Abstract
Context
Disability among older persons is a complex and highly dynamic process, with high rates of recovery and frequent transitions between states of disability. The role of intervening illnesses and injuries (i.e. events) on these transitions is uncertain.
Objectives
To evaluate the relationship between intervening events and transitions among states of no disability, mild disability, severe disability and death, and to determine the association of physical frailty with these transitions.
Design, Setting, and Participants
Prospective cohort study, conducted in greater New Haven, Connecticut, from March 1998 to December 2008, of 754 community-living persons, aged 70 years or older, who were nondisabled at baseline in four essential activities of daily living: bathing, dressing, walking, and transferring. Telephone interviews were completed monthly for more than 10 years to assess disability and ascertain exposure to intervening events, which included illnesses and injuries leading to either hospitalization or restricted activity. Physical frailty (defined as gait speed >10 seconds on the rapid gait test) was assessed every 18 months through 108 months.
Main Outcome Measure
Transitions between no disability, mild disability, and severe disability, and 3 transitions from each of these states to death, were evaluated each month.
Results
Hospitalization was strongly associated with 8 of the 9 possible transitions, with increased multivariable hazard ratios (HR) as high as 168 (95% confidence interval [CI], 118–239) for the transition from no disability to severe disability and decreased HRs as low as 0.41 (95% CI, 0.30–0.54) for the transition from mild disability to no disability. Restricted activity also increased the likelihood of transitioning from no disability to both mild and severe disability (HR [CI]: 2.59 [2.23–3.02] and 8.03 [5.28–12.21]), respectively, and from mild disability to severe disability (1.45 [1.14–1.84]), but was not associated with recovery from mild or severe disability. For all nine of the transitions, the presence of physical frailty accentuated the associations of the intervening events. For example, the absolute risk of transitioning from no disability to mild disability within one month after hospitalization for frail individuals was 12.4% (95% CI, 12.1%–12.7%) vs 4.9% (4.7%–5.1%) for non-frail individuals. Among the possible reasons for hospitalization, fall-related injury conferred the highest likelihood of developing new or worsening disability.
Conclusions
Among older persons, particularly those who were physically frail, intervening illnesses and injuries greatly increased the likelihood of developing new or worsening disability. Only the most potent events, i.e. those leading to hospitalization, reduced the likelihood of recovery from disability.
INTRODUCTION
Among older persons, disability in essential activities of daily living, such as bathing, dressing, walking, and transferring, is common and associated with increased mortality, institutionalization, and greater use of formal and informal home services (1). Active life expectancy, a metric that is often used by policy-makers to forecast the functional health of older persons is based on disability (2,3).
According to several conceptual models (4,5), disability is thought to arise when a vulnerable host is exposed to a new or worsening insult or intervening event. Supporting this theoretical framework, we have shown that intervening events, including illnesses and injuries leading to either hospitalization or restricted activity, are strongly associated with the development of disability in activities of daily living (6). These results, however, were based solely on the initial onset of disability. We have subsequently demonstrated that disability is a complex and highly dynamic process, with high rates of recovery and frequent transitions between states of disability (7,8). The role of intervening events on these transitions is uncertain.
The objectives of the current study were to evaluate the association of intervening events with transitions between states of no disability, mild disability, severe disability and death, and to determine the association of physical frailty, the vulnerability factor with the strongest epidemiologic link to disability (9,10), with these transitions. To accomplish our objectives, we used data from a longitudinal study that includes monthly assessments of intervening events and activities of daily living for more than 10 years in a cohort of community-living older persons.
METHODS
Study Population
Participants were members of the Precipitating Events Project, an ongoing longitudinal study of 754 community-living persons, aged 70 years or older, who were initially nondisabled (i.e. required no personal assistance) in 4 activities of daily living—bathing, dressing, walking inside the house, and transferring from a chair (11). Exclusion criteria included significant cognitive impairment with no available proxy (12), inability to speak English, diagnosis of a terminal illness with a life expectancy less than 12 months, and a plan to move out of the New Haven area during the next 12 months.
The assembly of the cohort, which took place between March 1998 and October 1999, is summarized in eFigure 1 and has been described in detail elsewhere (7,11). In brief, potential participants were identified from a computerized list of 3,157 age-eligible members of a large health plan in greater New Haven, Connecticut. Eligibility was determined during a screening telephone interview and was confirmed during an in-home assessment. Persons who were physically frail, as denoted by a timed score of greater than 10 seconds on the rapid gait test (i.e. walk back and forth over a 10-ft [3-m] course as quickly as possible), were oversampled to ensure a sufficient number of participants at increased risk for disability (9). In the absence of a gold standard, operationalizing physical frailty as slow gait speed is justified by its high face validity (13), clinical feasibility (14), and strong epidemiologic link to functional decline and disability (9,10,15). Only 4.6% of the 2,753 health plan members who were alive and could be contacted refused to complete the screening telephone interview, and 75.2% of the eligible members agreed to participate in the project. Persons who refused to participate did not differ significantly from those who were enrolled in terms of age or sex. The study protocol was approved by the Yale Human Investigation Committee, and all participants provided verbal informed consent.
Data Collection
Comprehensive home-based assessments were completed at baseline and subsequently at 18-month intervals for 108 months, while telephone interviews were completed monthly through December 2008 (median 111 months). For participants with significant cognitive impairment, the monthly telephone interviews were completed with a designated proxy. The accuracy of these proxy reports was high, with Kappa = 1.0 for disability, 1.0 for hospitalization, and .63 for restricted activity (6,12). Deaths were ascertained by review of the local obituaries and/or from an informant during a subsequent telephone interview. Four hundred and five (53.7%) participants died after a median follow-up of 68 months, while 35 (4.6%) dropped out of the study after a median follow-up of 24 months. Data were otherwise available for 99.2% of the 66,425 monthly telephone interviews, with no difference between the decedents and non-decedents.
Assessment of Physical Frailty and Covariates
During each of the comprehensive assessments, data were collected on physical frailty as previously described, and several covariates, including demographic characteristics, cognitive status as assessed by the Folstein Mini-Mental State Examination (MMSE) (16), depressive symptoms as assessed by the Center for Epidemiologic Studies Depression (CES-D) Scale (17), and 9 self-reported, physician-diagnosed chronic conditions: hypertension, myocardial infarction, congestive heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer. Data on these covariates were 100% complete at baseline and greater than 95% complete during the subsequent comprehensive assessments. Participants were considered to be cognitively impaired if they scored less than 24 on the MMSE (16) and to have significant depressive symptoms if they scored 20 or higher on the CES-D (18). Participants were asked by a trained nurse researcher to identify their race/ethnicity. These data were collected primarily for descriptive purposes and to fulfill federal regulations regarding the inclusion of minority participants in NIH-funded studies.
