Abstract
Brain function monitors have improved safety and efficiency in general anesthesia; however, they have not been adequately tested for guiding conscious sedation for periodontal surgical procedures. This study evaluated the patient state index (PSI) obtained from the SEDline monitor (Sedline Inc., San Diego, CA) to determine its capacity to improve the safety and efficiency of intravenous conscious sedation during outpatient periodontal surgery. Twenty-one patients at the periodontics clinic of Baylor College of Dentistry were admitted to the study in 2009 and sedated to a moderate level using midazolam and fentanyl during periodontal surgery. The PSI monitoring was blinded from the clinician, and the following data were collected: vital signs, Ramsay sedation scale (RSS), medications administered, adverse events, PSI, electroencephalography, and the patients' perspective through visual analogue scales. The data were correlated to evaluate the PSI's ability to assess the level of sedation. Results showed that the RSS and PSI did not correlate (r = −0.25) unless high values associated with electromyographical (EMG) activity were corrected (r = −0.47). Oxygen desaturation did not correlate with the PSI (r = −0.08). Satisfaction (r = −0.57) and amnesia (r = −0.55) both increased as the average PSI decreased. In conclusion, within the limits of this study, PSI appears to correlate with amnesia, allowing a practitioner to titrate medications to that effect. It did not provide advance warning of adverse events and had inherent inaccuracies due to EMG activity during oral surgery. The PSI has the potential to increase safety and efficiency in conscious sedation but requires further development to eliminate EMG activity from confounding the score.
While morbidity and mortality still exist in sedation today, the percentages have decreased dramatically with the use of monitoring devices such as the pulse oximeter (1). Unfortunately, adverse incidents have not been eliminated completely. In an effort to control the level of sedation and anesthesia more accurately and potentially lower the number of adverse incidents, brain function monitors have been introduced, particularly in operating rooms (2). These innovations are not common in office-based practices for monitoring sedation levels in patients undergoing surgical procedures, though their potential is advocated (3). The benefits of increased patient safety and effectiveness of sedation have been well documented for general anesthesia (4–6) and may be applicable to conscious sedation practices.
The primary hurdle for brain function monitoring in a light to moderate sedation procedure has been electromyographical (EMG) interference from the frontalis muscle immediately beneath the array electrodes. This very high frequency and low voltage signal can cause an artificially high score on the patient state index (PSI). These monitors include filters to account for this signal. However, during periodontal surgery, frontalis muscle EMG activity may be extremely high and not filtered out, causing an artifact-induced high PSI. The advancement of these monitors into the intensive care unit from the operating room demonstrates their capabilities in an environment where EMG activity may be more prevalent, as the patient is more awake and aware (8). The filtering and algorithms of the SEDline appear effective in that type of setting to overcome this impediment (9). However, while some cerebral function monitors have been investigated for light to moderate sedation involving outpatient surgery, the SEDline has not yet been investigated (10, 11). These sedation techniques are often performed outside of a hospital setting, yet the consequences of oversedation still exist, increasing the need for better monitoring.
The most common adverse events in sedation are oversedation, leading to hypoventilation and oxygen desaturation, or inadequate sedation, resulting in an uncomfortable patient (1). The pulse oximeter is an excellent monitor for detecting oxygen desaturation but loses some of its early detection of hypoventilation when supplemental oxygen is administered (12). A brain function monitor has the potential to help prevent oversedation and hypoventilation with oxygen desaturation. Inadequate sedation should also be easily recognized by a high PSI score, allowing practitioners a more objective method for drug titration (13). This study evaluated the ability of the SEDline monitor in providing a reliable indicator, the PSI, of cortex function that may be used to increase the safety and effectiveness of light to moderate sedation.
METHODS
Study population
Twenty-one patients volunteered to participate at the outpatient periodontics clinic of Baylor College of Dentistry. Each patient's treatment involved surgical therapy with conscious sedation via the intravenous route using midazolam and fentanyl, while monitored by the Ramsay Sedation Scale (RSS). The PSI was recorded, but the practitioner was blinded to the score during the procedure.
Patients received written and verbal instructions detailing the protocol before signing the informed consent document and participating in the research. The institutional review board at the Baylor College of Dentistry, Texas A&M University Health Science Center, approved the protocol for the study. Once entered into the program, patients were assigned a random number to remove any bias during analysis of the data.
