Abstract
Sensory systems create neural representations of environmental stimuli and these representations can be associated with other stimuli through learning. Are spike patterns the neural representations that get directly associated with reinforcement during conditioning? In the moth Manduca sexta, we found that odor presentations that support associative conditioning elicited only one or two spikes on the odor’s onset (and sometimes offset) in each of a small fraction of Kenyon cells. Using associative conditioning procedures that effectively induced learning and varying the timing of reinforcement relative to spiking in Kenyon cells, we found that odor-elicited spiking in these cells ended well before the reinforcement was delivered. Furthermore, increasing the temporal overlap between spiking in Kenyon cells and reinforcement presentation actually reduced the efficacy of learning. Thus, spikes in Kenyon cells do not constitute the odor representation that coincides with reinforcement, and Hebbian spike timing–dependent plasticity in Kenyon cells alone cannot underlie this learning.
The sense of smell is very flexible. For animals, odors can take on arbitrary meanings as warranted by the changing environment. Understanding how olfactory stimuli are represented in the brain is a prerequisite for studying how such representations become associated with other modalities.
The relatively simple structure of the insect brain makes it useful for studying the neural bases of sensory coding and associative learning. In insects, neural representations of odors begin in the antenna, where volatile molecules bind to olfactory receptor neurons, which respond with trains of action potentials and periods of inhibition1. These receptor neurons send processes to the antennal lobe, where new odor representations arise from the circuit interactions of the receptor neurons, local interneurons and projection neurons. In the antennal lobe, representations of any given odor are transformed into elaborate and enduring spiking patterns that are distributed across a large fraction of the projection neuron population2–5. The projection neurons, which provide the only output from the antennal lobe, send processes to the mushroom body, where another set of odor representations arise. Here, the output of hundreds of projection neurons, each contributing dense bursts of spontaneous and odor-elicited spikes, is transformed into something markedly sparse: rare single spikes on a nearly silent background in a tiny fraction of the tens of thousands of Kenyon cells6. The Kenyon cells then send processes to the lobes of the mushroom body.
The mushroom bodies have long been linked to associative learning and memory. In many insects, they are sites of multimodal convergence that include olfactory and gustatory inputs7–9. Furthermore, many types of studies indicate the mushroom bodies are important in olfactory learning. Insects that lack normally developed mushroom bodies suffer from learning and memory deficits10,11. Experimentally inactivating the mushroom bodies by cooling them12 or by conditionally blocking synaptic transmission from Kenyon cells13–15 prevents insects from forming or retaining associative memories. In Drosophila, work with mutants suffering from memory deficits found that proteins critical for memory are concentrated in the mushroom bodies16.
To understand how neural representations of odors become associated with reinforcement stimuli, we first sought to characterize the physiological responses of neurons along the olfactory pathway to odor pulses in the context of an associative learning procedure. The moth Manduca sexta has proved to be accessible for intracellular recording17 and is also capable of performing an appetitive olfactory learning task, proboscis extension reflex (PER) conditioning18. Thus, we examined neural representations of odor in the moth and performed PER training under identical conditions.
We used lengthy odor pulses (typically 4 s), as they correspond to odor exposures that moths encounter while feeding on flowers and because such pulses have often been used for studies of olfactory conditioning18–22. With intracellular recordings, we found that projection neurons in themoth’s antennal lobe responded to long odor pulses with extended and complex firing patterns that varied with the odor. We found, with intracellular and multiunit recordings, that Kenyon cells were almost silent at rest; odor responses typically consisted of single spikes in a small population of Kenyon cells. Notably, spiking in Kenyon cells occurred almost entirely on an odor pulse’s onset and sometimes offset, with few spikes occurring in between. For any given odor, the population of Kenyon cells responding to the stimulus onset was usually different from the population responding to the offset. This response feature allowed us to examine the ability of onset and offset spiking in Kenyon cells to support associative conditioning.
Having characterized the responses of Kenyon cells to these odor stimuli, we then used a set of behavioral studies to test whether pre- and postsynaptic neurons must both fire spikes nearly simultaneously, which is a key requirement of spike timing–dependent plasticity (STDP), a form of Hebbian learning. In the locust, STDP has been shown to occur between Kenyon cells and followers23. To test the relationship between odor-evoked spikes in Kenyon cells and olfactory learning in the Kenyon cells, we used several behavioral procedures with different intervals between odor and reward. Our results indicate that reinforcement that was delivered seconds after the conclusion of spiking responses in Kenyon cells was able to support the formation and recall of associative memory. Thus, the acquisition of short-term memory does not require the concurrence of spikes in Kenyon cells with activation of a reward pathway in the moth. Furthermore, we found that reinforcement provided specifically following the off response (spiking occurring in 1.5 s of odor offset) could not support associative learning. These results indicate that appetitive associative conditioning cannot occur by a Hebbian STDP mechanism alone in the Kenyon cells.