Assessment of Intervening Events
The intervening events included illnesses and injuries leading to either hospitalization or restricted activity. During the monthly telephone interviews, participants were asked whether they had stayed at least overnight in a hospital since the last interview, i.e. during the past month. The accuracy of these reports, based on an independent review of hospital records among a subgroup of 94 participants, was high, with Kappa = 0.94 (6). Participants who were hospitalized were also asked to provide the primary reason for their admission. These reasons were subsequently grouped into distinct diagnostic categories using a revised version of the protocol described by Ferrucci et al (19). Agreement relative to an independent review of hospital records among a subgroup of 172 admissions was 82% (6).
To ascertain less potent intervening events, participants were asked two questions related to restricted activity using a standardized protocol with high reliability, i.e. Kappa = .90 (11): 1) “Since we last talked on (date of last interview), have you cut down on your usual activities due to an illness, injury or other problem?” and 2) “Since we last talked on (date of last interview), have you stayed in bed for at least half a day due to an illness, injury or other problem?” Participants who answered “Yes” to one or both of these questions were considered to have restricted activity during a specific month (11). These participants were subsequently asked to identify the reason(s) for their restricted activity using a standardized protocol that included 24 prespecified problems and an open-ended response. We have previously demonstrated that older persons usually attribute their restricted activity to several concurrent health-related problems (11).
For participants who had died, a final interview was completed with a proxy informant who was asked about any hospitalizations since the prior interview. Given the nature of the questions, asking about restricted activity in the last month of life was not feasible. For each month during the follow-up period, exposure to the intervening events was categorized as hospitalization, restricted activity without hospitalization, referred to hereafter as simply restricted activity, or neither hospitalization or restricted activity.
Assessment of Disability
During the monthly telephone interviews, participants were assessed for disability using standard questions that were identical to those used during the screening telephone interview (12). For each of the four essential activities of daily living, we asked, “At the present time, do you need help from another person to (complete the task)?” Disability was operationalized as the need for personal assistance, and the severity of disability was denoted by the number of disabled activities of daily living (from 0 to 4) in a specific month. Disability in one or two activities of daily living was considered as mild, while disability in three or four activities of daily living was considered as severe (7,8). The reliability of our disability protocol was substantial (kappa=0.75) for reassessments completed within 48 hours and excellent (kappa=1.0) for reassessments performed the same day (12). Complete details regarding these procedures, including formal tests of reliability and accuracy, are provided elsewhere (7,12). To address the small amount of missing data on disability (i.e. less than 1% of observations), we used multiple imputation with 50 random draws per missing observation according to procedures described in an earlier report (20).
Functional Transitions
Based on prior research (8), four states were defined: no disability, mild disability, severe disability, and death. Transitions were possible among all of the non-decedent states, and from each non-decedent state to death.
Statistical Analysis
For each of the nine possible transitions, we calculated the cumulative incidence rate per 1000 person-months spent in the state immediately preceding the transition. We also calculated the median time (months) spent in a specific state before the next transition or end of follow-up for no disability, mild disability and severe disability, respectively. To quantify exposure to the two types of intervening events—hospitalization and restricted activity—we calculated the mean rate for each as the number of person-months exposed divided by all person-months observed. Confidence intervals for the transition rates and exposure rates were calculated by bootstrapping, using sampling with replacement. One thousand samples were created, and the 2.5th and 97.5th percentiles were used to form the confidence intervals. Bootstrap-derived confidence intervals provide non-parametric estimates of dispersion. For each of these analyses, the results were stratified according to physical frailty.
To evaluate the multivariable relationships between the independent variables and nine functional transitions, we used a competing risk Cox model for recurrent events (21). In this model, persons are simultaneously at risk for several “competing” outcomes. For example, participants with severe disability were at risk for transitions to no disability, mild disability, and death. The model calculates the respective associations based on the amount of time participants spend in a specific state prior to transitioning to another state. The competing risk Cox model accounts for the correlation among observations within individuals through the use of robust sandwich variance estimators for standard errors of the coefficients (22), is fairly robust to the distribution of time to event, and can be used for non-proportional hazards, which may occur with time-dependent variables (23). The independent variables included physical frailty, which was time-varying, and the two types of intervening events. For the intervening events, the calculated hazard ratio refers to the risk of making a specific transition between month t and month t+1 based on exposure to hospitalization or restricted activity, respectively, relative to participants who had no hospitalization or restricted activity, during this 1-month interval. We have previously shown that intervening events occurring prior to this 1-month interval are not independently associated with the development of disability (6). Similarly, the hazard ratio for physical frailty refers to the risk of making a specific transition between month t and month t+1 among participants who were physically frailty relative to those who are were not physically frail.
The multivariable model, which evaluated associations between the independent variables and each of the functional transitions, included three fixed covariates—sex, race/ethnicity, and years of education—and five time-varying covariates—age 85 years or older, living alone, number of chronic conditions, cognitive impairment, and depressive symptoms. These covariates represent important sociodemographic factors and pertinent clinical factors that have been linked to disability in prior studies (6). The time-varying covariates and physical frailty were updated using data from the comprehensive assessment that immediately preceded entry into the state. P values were adjusted for multiple comparisons assuming a false discovery rate of 5% (24). Potential interactions between physical frailty and the intervening events were tested and statistically significant interactions were retained in the final model. Because restricted activity could not be ascertained among decedents, transitions to death were not evaluated for this exposure.
To enhance the interpretability of our findings, we calculated the absolute risk per month of each functional transition for hospitalization, restricted activity, and no intervening event, respectively, in the presence and absence of physical frailty (25). Values for the absolute risk represent the probability of developing a specific outcome per unit of time in the setting of the competing outcomes. The coefficients used to calculate the absolute risks were obtained from a set of pooled logistic models (one for each transition) that approximate a simple exponential model over the entire follow-up period. Each logistic model included the three independent variables, eight covariates, and the transition-specific interaction terms that were statistically significant in the competing risk Cox model. The eight covariates were set as male sex, age less than 85, white race, greater than high school education, living alone, two or fewer chronic conditions, no cognitive impairment, and no depressive symptoms. In a separate set of analyses, the absolute risks were further stratified by sex and age, with the remaining covariates set as described above.
