affect subsequent fertility, at least in mice.[10,11]
Previous studies have shown that when an oocyte is challenged with radiation, germ cells die through upregulation of the p63 pathway.[12] Thus, p63 does not act during normal oocyte fate decisions but is upregulated in response to the exceptional damage created by external radiation.
Gonfloni et al.[6] now observe a similar effect with cisplatin: p63 serves to eliminate the germ cells that are irreparably injured by iatrogenic chemotherapy, ensuring that the chromosomal damage is not transferred to the subsequent generation.
Gonfloni et al.[6] hypothesized that inactivation of p63 during the treatment period would protect the ovarian reserve. The kinase responsible for activating p63 was unknown, and the authors show that the kinase c-Abl is activated in oocytes by cisplatin-mediated DNA damage.[6] Imatinib is a potent inhibitor of c-Abl and, when delivered with cisplatin to immature mice, blocked the immediate appearance of apoptotic oocytes. Furthermore, treatment of immature mice with cisplatin resulted in loss of the primordial follicle cohort and premature sterility in adults. Moreover, treatment of immature mice with imatinib and cisplatin in tandem resulted in normal-appearing follicles in adult ovarian tissue and a normal number of pups born to the treated mothers (Fig. 1).
Figure 1.
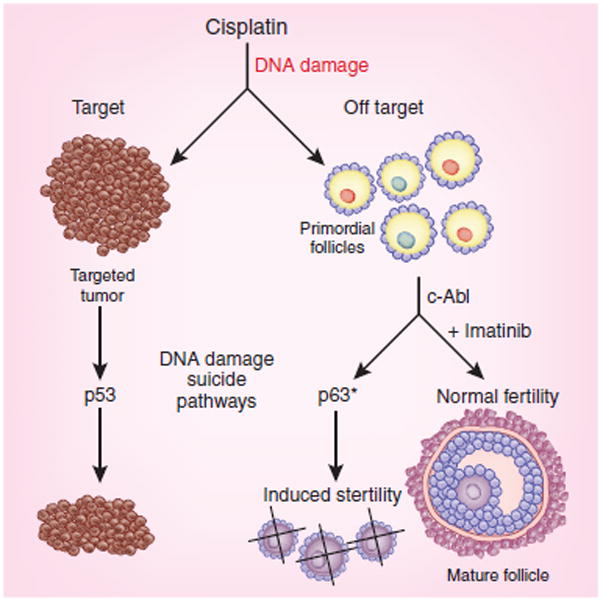
Imatinib protects primoridal follicles from cisplatin-mediated death. Cisplatin is a chemotherapeutic agent that kills tumor cells by inducing widespread DNA damage which activates a cascade of signaling pathways leading to cellular apoptosis. Unfortunately, the ovarian germ cells are also targeted by the drug and activate a parallel cell death pathway mediated by the kinase c-Abl and the tumor suppressor gene product p63. Imatinib blocks c-Abl activity and, when delivered with cisplatin, blocks the treatment-induced germ cell death, sparing the ovarian follicles during treatment. Adult mice treated with cisplatin alone become sterile, whereas mice treated with cisplatin plus imatinib are fertile6.
On the face of it, the new findings seem to be a breakthrough idea. But for individuals with cancer who wish to protect their fertility, selective inactivation of the p63 pathway comes at theoretical costs that must be weighed against other available options. Imatinib treatment does not change how cisplatin works, leading to the question of how healthy the remaining oocytes are. Does the endogenous repair mechanism right the wrong of the DNA adducts in a timely fashion? The lesson from aneuploidy studies is that badly damaged eggs can persist and create embryos, but miscarriage and babies with mild to devastating birth defects are the most common outcomes of chromosomal anomalies.[13] Moreover, imatinib has been shown to amplify the effects of cisplatin in other cancer cell types, leading to some caution and careful assessment of the new off target sites.[14] Finally, c-Abl is a common kinase activated in tumor suicide pathways.[7] Whether imatinib reduces the effectiveness of cisplatin on the main tumor target must be carefully explored.
These studies are sufficiently tantalizing to inspire additional basic research on the direct and indirect effects of fertoprotective adjuvant treatment, particularly in nonhuman primate models. The preclinical models should parallel clinical studies designed to assess tumor responsiveness and fertility preservation. Such clinical studies, like many in this field, will be challenging; they will require large, longitudinal cohorts to assess menstrual cycles, the time to delivery of offspring and the health of the babies. The health status of the mother and the healthiness of her uterus to bear a pregnancy must all be factored into the analysis.
Despite the hurdles, improved medical management plans for young people with cancer are urgently needed. The use of imatinib as an adjuvant to protect fertility in cisplatin-treated women is an intriguing idea that should be pursued.
References
- 1.Jeruss JS, Woodruff TK. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klosky JL, et al. Pediatr Hematol Oncol. 2009;26:252–260. doi: 10.1080/08880010902901294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Wolff M, et al. Eur J Cancer. 2009;45:1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, et al. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu M, et al. Hum Reprod. 2009;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonfloni S, et al. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Lippard SJ. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 8.Green DM, et al. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tingen C, Kim A, Woodruff TK. Mol Hum Reprod. 2009 August 26; doi: 10.1093/molehr/gap073. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Suh EK, et al. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 12.Livera G, et al. Reproduction. 2008;135:3–12. doi: 10.1530/REP-07-0054. [DOI] [PubMed] [Google Scholar]
- 13.Hassold T, Hall H, Hunt P. Hum Mol Genet. 2007;16(Spec No. 2):R203–R208. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- 14.Wang-Rodriguez J, et al. Laryngoscope. 2006;116:1409– 1416. doi: 10.1097/01.mlg.0000225895.40732.52. [DOI] [PubMed] [Google Scholar]


