Abstract
We report a case of splenic abscess with multiple brain abscesses caused by Streptococcus intermedius in a healthy young man without any identifiable risk factors, which resolved with percutaneous drainage and antibiotics. Streptococcus intermedius, a member of the Streptococcus anginosus group, is a common commensal organism of the oral cavity and gastrointestinal tract, and it is a known cause of deep-seated infections. Suppurative infections caused by Streptococcus anginosus group are sometimes associated with bacteremia, but hematogenous spread of infection from an occult source leading to concurrent splenic abscess and multiple brain abscesses has never been previously reported in a healthy young individual.
The association of the Streptococcus anginosus group (SAG) of bacteria with the tendency to form local suppurative infections has long been recognized, and these organisms are the most common pathogens associated with bacterial intracerebral abscesses (1–4). S. intermedius is the most pathogenic species of SAG and can cause deep abscesses, but this is a very uncommon occurrence in previously healthy individuals without any identifiable risk factors (4–6). Splenic abscess, in general, is very rare, with only 600 cases reported in the international literature, and abscesses are more frequently detected in middle-aged and older individuals with no obvious preference for either sex (7). Brain abscess occurs at any age with reported incidences much higher in men (8).
We report a case of splenic abscess with multiple brain abscesses caused by S. intermedius in a healthy young man without any identifiable risk factors. Resolution of the infection was achieved by percutaneous drainage and intravenous antibiotics. This case emphasizes that the current understanding about the pathogenicity and virulence of S. intermedius and SAG causing suppurative infections is limited. Bacteremia caused by SAG should alert physicians to look for foci of suppurative infections in multiple areas of the body.
CASE REPORT
A 21-year-old healthy Hispanic construction worker presented to the emergency department with a 1-week history of fever and persistent pain in the left upper quadrant of his abdomen. He reported malaise, chills, and decreased appetite for the previous month. He had no past medical history of any significant medical illnesses or immunocompromised states. His past surgical history was negative for any dental or gastrointestinal procedures performed in the previous 6 months. He denied a history of blunt trauma to the abdomen or intravenous drug abuse.
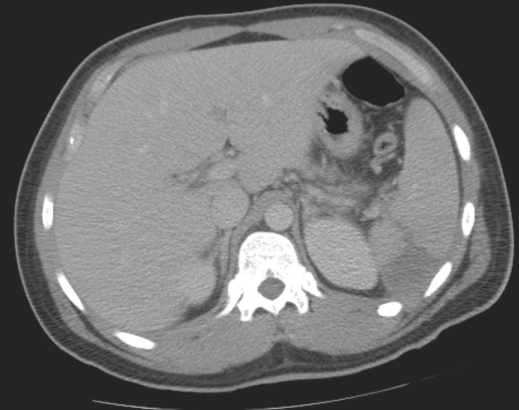
On admission, his vital signs were normal. Examination of the abdomen revealed tenderness in the left upper quadrant. His white blood cell count was 18.2 K/μL, with 76% neutrophil segments, 7% bands, 9% lymphocytes, 7% monocytes, and 1% metamyelocytes. Other laboratory findings were within normal limits. Computed tomography (CT) of the abdomen revealed a 2 × 3-cm low-density area with linear extension to the hilum of the spleen (Figure 1). The patient was started on intravenous ceftriaxone 2 g daily and vancomycin after obtaining two sets of blood cultures, one of which grew S. milleri (S. intermedius spp.). CT-guided needle aspiration of the lesion yielded 8 cc of purulent fluid, which also demonstrated S. milleri (S. intermedius spp.). Since the isolated bacteria were nonviable, antibiotic sensitivities were not performed. Repeat blood cultures after 48 hours were negative. Anaerobic, fungal, and acid-fast bacillus cultures from the splenic aspirate were negative.
Figure 1.
CT of the abdomen revealing a 2 × 3-cm low-density area with linear extension to the hilum of the spleen.
Further evaluation was performed to determine the source of the bacteremia and splenic abscess. Because S. anginosus is colonized in mucosal surfaces, a thorough search for oropharyngeal, upper respiratory, and gastrointestinal infections was performed through a detailed clinical exam and CT of the abdomen and pelvis, without any source being discovered. Examination of the oral cavity was normal with no evidence of dental caries, periodontitis, or oral abscesses. Transesophageal echocardiogram was negative for infective endocarditis. Laboratory evaluation for immune deficiency, including HIV and diabetes, was negative. He improved clinically and was discharged with intravenous vancomycin and ceftriaxone for 6 weeks.