To be conservative, power calculations were based on the lowest frequency of observed transitions out of each state. Covariates were assumed to contribute an R-Squared of 0.20. Using Cox regression and assuming 20% exposure for either physical frailty or hospitalization, we had 80% power with a Type I error of 5% to detect hazard ratios of 2.05, 2.35, and 1.85 for transitions from no disability, mild disability, and severe disability, respectively. For restricted activity, we had 80% or greater power to detect a hazard ratio of 1.5 or higher for all transitions except from severe disability to no disability, which had 46% power. All statistical tests were 2-tailed with P < .05 denoting statistical significance, and all analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
The baseline characteristics of the study participants are shown in Table 1. Participants who were physically frail at baseline were older; were more likely to be female, live alone, be cognitively impaired, and have depressive symptoms; were less likely to be Non-Hispanic white; and had less education and more chronic conditions than participants who were not physically frail. The median duration of follow-up was 81 (interquartile range [IQR]: 42–115) months for participants who were physically frail at baseline and 117 (77.5–123) months for those who were not physically frail.
Table 1.
Baseline Characteristics of Study Participants According to Physical Frailtya
| Overall | Physical Frailty | |||
|---|---|---|---|---|
| Characteristic | (n=754) | No (n=432) | Yes (n=322) | P Valueb |
| Age, y | ||||
| Median (IQR) | 78 (74–82) | 76 (73–80) | 80 (76–84) | <.001 |
| 85 or older | 102 (13.5) | 31 (7.2) | 71 (22.1) | <.001 |
| Female | 487 (64.6) | 260 (60.2) | 227 (70.5) | .003 |
| Non-Hispanic white | 682 (90.5) | 399 (92.4) | 283 (87.9) | .04 |
| Lives alone | 298 (39.5) | 148 (34.3) | 150 (46.6) | .001 |
| Education, median (IQR), y | 12 (10–14) | 12 (11–14) | 12 (9–12) | <.001 |
| Chronic conditions, median (IQR) | 2 (1–2) | 1 (1–2) | 2 (1–3) | <.001 |
| Mental status | ||||
| MMSE score, median (IQR) | 27 (25–29) | 28 (26–29) | 27 (25–28) | <.001 |
| Cognitive impairmentc | 86 (11.4) | 35 (8.1) | 51 (15.8) | .001 |
| Psychological status | ||||
| CES-D score, median (IQR) | 8 (3–14) | 5 (2–12) | 12 (5–18) | <.001 |
| Depressive symptomsd | 100 (13.3) | 38 (8.8) | 62 (19.3) | <.001 |
Abbreviations: IQR, interquartile range; MMSE, Mini-Mental State Examination; CES-D score, Center for Epidemiologic Studies Depression Scale.
Data are presented as No. (%) unless otherwise indicated.
The Wilcoxon rank-sum was used to evaluate differences in medians, while the chi-square or Fisher exact test was used to evaluate differences in percentages.
Defined as an MMSE score of less than 24.
Defined as a CES-D score of 20 or higher.
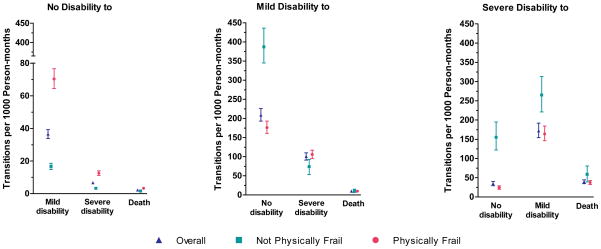
Of the 754 participants, 117 (15.5%) remained nondisabled and alive through the end of follow-up, and therefore made no transitions during a median (IQR) follow-up of 121 (117 – 124) months. Of these, 27 (23.1%) were physically frail at baseline. Figure 1 provides the rates of transitions between states of disability and death among the 637 participants who had at least one transition. Overall, the rates were highest for transitions from mild disability to no disability and from severe disability to mild disability. As compared with participants who were not physically frail, those who were physically frail had substantially higher rates of developing new or worsening disability and lower rates of recovering from mild and severe disability. While differences in rates among participants with vs without physical frailty were relatively small for those who died after having no disability or mild disability, the rate of death among those with severe disability was modestly lower among participants who were physically frail than those who were not physically frail. Participants who were physically frail spent a median (IQR) of 3 (1–12), 1 (2–3), and 1 (1–3) months, respectively, in a state of no disability, mild disability and severe disability before the next transition or end of follow-up. The corresponding values for participants who were not physically frail were 13 (3–49), 1 (1–1) and 1 (1–1) months, respectively.
Figure 1.
Rates of Functional Transitions per 1000 Person-months According to Physical Frailty. Point estimates are accompanied by 95% bootstrap confidence intervals. Physical frailty was updated every 18 months during the comprehensive assessments. Participants who did not make any transitions were included in the denominators for the calculation of rates.
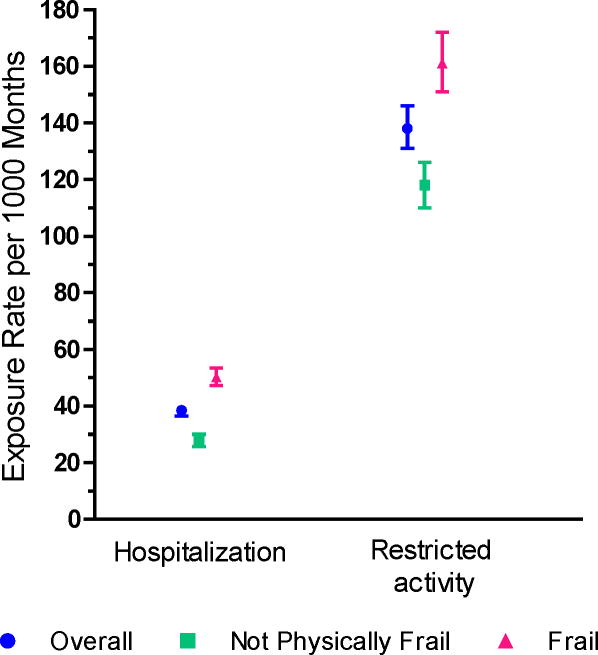
Among the 117 participants without a functional transition, 68 (58.1%) had at least one hospital admission and 107 (91.5%) had at least one month of restricted activity; they had a median (IQR) of 2 (1–3) hospital admissions and 7 (3–13) months of restricted activity. Among the 637 participants who had at least one functional transition, 578 (90.7%) had at least one hospital admission and 601 (94.3%) had at least one month of restricted activity during a median (IQR) follow-up of 102 (52–119) months; they had a median (IQR) of 3 (2–6) hospital admissions and 10 (5–18) months of restricted activity. As shown in Figure 2, the exposure rates to both hospitalization and restricted activity were considerably greater among participants who were physically frail than those who were not physically frail.