Subjects were required to be at least 18 years of age and medically able to proceed with both the surgery and sedation. Any patient with an allergy to midazolam, fentanyl, or adhesive was excluded from the study. Patients with prior brain injury, trauma resulting in facial scars, or neurologic conditions that would inhibit their ability to interact during the study were also excluded.
Protocol
All patients underwent outpatient periodontal surgery and light to moderate intravenous sedation using midazolam and fentanyl. Monitoring occurred at 5-minute intervals from the beginning of a given procedure to the time of discharge. Blood pressure, pulse, and oxygen saturation were recorded by a Criticare monitor as well as the RSS. Medications were recorded as administered, and all measurements normally made at 5-minute intervals were also recorded during any event requiring intervention by the attending health care team. The SEDline electrodes were connected to the patient before sedation, and the recording was synchronized according to the time of the Criticare monitor and wall clock. Oxygen was administered through a nasal cannula at 2 L/min during the course of the sedation.
Prior to local anesthetic injections, the patient was sedated, titrating each dose of benzodiazepine and narcotic to the desired target effect, defined as Verrill's sign, slurred speech, and a feeling of warmth or relaxation. This was maintained for each patient over the course of the procedure. These signs of sedation are widely accepted as a method of managing anesthesia in an outpatient dental or medical office (16).
To evaluate each patient's level of sedation during the procedure, the RSS was selected due to its clinical design. A moderate sedation plane of anesthesia was considered a 2 or 3 on the RSS, with a 4 progressing into deep sedation.
As with most brain function monitors, the PSI was developed from a proprietary algorithm, and a dimensionless scale from 0 to 100 was applied. A score of 0 represents total cortical silence, and the number continues upwards in a nonlinear path to 100 with increasing cortical activity. Evaluations of the PSI and RSS in studies in intensive care units have shown good correlation between the two scales (14, 15).
During the course of the sedation, any adverse events were recorded and appropriate intervention performed. Hypoxia was defined as an oxygen saturation of <93%. The appropriate response involved stimulating the patient to voluntarily breathe or to open the airway by a head-tilt chin-lift. No further intervention was required in the study to treat hypoxia. Agitation was defined as a patient reaching an RSS of 1 during the procedure. Additional sedation was provided for this adverse event. All other events were defined and treated according to protocols accepted by the Periodontics Department, Baylor College of Dentistry.
Each patient was provided a self-addressed envelope containing a survey. Both written and oral survey instructions were provided. For participation in the study, patients received a $50 allowance towards the cost of the sedation at the time of the surgery. The survey consisted of a modified Iowa Satisfaction with Sedation Survey (ISSS) and visual analogue scales for pain, amnesia, and satisfaction. The ISSS was modified only by removing components relating specifically to general anesthesia.
Data analysis
Participants were evaluated based on the three sources of data obtained during the study: clinical, PSI/electroencephalography [EEG], and patient perspective. We compared the RSS and PSI to check for consistency and determine which method provided a more effective means of monitoring a patient's level of sedation. All adverse events were examined to ascertain which method of monitoring provided the most advance warning and effective detection of the problem. Data from the visual analogue scale and survey were compared with the PSI to determine if the PSI data correlated with the clinical picture and could act as an objective monitor of sedation in patients undergoing periodontal surgery.
The data were evaluated using scatter plots and Spearman correlations given their nonparametric nature, making use of the software package SPSS (IBM, Chicago, IL) and the Polyman EEG analysis software (Roessen M, Kemp B). The level of significance for all statistical tests was set at P < 0.05.
RESULTS
Nineteen of the 21 enrolled patients completed the full protocol. Two patients had partial data and were excluded from the statistics where they lacked data. One patient chose not to return the survey, simply stating dissatisfaction with the sedation due to undersedation. A second patient did not have complete data from the brain function monitor. In that patient, two different electrodes were used, but a clear signal was established for only 2 minutes. The patient had significant amounts of hair, making electrode placement difficult and an adequate signal impossible. It was discovered during the study that one patient purposely falsified her survey. This patient's survey was excluded from the results and statistics.