RESULTS
Odor representation in the antennal lobe and mushroom body
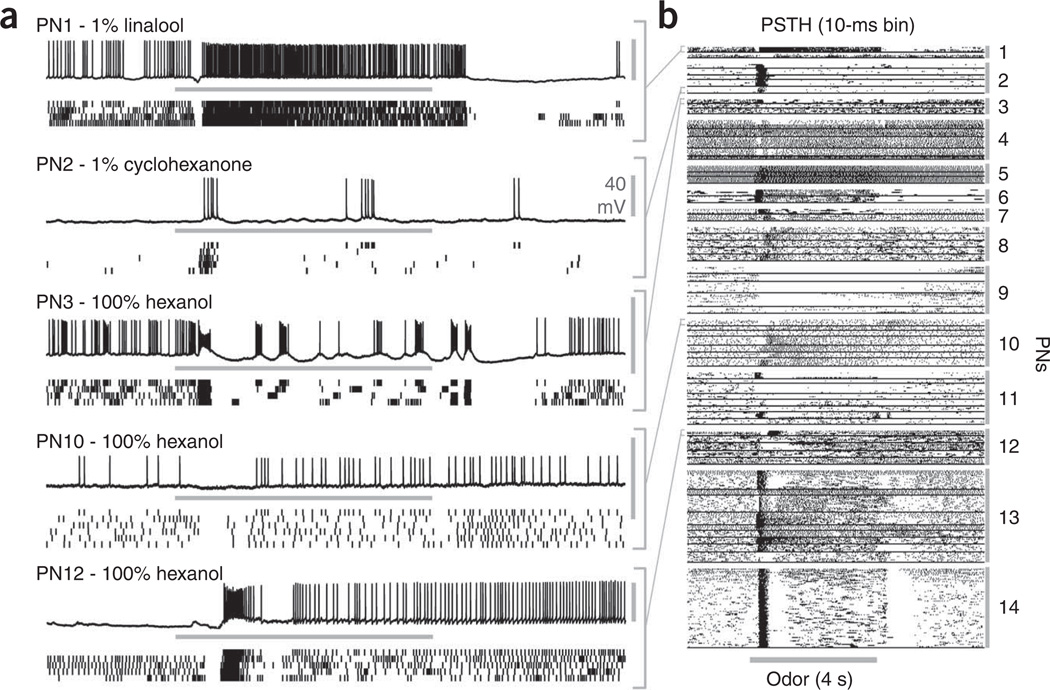
To characterize odor representations in the antennal lobe, we made intracellular recordings from projection neurons and analyzed their responses to odor pulses presented to the antenna. In all cases, we confirmed the cell type by dye injection and subsequent histological analysis (Supplementary Fig. 1 online). Consistent with earlier studies in locusts2,4,5, moths3,24 and Drosophila25,26, we found that, over the course of an odor pulse, different projection neurons responded with slowly changing temporal patterns of spikes and periods of inhibition (Fig. 1). These distributed, time-varying firing patterns were reliable over repeated trials and varied greatly with the odor. A standard test for information content4 showed that these odor-elicited patterns were sufficiently reliable and distinctive to allow for classification far exceeding chance (Supplementary Fig. 2 online); thus, these firing patterns could carry information about the odors. We were particularly interested in characterizing responses to relatively lengthy pulses of odor, which match the conditions in which moths naturally learn about food sources and which have often been used to test perception, learning and memory in insects, including moths18,20 and honeybees19,21. In projection neurons, responses to 4-s odor pulses generally consisted of lengthy trains of spikes, with 51% of odor-evoked spikes occurring in the first 0.6 s after odor arrived at the antenna (Fig. 1; timing determined by reference to an electroantennogram, data not shown). We also found that odors evoked the oscillatory synchronization of projection neurons, which, in turn, regulated the fine timing of spiking in the Kenyon cells (I. Ito et al., Soc. Neurosci. Abstr. 541.8, 2006).
Figure 1.
Projection neurons respond reliably to odors, and different odors evoke different temporally structured patterns of activity. (a) Examples of intracellular recordings of projection neurons (PN) responding to 4-s odor pulses (stimulus duration indicated by horizontal bars). Top, intracellular record of 1 trial. Bottom, rasters showing spikes from multiple trials. In PN1, 1% linalool induced brief inhibition followed by sustained spiking that outlasted the stimulus and a prolonged period of inhibition at the offset. In PN2, 1% cyclohexanone evoked only brief excitation. PN3, PN10 and PN12 showed distinct patterns to the same odor (100% hexanol). PN3 and PN10 showed excitatory off responses as well. Vertical scale bars represent 40 mV. (b) Peri-stimulus time histograms (PSTHs) showed reliable odor responses in projection neurons to 4-s odor pulses. These firing patterns contained information about odors (see Supplementary Fig. 2). Spikes were binned (10 ms) and bins with at least one spike are indicated by a black dot. One row represents one trial, and 62 projection neuron–odor combinations, each separated by a horizontal black bar, are shown. All projection neurons (except PN14) were tested with more than one odor.
To systematically examine the neural representation of odors by populations of Kenyon cells in the moth, we made intracellular recordings from Kenyon cells and extracellular recordings from the mushroom body with tetrodes (see Methods). Using 4-s pulses of each of a panel of 21 odors, we tested a set of 117 Kenyon cells (recorded extracellularly, 2,457 Kenyon cell–odor combinations, 10 trials per odor, each trial was 12 s long with an intertrial interval of 20 s, Fig. 2a; a smaller set of intracellular recordings from Kenyon cells revealed the same response properties, Fig. 2b).We detected extremely little spontaneous activity in Kenyon cells in the pre-stimulation period (2 s) of each trial; in 24,570 trials (49,140 s), we observed only 203 spikes. This spontaneous firing rate (mean ± s.d., 0.0041 ± 0.0122 Hz; range, 0–0.1696 Hz; n = 117) was ~2,000-fold lower than the spontaneous firing rate that we observed in the projection neuron population (measured from intracellular recordings; mean ± s.d., 8.046 ± 5.899 Hz; range, 0–26Hz; n = 15). Despite the strong and constant convergent and excitatory drive from spontaneously active projection neurons, Kenyon cells remained inactive.
Figure 2.