Figure 2.
Exposure Rates to Intervening Events per 1000 Months According to Physical Frailty. Point estimates are accompanied by 95% bootstrap confidence intervals. Physical frailty was updated every 18 months during the comprehensive assessments. As described in the Methods, the two intervening events are mutually exclusive.
The multivariable associations between the 3 independent variables — physical frailty, hospitalization, and restricted activity—and each of the functional transitions are shown in Table 2. Participants who were frail were more likely to transition to new or worsening disability and less likely to recover from mild and severe disability, but frailty was associated with the transition to death only for those with no disability. Hospitalization was associated with disability for 8 of the 9 transitions (the ninth being severe disability to no disability), with hazard ratios as high as 168 for the transition from no disability to severe disability and as low as 0.41 for the transition from mild disability to no disability. Restricted activity increased the likelihood of transitioning from no disability to both mild and severe disability, respectively, and from mild disability to severe disability, but was not associated recovery from mild or severe disability.
Table 2.
Associations of Physical Frailty, Hospitalization, and Restricted Activity with Functional Transitionsa
| Physical Frailty
|
Hospitalizationb |
Restricted Activityb,c |
||||
|---|---|---|---|---|---|---|
| Transition | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| No disability to | ||||||
| Mild disability | 4.34 (3.58–5.27) | <.001 | 8.90 (7.05–11.22) | <.001 | 2.59 (2.23–3.02) | <.001 |
| Severe disability | 3.53 (2.68–4.63) | <.001 | 168 (118–239) | <.001 | 8.03 (5.28–12.21) | <.001 |
| Death | 1.79 (1.20–2.68) | .005 | 23.8 (15.9–35.7) | <.001 | --- | --- |
| Mild disability to | ||||||
| No disability | 0.30 (0.21–0.41) | <.001 | 0.41 (0.30–0.54) | <.001 | 0.95 (0.77–1.17) | .62 |
| Severe disability | 2.15 (1.51–3.04) | <.001 | 7.73 (5.47–10.9) | <.001 | 1.45 (1.14–1.84) | .002 |
| Death | 1.26 (0.59–2.67) | .55 | 10.9 (6.70–17.7) | <.001 | --- | --- |
| Severe disability to | ||||||
| No disability | 0.13 (0.08–0.21) | <.001 | 1.04 (0.65–1.66) | .87 | 0.78 (0.46–1.32) | .35 |
| Mild disability | 0.57 (0.39–0.83) | .003 | 0.70 (0.51–0.95) | .02 | 0.93 (0.69–1.27) | .66 |
| Death | 0.87 (0.51–1.49) | .61 | 6.40 (4.49–9.12) | <.001 | --- | --- |
Abbreviations: HR, hazard ratio; CI, confidence interval.
As described in the Methods, a single multivariable model was run that included three fixed covariates—sex, race/ethnicity, and years of education, five time-dependent covariates—age 85 years or older, living alone, number of chronic conditions, cognitive impairment, and depressive symptoms, and the transition-specific interaction terms that were statistically significant in the competing risk Cox model. For hospitalization, significant interactions with physical frailty were observed for the transitions from no disability to mild disability and from no disability to severe disability. For restricted activity, a significant interaction with physical frailty was observed only for the transition from no disability to mild disability. Physical frailty and the time-dependent covariates were updated every 18 months during the comprehensive assessments. P values were adjusted for multiple comparisons assuming a false discovery rate of 5%.
Hazard ratios refer to the risk of making the specific transitions between month t and month t+1 based on exposure to hospitalization or restricted activity, respectively, during this 1-month interval.
Values for the transitions to death were not calculated because restricted activity could not be ascertained in the last month of life.
Table 3 provides the absolute risk per month of having a functional transition, based on exposure to intervening events and stratified by physical frailty. Regardless of intervening event, the absolute risk of transitions to new or worsening disability or death was consistently greater in participants with frailty, while transitions representing functional recovery were consistently greater in those who were not frail. As shown in eTable 1, similar results were observed for those 85 years and older vs those younger than 85, although the differences in absolute risk were generally smaller than the differences in absolute risk observed for frailty. The absolute risk of death was consistently higher in men than women, while the functional outcomes related to both hospitalization and restricted activity were worse in women than men, with few exceptions (e.g. severe disability to mild disability). These differences were generally small. Regardless of sex or age, the absolute risks for new or worsening disability or death were greatest for those who were frail and hospitalized, with probabilities as high as 20.2% for the transition from mild disability to severe disability among women aged 85 years or older. In contrast, for those without an intervening event or physical frailty, the absolute risk of transitioning to new or worsening disability or dying was very low, while the absolute risk of functional recovery ranged — widely—from 17.6% for the transition from severe disability to no disability among women older than 85 years to 44.1% for the transition from mild disability to no disability among men younger than 85 years.
Table 3.