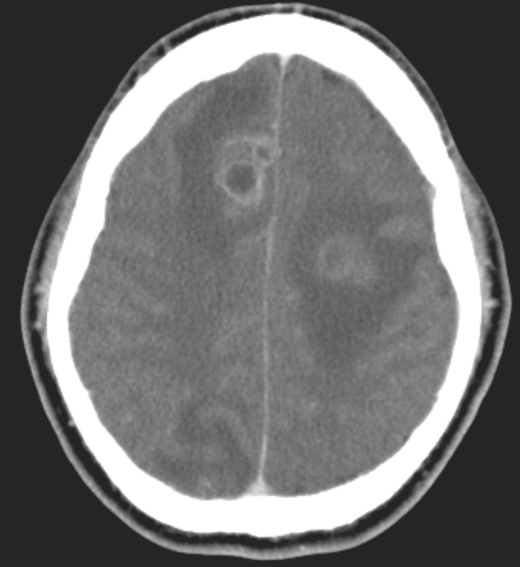
The patient presented to the emergency department 2 weeks later with an episode of secondarily generalized seizure. On further questioning, he revealed that a similar episode of seizure occurred before his first hospital admission, although he had not provided this information at that time. CT of the brain detected three ring-enhancing lesions at the junction of the gray and white matter, in the frontal lobe and occipital lobe (Figure 2). A CT-guided stereotactic biopsy of the brain lesion was performed, and the fibrinous material obtained was sent for cultures. CT of the abdomen showed abscess collection in the spleen unchanged from the previous exam. A repeat percutaneous drainage of the lesion was performed, and a 10F pigtail catheter was placed. Vancomycin was continued and ceftriaxone was increased to 2 g intravenously every 12 hours. Cultures from the brain abscesses and splenic abscess were negative for S. anginosus. The patient's clinical presentation improved and the pigtail catheter was removed after 2 days. Treatment with levetiracetam was initiated for the seizures. He was discharged home with ertapenem for 6 weeks in addition to levetiracetam. The brain abscesses were monitored with serial CT scans every 2 weeks, which showed gradual resolution. Antibiotic treatment was stopped at 6 weeks based on clinical improvement and the decreased size of the abscesses in the CT scan. Repeat CT scan at 4 months showed complete resolution of the abscesses with no underlying structural anomalies in the brain. The patient recovered without any permanent sequelae.
Figure 2.
CT of the brain showing three ring-enhancing lesions at the junction of the gray and white matter, in the frontal lobe and occipital lobe.
DISCUSSION
Suppurative infections caused by the SAG (or “milleri group”) are very rarely reported in previously healthy patients (4–6, 9–12). In fact, hematogenous spread of infection from an occult source leading to multiple abscesses in the brain and spleen has never been previously reported in a healthy individual, although it has occurred in immunocompromised patients (5, 11, 13).
SAG
Streptococci in the mouth, upper respiratory tract, and gastrointestinal tract are generally harmless commensals. These commensals are distinct from the obvious pathogens S. pyogenes and S. pneumoniae, which frequently cause acute infections and may also be found on healthy mucosa. However, one group of commensal streptococcus, called either SAG or the S. milleri group, has been referred to as “a significant pathogen causing suppurative infections” (4, 10, 11, 13, 14). These streptococci are common inhabitants of the mouth, nasopharynx, gastrointestinal tract, and vagina, with an isolation rate of 15% to 30% (10).
SAG consists of the species S. intermedius, S. anginosus, and S. constellatus (15). These bacteria are usually alpha-hemolytic but can occasionally be beta-hemolytic or nonhemolytic. While these organisms are commonly associated with purulent infections and abscess formation, they are less commonly the cause of endocarditis than the other viridans streptococci (13). SAG is one of the most common causes of brain abscesses (1, 3, 5, 11, 13). Most studies that have identified SAG to the species level have suggested that S. intermedius is the least common isolate but the most pathogenic species within SAG (9, 15). In many recent studies, S. intermedius was found as a solitary isolate in specimens, and abscesses caused by this species tended to be associated with hematogenous spread or were deep seated and extensive in comparison with the infections caused by the other two species, where it was polymicrobial and more often superficial (15). In our case, S. intermedius was the only isolate from the blood and splenic abscess aspirate. The propensity for this organism to cause abscesses in multiple sites demonstrates its high-grade pathogenicity.
It has been suggested that S. anginosus bacteremia is a significant indicator for occult abscesses (9). Multiple positive blood cultures and polymicrobial bacteremia strongly suggest a suppurative focus of infection and should prompt clinicians to initiate a thorough investigation of the abdominal and thoracic cavities. However, local trauma (e.g., through enemas or colonoscopy) to the mucosal barrier where the SAG is normally found may result in bacteremia. These transient bacteremias should not be overlooked, since they may be complicated by metastatic purulent lesions.
