Abstract
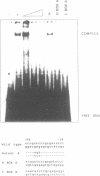
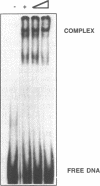
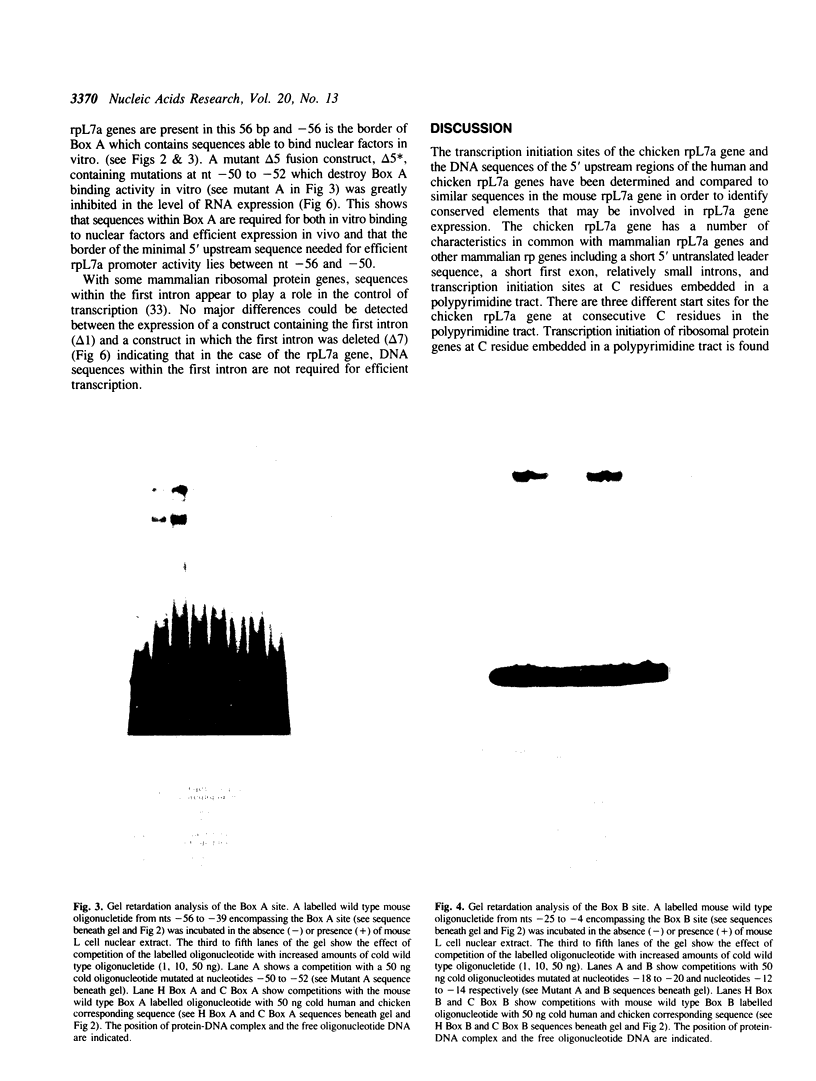
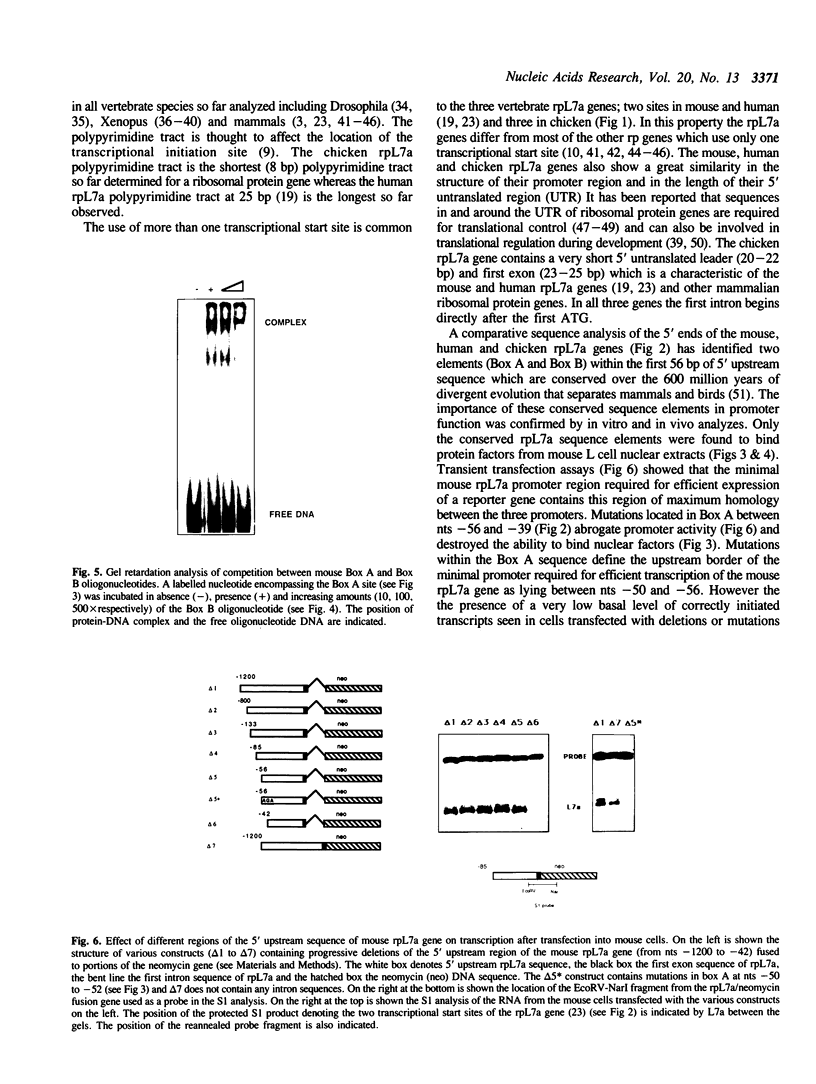
The transcriptional initiation sites of the chicken ribosomal protein L7a (rpL7a) gene have been determined and found to occur at three consecutive cytidine residues at the start of a polypyrimidine tract of 8 base pairs (bp). A comparative analysis of the 5' upstream regions of the mouse, human and chicken rpL7a genes identified two sequence elements (Box A and Box B) conserved over the 600 million years of divergent evolution that separate mammals and birds. Only Box A (nts - 56 to - 39) and Box B (nts - 25 to - 4) sequences were detected to bind nuclear factors from mouse nuclear extracts in an analysis of the mouse rpL7a 5' upstream sequence. Box A and Box B bind different nuclear factors and the factor binding to mouse Box A and mouse Box B sequences could be effectively competed by corresponding homologous sequences from the human and chicken rpL7a promoters. These results indicate that elements of the rpL7a promoter region are conserved between mammals and birds. An in vivo analysis of the mouse rpL7a 5' upstream sequence required for efficient transcription identified the 5' border of the minimal promoter region as lying between nts - 50 and - 56. Constructs containing 56 bp of 5' upstream DNA and the first 25 bp rpL7a exon were very efficiently transcribed indicating that sequences within the first intron are not required for gene expression. No sequence similarity was detected between the rpL7a promoter elements and described promoter elements of other eukaryotic ribosomal protein genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arevalo S. G., Warner J. R. Ribosomal protein L4 of Saccharomyces cerevisiae: the gene and its protein. Nucleic Acids Res. 1990 Mar 25;18(6):1447–1449. doi: 10.1093/nar/18.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison M. L., Meyuhas O., Perry R. P. Localization of transcriptional regulatory elements and nuclear factor binding sites in mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2067–2074. doi: 10.1128/mcb.9.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C., Mariottini P., Annesi F., Amaldi F. Structure of Xenopus laevis ribosomal protein L32 and its expression during development. Nucleic Acids Res. 1990 Aug 11;18(15):4423–4426. doi: 10.1093/nar/18.15.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari E., Mazzetti P., Mileo A., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Sequences coding for the ribosomal protein L14 in Xenopus laevis and Xenopus tropicalis; homologies in the 5' untranslated region are shared with other r-protein mRNAs. Nucleic Acids Res. 1986 Oct 10;14(19):7633–7646. doi: 10.1093/nar/14.19.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ishai R., Scharf R., Sharon R., Kapten I. A human cellular sequence implicated in trk oncogene activation is DNA damage inducible. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6039–6043. doi: 10.1073/pnas.87.16.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M. J. Phylogeny of the major tetrapod groups: morphological data and divergence dates. J Mol Evol. 1990 May;30(5):409–424. doi: 10.1007/BF02101113. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Wool I. G. The structure of a gene containing introns and encoding rat ribosomal protein P2. Nucleic Acids Res. 1991 Sep 25;19(18):4895–4900. doi: 10.1093/nar/19.18.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G. D., Wadsworth H. L., Rapoport B. Thyrotropin-induced expression of a gene for a ribosomal protein related to the trk oncogene. Mol Cell Endocrinol. 1990 Jan 2;68(1):R25–R30. doi: 10.1016/0303-7207(90)90177-a. [DOI] [PubMed] [Google Scholar]
- Chen I. T., Roufa D. J. The transcriptionally active human ribosomal protein S17 gene. Gene. 1988 Oct 15;70(1):107–116. doi: 10.1016/0378-1119(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo P., Yon J., Fried M. The organization and expression of the human L7a ribosomal protein gene. Biochim Biophys Acta. 1991 Dec 2;1129(1):93–95. doi: 10.1016/0167-4781(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Davies B., Feo S., Heard E., Fried M. A strategy to detect and isolate an intron-containing gene in the presence of multiple processed pseudogenes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6691–6695. doi: 10.1073/pnas.86.17.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. Properties of a mouse ribosomal protein promoter. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8545–8549. doi: 10.1073/pnas.83.22.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Giallongo A., Yon J., Fried M. Ribosomal protein L7a is encoded by a gene (Surf-3) within the tightly clustered mouse surfeit locus. Mol Cell Biol. 1989 Jan;9(1):224–231. doi: 10.1128/mcb.9.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. L., Merrick W., Bowman L. H. Sequences mediating the translation of mouse S16 ribosomal protein mRNA during myoblast differentiation and in vitro and possible control points for the in vitro translation. Genes Dev. 1991 Sep;5(9):1723–1736. doi: 10.1101/gad.5.9.1723. [DOI] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Equipotent mouse ribosomal protein promoters have a similar architecture that includes internal sequence elements. Genes Dev. 1989 Nov;3(11):1789–1800. doi: 10.1101/gad.3.11.1789. [DOI] [PubMed] [Google Scholar]