Absolute Risk (%) of Functional Transitions Per Month, Based on Intervening Events and Stratified by Physical Frailtya
| Hospitalizationb | Restricted Activityb,c | No Intervening Event | ||||
|---|---|---|---|---|---|---|
| Transition | Physical Frailty Present | Physical Frailty Absent | Physical Frailty Present | Physical Frailty Absent | Physical Frailty Present | Physical Frailty Absent |
| Percent (95% Confidence Interval) | ||||||
| No disability to | ||||||
| Mild disability | 12.4 (12.1–12.7) | 4.9 (4.7–5.1) | 6.7 (6.5–6.9) | 1.5 (1.4–1.6) | 2.2 (2.1–2.3) | 0.61 (0.54–0.68) |
| Severe disability | 12.0 (11.7–12.3) | 3.3 (3.1–3.4) | 0.88 (0.80–0.96) | 0.17 (0.13–0.20) | 0.07 (0.04–0.09) | 0.02 (0.00–0.03) |
| Death | 5.1 (4.9 – 5.3) | 3.9 (3.7–4.0) | --- | --- | 0.06 (0.04–0.09) | 0.04 (0.03–0.06) |
| Mild disability to | ||||||
| No disability | 14.6 (13.8–15.3) | 27.6 (26.6–28.6) | 29.6 (28.6–30.6) | 43.8 (42.7–44.9) | 30.0 (29.0–31.0) | 44.1 (43.0–45.2) |
| Severe disability | 18.1 (17.2–18.9) | 11.3 (10.6–11.9) | 4.6 (4.2–5.1) | 2.6 (2.2–2.9) | 3.3 (2.9–3.7) | 1.8 (1.5–2.1) |
| Death | 6.1 (5.6–6.7) | 4.5 (4.1–5.0) | --- | --- | 0.12 (0.04–0.19) | 0.08 (0.02–0.15) |
| Severe disability to | ||||||
| No disability | 8.4 (7.8–9.0) | 24.2 (23.3–25.1) | 7.4 (6.8–7.9) | 22.6 (21.7–23.5) | 9.7 (9.0–10.3) | 27.1 (26.1–28.1) |
| Mild disability | 14.5 (13.7–15.2) | 17.5 (16.6–18.3) | 19.8 (18.9–20.6) | 23.9 (23.0–24.9) | 21.2 (20.3–22.1) | 26.1 (25.2–27.1) |
| Death | 14.0 (13.2–14.7) | 12.8 (12.0–13.5) | --- | --- | 0.61 (0.44–0.77) | 0.55 (0.39–0.72) |
As described in the Methods, the absolute risks were calculated using coefficients obtained from a set of pool logistic regression models (one for each transition) that included the three independent variables, eight covariates, and the transition-specific interaction terms that were statistically significant in the competing risk Cox model. For hospitalization, significant interactions with physical frailty were observed for the transitions from no disability to mild disability and from no disability to severe disability. For restricted activity, a significant interaction with physical frailty was observed only for the transition from no disability to mild disability. Physical frailty and the time-dependent covariates were updated every 18 months during the comprehensive assessments.
Values refer to the absolute risk of making the specific transitions between month t and month t+1 based on exposure to hospitalization or restricted activity, respectively, during this 1-month interval.
Values for the transitions to death were not calculated because restricted activity could not be ascertained in the last month of life.
The primary reasons that individuals were hospitalized are provided in Table 4. While cardiac causes (coronary heart disease, congestive heart failure, arrhythmia, etc.) and infection were the most common diagnostic categories (as seen in the total columns), fall-related injury conferred the highest likelihood of developing new or worsening disability. For example, of the 97 hospital admissions for a fall-related injury among nondisabled participants, 32 (33.0%) and 43 (44.3%) resulted in transitions to mild and severe disability, respectively. Table 5 provides comparable information for episodes of restricted activity. For each set of transitions, fatigue was the most common reason for restricted activity. Of all the reasons, however, a fall or injury conferred the highest likelihood of transitioning from no disability to mild and severe disability, respectively, and the second highest likelihood (after problem with memory or difficulty thinking) of transitioning from mild disability to severe disability. For example, of the 665 episodes of restricted activity attributed to a fall or injury among nondisabled participants, 100 (15.0%) and 25 (3.8%) resulted in transitions to mild and severe disability, respectively.
Table 4.
Reasons for Hospitalization According to Functional Transition
| No. (%) of Transitions from No Disabilityb |
No. (%) of Transitions from Mild Disabilityb |
No. (%) of Transitions from Severe Disabilityb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reason for Hospitalizationa | Total (n = 1528) | to Mild Disability | to Severe Disability | to Death | Total (n = 562) | to No Disability | to Severe Disability | to Death | Total (n = 473) | to No Disability | to Mild Disability | to Death |
| Cardiac | 355 (23.2) | 62 (17.5) | 25 (7.0) | 11 (3.1) | 99 (17.6) | 11 (11.1) | 32 (32.3) | 6 (6.1) | 70 (14.8) | 5 (7.1) | 10 (14.3) | 6 (8.6) |
| Infection | 198 (13.0) | 45 (22.7) | 36 (18.2) | 10 (5.1) | 108 (19.2) | 6 (5.6) | 37 (34.3) | 9 (8.3) | 131 (27.7) | 6 (4.6) | 15 (11.5) | 24 (18.3) |
| Fall-related injury | 97 (6.4) | 32 (33.0) | 43 (44.3) | 2 (2.1) | 54 (9.6) | 6 (11.1) | 31 (57.4) | 1 (1.9) | 24 (5.1) | 0 (0) | 4 (16.7) | 5 (20.8) |
| Stroke | 80 (5.2) | 15 (18.8) | 16 (20.0) | 4 (5.0) | 11 (2.0) | 0 (0) | 5 (45.5) | 3 (27.3) | 22 (4.7) | 1 (4.5) | 4 (18.2) | 2 (9.1) |
| Arthritis | 69 (4.5) | 22 (31.9) | 21 (30.4) | 0 (0) | 24 (4.3) | 3 (12.5) | 13 (54.2) | 0 (0) | 16 (3.4) | 4 (25.0) | 2 (12.5) | 1 (6.3) |
| Cancer | 66 (4.3) | 18 (27.3) | 12 (18.2) | 10 (15.2) | 18 (3.2) | 3 (16.7) | 5 (27.8) | 4 (22.2) | 10 (2.1) | 2 (20.0) | 1 (10.0) | 2 (20.0) |
| Gastrointestinal tract bleeding | 53 (3.5) | 8 (15.1) | 11 (20.8) | 2 (3.8) | 15 (2.7) | 2 (13.3) | 4 (26.7) | 0 (0) | 10 (2.1) | 0 (0) | 2 (20.0) | 2 (20.0) |
| Other | ||||||||||||
| Medical | 396 (25.9) | 71 (17.9) | 53 (13.4) | 10 (2.5) | 164 (29.2) | 20 (12.2) | 56 (34.1) | 11 (6.7) | 138 (29.2) | 8 (5.8) | 23 (16.7) | 23 (16.7) |
| Surgical | 184 (12.0) | 35 (19.0) | 22 (12.0) | 2 (1.1) | 40 (7.1) | 6 (15.0) | 15 (37.5) | 3 (7.5) | 34 (7.2) | 1 (2.9) | 8 (23.5) | 6 (17.7) |
| Otherc | 30 (2.0) | 7 (23.3) | 4 (13.3) | 0 (0) | 29 (5.2) | 6 (20.7) | 9 (31.0) | 1 (3.4) | 18 (3.8) | 2 (11.1) | 6 (33.3) | 1 (5.6) |
Grouped into distinct diagnostic categories, as described in the Methods; the first seven categories are presented in order of frequency of occurrence, from highest to lowest, for total transitions from no disability.