Odor-elicited spiking in Kenyon cells is brief and sparse. (a) Examples of Kenyon cells (KC) responding to a panel of 21 odors. KC50a, KC41a and KC21a responded very sparsely, with either spikes at odor onset or offset. KC3a responded to a broader set of odors. KC1a was the most responsive cell in our set and fired reliably at different points in time for different odors. Ten trials were carried out for each odor. Rasters indicate spike times and the gray blocks indicate odor stimulation (4 s). See Methods for odors. (b) Spiking and subthreshold depolarization in Kenyon cells occurred mainly on odor onset and offset. The top trace indicates the intracellular voltage record and the dark horizontal line indicates odor delivery (4 s). The subsequent lines indicate the number of trials (one line per trial), and the rasters indicate spikes. Insets, enlarged membrane potential, averaged over first five trials, for on and off responses (times indicated as horizontal lines below rasters). (c) Histogram of Kenyon-cell firing probability (117 Kenyon cells, 10 trials each of 21 odors). The top brackets indicate the percentage of spikes during onset, middle and offset periods. The bottom brackets indicate the analysis bins used in subsequent panels. (d,e) Responses of Kenyon cells to odors were sparse. (e) Odor responses usually consisted of a single spike. Frequency distributions of odor-evoked spikes per trial measured over the full analysis bin are shown. (f) Different Kenyon cell ensembles were usually active during on, middle and off responses (ON, MD and OFF, respectively). MD&ON, overlap in spiking between middle and on responses; OFF&ON, overlap between off and on responses; OFF&MD, overlap with off and middle responses.
We found that Kenyon cells responded mainly to the onset of lengthy odor pulse: 72% of spikes evoked by an odor occurred in the first 0.6 s of a 4-s odor presentation (we refer to these early spikes as the ‘on response’) (Fig. 2c). Additional spikes sometimes occurred just after an odor’s offset (21% of spikes were off responses) and very few spikes occurred between these on and off responses (7% in the 3.4-s ‘middle response’ period). During the on responses, the mean firing rate, averaged over odors and trials, significantly increased (P < 0.0001, Wilcoxon signed rank test, n = 117 Kenyon cells, 0.6-s response bracket) about 21.5-fold from the basal firing level (activity during 2 s before odor stimulation). The mean firing rate during the off responses increased 3.5-fold (P < 0.0001, 3.4-s bracket) and increased by 1.3-fold during the middle responses (P < 0.005, 1.5-s bracket).
Most Kenyon cells responded to only a few of the 21 odors that we tested, although a subset of Kenyon cells responded to a broader range (Fig. 2a). In some experiments, we presented pulses of clean air as control stimuli. These presentations evoked no reliable responses (see Methods) in any of the 42 Kenyon cells that we tested this way. To characterize odor responses across the Kenyon cell population, we computed population sparseness (SP) and lifetime sparseness (SL)6,27 (see Methods). These measures, which take into account all of the odor-evoked spikes in all of the tested Kenyon cells, range from 0 to 1, where 1 is sparsest. Mean population sparseness SP (full) was 0.79 (Fig. 2d), indicating that a given odor elicited responses in very few cells. Similarly, mean lifetime sparseness was 0.72 (Fig. 2d), indicating that a given cell responded to a narrow range of odors, although a subset of Kenyon cells was more broadly tuned, as in the locust6. Most odor responses consisted of a single spike per trial and the maximum number of spikes in one responsive trial was 5 (Fig. 2e). These results indicate that odor representations in the moth mushroom body are extremely sparse: they consist of very few spikes in very few neurons.
Spatiotemporal odor representations in Kenyon cells
When driven by a lengthy odor stimulus, the great majority of spikes that form the odor representation in the mushroom body occur at the onset and to a lesser extent the offset of an odor pulse (Fig. 2c). Are the Kenyon cells that fire at the odor onset the same ones that fire at odor offset? To analyze how spiking patterns in the mushroom body change over time, we divided the odor response time into three 1.5-s periods that together captured about 95% of all spikes (Fig. 2c,f). We chose to focus on responses of Kenyon cell–odor combinations that were relatively strong and reliable, which consisted of at least three responsive trials out of ten (see Methods for rate and reliability criteria). Our set of Kenyon cell–odor combinations elicited 145 reliable on and 39 reliable off responses; of these, an odor elicited reliable spiking in the same Kenyon cell both during onset and offset in only six cases. We observed only 13 reliable Kenyon cell–odor combinations during the middle time period, with two overlaps with the on response and two overlaps with the off response (Fig. 2f). Together, these findings indicate that odor responses in the mushroom body are spatially distributed and vary over the course of the stimulus. Thus, the moth olfactory system appears to use a time-varying, distributed spatiotemporal code to represent odors both in the antennal lobe and in the mushroom body.
To examine the effect of odor-pulse duration on Kenyon cells, we analyzed all of the spikes that we observed in another set of experiments (Fig. 3) and found that the probability of off response spiking increased with the length of the odor pulse. We almost never observed off responses following odor pulses of less than 750 ms (examples of Kenyon cells selected for their prominent off responses are shown in Fig. 3a). Odor pulses of at least 4 s produced the most off responses (Fig. 3b–d). On and off responses elicited in Kenyon cells by long odor pulses (4 and 18 s) were distributed almost exclusively around two narrow time ranges, 0–600 ms after the odor arrived at the antenna (determined by reference to electroantennogram recordings, data not shown) and 0–800 ms after the odor was removed by vacuum (Fig. 3c,d). On response spiking was maximal at around 65 ms after odor arrival.
Figure 3.
Kenyon cells responded only to the onset of brief odor pulses and to the onset and offset of long pulses. (a) Kenyon cell responses varied with odor pulse duration. Pulses at least 4 s long were most likely to induce odor-specific off responses. Briefer odor pulses generally elicited weaker or no off responses (see also b). Examples shown were selected for prominent off responses. Trials are shown from top to bottom (20 trials of 4-s odor pulses, then 6 shorter pulses, 3 trials each). (b) Off response probability increased with stimulus duration. Multiunit recordings of Kenyon cells (including 117 sorted cells from 16 animals, see Methods) responding to odor pulses of different durations (gray bars, tested in a randomized order, ten trials each). The histogram (bin size, 1 ms) combines the responses to five odors. (c) Long 4-s (black) and 18-s (gray) odor pulses evoked comparable onset and offset responses (arrows indicate the corresponding off responses). Multiunit recordings of Kenyon cells averaged across the four odors shown in d and across multiple trials are shown. (d) Examples of Kenyon cells responding to the offset of 18-s odor pulses. See Methods for the odor labels in a and d.