Splenic abscess
Splenic abscess is an uncommon condition with a high mortality rate due to delayed detection and treatment. It is particularly uncommon in healthy young adults (16). Most of the reported cases have been in patients with recognized risk factors (17–22). These include metastatic infection from other sites in the body, such as bacterial endocarditis; immunocompromised states; hemoglobinopathies; secondary infection from splenic trauma; and direct extension from infection of contiguous organs (7, 23, 24). A solitary splenic abscess in the absence of risk factors is very rare.
The clinical presentation of a splenic abscess is nonspecific, making the diagnosis difficult (Table Table 1) (14, 25). The most frequently seen symptoms and signs are fever, abdominal pain, tenderness over the left upper quadrant, splenomegaly, leukocytosis, and left lower chest abnormalities. These nonspecific clinical pictures should be thoroughly investigated (17). CT should be used whenever a splenic abscess is suspected. The most common pathogens detected include streptococcus and staphylococcus. Early diagnosis is the most important factor in successful treatment. Even though splenectomy is the standard treatment, CT-guided drainage of the splenic abscess is a safe and effective alternative to surgery, and it should be considered when the abscess is <3 cm and unilocular with thin liquid content, the patient has significant risk factors for a standard surgical approach, and a safe drainage window is present (21, 26–28).
Table 1.
Differential diagnosis∗
| Category | Differential diagnosis of ring-enhancing brain lesions | Differential diagnosis of splenic lesions |
|---|---|---|
| Infections |
|
|
| Neoplasms |
|
|
| Vascular lesions |
|
|
| Other |
|
|
Adapted from Friedlander, Gonzalez, Afridi, and Pfannl, 2003 (14) and Kamaya, Weinstein, and Desser, 2006 (25).
Brain abscess
Brain abscesses are serious, life-threatening conditions. Patients who survive may be left with a severe neurological deficit. Abscesses may occur as a result of spread from a contiguous focus of infection or by hematogenous spread from a distant focus, or they may be of unknown origin (Table Table 1). Abscesses that develop due to hematogenous spread from distant locations are located in proportion to blood flow; they occur more commonly in the area of the middle cerebral artery at the junction of gray and white matter, and they are more likely to occur as multiple abscesses, as in our case.
Headache is reported as the most common presenting symptom of brain abscess (8, 29). Results from several series warn against relying on the presence of fever as a diagnostic clue, since some studies report an incidence of <50% (8, 29). Other important symptoms are altered mental status, focal neurological deficits, nausea, vomiting, and seizures. The diagnosis of cerebral abscess should be considered in the presence of a ring-enhancing lesion with perilesional edema on CT scan.
The most important microbiological investigation in the management of brain abscess is culture of the abscess fluid (Table Table 2). Cerebrospinal fluid studies do not contribute to the diagnosis. In fact, a lumbar puncture may cause brain stem herniation and death in the setting of raised intracranial pressure. Blood culture is indicated, especially if the abscess is thought to be the result of hematogenous spread. Early blood culture may contribute helpful information in patients whose abscess culture is negative. Among intracerebral abscesses, 24% to 40% produce negative culture results, due in large part to patients receiving antimicrobial therapy. Gene amplification and sequencing tests may aid in the diagnosis of culture-negative brain abscesses, allowing for more targeted antibiotic therapy (1, 30).
Table 2.
Microbiologic pathogens in brain abscesses, according to major primary source of infection∗
| Source of infection | Pathogens |
|---|---|
| Paranasal sinuses | Streptococcus (especially Streptococcus milleri), haemophilus, bacteroides fusobacterium |
| Odontogenic sources | Streptococcus, bacteroides, prevotella fusobacterium, haemophilus |
| Otogenic sources | Enterobacteriaceae, streptococcus pseudomonas, bacteroides |
| Lungs | Streptococcus, fusobacterium actinomyces |
| Urinary tract | Pseudomonas, enterobacter |
| Penetrating head trauma | Staphylococcus aureus, enterobacter clostridium |
| Neurosurgical procedure | Staphylococcus, streptococcus pseudomonas, enterobacter |
| Endocarditis | Viridans streptococcus, S. aureus |
| Congenital cardiac malformations (especially right-to-left shunts) | Streptococcus |
Reproduced from Friedlander, Gonzalez, Afridi, Pfannl, 2003 (14) with permission Copyright © 2003 Massachusetts Medical Society.