- Hariharan N., Perry R. P. A characterization of the elements comprising the promoter of the mouse ribosomal protein gene RPS16. Nucleic Acids Res. 1989 Jul 11;17(13):5323–5337. doi: 10.1093/nar/17.13.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Perry R. P. Functional dissection of a mouse ribosomal protein promoter: significance of the polypyrimidine initiator and an element in the TATA-box region. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1526–1530. doi: 10.1073/pnas.87.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Fried M. The mouse rpL7a gene is typical of other ribosomal protein genes in it's 5' region but differs in being located in a tight cluster of CpG-rich islands. Nucleic Acids Res. 1990 Sep 25;18(18):5353–5357. doi: 10.1093/nar/18.18.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Fried M. The mouse surfeit locus contains a cluster of six genes associated with four CpG-rich islands in 32 kilobases of genomic DNA. Mol Cell Biol. 1990 Feb;10(2):605–614. doi: 10.1128/mcb.10.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Williams T., Fried M. One of the tightly clustered genes of the mouse surfeit locus is a highly expressed member of a multigene family whose other members are predominantly processed pseudogenes. Mol Cell Biol. 1988 Sep;8(9):3898–3905. doi: 10.1128/mcb.8.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar R. L., Kakegawa T., Cranston H., Morris D. R., White M. W. A regulatory cis element and a specific binding factor involved in the mitogenic control of murine ribosomal protein L32 translation. J Biol Chem. 1992 Jan 5;267(1):508–514. [PubMed] [Google Scholar]
- Kozma S. C., Redmond S. M., Fu X. C., Saurer S. M., Groner B., Hynes N. E. Activation of the receptor kinase domain of the trk oncogene by recombination with two different cellular sequences. EMBO J. 1988 Jan;7(1):147–154. doi: 10.1002/j.1460-2075.1988.tb02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Avni D., Hariharan N., Perry R. P., Meyuhas O. Oligopyrimidine tract at the 5' end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreni F., Ruberti I., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Nucleotide sequence of the L1 ribosomal protein gene of Xenopus laevis: remarkable sequence homology among introns. EMBO J. 1985 Dec 16;4(13A):3483–3488. doi: 10.1002/j.1460-2075.1985.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager W. H. Control of ribosomal protein gene expression. Biochim Biophys Acta. 1988 Jan 25;949(1):1–15. doi: 10.1016/0167-4781(88)90048-6. [DOI] [PubMed] [Google Scholar]
- Maki C., Rhoads D. D., Stewart M. J., Van Slyke B., Roufa D. J. The Drosophila melanogaster RPS17 gene encoding ribosomal protein S17. Gene. 1989 Jul 15;79(2):289–298. doi: 10.1016/0378-1119(89)90211-4. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Amaldi F. The 5' untranslated region of mRNA for ribosomal protein S19 is involved in its translational regulation during Xenopus development. Mol Cell Biol. 1990 Feb;10(2):816–822. doi: 10.1128/mcb.10.2.