STDP alone cannot mediate odor learning in Kenyon cells
Hebbian STDP mechanisms require the temporal convergence of activated neural pathways. Do the spikes that we observed in Kenyon cells constitute the odor representation that coincides with reinforcement that supports learning? To test this, we examined the relative timing of odor-elicited spiking in Kenyon cells and sucrose reinforcement in the context of a learning procedure. We trained several groups of moths and compared the amount of learning elicited by procedures in which we varied the temporal intervals between the odor and the reward (Fig. 4).
Figure 4.
Greater temporal overlap between odor-elicited spiking in Kenyon cells and reinforcement delivery did not lead to more learning. (a) Diagrams illustrate PER conditioning procedures used to vary temporal overlap between spiking in Kenyon cells and sucrose delivery. Black traces represent time course of Kenyon cell spike response probability and gray boxes indicate analysis time windows used to compute conditioned stimulus (CS, odor)-elicited Kenyon cell spike probability concurrent with the unconditioned stimulus (US, sucrose) presentation shown in b (right ordinate). Conditioned stimulus was always paired with the unconditioned stimulus five times with 5 min between trials. The unconditioned stimulus duration was always 3 s. Short-term memory was tested 5 min after training by presenting the conditioned stimulus without the unconditioned stimulus. (b,c) Bar graphs in b and c show the PER probability for short-term memory tests. Asterisks indicate significant difference (P < 0.05, Fisher’s exact test with Bonferroni correction). More Kenyon cell spikes in the unconditioned stimulus period did not result in better learning. Open circles indicate normalized numbers of Kenyon cell spikes during the unconditioned stimulus presentation period (see Fig. 3b); spike counts were normalized with respect to the maximum elicited during the on response procedure (0.25-s ISI). (c) Reinforcement provided following off response spiking in Kenyon cells does not support learning. (d) The most effective conditioning occurred when the unconditioned stimulus followed the burst of onset spiking in Kenyon cells by a delay of several seconds. A delay of 20 s elicited no learning. The graph shows PER probability during the short-term memory test for different on response procedure groups.
Effective appetitive conditioning in honeybees19,21,28 and moths18,20,22 generally occurs when the unconditioned stimulus, a sucrose reward, is presented a few seconds after the onset of a lengthy conditioned stimulus, an odor pulse. Using a computer-controlled delivery system identical to (and frequently calibrated with) the olfactometer used for our electrophysiology experiments (see Methods), we precisely regulated the timing of both the conditioned and unconditioned stimuli in all procedures (Fig. 4a).
The control ‘unconditioned stimulus alone’ procedure group received five trials of 3-s unconditioned stimulus presentations alone (n = 33; Fig. 4a). This repeated delivery of sucrose alone may have caused some sensitization, as the spontaneous PER probability slightly increased from the baseline of 0 to 6.1% (not significant, P = 0.5, McNemar’s exact test; Fig. 4b).
For all associative conditioning procedures, the unconditioned stimulus duration was 3 s and the conditioned stimulus was paired with the unconditioned stimulus five times with 5-min intertrial intervals. Short-term memory was assessed 5 min after training by delivering only the conditioned stimulus. Our ‘on/off response’ procedure (Fig. 4a), one that is commonly used for training honeybees and moths, consisted of a 4-s conditioned stimulus18,21 and a 2-s inter-stimulus interval (ISI) from the onset of conditioned stimulus to the onset of unconditioned stimulus18,21,28. Moths in the on/off response group (2-s ISI, n = 64) attained a 34.4% PER probability (Fig. 4b). This amount of appetitive learning is typical for moths18,20,22, which, having fattened as caterpillars, do not need to eat as much as adults. Another group of moths trained with the on/off response procedure (2-s ISI, n = 23) and then tested with a different, non-trained odor did not respond to the different odor (Supplementary Fig. 3 online). This result indicates that learning was specific; moths learned to associate the odor, rather than unintended cues, with the reward. The amount of learning elicited by the on/off response procedure (2-s ISI) was significantly greater than that of the control, unconditioned stimulus alone procedure group (Fisher’s exact test, P = 0.0024).
Notably, in this effective and commonly used learning procedure, sucrose reinforcement was delivered ~1.2 s after the end of the on response in the Kenyon cells, as we knew from our physiology experiments. Thus, successful conditioning occurred in the absence of any overlap between odor-elicited on response spikes in Kenyon cells and the sucrose reward.
To further explore the timing relationship of on-response spikes in Kenyon cells and sucrose reinforcement, we then used an on/off response procedure (3.75 s ISI) in which conditioned stimulus and unconditioned stimulus were spaced further apart in time (4 s conditioned stimulus duration, 3.75 s ISI). We found that this group (n = 58) learned as well as that receiving the on/off response procedure with 2 s ISI (34.5%, Fig. 4b), a level of learning significantly greater than that shown by the control, “unconditioned stimulus alone” procedure group (Fisher’s exact test, P = 0.0021).
Our matching electrophysiology experiments found that brief odor pulses elicited only on response spikes in Kenyon cells (Fig. 3b). To test the importance of overlapping on response spikes in Kenyon cells with sucrose reward, we conditioned a group of moths with brief (0.5 s) odor pulses, which were followed 0.25 s later by reinforcement (on response procedure, 0.25-s ISI, n = 61). Shifting the timing of the reward presentation closer to the on response spikes in Kenyon cells actually resulted in decreased learning (18.0% PER probability, which was not significantly different from that elicited by the unconditioned stimulus alone procedure, P = 0.1299; Fig. 4b).