The management of brain abscess generally requires a combination of neurosurgical intervention, antimicrobials, and eradication of any primary foci. Conservative management with antibiotics may be appropriate in patients with significant risk factors for surgery or if small cerebral lesions <5 mm in diameter are present on CT imaging (2). CT-guided stereotactic aspiration of brain abscesses is a minimally invasive procedure with low morbidity and mortality and allows for rapid and effective surgical drainage, especially for small and deep-seated abscesses (8). This procedure, which enables even the very sick patient to undergo aspiration of the abscess and identification of the infecting organism, has contributed to the improved prognosis of brain abscesses. Craniotomy and excision of the abscess is usually done when the abscess is superficial.
Prospective controlled trials of antibiotic therapy for brain abscess are unlikely to be undertaken because the number of cases is too small. SAG bacteria are generally susceptible to beta-lactam antibiotics and vancomycin. The guidelines of the British Society of Antimicrobial Chemotherapy recommend a third-generation cephalosporin (and/or other beta-lactam agents) together with metronidazole as an empirical treatment, depending on the intracerebral location and source of infection (2, 8, 31). Vancomycin should be included in the initial regimen when Staphylococcus aureus is suspected (e.g., in cases of trauma and in postneurosurgery cases) until culture results are available (31). The recommended duration of parenteral antibiotics is 3 to 4 weeks when abscesses are excised. Four to 6 weeks of antibiotics is recommended for abscesses that are aspirated. In our case, since we selected ertapenem, which is a broad-spectrum penicillin with good anaerobic coverage, to treat the brain abscess, we did not feel additional anaerobic coverage with metronidazole was warranted.
CONCLUSION
Our current understanding about the pathogenicity and virulence of S. intermedius (SAG) and its association with suppurative infections is limited. Bacteremia caused by SAG should alert physicians to look for foci of suppurative infections. Splenic abscess is a rather rare entity but can be fatal if left untreated. CT is the most sensitive diagnostic tool, and it should be used whenever splenic abscess is suspected. Percutaneous drainage of splenic abscess is a safe and effective alternative for splenectomy, allowing preservation of the spleen, and should be considered in selected patients. Early diagnosis and improved treatment options have likewise decreased the mortality rate from brain abscess, yet it remains a serious, life-threatening condition and further research is warranted to advance understanding of its optimal treatment.
References
- 1.Petti CA, Simmon KE, Bender J, Blaschke A, Webster KA, Conneely MF, Schreckenberger PC, Origitano TC, Challapalli M. Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: a case series. Clin Infect Dis. 2008;46(10):1578–1580. doi: 10.1086/587655. [DOI] [PubMed] [Google Scholar]
- 2.Kowlessar PI, O'Connell NH, Mitchell RD, Elliott S, Elliott TS. Management of patients with Streptococcus milleri brain abscesses. J Infect. 2006;52(6):443–450. doi: 10.1016/j.jinf.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Singh KP, Morris A, Lang SD, MacCulloch DM, Bremner DA. Clinically significant Streptococcus anginosus (Streptococcus milleri) infections: a review of 186 cases. N Z Med J. 1988;101(859):813–816. [PubMed] [Google Scholar]
- 4.Molina JM, Leport C, Bure A, Wolff M, Michon C, Vilde JL. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand J Infect Dis. 1991;23(6):659–666. doi: 10.3109/00365549109024289. [DOI] [PubMed] [Google Scholar]
- 5.Shlaes DM, Lerner PI, Wolinsky E, Gopalakrishna KV. Infections due to Lancefield group F and related Streptococci (S. milleri, S. anginosus) Medicine (Baltimore) 1981;60(3):197–207. doi: 10.1097/00005792-198105000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Rashid RM, Salah W, Parada JP. ‘Streptococcus milleri' aortic valve endocarditis and hepatic abscess. J Med Microbiol. 2007;56(Pt 2):280–282. doi: 10.1099/jmm.0.46781-0. [DOI] [PubMed] [Google Scholar]
- 7.Fotiadis C, Lavranos G, Patapis P, Karatzas G. Abscesses of the spleen: report of three cases. World J Gastroenterol. 2008;14(19):3088–3091. doi: 10.3748/wjg.14.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26(1):1–11. doi: 10.1007/s10096-006-0236-6. [DOI] [PubMed] [Google Scholar]