For the total columns, the numerator includes the number of admissions during the follow-up period for the specific reason, while the denominator includes the number of admissions for all reasons combined. The percentages may not add up to 100 because of rounding. For the other columns, the numerator includes the number of admissions leading to the relevant disability outcome for the specific reason, while the denominator includes the number of admissions for the specific reason during the relevant follow-up period.
Includes psychiatric admissions and responses that could not otherwise be categorized.
Table 5.
Reasons for Restricted Activity According to Functional Transition
| No. (%) of Transitions from No Disabilityb |
No. (%) of Transitions from Mild Disabilityb |
No. (%) of Transitions from Severe Disabilityb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reason for Restricted Activitya | Total (n = 6906) | to Mild Disability | to Severe Disability | to Death | Total (n = 1430) | to No Disability | to Severe Disability | to Death | Total (n = 834) | to No Disability | to Mild Disability | to Death |
| Fatigued (no energy/tired) | 4326 (62.6) | 323 (7.5) | 45 (1.0) | 8 (0.2) | 983 (68.7) | 206 (20.9) | 113 (11.5) | 11 (1.1) | 558 (66.9) | 11 (2.0) | 93 (16.7) | 31 (5.6) |
| Pain or stiffness in joints | 2695 (39.0) | 229 (8.5) | 38 (1.4) | 1 (0.0) | 615 (43.0) | 153 (24.9) | 59 (9.6) | 7 (1.1) | 323 (38.7) | 12 (3.7) | 73 (22.6) | 10 (3.1) |
| Pain or stiffness in back | 2212 (32.0) | 170 (7.7) | 30 (1.4) | 2 (0.1) | 528 (36.9) | 140 (26.5) | 50 (9.5) | 4 (0.8) | 229 (27.5) | 11 (4.8) | 47 (20.5) | 6 (2.6) |
| Dizziness or unsteadiness on feet | 1955 (28.3) | 190 (9.7) | 36 (1.8) | 5 (0.3) | 492 (34.4) | 98 (19.9) | 80 (16.3) | 5 (1.0) | 259 (31.1) | 8 (3.1) | 53 (20.5) | 8 (3.1) |
| Cold or flu symptoms | 1693 (24.5) | 77 (4.6) | 8 (0.5) | 1 (0.1) | 274 (19.2) | 46 (16.8) | 19 (6.9) | 1 (0.4) | 163 (19.5) | 5 (3.1) | 33 (20.3) | 3 (1.8) |
| Leg pain during walking | 1475 (21.4) | 109 (7.4) | 22 (1.5) | 2 (0.1) | 396 (27.7) | 85 (21.5) | 53 (13.4) | 5 (1.3) | 154 (18.5) | 6 (3.9) | 38 (24.7) | 5 (3.3) |
| Fear of falling | 1475 (21.4) | 163 (11.1) | 26 (1.8) | 3 (0.2) | 435 (30.4) | 94 (21.6) | 64 (14.7) | 6 (1.4) | 203 (24.3) | 5 (2.5) | 55 (27.1) | 6 (3.0) |
| Nausea, vomiting, diarrhea, or other stomach (abdominal) problem | 1347 (19.5) | 87 (6.5) | 14 (1.0) | 1 (0.1) | 302 (21.1) | 53 (17.6) | 32 (10.6) | 3 (1.0) | 178 (21.3) | 6 (3.4) | 30 (16.9) | 8 (4.5) |
| Difficulty breathing or shortness of breath | 1334 (19.3) | 110 (8.3) | 16 (1.2) | 2 (0.2) | 331 (23.1) | 67 (20.2) | 46 (13.9) | 7 (2.1) | 232 (27.8) | 6 (2.6) | 33 (14.2) | 16 (6.9) |
| Difficulty sleeping | 1317 (19.1) | 112 (8.5) | 16 (1.2) | 0 (0.0) | 306 (21.4) | 72 (23.5) | 29 (9.5) | 6 (2.0) | 106 (12.7) | 3 (2.8) | 27 (25.5) | 3 (2.8) |
| Depressed | 1059 (15.3) | 124 (11.7) | 20 (1.9) | 2 (0.2) | 343 (24.0) | 80 (23.3) | 57 (16.6) | 7 (2.0) | 218 (26.1) | 4 (1.8) | 29 (13.3) | 8 (3.7) |
| Weakness of arms or legs | 883 (12.8) | 94 (10.6) | 24 (2.7) | 1 (0.1) | 299 (20.9) | 50 (16.7) | 57 (19.1) | 8 (2.7) | 362 (43.4) | 2 (0.6) | 37 (10.2) | 22 (6.1) |
| Anxious or worried | 877 (12.7) | 86 (9.8) | 18 (2.1) | 3 (0.3) | 269 (18.8) | 61 (22.7) | 47 (17.5) | 7 (2.6) | 172 (20.6) | 5 (2.9) | 24 (14.0) | 8 (4.7) |
| Swelling in feet or ankles | 860 (12.5) | 88 (10.2) | 19 (2.2) | 1 (0.1) | 255 (17.8) | 54 (21.2) | 30 (11.8) | 4 (1.6) | 136 (16.3) | 3 (2.2) | 27 (19.9) | 4 (2.9) |
| Fall or injury | 665 (9.6) | 100 (15.0) | 25 (3.8) | 3 (0.5) | 190 (13.3) | 27 (14.2) | 41 (21.6) | 3 (1.6) | 82 (9.8) | 1 (1.2) | 21 (25.6) | 2 (2.4) |
| Poor or decreased vision | 634 (9.2) | 56 (8.8) | 9 (1.4) | 3 (0.5) | 158 (11.0) | 41 (25.9) | 13 (8.2) | 2 (1.3) | 88 (10.6) | 2 (2.3) | 21 (23.9) | 1 (1.1) |
| Change in medications | 621 (9.0) | 53 (8.5) | 12 (1.9) | 2 (0.3) | 165 (11.5) | 25 (15.2) | 21 (12.7) | 6 (3.6) | 101 (12.1) | 4 (4.0) | 18 (17.8) | 7 (6.9) |
| Chest pain or tightness | 552 (8.0) | 48 (8.7) | 9 (1.6) | 2 (0.4) | 121 (8.5) | 28 (23.1) | 14 (11.6) | 4 (3.3) | 68 (8.2) | 5 (7.4) | 19 (27.9) | 4 (5.9) |
| Lost control of urine and wet self | 430 (6.2) | 47 (10.9) | 7 (1.6) | 1 (0.2) | 199 (13.9) | 36 (18.1) | 24 (12.1) | 2 (1.0) | 184 (22.1) | 3 (1.6) | 17 (9.2) | 15 (8.2) |
| Frequent or painful urination | 421 (6.1) | 52 (12.4) | 8 (1.9) | 1 (0.2) | 119 (8.3) | 23 (19.3) | 16 (13.4) | 1 (0.8) | 92 (11.0) | 2 (2.2) | 8 (8.7) | 4 (4.4) |
| Family member or friend became seriously ill or was injured | 283 (4.1) | 16 (5.7) | 3 (1.1) | 1 (0.4) | 48 (3.2) | 10 (21.7) | 3 (6.5) | 0 (0.0) | 26 (3.1) | 1 (3.9) | 4 (15.4) | 1 (3.9) |
| Problem with memory or difficulty thinking | 281 (4.1) | 42 (14.9) | 9 (3.2) | 3 (1.1) | 173 (12.1) | 31 (17.9) | 41 (23.7) | 3 (1.7) | 223 (26.7) | 2 (0.9) | 28 (12.6) | 13 (5.8) |
| Experienced death or loss of a family member or friend | 219 (3.2) | 18 (8.2) | 7 (3.2) | 2 (0.9) | 54 (3.8) | 16 (29.6) | 8 (14.8) | 0 (0.0) | 23 (2.8) | 1 (4.4) | 6 (26.1) | 1 (4.4) |
| Problem with alcohol | 4 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other reason | 1745 (25.3) | 124 (7.1) | 16 (0.9) | 3 (0.2) | 341 (23.8) | 97 (28.4) | 27 (7.9) | 5 (1.5) | 245 (29.4) | 4 (1.6) | 38 (15.5) | 15 (6.1) |
Presented in order of frequency of occurrence, from highest to lowest, for total transitions from no disability, with the exception of other reason.