Among these three groups, only the on response procedure (0.25-s ISI) elicited exclusively on response spikes in Kenyon cells, and was ineffective for learning. This raised the possibility that off response spiking in Kenyon cells (and possibly some middle response spiking) in the other two groups (on/off response procedures with 2-s and 3.75-s ISIs) may have contributed substantially to successful conditioning. To test this, we trained moths with a brief odor pulse in a trace procedure (on response procedure with 3.75-s ISI, 0.5-s conditioned stimulus duration, n = 23). Notably, conditioning with this procedure yielded learning (43.5%, significantly different from unconditioned stimulus alone procedure, Fisher’s exact test, P = 0.0018) that was similar to that elicited by other conditioning procedures including off response spikes in Kenyon cells (on/off response procedures with 2-s and 3.75-s ISIs; Fig. 4b). This suggests that the off response spikes contributed little or nothing to conditioning efficacy. We counted the number of spikes evoked in Kenyon cells during the time of unconditioned stimulus presentation in these procedures (Fig. 3b). The on response procedure (3.75-s ISI) group, which elicited the highest learning rate, corresponded to the fewest spikes in Kenyon cells during the time of reinforcement (Fig. 4b). To examine the limits of the interval between on response spikes in Kenyon cells and reinforcement to effectively support conditioning, we tried spacing the conditioned and unconditioned stimuli further and further apart. When we set the conditioned stimulus duration to 0.5 s to induce almost exclusively on response spikes (Fig. 3) and gradually increased the interval between the conditioned and unconditioned stimuli, we found that the PER probability peaked at the 3.75-s ISI (43.5%) and gradually decreased (at the 9.75-s ISI, 31.8%, n = 22, not significantly different from the unconditioned stimulus alone procedure, Fisher’s exact test, P = 0.022, not significant after Bonferroni correction) and reached the control level at around the 20-s ISI (9.1%, n = 22, not significantly different from the unconditioned stimulus alone procedure, Fisher’s exact test, P = 1; Fig. 4d).
Notably, effective conditioning was possible even when sucrose reinforcement was delivered many seconds after the spiking responses in Kenyon cells had returned to baseline levels. These results indicate that appetitive olfactory conditioning in the moth Kenyon cells cannot be mediated by a Hebbian STDP process that requires the near-overlap of spikes elicited by the odor stimulus and spikes elicited by the reinforcement; spiking in Kenyon cells cannot be the representation that coincides with appetitive reinforcement during associative conditioning.
Finally, we asked whether off response spikes alone in Kenyon cells could support associative learning. Drawing on the results of our on response procedures, we used an ISI that was long enough to separate the onset and offset spiking in Kenyon cells by an interval that exceeded that which can support trace conditioning (Fig. 4c,d); we used an extra-long conditioned stimulus (18 s, which induced small off responses similar to those elicited by 4-s odor pulses; Fig. 3c,d) and delivered the unconditioned stimulus at a 20-s ISI (2 s after the beginning of the off response). This allowed us to selectively reinforce the off response spikes, but not the on response spikes (off response procedure group, 6.7%, n = 30; Fig. 4c). This procedure did not lead to PER conditioning that was significantly different from the control level (Fisher’s exact test, P = 1). Therefore, we concluded that off response spiking alone cannot support learning. This absence of learning may result because off responses were generally small, consisting of far fewer spikes than the on responses (Fig. 3b,c). As middle response spikes were much less frequent than off response spikes, we conclude that only the on response spikes contributed substantially to learning. Responses occurring after the on response could be important for other tasks that require temporal integration. The apparent importance of odor onset for associative conditioning suggests that, at least for our simple learning task, moths were prepared to make rapid behavioral choices. Consistent with this analysis, we found that moths tended to respond rapidly with proboscis extension on the onset of an odor pulse regardless of its duration or time of reinforcement during training (Supplementary Fig. 4 online).
DISCUSSION
Odor representations in the moth olfactory system
Olfaction, with only a few layers of neurons separating input from output, provides a useful model for understanding a succession of neural representations of sensory events. Consistent with earlier findings in moths3 and locusts5, our intracellular recordings from projection neurons revealed high spontaneous firing rates and odor-specific, temporally complex patterns of robust spiking and inhibition (Fig. 1). We provide, to the best of our knowledge, the first characterization of odor responses in the Kenyon cells of moths; they showed markedly low background firing rates and typically responded to odors with one spike at the odor onset or, less often, at the offset (Fig. 2). Thus, in the moth, as has been observed in locusts6, honeybees29 and Drosophila30,31, dense spatiotemporal patterns in the projection neurons were transformed into sparse representations in the Kenyon cells. We found that largely distinct ensembles of Kenyon cells spiked at the onset and offset of odor pulses in the moth, with a very low level of spiking in between onset and offset (Figs. 2 and 3).
In moths, as in other animals, the meanings of odors are readily adjusted by learning experiences. Needless to say, the odorants themselves are not matched with conditioning reinforcements in the brain, but rather neural representations of odors, presumably spiking activity in olfactory neurons, must undergo this matching process. Having characterized the responses of olfactory neurons to odor stimuli such as those used in conditioning procedures, we asked what was the neural representation of the odor that coincides with the reward. Memory traces are, in general, distributed across multiple neural populations. Specifically, short-term memory induced by appetitive olfactory conditioning, such as the PER procedures that we used here, appears to involve both the antennal lobe and the mushroom body28,32 and possibly other areas. We focused on the timing of odor-elicited firing patterns of the intrinsic neurons of the mushroom bodies, the Kenyon cells, and the timing of reinforcement stimuli that leads to effective associative conditioning. In a number of conditioning procedures, we found that reinforcement stimuli that were delivered at times that did not coincide with odor-elicited spiking in Kenyon cells could still effectively support associative conditioning (Fig. 4).