RSS versus PSI
The PSI values corresponding to each RSS taken clinically were compared using Spearman's correlation. Overall, the comparison revealed a poor correlation (r = −0.25; P < 0.001) between the two values due to a wide distribution of PSI values within each RSS. The PSI values were skewed upwards for each RSS, with a small percentage of low values well outside of the RSS value assigned. For example, the PSI values for an RSS of 2 ranged from 45 to 99, exceeding the normal range of 80 to 95. However, if PSI values that correspond to EMG activity ≥50% were removed, the Spearman correlation for PSI and RSS rose to a significant level (r = −0.49; P < 0.001). This serves as confirmation that muscular interference alters PSI values.
Adverse events
Four types of events were reported that required intervention during the sedations. Seven incidents of oxygen desaturation, a severe pain event, one incident of oversedation requiring reversal agents, and six incidents of undersedation were found over the course of the study.
An evaluation of the seven events of oxygen desaturation below 93% found no correlation with PSI (r = −0.08; P = 0.064) or RSS (r = 0.11; P = 0.008). The PSI ranged from 67 to 95 at the time of clinical recognition, when the patient had desaturated to a point requiring intervention.
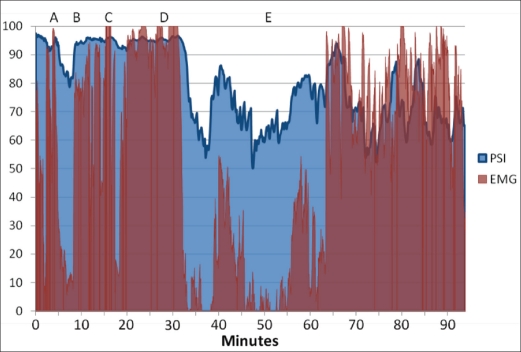
One severe pain event resulting in oversedation was detected during the procedures. It involved a 19-year-old woman having four third molars extracted. The patient seemed overly responsive during the extractions, and additional sedation was provided. However, the reason for the responsiveness was likely inadequate local anesthesia rather than inadequate sedation. In Figure 2, the progression of the case is illustrated. At point A, the patient was initially sedated using midazolam and fentanyl. Between points A and B, the surgeon attempted a local block of the right inferior alveolar nerve. At point B, the extraction was initiated, and due to significant agitation from the patient, additional midazolam was provided at point C. This reduced the EMG associated with the responsiveness of the patient for a short time; however, the pain was substantial enough for the EMG to return to near 100 levels. At point D, it was clear that the patient was experiencing pain associated with an inadequate inferior alveolar nerve block. Additional midazolam and fentanyl were administered and the surgeon also provided additional local anesthesia. At point E, the patient was noted to have an RSS of 4, which was deemed too deep for conscious sedation. Reversal agents, flumazenil and naloxone, were administered until the patient had regained an RSS of 2. The procedure was completed without additional intervention, although the EMG activity was notably high.
Figure 2.
Patient state index (PSI) and electromyographic (EMG) results of an inadequately sedated patient.
One patient appeared clinically to be undersedated; however, five additional patients noted less amnesia on their survey compared with the other 15 patients and would have preferred additional medication. The patient who was clinically undersedated had an average PSI of 95 despite receiving 7 mg of midazolam and 75 μg of fentanyl over a 12-minute period. Figure 2 illustrates her lack of sedation. The other patients who stated a preference for additional medication had an average PSI of 90 versus 83 for those satisfied with the amount they received. In these patients, the PSI was an objective confirmation of their light sedation.
Trends
Each patient filled out a visual analogue scale for pain, amnesia, and satisfaction based on their experience during the procedure. Patients' satisfaction (r = −0.57; P = 0.012) and pain (r = −0.73; P = 0.006) appeared to correlate well with amnesia.
DISCUSSION
The PSI generated by the brain function monitor did not correlate to the RSS, as previously demonstrated in other studies (8, 17, 18). Many of those studies were done in intensive care unit settings, when there was no procedure that might stimulate the patient and cause contraction of the frontalis muscle adjacent to the EEG leads.