For the total columns, the numerator includes the number of restricted activity episodes during the follow-up period for the specific reason, while the denominator includes the number of restricted activity episodes for all reasons combined. Because participants could provide more than one reason for their restricted activity, the percentages do not add up to 100. For the other columns, the numerator includes the number of restricted activity episodes leading to the relevant transition for the specific reason, while the denominator includes the number of restricted activity episodes for the specific reason during the follow-up period.
COMMENT
In this prospective cohort study of community-living older persons, we found that illnesses and injuries leading to hospitalization were associated with worsening functional ability for nearly all transitions between states of no disability, mild disability, severe disability and death over the course of more than ten years. Furthermore, illnesses and injuries leading to restricted activity but not hospitalization increased the likelihood of transitioning from no disability to both mild and severe disability and from mild disability to severe disability, but were not associated with recovery from mild or severe disability. Finally, these associations of hospitalization and restricted activity with functional transitions were accentuated by the presence of physical frailty. These results provide strong evidence that intervening events play an important role in precipitating and, subsequently, perpetuating the disabling process.
Unlike conditions that invariably progress, such as Alzheimer Disease, disability is a recurrent disorder, characterized by high rates of recovery (7). The dynamic nature of disabilty has only recently been elucidated (8), and relatively little is known about the factors that are associated with clinically relevant transitions in functional status. We have previously shown that illnesses and injuries leading to either hospitalization or restricted activity are strongly associated with the initial onset of disability (6). The current study extends this earlier work by demonstrating that exposure to these intervening illnesses and injuries is also associated with the subsequent course of disability.
Hospitalization was associated with a particularly pronounced risk, with relative risks for developing new and worsening disability much greater than those for physical frailty, which is the single strongest risk factor for disability and functional decline (9,10). For the transition from no disability to severe disability, the high hazard ratio associated with hospitalization is likely attributable not only to the potent disabling effects of serious illness, and hospitalization itself (26), but also to the low incidence of severe disability in the comparison group of nondisabled participants without an acute hospital admission or restricted activity. Our results support the hypothesis that illnesses and injuries leading to hospitalization act not only to precipitate and worsen disability, but also to hasten death and to impede recovery from disability, thereby prolonging the disabling process. Hospitalization was not associated with the transition from severe disability to no disability; this was likely due to the short duration of severe disability among participants who regained independence (median [interquartile range], 1 [1-1] month) and, for participants with severe disability, the strong association of hospitalization with the 2 competing outcomes of mild disability and death.
Illnesses and injuries leading to restricted activity were also associated with developing new and worsening disability, although these associations were not as strong as those for acute hospital admissions. Because restricted activity was much more common than hospitalization, the overall magnitude of its effects could be heightened. We were unable to calculate population attributable fractions, but in our earlier report (6), which focused only on the initial onset of disability, the population attributable fractions were considerably greater for hospitalization than restricted activity for three distinct disability outcomes. In the current study, we found that episodes of restricted activity were not associated with transitions from mild disability to no disability or from severe disability to mild disability, suggesting that intervening events less potent than those leading to hospitalization do not impede recovery from disability.
Physical frailty was independently associated with each of the six transitions between no disability, mild disability and severe disability and accentuated the associations of the intervening events in absolute terms for all nine of the possible transitions. Because physical frailty was assessed every 18 months but could have changed over shorter periods of time, it is possible that its effects were underestimated. Nonetheless, in the setting of an intervening event, the change in absolute risks associated with physical frailty on transitions to new or worsening disability was greater than that of sex and age.
We determined absolute risks for subgroups defined on the basis of physical frailty, sex, and age, which greatly enhances the clinical relevance of our findings. For example, in the setting of an acute illness or injury leading to hospitalization, the absolute risk of transitioning from no disability to severe disability within one month ranged from only 3.3% in men younger than 85 years without physical frailty to 16.6% in physically frail women aged 85 years or older. Although the multivariate nature (i.e. nine different outcomes) of our analytic strategy did not permit us to evaluate the risks associated with specific reasons for hospitalization or restricted activity, we found that falls and fall-related injuries almost invariably conferred the highest likelihood for developing new or worsening disability.