Recent work in vertebrates and insects has focused on the role of STDP, a form of Hebbian learning, which requires precise, millisecond-scale correlation between spiking in pre- and postsynaptic neurons that undergo plasticity. Notably, we found that the most behaviorally effective reinforcement occurred long after, sometimes seconds past, the cessation of all odor-elicited spiking in the Kenyon cells. Thus, it is not possible for spikes in Kenyon cells to interact, in a STDP temporal window, with spikes arriving via any pathway bearing the reinforcement. Plasticity cannot occur in these cells through any type of Hebbian mechanism that requires spiking in both pre- and postsynaptic neurons to occur in a temporal window of less than several hundred milliseconds. Plasticity here must occur through a different mechanism.
Neurotransmission from Kenyon cells is required for memory retrieval, as shown by behavioral studies in transgenic flies in which neurotransmission from a subset of Kenyon cells (αβ neurons) was conditionally regulated by temperature shifts13,14. A recent study investigating the role of another subset of Kenyon cells, the α‘β’ neurons15,16, indicated that neurotransmission from α‘β’ neurons is required during the acquisition of memory and contributes to stabilizing the memory. These results are consistent with an earlier finding in honeybees, where the neuromodulator octopamine, injected specifically into the mushroom body, can induce olfactory learning by substituting for the sucrose reward28. Studies such as these show that Kenyon cells are involved in memory acquisition (plasticity in the antennal lobe may be involved as well)28,32. However, the precise mechanism by which Kenyon cell activity contributes to the acquisition of associative memory remains unknown. Any such mechanism would require the temporal convergence of the neural representations of the odorant and sucrose.
Sparse coding and associative learning
Accumulating evidence shows that organisms spanning locusts6 to humans33 make use of sparse neural coding strategies to represent stimuli. Sparse codes, in which stimuli elicit very low spike rates in a small fraction of a large population of mostly silent neurons, maximize coding space between representations of different sensory stimuli34. This increases associative memory capacity and also readily allows for efficient formation of learned associations via a local rule. Hebbian mechanisms, through which synapses are strengthened if spikes in the presynaptic neurons contribute to produce an action potential in the postsynaptic neurons, seem ideally suited for efficiently modifying sparsely coded neural representations of stimuli35,36. Thus, Hebbian plasticity has become a common component of associative neural network models, particularly in the context of sparse codes34.
Indeed, a recent study found millisecond-scale STDP in the olfactory pathway of the locust, demonstrating that insects have synapses that exemplify Hebb’s rule23. Behavioral studies in Drosophila have revealed the sort of bidirectional plasticity that is typical of STDP, but with conditioned stimulus–unconditioned stimulus pairing time scales on the order of seconds rather than milliseconds37. Computational studies suggest that the time scale mismatch between behavioral and physiological STDP characteristics can be resolved if the pre- and postsynaptic neurons responding to conditioned and unconditioned stimuli show sustained firing that slowly decays38. However, our finding that Kenyon cells respond only sparsely and very briefly to odor pulses that support conditioning is not consistent with this model. It is possible that STDP mechanisms may contribute to olfactory conditioning when combined with slower biochemical processes39. Might the responses of Kenyon cells to odor become altered by conditioning such that spikes then temporally overlap with the reward? This seems to be an unlikely explanation for our results. First, we found that the most learning occurred during the first training trial; that is, before any potential learning-induced changes could have occurred (on/off response procedure with 2-s ISI; Supplementary Fig. 3). Second, odor responses of Kenyon cells in moths that had been successfully trained to associate that odor with reward were no less sparse than responses from Kenyon cells in untrained moths (data not shown). Therefore, we conclude that STDP mechanisms alone cannot account for the learning in Kenyon cells that we observed in moths.
Our physiological and behavioral studies indicate that spikes in Kenyon cells cannot, in and of themselves, constitute the odor representation that coincides with appetitive reinforcement. We suggest instead that the odor representation in Kenyon cells that is paired with reward may be a sustained biochemical process, perhaps second messenger responses13,14,40 that are triggered by very transient spiking. The situation may be different in other neurons or species. Recent recordings from Drosophila Kenyon cells found that odor-elicited somatic subthreshold excitatory postsynaptic potentials are close in amplitude to those attained by spikes31. If excitatory postsynaptic potentials alone suffice to activate voltage-dependent calcium channels, for example, reinforceable odor representations might include neurons that are not firing spikes.
METHODS
Experimental animals
Moths (Manduca sexta) were reared from eggs (purchased from the NCSU Insectary) in our laboratory on an artificial diet41 under a long-day photoperiod at 26 °C, and at more than 70% relative humidity.
Olfactory stimulation
The odor-stimulation method that we used was modified from our previous study4. Briefly, the odorized headspace in 60-ml glass bottles above mineral oil–diluted odorant solution was pushed by a controlled volume of humidified air (0.1 l min−1) into an activated carbon–filtered, humidified air stream (0.75 l min−1) that flowed continuously across the antenna. The inner diameter of the odor delivery tube was 6.5 mm and the air speed at the end of tube was about 9.4 cm s−1. Excess odorants were continuously drawn by vacuum from the back of the preparation. All chemicals were purchased from Sigma-Aldrich unless otherwise noted. The odorants that we used were benzylalcohol (Bzalc), benzaldehyde (Bzald), (+)-β-citronellene (βcit, Fluka Chemika), cyclohexanone (Cychex), geraniol (Ger), hexanol (1hex, 10hex and 100hex), cis-3-hexenyl acetate (Cis3ha), (±)linalool (Lin, Aldrich), methyl salicylate (Mes), methyl jasmonate (Mej), 1-octanol (Oct, Fluka Chemika), trans-2-hexenal (T2hal), trans-2-hexen-1-ol (T2h-1-ol), oil extracts, strawberry (Strwb), cinnamon (Cinn), peach, lime, jasmine (Jasm, Balducci’s), thyme (Thyme Red, Saidel) and wintergreen (Wntgr, Wagner’s). Monomolecular odorant solutions were diluted to 1% (vol/vol) in mineral oil unless otherwise noted. Oil extracts were used undiluted. The odor vapor drawn from the headspace was further diluted when mixed into the constant air stream.