- 9.Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385–387. doi: 10.1086/514658. [DOI] [PubMed] [Google Scholar]
- 10.Van der Auwera P. Clinical significance of Streptococcus milleri. Eur J Clin Microbiol. 1985;4(4):386–390. doi: 10.1007/BF02148688. [DOI] [PubMed] [Google Scholar]
- 11.Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257–285. doi: 10.1093/clinids/10.2.257. [DOI] [PubMed] [Google Scholar]
- 12.Matsubayashi T, Matsubayashi R, Saito I, Tobayama S, Machida H. Splenic abscess in an infant caused by Streptococcus intermedius. J Infect Chemother. 2007;13(6):423–425. doi: 10.1007/s10156-007-0561-4. [DOI] [PubMed] [Google Scholar]
- 13.Tran MP, Caldwell-McMillan M, Khalife W, Young VB. Streptococcus intermedius causing infective endocarditis and abscesses: a report of three cases and review of the literature. BMC Infect Dis. 2008;8:154. doi: 10.1186/1471-2334-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander RM, Gonzalez RG, Afridi NA, Pfannl R. Case 16-2003. A 58-year-old woman with left-sided weakness and a right frontal brain mass. N Engl J Med. 2003;348(21):2125–2132. doi: 10.1056/NEJMcpc030011. [DOI] [PubMed] [Google Scholar]
- 15.Claridge JE, 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32(10):1511–1515. doi: 10.1086/320163. [DOI] [PubMed] [Google Scholar]
- 16.Saber A. Multiple splenic abscesses in a rather healthy woman: a case report. Cases J. 2009;2:7340. doi: 10.4076/1757-1626-2-7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang IS, Lin TJ, Chiang IC, Tsai MS. Splenic abscesses: review of 29 cases. Kaohsiung J Med Sci. 2003;19(10):510–515. doi: 10.1016/S1607-551X(09)70499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faught WE, Gilbertson JJ, Nelson EW. Splenic abscess: presentation, treatment options, and results. Am J Surg. 1989;158(6):612–614. doi: 10.1016/0002-9610(89)90205-5. [DOI] [PubMed] [Google Scholar]
- 19.Westh H, Reines E, Skibsted L. Splenic abscesses: a review of 20 cases. Scand J Infect Dis. 1990;22(5):569–573. doi: 10.3109/00365549009027098. [DOI] [PubMed] [Google Scholar]
- 20.Ooi LL, Leong SS. Splenic abscesses from 1987 to 1995. Am J Surg. 1997;174(1):87–93. doi: 10.1016/s0002-9610(97)00030-5. [DOI] [PubMed] [Google Scholar]
- 21.Llenas-García J, Fernández-Ruiz M, Caurcel L, Enguita-Valls A, Vila-Santos J, Guerra-Vales JM. Splenic abscess: a review of 22 cases in a single institution. Eur J Intern Med. 2009;20(5):537–539. doi: 10.1016/j.ejim.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Ng CY, Leong EC, Chng HC. Ten-year series of splenic abscesses in a general hospital in Singapore. Ann Acad Med Singapore. 2008;37(9):749–752. [PubMed] [Google Scholar]
- 23.Sinha S, Sharma DC, Miri B, Gupta V, Chattopadhyay TK. Splenic abscess—case report and review of literature. Trop Gastroenterol. 1997;18(3):134–135. [PubMed] [Google Scholar]
- 24.Kogo H, Yoshida H, Mamada Y, Taniai N, Bando K, Mizuguchi Y, Ishikawa Y, Yokomuro S, Akimaru K, Tajiri T. Successful percutaneous ultrasound-guided drainage for treatment of a splenic abscess. J Nippon Med Sch. 2007;74(3):257–260. doi: 10.1272/jnms.74.257. [DOI] [PubMed] [Google Scholar]
- 25.Kamaya A, Weinstein S, Desser TS. Multiple lesions of the spleen: differential diagnosis of cystic and solid lesions. Semin Ultrasound CT MR. 2006;27(5):389–403. doi: 10.1053/j.sult.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Taşar M, Uğurel MS, Kocaoğlu M, Sağlam M, Somuncu I. Computed tomography-guided percutaneous drainage of splenic abscesses. Clin Imaging. 2004;28(1):44–48. doi: 10.1016/S0899-7071(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferraioli G, Brunetti E, Gulizia R, Mariani G, Marone P, Filice C. Management of splenic abscess: report on 16 cases from a single center. Int J Infect Dis. 2009;13(4):524–530. doi: 10.1016/j.ijid.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Zerem E, Bergsland J. Ultrasound guided percutaneous treatment for splenic abscesses: the significance in treatment of critically ill patients. World J Gastroenterol. 2006;12(45):7341–7345. doi: 10.3748/wjg.v12.i45.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25(4):763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 30.Petti CA. Detection and identification of microorganisms by gene amplification and sequencing. Clin Infect Dis. 2007;44(8):1108–1114. doi: 10.1086/512818. [DOI] [PubMed] [Google Scholar]
- 31.Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect. 2003;36(2):129–136. [PubMed] [Google Scholar]