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariottini P., Bagni C., Annesi F., Amaldi F. Isolation and nucleotide sequences of cDNAs for Xenopus laevis ribosomal protein S8: similarities in the 5' and 3' untranslated regions of mRNAs for various r-proteins. Gene. 1988 Jul 15;67(1):69–74. doi: 10.1016/0378-1119(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Baldin V., Bouche G., Amalric F. Glucocorticoids repress ribosome biosynthesis in lymphosarcoma cells by affecting gene expression at the level of transcription, posttranscription and translation. Biochim Biophys Acta. 1990 May 24;1049(1):38–44. doi: 10.1016/0167-4781(90)90082-d. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Klein A. The mouse ribosomal protein L7 gene. Its primary structure and functional analysis of the promoter region. J Biol Chem. 1990 Jul 15;265(20):11465–11473. [PubMed] [Google Scholar]
- Moura-Neto R., Dudov K. P., Perry R. P. An element downstream of the cap site is required for transcription of the gene encoding mouse ribosomal protein L32. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3997–4001. doi: 10.1073/pnas.86.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Tanaka T., Ishikawa K. Nucleotide sequence of cloned cDNA specific for rat ribosomal protein L7a. Nucleic Acids Res. 1989 Jun 26;17(12):4875–4875. [PMC free article] [PubMed] [Google Scholar]
- Patel R. C., Jacobs-Lorena M. Cis-acting sequences in the 5'-untranslated region of the ribosomal protein A1 mRNA mediate its translational regulation during early embryogenesis of Drosophila. J Biol Chem. 1992 Jan 15;267(2):1159–1164. [PubMed] [Google Scholar]
- Qian S., Zhang J. Y., Kay M. A., Jacobs-Lorena M. Structural analysis of the Drosophila rpA1 gene, a member of the eucaryotic 'A' type ribosomal protein family. Nucleic Acids Res. 1987 Feb 11;15(3):987–1003. doi: 10.1093/nar/15.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Roufa D. J. A cloned human ribosomal protein gene functions in rodent cells. Mol Cell Biol. 1987 Oct;7(10):3767–3774. doi: 10.1128/mcb.7.10.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. E1A control of gene expression is mediated by sequences 5' to the transcriptional starts of the early viral genes. Mol Cell Biol. 1983 Jul;3(7):1222–1234. doi: 10.1128/mcb.3.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Yon J., Huxley C., Fried M. The mouse surfeit locus contains a very tight cluster of four "housekeeping" genes that is conserved through evolution. Proc Natl Acad Sci U S A. 1988 May;85(10):3527–3530. doi: 10.1073/pnas.85.10.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon J., Giallongo A., Fried M. The organization and expression of the Saccharomyces cerevisiae L4 ribosomal protein genes and their identification as the homologues of the mammalian ribosomal protein gene L7a. Mol Gen Genet. 1991 May;227(1):72–80. doi: 10.1007/BF00260709. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Müller R. G., Fu X. C., Hynes N. E., Kozma S. Oncogenic activation of the human trk proto-oncogene by recombination with the ribosomal large subunit protein L7a. EMBO J. 1990 Jan;9(1):191–196. doi: 10.1002/j.1460-2075.1990.tb08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]