Electrophysiology
Physiological data were obtained from 38 adult moths of both sexes. Adults that were 1 d post-eclosion or older were dissected following a procedure described for locusts42. The brain was treated for 1–3 min with 3% collagenase-dispase (Roche Diagnostics) that had been dissolved in saline. The antennal lobe and mushroom body were then carefully desheathed with fine forceps. The head capsule was superfused with moth physiological saline17 at room temperature (about 25 °C).
Intracellular recordings were made using sharp glass micropipettes pulled horizontally (P87, Sutter Instrument Company) to yield 50–150 MΩ electrodes for antennal lobe neurons and 50–200 MΩ electrodes for Kenyon cells when filled with one of the internal solutions (details are given in Supplementary Fig. 1). Multiunit recordings from Kenyon cells were made using 8-channel, custom-made, twisted wire tetrodes6, amplified with a custom 16-channel amplifier (Biology Electronics Shop, Caltech) and digitized at 15 kHz (details on spike sorting43 are given in Supplementary Fig. 5 online).
Behavioral experiments
A total of 336 moths were used for behavioral experiments. Moths that were 1–4 d post eclosion were restrained in plastic tubes (inner diameter, 1.5 cm.) with the head protruding. The proboscis was made to extend partially by threading it through flexible polyethylene tubing (inner diameter, 0.86 mm.) with the proboscis tip exposed to allow sucrose application. To eliminate visual cues during training and testing, the compound eyes were covered with black ink at least 15 min before training began.
Moths were classically conditioned during the dark photoperiod with the four types of training procedures described above (shown in Fig. 4a). Time and pressure-regulated odor stimuli (1% cyclohexanone or 1% benzaldehyde) were pulsed onto one antenna as described above. These two odors, as with the others in our set, evoked mainly on response spiking and weaker off response spiking in Kenyon cells (Supplementary Fig. 6 online). The equipment that we used for odor presentation in these behavioral experiments was identical to, and with settings daily cross-calibrated with, the equipment that we used for the physiology experiments. For taste reward presentation, air driven by a picopump pushed ~10 µl of sucrose solution (40% wt/vol in water) from a glass capillary (inner diameter: 0.058 mm) to the tip region of the proboscis. The two pneumatic picopumps used for odor and sucrose stimuli were controlled by a programmable pulse generator (Master-8, A.M.P. Instruments). Proboscis extension was monitored visually by an investigator. Responses were recorded if PER occurred within 1 min of the odor onset. For some experiments, response latency was measured from video images (details of video analysis are given in the Supplementary Methods online).
Data analysis
All analyses, except for spike sorting, were carried out using custom programs in MATLAB (MathWorks). Given the sparseness of Kenyon cell spiking, it was not always clear when a Kenyon cell was responding to an odor. Therefore, for some analyses of our extracellular Kenyon cell records (Fig. 2e,f) we used rate and reliability criteria modified from an earlier study6. To meet our rate criterion, Kenyon cell firing was averaged over all ten trials in each Kenyon cell–odor combination set and segmented into successive, non-overlapping 250-ms bins that spanned the 5.5-s full analysis window. Firing had to exceed 3.5 s.d. of the mean baseline rate (2 s before stimulation) in at least one of the bins; background activity was so low that in almost all cases a single spike in any bin in any trial sufficed. Therefore, we defined a responsive trial as one with at least one spike. To meet the reliability criterion, the response probability (number of trials showing at least one spike divided by the number of trials tested) had to exceed 30%. We evaluated the usefulness of this threshold by estimating the probability that at least one spike in our dataset would occur during the pre-stimulus period (in the absence of odorant, 2 s). We made this estimate for each Kenyon cell (normalizing for test windows of different duration) using all 210 trials. These probabilities for individual Kenyon cells had a median of 0 and a mean of 0.0058. Except for two extremes (0.125 and 0.0929) all Kenyon cells showed a probability lower than 0.0607. At P = 0.0607, the binomial probability theorem shows that the probability that at least one spike would occur in the absence of odorant in more than three trials in 10 (30% reliability) was less than 0.02. For the two extreme cases, the probabilities estimated in the same way were just above the 0.05 level of significance (P < 0.12 or 0.06). Thus, we judged our 30% threshold to be appropriate for detecting odor responses in Kenyon cells. We also provide results using a 50% criterion to allow for comparison with results obtained in locusts6.
The distribution of spike number per trial was analyzed by counting the number of spikes occurring within 5.5 s of odor onset using only the cell-odor pairs that included odor responses (that is, met the response reliability criteria given above). To compute the sparseness of Kenyon cell responses, we used measures of population (SP) and lifetime (SL) sparseness6,27. SP estimates the proportion of cells not responding to each stimulus:
where N is the total number of Kenyon cells and rj is the number of spikes detected in cell j over ten trials. SP takes values from 0 to 1, with SP = 1 being sparsest. To estimate the response intensity (rj), we segmented the 5.5-s full response window into 250-ms bins and calculated the mean spike count in each bin averaged over ten trials for each cell-odor pair. The mean baseline activity in the 2-s pre-stimulus period was then subtracted from all of the bins. Finally, only the bins showing more than the mean basal activity (values greater than 0) were added to obtain rj. SL estimates the range of responses of each cell and was calculated in the same way as SP, except that index j corresponds to each odor and N to the total number of odors tested with each cell.