In this study, RSS values of 2 were designated 82% of the time and only one RSS of 4, moderate to deep sedation, was noted with a corresponding PSI of 64. Interestingly, the distribution of PSI values tended to be skewed upwards for all RSS values (Table). This positive skew was likely due to excessive EMG interference, which was noted in all 20 cases with high PSI values and EMG pattern recognition on the cerebral function monitor screen. Once the values associated with ≥50% EMG were excluded, the relation between RSS and PSI became significant. In light to moderate sedation, particularly during an intense procedure, it is difficult to reduce the EMG impact on an EEG monitor (18). While Matsuzaki and Tanaka (2004) showed a useable signal for the bispectral index, the filters for EMG may not be adequate to eliminate frontalis muscle activity during periodontal surgery and establish a reliable PSI in this range of sedation at all times (7). Given that situation, PSI values are more reliable when they are lower and cannot be implicitly relied on when they are high. For a device to be useful in a clinical setting, it should be reliable for all values of PSI. Therefore, it may be more useful to move away from an oversimplified approach that summarizes all the data obtained in an EEG to a PSI and, instead, use pattern recognition of the EEG to discern the patient's level of sedation.
Table.
Patient state index quartiles at respective Ramsay sedation scale (RSS) levels
| Patient State Index |
|||||
|---|---|---|---|---|---|
| RSS | Min | Quartile 2 | Median | Quartile 3 | Max |
| 1 | 64.50 | 90.42 | 93.88 | 96.01 | 98.68 |
| 2 | 45.39 | 77.69 | 89.09 | 94.28 | 98.75 |
| 3 | 59.33 | 69.76 | 84.97 | 93.15 | 95.56 |
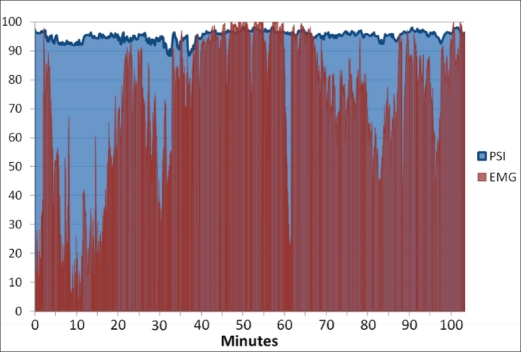
The high EMG noted during a pain event (Figure 1) illustrates the difficulty in obtaining an accurate PSI value, but also indicates that the cerebral function monitor may help by acting as a pain or discomfort indicator through the EMG display. It is well known that in response to a painful stimulus, the patient withdraws from the pain and alters his or her normal facial expression (19). However, such activity is unlikely to provide an early indicator of pain when the patient's face is obscured, as is typical during dental procedures.
Figure 1.
Patient state index (PSI) and electromyographic (EMG) results during moderate sedation.
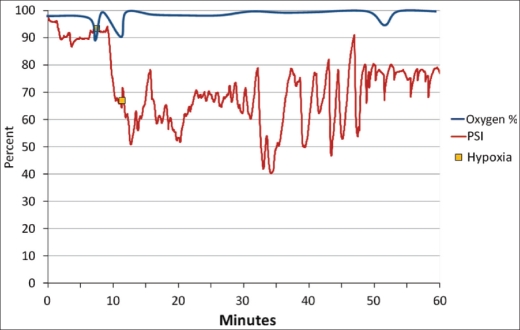
During light to moderate sedation, three monitors are typically in place to ensure patient safety: the pulse oximeter, blood pressure monitor, and EKG. No other noninvasive devices have been found to precisely measure the cardiovascular system and provide an easily understood readout or audible alarm for the practitioner. The brain function monitor has the potential to provide advance warning of a patient who is oversedated before the vulnerable airway is lost. Unfortunately, PSI values as high as 95 were found when patients' oxygen saturation had decreased below 93% (Figure 3). These high readings are likely caused by high EMG signals masking a much lower PSI. However, the PSI values may be accurate and the oxygen desaturation may be a result of occlusion of the airway due to positioning. While practitioners are careful with the physical manipulation of patients, it is common for the mandible to be depressed and intruded during a procedure, placing pressure on the airway. A third possibility relates to the use of an opioid during sedation. Fentanyl does not have a significant effect on the PSI, but does affect the respiratory drive (20).
Figure 3.
Patient state index (PSI) and oxygen saturation.