The results of the current study, coupled with those of our earlier report (6), provide strong evidence that disability among older persons is driven largely by illnesses and injuries leading to hospitalization or restricted activity. Both types of intervening events greatly increased the likelihood of developing new or worsening disability, while only the most potent events, i.e. those leading to hospitalization, reduced the likelihood of recovery from disability. Given the central role of intervening illnesses and injuries on the disabling process, more aggressive efforts are warranted to prevent their occurrence (27–30), to manage them more effectively and reduce subsequent complications, especially in the hospital setting (31–34), and, post event, to enhance restorative interventions in the subacute, home care, and outpatient settings (35–37).
Although causality cannot be established by an observational study, the frequency of our assessments increases the likelihood that the intervening events at least preceded the functional transitions. However our data do not allow us to determine how often the intervening events resulted immediately in new or worsening disability, as may occur with a sudden acute process such as a stroke or hip fracture. The validity of our results is strengthened by the nearly complete ascertainment of intervening events and disability, by the high reliability and accuracy of these assessments, by the low rate of attrition, and by adjustment for several relevant covariates at 18-month intervals with few missing data.
Our study has at least two additional limitations. First, information was not available on the duration of the intervening events. It is possible that the likelihood of recovery may be reduced by long episodes of restricted activity, but not by short episodes. Second, because our study participants were members of a single health plan in a small urban area and were oversampled for slow gait speed, our results may not be generalizable to older persons in other settings. However, the demographic characteristics of our cohort did reflect those of older persons in New Haven County, Connecticut, which are similar to the characteristics of the U.S. population as a whole, with the exception of race or ethnic group (38). The generalizability of our results is enhanced by our high participation rate, which was greater than 75%.
Despite the reductions observed in the prevalence of disability over the past two decades (39), the absolute number of disabled older Americans could increase substantially in the coming years with the aging of the baby boom generation (40). To obviate this increase, more aggressive efforts will be needed to prevent and manage intervening illnesses and injuries, given their apparent role in precipitating and perpetuating the disabling process.
Supplementary Material
Acknowledgments
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Paula Clark, RN, Martha Oravetz, RN, Shirley Hannan, RN, Barbara Foster, Alice Van Wie, BSW, Patricia Fugal, BS, Amy Shelton, MPH, and Alice Kossack for assistance with data collection; Wanda Carr and Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH for design and development of the study database and participant tracking system; Linda Leo-Summers, MPH for assistance with Figures 1 and 2; Haiqun Lin MD, PhD for advice on the statistical methods; and Joanne McGloin, MDiv, MBA for leadership and advice as the Project Director.
Funding/Support: The work for this report was funded by grants from the National Institute on Aging (R37AG17560, R01AG022993). The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
Role of the Sponsors: The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Gill had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The specific contributions are enumerated in the authorship, financial disclosure, and copyright transfer form.
References
- 1.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 2.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 3.Manton KG, Stallard E, Liu K. Forecasts of active life expectancy: policy and fiscal implications. J Gerontol. 1993;48(Spec No):11–26. doi: 10.1093/geronj/48.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 4.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150:489–495. [PMC free article] [PubMed] [Google Scholar]
- 6.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 7.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 8.Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161:575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 9.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 11.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Gill TM, Hardy SE, Williams CS. Underestimation of disability among community-living older persons. J Am Geriatr Soc. 2002;50:1492–1497. doi: 10.1046/j.1532-5415.2002.50403.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin JS. Ambling towards Nirvana. Lancet. 2002;359:1358. doi: 10.1016/S0140-6736(02)08318-6. [DOI] [PubMed] [Google Scholar]
- 14.Gill TM, McGloin JM, Gahbauer EA, Shepard DM, Bianco LM. Two recruitment strategies for a clinical trial of physically frail community-living older persons. J Am Geriatr Soc. 2001;49:1039–1045. doi: 10.1046/j.1532-5415.2001.49206.x. [DOI] [PubMed] [Google Scholar]
- 15.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 18.Barry LC, Allore HG, Guo Z, Bruce ML, Gill TM. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psychiatry. 2008;65:172–178. doi: 10.1001/archgenpsychiatry.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277:728–734. [PubMed] [Google Scholar]
- 20.Gill TM, Guo Z, Allore HG. Subtypes of disability in older persons over the course of nearly 8 years. J Am Geriatr Soc. 2008;56:436–443. doi: 10.1111/j.1532-5415.2007.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Guo Z, Peduzzi PN, Gill TM, Allore HG. A semiparametric transition model with latent traits for longitudinal multistate data. Biometrics. 2008;64:1032–1042. doi: 10.1111/j.1541-0420.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Am Stat Soc. 1989;84:1074–1078. [Google Scholar]
- 23.Allison PD, editor. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute Inc; 1995. [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Contolling the false discovery rate - A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 25.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990;46:813–826. [PubMed] [Google Scholar]
- 26.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Tinetti ME, Baker DI, King M, et al. Effect of dissemination of evidence in reducing injuries from falls. N Engl J Med. 2008;359:252–261. doi: 10.1056/NEJMoa0801748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straus SE, Majumdar SR, McAlister FA. New evidence for stroke prevention: clinical applications. JAMA. 2002;288:1396–1398. doi: 10.1001/jama.288.11.1396. [DOI] [PubMed] [Google Scholar]
- 29.Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371:725–735. doi: 10.1016/S0140-6736(08)60342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2010. Ann Intern Med. 2010;152:36–39. doi: 10.7326/0003-4819-152-1-201001050-00008. [DOI] [PubMed] [Google Scholar]
- 31.Rich MW. Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol Med Sci. 2001;56A:M88–M96. doi: 10.1093/gerona/56.2.m88. [DOI] [PubMed] [Google Scholar]
- 32.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 33.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 34.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 35.Hoenig H, Nusbaum N, Brummel-Smith K. Geriatric rehabilitation: state of the art. J Am Geriatr Soc. 1997;45:1371–1381. doi: 10.1111/j.1532-5415.1997.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 36.Tinetti ME, Baker D, Gallo WT, Nanda A, Charpentier P, O’Leary J. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA. 2002;287:2098–2105. doi: 10.1001/jama.287.16.2098. [DOI] [PubMed] [Google Scholar]
- 37.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292:837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 38.American FactFinder. U.S. Census Bureau; [Accessed May 29, 2003]. Available at: http://factfinder.census.gov. [Google Scholar]
- 39.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci USA. 2006;103:18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.