Statistical tests were made using SAS version 9.0 (SAS Institute) and R version 2.4.1 (http://www.r-project.org/) for behavioral data and using Statistical toolbox version 5.2 for MATLAB for physiological data. All of the statistical tests for physiology were two-tailed and significance was judged at P = 0.05. To make conservative multiple comparisons of results from behavioral experiments, we judged significance by more stringent, Bonferroni-corrected P values. For evaluation of sensitization, spontaneous PER probability was estimated using the 1-min window before the beginning of the unconditioned stimulus alone procedure and compared with the PER probability of the test period with McNamar’s exact test. PER probabilities between different procedures were compared with Fisher’s exact test.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to members of the Stopfer laboratory for helpful discussions. We especially thank K. Sun for her excellent animal care. Micrographs were made at the Microscopy and Imaging Core (US National Institute of Child Health and Development, NICHD) with the assistance of V. Schram. We thank C. Wu in the Biometry and Mathematical Statistics Branch, US National Institutes of Health (NIH)/NICHD for his advice on the statistical analysis of the behavioral experiments. This work was supported by grants from the Japan Society for the Promotion of Science (00169, 70510) to I.I., a joint NIH–National Institutes of Standards and Technology postdoctoral fellowship award by the National Research Council to B.R. and an intramural grant from NIH-NICHD to M.S.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Daly KC, Wright GA, Smith BH. Molecular features of odorants systematically influence slow temporal responses across clusters of coordinated antennal lobe units in the moth Manduca sexta. J. Neurophysiol. 2004;92:236–254. doi: 10.1152/jn.01132.2003. [DOI] [PubMed] [Google Scholar]
- 4.Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat. Neurosci. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- 5.Mazor O, Laurent G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron. 2005;48:661–673. doi: 10.1016/j.neuron.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 7.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 8.Schröter U, Menzel R. A new ascending sensory tract to the calyces of the honeybee mushroom body, the subesophageal-calycal tract. J. Comp. Neurol. 2003;465:168–178. doi: 10.1002/cne.10843. [DOI] [PubMed] [Google Scholar]
- 9.Dacks AM, Christensen TA, Agricola H, Wollweber L, Hildebrand JG. Octopamine-immunoreactive neurons in the brain and subesophageal ganglion of the hawkmoth Manduca sexta. J. Comp. Neurol. 2005;488:255–268. doi: 10.1002/cne.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J. Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 11.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 12.Erber J. Retrograde amnesia in honeybees (Apis mellifera carnica) J. Comp. Physiol. Psychol. 1976;90:41–46. doi: 10.1037/h0077258. [DOI] [PubMed] [Google Scholar]
- 13.Heisenberg M. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 15.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 17.Christensen TA, Hildebrand JG. Male-specific, sex pheromone–selective projection neurons in the antennal lobes of the moth Manduca sexta. J. Comp. Physiol. [A] 1987;160:553–569. doi: 10.1007/BF00611929. [DOI] [PubMed] [Google Scholar]
- 18.Daly KC, Smith BH. Associative olfactory learning in the moth Manduca sexta. J. Exp. Biol. 2000;203:2025–2038. doi: 10.1242/jeb.203.13.2025. [DOI] [PubMed] [Google Scholar]
- 19.Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J. Comp. Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- 20.Fan RJ, Anderson P, Hansson B. Behavioural analysis of olfactory conditioning in the moth spodoptera littoralis (Boisd.) (Lepidoptera: noctuidae) J. Exp. Biol. 1997;200:2969–2976. doi: 10.1242/jeb.200.23.2969. [DOI] [PubMed] [Google Scholar]
- 21.Müller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 22.Skiri HT, Stranden M, Sandoz JC, Menzel R, Mustaparta H. Associative learning of plant odorants activating the same or different receptor neurones in the moth Heliothis virescens. J. Exp. Biol. 2005;208:787–796. doi: 10.1242/jeb.01431. [DOI] [PubMed] [Google Scholar]
- 23.Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–713. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson MA, Knüsel P, Verschure PFMJ, Hansson BS. Spatio-temporal Ca2+ dynamics of moth olfactory projection neurones. Eur. J. Neurosci. 2005;22:647–657. doi: 10.1111/j.1460-9568.2005.04239.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287:1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- 28.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 29.Szyszka P, Ditzen M, Galkin A, Galizia CG, Menzel R. Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J. Neurophysiol. 2005;94:3303–3313. doi: 10.1152/jn.00397.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein–based Ca2+ imaging. J. Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2007;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 32.Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J. Neurosci. 2007;27:11132–11138. doi: 10.1523/JNEUROSCI.2712-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 34.Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Willshaw DJ, Buneman OP, Longuet-Higgins HC. Non-holographic associative memory. Nature. 1969;222:960–962. doi: 10.1038/222960a0. [DOI] [PubMed] [Google Scholar]
- 36.Marr D. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- 38.Drew PJ, Abbott LF. Extending the effects of spike timing–dependent plasticity to behavioral time scales. Proc. Natl. Acad. Sci. USA. 2006;103:8876–8881. doi: 10.1073/pnas.0600676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izhikevich EM. Solving the distal reward problem through linkage of STDP and dopamine signaling. Cereb. Cortex. 2007;17:2443–2452. doi: 10.1093/cercor/bhl152. [DOI] [PubMed] [Google Scholar]
- 40.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell RA, Joachim FA. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms lepidoptera-sphingidae-gelechiidae. Ann. Entomol. Soc. Am. 1976;69:365–373. [Google Scholar]
- 42.Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. J. Neurosci. 1994;14:2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pouzat C, Mazor O, Laurent G. Using noise signature to optimize spike-sorting and to assess neuronal classification quality. J. Neurosci. Methods. 2002;122:43–57. doi: 10.1016/s0165-0270(02)00276-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.