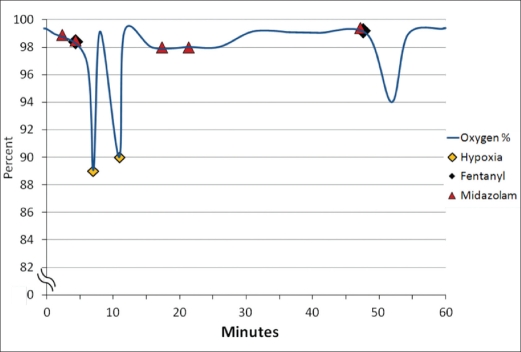
As the case in Figure 4 illustrates, two incidents of oxygen desaturation closely followed the initial administration of 50 μg of fentanyl. The second 25-μg dose of fentanyl corresponded to a decrease in the oxygen saturation to 95%. The hypoventilation events appeared to be closely related to the administration of fentanyl in conjunction with midazolam.
Figure 4.
Medications administered and oxygen saturation.
The brain function monitors are not capable of providing a reliable warning of a hypoventilating patient. The oximeter is far more effective at detecting an apneic patient. Unfortunately, the common practice of administering oxygen during sedation delays the responsiveness of an oximeter (12). In this circumstance, capnography may be the more effective method of monitoring a patient. However, it is difficult to measure end-tidal carbon dioxide during a procedure involving the head and neck (21).
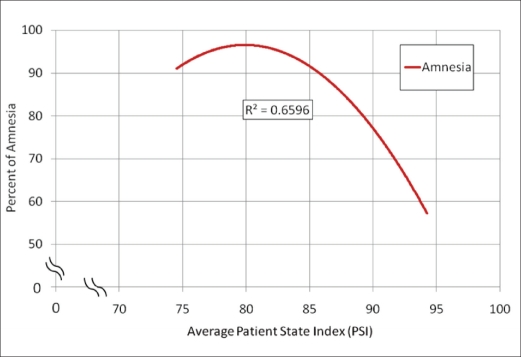
Traditionally, amnesia can be difficult to predict based on clinical evaluation since the data used for evaluation are subjective. From this study, it appears that the PSI may be capable of determining the point of amnesia. This would enable the cerebral function monitor to help guide a sedation technique, increasing the overall safety and efficiency. By taking the percentage of amnesia and comparing it to the average PSI of each patient, a significant correlation (r = −0.55; P = 0.015) was revealed. Extrapolating from our findings, a PSI value of 85 appears to be the threshold for patients to experience at least 90% amnesia over the course of sedation (Figure 5).
Figure 5.
Perception of amnesia and average patient state index.
Unfortunately, the ambiguity created by EMG interference makes it difficult to use PSI as a guide for sedation, but the combination of PSI with EEG interpretation appears to be a useful tool. The positive skewing of the PSI by EMG when used alone without the EEG screen could create the false impression of an undersedated patient, thus leading to oversedation.
The brain function monitors using only the algorithm-generated score do not add significantly to light and moderate sedation management for periodontal surgery. However, when EMG is identified by direct visualization and the depth of sedation assessed by looking at the EEG signal, then these monitors may be useful as a guide for sedation management. In summary, the most important monitor of the patient remains the practitioner, who observes the patient directly and understands the information provided by the equipment.
Acknowledgments
This study was supported financially by the Graduate Studies Department, Baylor College of Dentistry, Texas A&M Health Science Center. The SEDline monitor was provided for the duration of the study by the Department of Anesthesiology and Pain Management, Baylor University Medical Center at Dallas. We thank Jan Steele, RN, for her assistance with the collection of data without compensation. We appreciate the statistical advice of Jay Shulman, DMD, MA, MSPH (Dental Public Health Residency Program, Texas A&M Baylor College of Dentistry, Dallas) during the analysis of data, provided without compensation.
References
- 1.Bhananker SM, Posner KL, Cheney FW, Caplan RA, Lee LA, Domino KB. Injury and liability associated with monitored anesthesia care: a closed claims analysis. Anesthesiology. 2006;104(2):228–234. doi: 10.1097/00000542-200602000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Loskota WJ. Intraoperative EEG monitoring. J Crit Care. 2005;24(4):176–185. [Google Scholar]
- 3.Green SM, Mason KP. Reformulation of the sedation continuum. JAMA. 2010;303(9):876–877. doi: 10.1001/jama.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, Manberg P. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. BIS Utility Study Group. Anesthesiology. 1997;87(4):808–815. doi: 10.1097/00000542-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Bannister CF, Brosius KK, Sigl JC, Meyer BJ, Sebel PS. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92(4):877–881. doi: 10.1097/00000539-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 6.White PF, Ma H, Tang J, Wender RH, Sloninsky A, Kariger R. Does the use of electroencephalographic bispectral index or auditory evoked potential index monitoring facilitate recovery after desflurane anesthesia in the ambulatory setting? Anesthesiology. 2004;100(4):811–817. doi: 10.1097/00000542-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki S, Tanaka H. The feasibility of bispectral index monitoring for intravenous sedation during dental treatment. Anesth Prog. 2004;51(2):52–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsay MA, Huddleston P, Hamman B, Tai S, Matter G. The patient state index correlates well with the Ramsay sedation score in ICU patients. Anesthesiology. 2004;101:A338. [Google Scholar]
- 9.Chen X, Tang J, White PF, Wender RH, Ma H, Sloninsky A, Kariger R. A comparison of patient state index and bispectral index values during the perioperative period. Anesth Analg. 2002;95(6):1669–1674. doi: 10.1097/00000539-200212000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson M, Goodchild JH. Use of bispectral index system (BIS) to Monitor Enteral Conscious (moderate) sedation during general dental procedures. J Can Dent Assoc. 2009;75(10):709. [PubMed] [Google Scholar]
- 11.Hata K, Andoh A, Hayafuji K, Ogawa A, Nakahara T, Tsujikawa T, Fujiyama Y, Saito Y. Usefulness of bispectral monitoring of conscious sedation during endoscopic mucosal dissection. World J Gastroenterol. 2009;15(5):595–598. doi: 10.3748/wjg.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126(5):1552–1558. doi: 10.1378/chest.126.5.1552. [DOI] [PubMed] [Google Scholar]
- 13.Prichep LS, Gugino LD, John ER, Chabot RJ, Howard B, Merkin H, Tom ML, Wolter S, Rausch L, Kox WJ. The Patient State Index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth. 2004;92(3):393–399. doi: 10.1093/bja/aeh082. [DOI] [PubMed] [Google Scholar]
- 14.Adesanya AO, Wiley J, Joshi GP. A comparison of patient state index, bispectral index, and modified Ramsay sedation scores in mechanically ventilated critically ill patients. Chest. 2006;130(4):203S. [Google Scholar]
- 15.Chisholm CJ, Zurica J, Mironov D, Sciacca RR, Ornstein E, Heyer EJ. Comparison of electrophysiologic monitors with clinical assessment of level of sedation. Mayo Clin Proc. 2006;81(1):46–52. doi: 10.4065/81.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson D, Harrop TJ, Klienknecht RE. An objective evaluation of clinical signs used to assess sedation with intravenous diazepam. Anesth Prog. 1980;27(1):18–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Drover DR, Lemmens HJ, Pierce ET, Plourde G, Loyd G, Ornstein E, Prichep LS, Chabot RJ, Gugino L. Patient state index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 2002;97(1):82–89. doi: 10.1097/00000542-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Schneider G, Heglmeier S, Schneider J, Tempel G, Kochs EF. Patient state index (PSI) measures depth of sedation in intensive care patients. Intensive Care Med. 2004;30(2):213–216. doi: 10.1007/s00134-003-2092-5. [DOI] [PubMed] [Google Scholar]
- 19.Prkachin KM. Assessing pain by facial expression: facial expression as nexus. Pain Res Manag. 2009;14(1):53–58. doi: 10.1155/2009/542964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowdle TA. Depth of anesthesia monitoring. Anesthesiol Clin. 2006;24(4):793–822. doi: 10.1016/j.atc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Bennett J. A case against capnographic monitoring as a standard of care. J Oral Maxillofac Surg. 1999;57(11):1348–1352. doi: 10.1016/s0278-2391(99)90875-3. [DOI] [PubMed] [Google Scholar]